Abstract
Background
The purpose of this study was to re-examine intragastric ethanol intubation as a dependence induction method that effectively induces physical dependence upon ethanol over a short time period, is devoid of intrinsic stress artifacts, inexpensive, and easy to implement.
Methods
Male Wistar rats were subjected to ethanol dependence induction via intragastric ethanol intubation. Ethanol solution (final concentration 20%, made up in a dietary liquid vehicle consisting of powdered milk, sucrose, and water) was intubated four times per day, at 4 h intervals, for 6 consecutive days (for a total of 10 g/kg/day). The utility of this procedure was evaluated for inducing physical dependence, determined by daily and final withdrawal ratings. Anxiety-like behavior associated with ethanol dependence history was examined using the elevated plus-maze (EPM) test, conducted five days after ethanol withdrawal. To evaluate whether potential stress-like effects of intragastric intubation per se produce lasting effects on behavior, experimentally naive rats were compared to vehicle-intubated rats for anxiety-like behavior on the EPM.
Results
Blood alcohol levels reached stable levels of 200–250 mg%, measured 1 h after the second and third ethanol intubation on days 2, 4, and 6. Ethanol-treated rats developed significant somatic withdrawal signs, recorded daily between 10–12 h after the last ethanol administration. At 5 days post-withdrawal, ethanol-treated rats showed significant anxiety-like behavior, measured by decreased open arm time and open arm entries on the EPM, compared with vehicle controls. Additionally, ethanol post-dependent rats showed decreased open arm time compared to experimentally naive rats. EPM performance did not differ between vehicle-intubated and naive rats. No withdrawal seizures were observed and mortality rate was near zero.
Conclusions
These findings suggest that intragastric ethanol administration produces a behavioral profile consistent with ethanol dependence (i.e., significant withdrawal signs after termination of ethanol exposure and elevated anxiety-like behavior persisting beyond completion of physical withdrawal), and that the intubation procedure itself does not produce lasting nonspecific anxiety-like effects. Thus, under the conditions employed here, this procedure provides an effective tool for inducing and evaluating the consequences of ethanol dependence in animal models of ethanol reward and motivation.
Keywords: alcohol dependence, intragastric administration, blood alcohol levels, withdrawal, anxiety
Introduction
A major procedural and conceptual issue in experimental studies of alcohol addiction is the need for reliance on alcohol dependence induction procedures. In particular, rats do not voluntarily consume sufficient amounts of ethanol to produce intoxication, much less dependence - with the exception of several genetically selected rodent lines (Eriksson, 1968; Li et al., 1987; McBride and Li, 1998; Rodd et al., 2004). The most frequently employed methods to induce ethanol dependence all involve some form of involuntary or forced ethanol administration, including delivery of ethanol by intragastric intubation (Majchrowicz, 1975), ethanol vapor inhalation (O’Dell et al., 2004), and consumption of ethanol in a liquid-diet available as the animal’s sole source of fluid and nutrition (Lieber and DeCarli, 1982). Each procedure has its respective advantages and disadvantages. For instance, intragastric ethanol administration, widely employed in early studies of ethanol intoxication and withdrawal (Majchrowicz, 1975), rapidly induces physical dependence over a short period of time (four days) and leads to significant overt withdrawal signs upon termination of ethanol administration. However, this method produces excessively high blood alcohol levels (BALs) resulting in high mortality rates. Nonetheless, this procedure continues to be successfully used for the investigation of withdrawal seizures and kindling (Feng et al., 2007). Ethanol vapor inhalation (O’Dell et al., 2004) provides precise control over ethanol dose, duration, and pattern of exposure, thus reliably producing ethanol intoxication and overt signs of withdrawal upon termination of ethanol vapor exposure. However, this procedure is expensive and requires appropriate instrumentation, institutional space, and dedicated personnel for efficient operation and maintenance. With the liquid-diet procedure (Lieber and DeCarli, 1982), animals will consume significant quantities of ethanol (as part of a nutritionally balanced liquid diet that needs to be consumed by the animal to meet and maintain nutritional and hydration needs) to develop physical dependence with the emergence of withdrawal signs when ethanol is omitted from the diet. However, the liquid-diet procedure provides less rigorous control over ethanol exposure than intragastric ethanol administration or ethanol vapor inhalation because animals vary in terms of the daily quantity of ethanol-liquid diet consumed as well as the duration and diurnal distribution of drinking bouts, resulting in often marked variability in BALs and withdrawal ratings.
