Abstract
Context
Untreated sleep apnea is a prevalent but treatable condition of breathing pauses during sleep. With approximately 15% of the US population affected, understanding of the total health burden is necessary to guide policy, population initiatives, and clinical practice to reduce the prevalence of this condition.
Objective
To outline the history and need for a population approach to understanding sleep apnea and provide a review of the first longitudinal population study of this disorder.
Data Source
The results of cross-sectional and longitudinal data from 1500 participants in the Wisconsin Sleep Cohort, initiated 2 decades ago, illustrate the population burden of sleep apnea.
Results
The prevalence of sleep apnea is increasing with trends of increased obesity. Prospective findings from 4- to 15-year follow-up data indicate untreated sleep apnea predicts increased blood pressure, hypertension, stroke, depression, and mortality.
Conclusions
The high prevalence of untreated sleep apnea and links to serious morbidity and mortality underscore the population burden of this condition and the need for greater clinical recognition and strategies to reduce prevalence.
Introduction
Breathing abnormalities induced by sleep—ie, sleep disordered breathing (SDB)—is now recognized as a prevalent condition with serious adverse consequences.1-2 The most common form of SDB is obstructive sleep apnea (OSA), characterized by frequent breathing pauses due to sleep-related changes in muscle tone; the increased airway resistance leads to partial (hypopnea) or complete (apnea) upper airway collapse. The acute effects of these events include repeated drops in oxyhemoglobin saturation, cardiovascular perturbations, and cortical arousals that fragment sleep. Over time, nightly exposure to SDB, with up to 90 pauses per hour of sleep, is thought to contribute to impaired daytime function including excessive daytime sleepiness, behavioral and cardiovascular morbidity, and mortality.
OSA was documented centuries ago by scholars using colorful case descriptions of patients with loud snoring punctuated by suffocating gasps, usually combined with the common symptom of daytime sleepiness.3-4 In 1966, European researchers defined the clinical entity of sleep apnea syndrome as the combination of episodes of obstructive apnea and extreme daytime sleepiness.5 However, diagnosis of SDB required overnight monitoring of several sleep and breathing signals (polysomnography [PSG]), and the only effective treatment was a tracheotomy to provide a patent airway. With such invasive treatment, only the most life-threatening cases of sleep apnea were likely to receive medical attention.
In 1981, treatment with continuous positive air pressure (CPAP), delivered by a facial mask, became available.6 CPAP effectively kept the upper airway patent and prevented apneas. With an acceptable, effective treatment, the clinical significance of SDB greatly increased.7 The US Congress mandated a task force8 to determine the overall public burden of sleep disorders (including SDB). In 1986, NIH sought applications for Specialized Centers of Research (SCOR) in Cardiopulmonary Disorders of Sleep comprising clinical, experimental, and epidemiological research projects. As the epidemiological project of the SCOR application from the University of Wisconsin, we proposed the Wisconsin Sleep Cohort Study (WSCS), a longitudinal epidemiology study designed to investigate the natural history of SDB by conducting overnight PSG, repeated at 4-year intervals, on a random sample of the general population.
In the past, SDB was not recognized as a medical problem. With symptoms of snoring and daytime sleepiness, this condition was seen as comical, or as a nuisance, at best. SDB was most likely to be diagnosed coincidentally, in patients with serious comorbid conditions. The stereotypical patient was overweight, sleepy, middle-aged, snoring, and male. These and other selection biases served to build in spurious associations of SDB with characteristics and comorbidity in clinic samples of SDB patients. Thus, epidemiology studies of SDB using population samples, free of clinic selection biases, were critical to determine the occurrence and health burden of SDB. A major limitation for population studies was the resource-intense in-laboratory PSG needed to determine SDB status according to clinical standards. However, with strong NIH support, the WSCS became the first large longitudinal population study of SDB based on repeated in-laboratory PSG. Now in its 20th year, the WSCS remains unique. With the advent of portable monitors, varying in sophistication, other population studies of SDB were initiated subsequently worldwide. Several studies, such as the Sleep Heart Health Study,9 added in-home PSG to protocols of established cohorts with a large sample size. Consequently, the previously sparse literature on the epidemiology of SDB has greatly increased over the past decade.10-11
Design of the Wisconsin Sleep Cohort Study
Because SDB was considered rare, we chose a 2-stage probability sampling scheme to yield a final cohort sample (n=1500) enriched for sufficient variability in SDB. Payroll files of Wisconsin state employees served as the sampling frame. Among the advantages, the sampling frame comprised a complete range of job titles from unskilled to professional and included sociodemographic data for targeted recruitment and eventual comparison of responders and non-responders. Like other employed groups, the sample would be traced more easily. All employees had equal access to health care, an advantage in reducing potential bias in health outcomes.