The purpose of this study was to re-evaluate the utility of intragastric ethanol administration as a method that (i) effectively and reliably induces physical dependence on ethanol over a short period of time, (ii) is cost-effective and easy to implement, (iii) is devoid of stress artifacts, (iv) mimics the diurnal ethanol intake cycle in alcoholics, (v) is associated with minimal mortality rates, and (vi) is suitable for use especially in the context of behavioral and neurobiological studies of ethanol reward and motivation.
Materials and Methods
Animals
Male Wistar rats (Charles River, Raleigh, NC), weighing 250–300 g at the beginning of experiments, were housed two per cage on a reversed 12 h light/dark cycle (lights off at 10:00 h) in a temperature- and humidity-controlled vivarium. Rats were handled daily for 5 min during the first week after arrival and had ad libitum access to standard rat chow and water throughout the course of the study. All intragastric intubation and elevated plus-maze test sessions were initiated during the dark cycle. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the European Community Directive for the Care and Use of Laboratory Animals.
Ethanol solution preparation
Ethanol intubation solution (final concentration, 20% w/v) was prepared by diluting 95% ethanol in a solution consisting of powdered milk (baby formula), sucrose, and water. Specifically, 1 liter of solution contained 166 g powdered milk, 60 g sucrose, 211 ml 95% ethanol, and 250 ml water. The solution was gently warmed and stirred until the powdered milk and sucrose were completely dissolved. Water was then added for a final volume of 1 liter. Preparation of the vehicle solution was identical, with the exception that ethanol was substituted with an equicaloric dose of sucrose. All solutions were freshly prepared daily and administered by intragastric intubation via a standard 10 ml syringe equipped with PE 50 tubing (5–6 cm length) connected to the tip of a blunted 18-gauge needle.
Intragastric intubation procedure
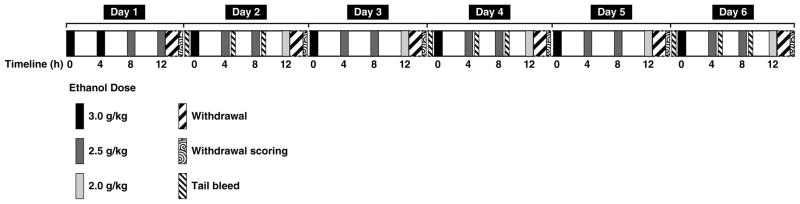
Rats were subjected to induction of ethanol dependence (ethanol group, n = 12) via repeated intragastric ethanol intubation (see Fig. 1 for details). Ethanol was administered four times per day for six consecutive days. On the first day, rats were intubated with a total of 11.0 g/kg ethanol in four fractional doses of 3.0, 3.0, 2.5, and 2.5 g/kg ethanol administered at 4 h intervals. On days 2–6, 12 h after the last intubation on the preceding day, rats received a total of 10.0 g/kg ethanol in four fractional doses of 3.0, 2.5, 2.5, and 2.0 g/kg, again separated by 4 h intervals. Rats serving as nondependent controls (vehicle group, n = 12) received intragastric administration of vehicle in a total volume of 22 ml/kg/day at time intervals and relative volumes identical to those in ethanol-treated rats. All rats were weighed daily.
Figure 1.
Diagram illustrating the procedure for inducing ethanol dependence. On day 1, rats were administered a total of 11.0 g/kg ethanol in four fractional doses of 3.0, 3.0, 2.5, and 2.5 g/kg ethanol, with doses administered at 4 h intervals. On days 2–6, 12 h after the last intubation on the preceding day, rats received a total of 10.0 g/kg ethanol in four fractional doses of 3.0, 2.5, 2.5, and 2.0 g/kg, again separated by 4 h intervals. Control rats received intragastric administration of vehicle at volumes and time intervals identical to those in ethanol-intubated rats. Tail bleeds were collected on days 2, 4, and 6 immediately before the first daily ethanol intubation and 1 h after the second and third daily intubation. Between 10–12 h after the last ethanol intubation, behavioral signs of withdrawal were rated daily.
Measurement of blood alcohol levels
Tail blood (approximately 200 μl) was collected on days 2, 4, and 6 immediately before the first daily dose of ethanol and 1 h after the second and third daily doses. Samples were collected on ice and then immediately centrifuged (10 min, 5000 rpm). Ethanol content was then assayed from 5 μl plasma aliquots using an oxygen-rate alcohol analyzer (Analox Instruments, Lunenburg, MA).
Measurement of ethanol withdrawal signs
Rats were examined daily for physical signs of withdrawal between 10–12 h after the last ethanol intubation by an experimenter blind to treatment conditions. Using a withdrawal rating scale adapted from Macey and colleagues (1996), ethanol withdrawal signs, including ventro-medial limb retraction (VLR), irritability to touch (vocalization), tail rigidity, and body tremors, were scored. Each sign was assigned a score of 0–2, based on the following severity scale: 0 = no sign, 1 = moderate, 2 = severe. The sum of the four observation scores (0–8) was used as a quantitative measure of withdrawal severity. For these behavioral observations, animals were individually transferred from their home cages to a quiet observation room to avoid excessive stimulation.
Elevated plus-maze test
To examine the effects of ethanol dependence on anxiety-like behavior, a separate group of rats was subjected to the intragastric ethanol (n = 10) or vehicle (n = 10) intubation regimen. An additional group of rats (experimentally naive, n = 10) was not subjected to the intubation procedure but handled daily. Five days after completion of the ethanol dependence induction regimen (and withdrawal in the ethanol-treated group), all three groups of rats (ethanol, vehicle, and naive groups) were tested for anxiety-like behavior on the elevated plus-maze. The elevated plus-maze apparatus was made of Plexiglas and consisted of four arms (10 × 50 cm) positioned at right angles and elevated 50 cm above the floor. Two arms were fitted with 40 cm high dark walls (enclosed arms). The other two arms had 0.5 cm high ledges (open arms). The elevated plus-maze was located in a quiet room that provided 1.5–2.0 lux of illumination for the open arms and <1 lux for the enclosed arms. On the test day, rats were transferred in their home cages to a quiet area adjacent to the elevated plus-maze procedure room where they remained undisturbed for at least 2 h in the presence of white noise (70 dB). Rats were then placed individually onto the center of the maze facing a closed arm, with white noise (70 dB) continuing to be present throughout testing. The apparatus was wiped clean with water and dried after each subject. During the 5 min elevated plus-maze tests, rats were observed through a window in the door, and behavior was recorded by video camera using a low-light recording mode. Video recordings were later scored under blind conditions for the number of open arm, closed arm, and center platform entries (with entries defined as placement of all four paws into the respective area), as well as the time spent in the open arms, closed arms, and center platform. From these data, the percentage of time spent in the open arms was calculated as (seconds in open arms/[seconds in open arms + seconds in closed arms]) × 100.
Statistical analysis