In Stage 1, a mailed survey on sociodemographic factors, lifestyle, health history, and sleep characteristics was sent to a random sample of 6000 state employees aged 30-60 years (84% response). Survey respondents were then ranked on risk of SDB based on self-report of snoring. All high-risk respondents were selected for recruitment, along with an age- and sex-frequency matched random sample of low-risk respondents.
The sample design and baseline protocol is shown in Figure 1. Participation for the baseline protocol was 51% and has been more than 80% for repeated studies. Detailed survey data on the entire cohort sampling frame has been vital in assessing participation and retention bias. Although the focus of the WSCS was sleep apnea, data have been collected on other sleep disorders including restless legs, insomnia, and narcolepsy, as well as sleep patterns. Several ancillary studies, some of which are presented in this volume of the Wisconsin Medical Journal, have been conducted, and collaborations with intra- and extramural researchers are ongoing. Over the next 5 years, a data-sharing plan will be developed to enable other partnerships.
Figure 1.
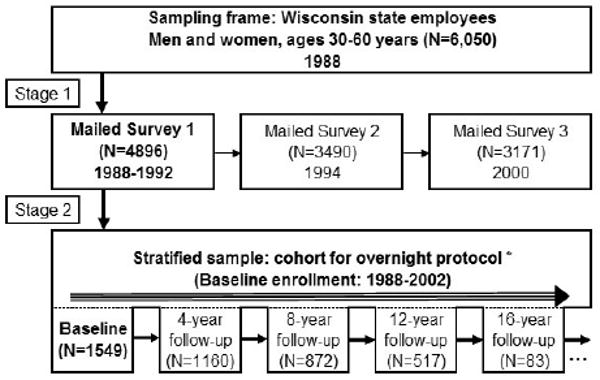
The basic design and study protocol of the Wisconsin Sleep Cohort, which included the following: 18 channel poly-somnography; health history, medication, lifestyle, demographics interview; depression, anxiety scales; Epworth Sleepiness Scale; sleep problems (insomnia, RLS, sleepiness, etc) inventory; blood pressure: standard seated, arm: ankle index, 24-hour ambulatory monitoring; body habitus measures: weight, height, circumferences, skin fold; neuropsychological test battery; fasting blood sample; cardiovascular function, eg, carotid IMT; objective sleepiness (MSLT); Performance, reaction time (PVT); Horne-Ostberg, sleep diaries, DMV accident reports, death records, quality of life (SF36); genetic analyses, 24-hour ambulatory blood pressure. Abbreviations: RLS, Restless Leg Syndrome; IMT, Intima Media Thickness; MSLT, Multiple Sleep Latency Test; PVT, Psychomotor Vigilance Task; DMV, Department of Motor Vehicles.
Summary of Findings
Prevalence of SDB
After accounting for the weighted sampling procedure used to increase SDB variability, we estimated the occurrence of SDB, based on the commonly used metric of the number of apnea and hypopnea events per hour of sleep (apnea-hypopnea index [AHI]). The severity spectrum was wide, with AHI ranging from 0 to 92. Using commonly used cutpoints (AHI at 5 and 15) to indicate mild, moderate, and severe SDB, we estimated the age- and sex-specific prevalence as weighted averages from the high- and low-risk strata.
In contrast to the belief that SDB was a rare disorder of men, the estimated prevalence of this condition was surprisingly high for both men and women.12 Prevalence (95% confidence interval) of AHI >5 was 9% (5.6, 12) for women and 24% (19, 28) for men; prevalence of AHI>15 was 4% (1.5, 6.6) for women and 9% (6.4, 11) for men.