Blood alcohol levels on days 2, 4, and 6 were each analyzed by mixed-factorial analysis of variance (ANOVA) with one between-subjects factor (treatment) and two within-subjects factors (days and bleeds), followed by Simple Effects analysis. Body weights were analyzed by mixed-factorial ANOVA with “treatment” (ethanol/vehicle) as the between-subjects factor and “days” as the within-subjects factor. EPM data were analyzed by one-way between-subjects ANOVA. Significant main effects or interactions were confirmed by Newman-Keuls post hoc tests. Withdrawal scores were analyzed by the nonparametric Wilcoxon test.
Results
Effects of ethanol intoxication on blood alcohol levels, withdrawal ratings, and body weights
Blood alcohol levels
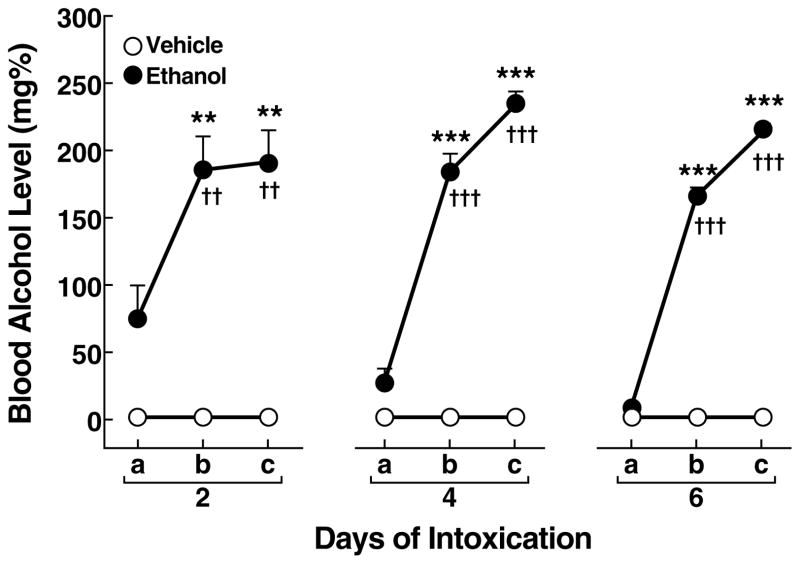
Intragastric administration of ethanol produced significant BALs measured on days 2, 4, and 6 (Fig. 2) with an overall main effect of treatment (F1,42 = 82.6, p < 0.001) and bleeds (F2,42 = 84.2, p < 0.001). The average BALs (mean ± SEM) in ethanol-treated rats after the second and third daily intubations were 187.6 ± 17.3 mg% (day 2), 209.2 ± 9.2 mg% (day 4), and 190.4 ± 6.6 mg% (day 6). Blood alcohol levels in ethanol-treated rats significantly increased after the second and third daily intubation on day 2 (p < 0.05; Newman-Keuls post hoc test after ANOVA: F2,35 = 6.8, p < 0.01), day 4 (p < 0.05; Newman-Keuls post hoc test after ANOVA: F2,35 = 98.0, p < 0.001), and day 6 (p < 0.05; Newman-Keuls post hoc test after ANOVA: F2,35 = 493.7, p < 0.001) compared with BALs measured before the first scheduled ethanol intubation (Fig. 2). On day 2, modest residual morning BALs of 74.1 ± 25.3 mg% were present, measured before the first scheduled ethanol intubation. In contrast, on day 2, residual morning BALs significantly decreased to 26.5 ± 11.0 mg% on day 4 and 7.5 ± 0.7 mg% on day 6 (p < 0.05; Newman-Keuls post hoc test after ANOVA: F2,35 = 4.6, p < 0.05).
Figure 2.
Blood alcohol levels measured on days 2, 4, and 6 immediately before the first daily intubation (a) and 1 h after the second (b) and third (c) daily intubation. **p < 0.01, ***p < 0.001, different from vehicle group; ††p < 0.01, †††p < 0.001, different from the first tail bleed of the ethanol group.
Ethanol withdrawal
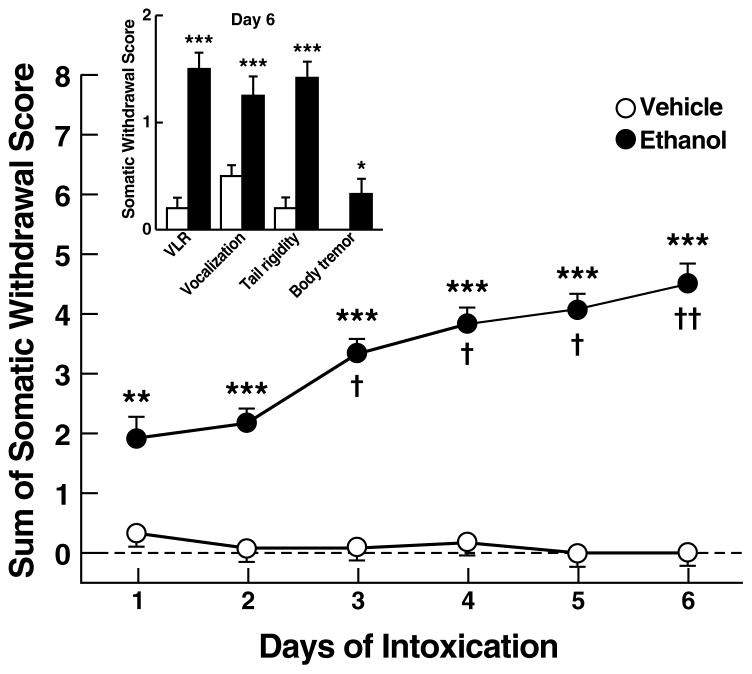
Ethanol-treated rats showed significant withdrawal signs (compared with vehicle-treated rats), measured 12 h after each final daily ethanol treatment (Fig. 3). The sum of the four observation scores (VLR, vocalization, tail rigidity, and body tremor) progressively increased from 1.9 ± 0.4 on day 1 to 4.5 ± 0.4 on day 6 (Wilcoxon W = 2770.0, p < 0.001; Fig. 3), with significant differences (compared with day 1) on day 3 (p < 0.05), day 4 (p < 0.05), day 5 (p < 0.05), and day 6 (p < 0.01), confirming the presence of significant overall withdrawal severity. All individual withdrawal ratings (compared with vehicle-treated rats), measured on day 6 (Fig. 3 inset), were significantly elevated as confirmed by a significant increase in VLR (Wilcoxon W = 78.0, p < 0.001), vocalization (W = 84.0, p < 0.001), tail rigidity (W = 78.0, p < 0.001), and body tremor (W = 126.0, p < 0.05).
Figure 3.