Since our report, several other studies, using comparable methods, have reported similar SDB prevalence.13-15 The high prevalence of screen-detected SDB in the general population, compared with the small number of patients diagnosed with SDB, indicated that a large proportion of people with SDB who would meet clinical criteria for treatment were not being diagnosed.16 Furthermore, the findings revealed a gender bias in SDB diagnosis. The ratio of men to women diagnosed with SDB was widely reported as approximately 9:1, but in our cohort, the ratio was 2-3 to 1. This difference, since found in other epidemiology studies, indicated that there had been a strong bias against women for diagnosis of SDB.10-11,14,17
The Cost of SDB: Adverse Health Outcomes
With cross-sectional data from the WSCS and other studies, SDB has been linked with significant health outcomes.2,10-11 As our sets of longitudinal data have increased, we have been able to explore the role of SDB in the development of cardiovascular and behavioral morbidity and mortality (Table 1).
Table 1.
Longitudinal Associations of Baseline Sleep-Disordered Breathing with Development of Adverse Health Outcomes
| Outcome | Follow-up time (mean) | Adjustment Variables | Odds Ratio (95% Confidence Interval) for Outcome and SDB severity levela | |
|---|---|---|---|---|
| Moderate vs None | Severe vs None | |||
| Incident Hypertension18 >140/90 mm Hg or use of antihypertensives | 4 years | Age, sex, BMI, waist, hip girth, health hx, BP, smoking, alcohol | 2.0 (1.2,3.2) | 2.9 (1.5,5.6) |
| Incident “non-dipping”19 loss of >10% drop in systolic BP from wake to sleep | 4 years | Age, sex, BMI, BP, smoking, alcohol, sleep duration, antihypertensive meds | 3.1 (1.3,7.7) | 4.4 (1.2,16) |
| Incident Depression21 Zung score>50 | 4 years | Age, sex, BMI, alcohol, education | 2.0 (1.4, 2.9) | 2.6 (1.7, 3.9) |
| Incident stroke 22 | 4 years | Age, sex | — | 4.5(1.3,,15) |
| All-cause mortality23 | 14 years | Age, sex, BMI | — | 3.0 (1.4,6.3) |
| All cause mortality,23 CPAP users excluded | 14 years | Age, sex, BMI | — | 3.8 (1.6,9.0) |
| Cardiovascular mortality,23 CPAP users excluded | 14 years | Age, sex, BMI | — | 5.2 (1.4, 19) |
No SDB was defined as AHI <5; moderate SDB was defined as AHI 5-15; and severe SDB was defined as AHI >30 for mortality outcomes, AHI >20 for stroke, and AHI >15 for all other outcomes. Odds ratios were estimated with AHI <5 as the reference category.
Abbreviations: SDB, sleep disordered breathing; AHI, apnea-hypopnea index.
Regardless of how blood pressure (BP) was measured, we have found significant associations between SDB and hypertension or elevated BP. With a prospective design, we determined the 4- to 8-year incidence of new hypertension defined as measured BP of >140/90 mm Hg or treatment with antihypertensive medication.18 After controlling for age, sex, BMI, initial BP, and other confounding factors, we found a dose-response increased risk of developing hypertension with SDB. The 4-year incidence of hypertension was 2.9 greater for participants with AHI>15 versus <5 at baseline.
Using 24-hour ambulatory BP monitoring, we found participants with SDB had higher BP levels before, during, and after sleep, compared to those without SDB.19 Longitudinally, we repeated ambulatory blood pressure monitoring at 4-year intervals to determine the incidence of developing an abnormal nighttime BP pattern, described by the lack of 10% or greater dip in BP with sleep. SDB severity at baseline predicted an increase in the incidence of non-dipping: participants with AHI>15 versus <5 at baseline had a 4-fold greater odds of developing non-dipping nocturnal BP.20
Other longitudinal analyses of the WSCS data have linked SDB with the development of depression21 and incident stroke.22 Most recently, we have assessed the 18-year mortality rate by SDB status at baseline. The rate of all-cause mortality was 3-fold higher for participants with severe SDB, with AHI>30, compared to those without SDB.23
Corroboration from other population studies is needed, but our findings suggest that the burden of SDB is large, due to a high prevalence of untreated SDB and potentially high attributable risk for significant adverse health outcomes.
Effect Modifiers
Factors that alter the prevalence and progression of SDB are important in determining the total societal burden. Body weight is an established risk factor for SDB.10-12,15 Longitudinal analyses indicate that weight is a modifiable risk factor of SDB.24-25 In the WSCS, relative to stable weight over a 4-year period, a 10% loss in weight was associated with a decrease in SDB severity of a 23% reduction in AHI; a 10% gain in weight was associated with a 6-fold (2.2, 17) greater risk of developing moderate or worse SDB, and a 32% increase in AHI progression.24 As a modifiable risk factor, weight loss should hold the greatest promise for reducing SDB prevalence. However, as a result of the ongoing strong trends in weight gain in both adults and children in the population,26-27 the opposite result is likely: The population prevalence of SDB is bound to increase.
Conclusion
The prevalence of untreated SDB is large, with at least 12-18 million adults affected. Plus, the prevalence is rising due to an increase in obesity raising public health concerns. Similarly, as the US population ages, prevalence of SDB will increase, due to accumulation of cases and the likelihood that incidence is higher in older age.
Limited longitudinal findings from the WSCS and some clinical studies support a causal role of SDB in increased morbidity and mortality, suggesting that the cost of untreated SDB is significant. SDB is likely to contribute to increased cases of hypertension, cardiovascular disease, stroke, depression, and to mortality.
The total burden of SDB reflected by the large number of persons with this disorder multiplied by the cost of adverse consequences that can be attributed to SDB is likely to be staggering. The burden could be decreased by preventing SDB through risk factor reduction, with weight loss as the most likely starting point. However, national and international trends show that the prevalence of obesity is increasing, so it is likely that the burden of SDB will increase in the near future, as well.
Acknowledgments
Funding/Support: This work was supported by grants R01HL62252, RR03186, R01AG14124, and 1UL1FF025011 from the National Institutes of Health.
Footnotes
Financial Disclosures: None declared.
Contributor Information
Terry Young, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wis.
Mari Palta, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wis.
Jerome Dempsey, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wis.
Paul E. Peppard, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wis.
F. Javier Nieto, Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wis.
K. Mae Hla, Department of Medicine, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wis.
References
- 1.Colten H, Altevogt B, editors. Committee on Sleep Medicine and Research, Board on Health Sciences Policy: Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington DC: Institute of Medicine/National Academies Press; 2006. pp. 20–32. [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease an American Heart Association/American College of Cardiology Foundation scientific statement. Circulation. 2008 [Google Scholar]
- 3.Kryger MH. Sleep apnea. from the needles of Dionysius to continuous positive airway pressure. Arch Intern Med. 1983;143(12):2301–2303. doi: 10.1001/archinte.143.12.2301. [DOI] [PubMed] [Google Scholar]
- 4.Lavie P. Who was the first to use the term Pickwickian in connection with sleepy patients? history of sleep apnoea syndrome. Sleep Med Rev. 2008;12(1):5–17. doi: 10.1016/j.smrv.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1(2):167–186. doi: 10.1016/0006-8993(66)90117-x. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 7.Shepard JW, Jr, Buysse DJ, Chesson AL, Jr, et al. History of the development of sleep medicine in the United States. J Clin Sleep Med. 2005;1(1):61–82. [PMC free article] [PubMed] [Google Scholar]
- 8.National Commission on Sleep Disorders Research. Wake up America: a National Sleep Alert. 1993 [Google Scholar]
- 9.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 10.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 13.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 14.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 15.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ball EM, Simon RD, Jr, Tall AA, Banks MB, Nino-Murcia G, Dement WC. Diagnosis and treatment of sleep apnea within the community. the Walla Walla Project. Arch Intern Med. 1997;157(4):419–424. [PubMed] [Google Scholar]
- 17.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. are women missed because they have different symptoms? Arch Intern Med. 1995;156:2445–2451. [PubMed] [Google Scholar]
- 18.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 19.Hla KM, Young TB, Bidwell T, Palta M, Skatrud J, Dempsey J. Association of sleep apnea and hypertension in a population-based study. Ann Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 22.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: 18-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 24.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 25.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 26.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35(1):93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- 27.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]