Overall withdrawal severity (sum of somatic withdrawal scores across the four behavioral signs of ethanol withdrawal) measured between 10–12 h after the last daily ethanol intubation during the six-day ethanol dependence procedure. (Inset) Somatic withdrawal signs measured on day 6 of the ethanol dependence procedure. *p < 0.05, **p < 0.01, ***p < 0.001, different from vehicle group; †p < 0.05, ††p < 0.01, different from overall withdrawal score of the ethanol group rated on day 1. VLR, ventro-medial limb retraction.
Body weights
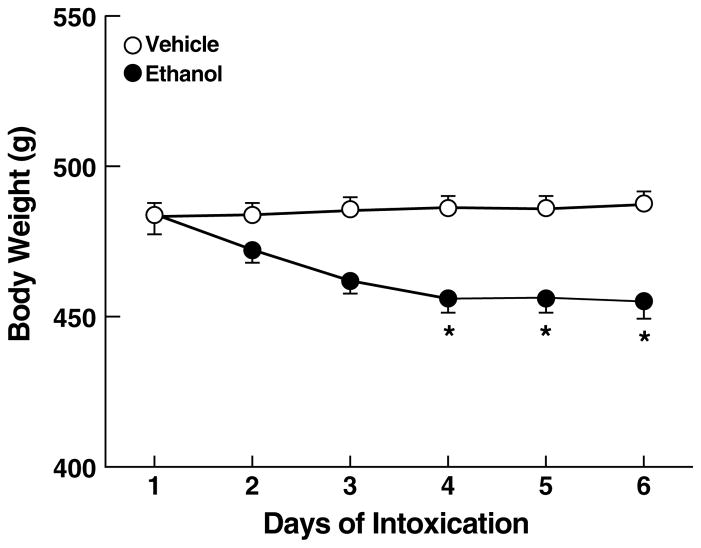
The average body weight at the beginning of the intubation procedure was 484.1 ± 5.1 g and remained stable in vehicle-treated rats (Fig. 4). Body weights of ethanol-treated rats decreased over the 6-day intubation period to 455.1 ± 5.3 g, as reflected by a significant main effect of days (F5,110 = 15.5, p < 0.001) and a day x treatment interaction (F5,110 = 24.8, p < 0.001); however only a marginally significant main effect of treatment (F1,22 = 3.2, 0.1 > p > 0.05) was evident. Simple effects analyses indicated that the decrease in body weight of ethanol-treated rats reached peak on day 4, with body weights remaining stable throughout the remainder of the ethanol intoxication period (days 4–6: p < 0.05 vs. vehicle-intubated rats; Fig. 4).
Figure 4.
Effect of intragastric ethanol intubation on body weight over the six-day ethanol dependence procedure in rats. *p < 0.05, different from vehicle group.
Mortality rate
Two animals died during the course of the experimental procedures due to improper intragastric intubation resulting in a mortality rate of 4.3%.
Effects of ethanol withdrawal on anxiety-like behavior
Elevated plus-maze
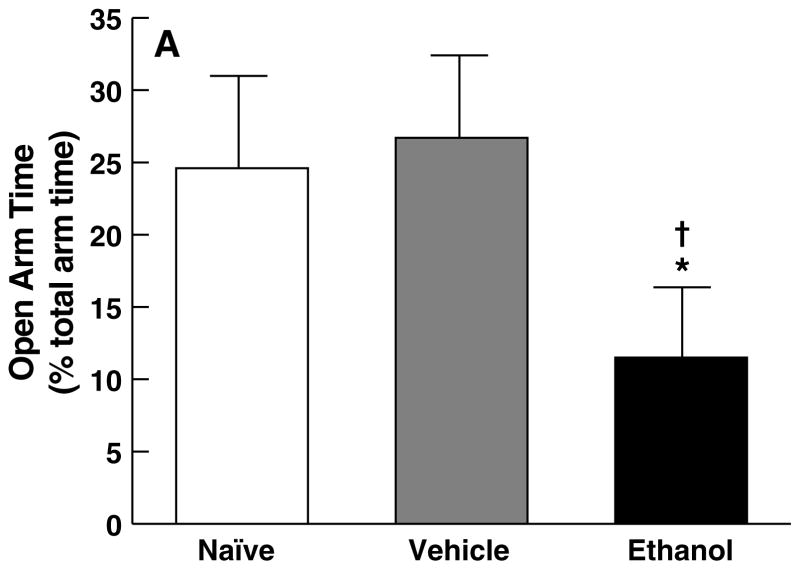
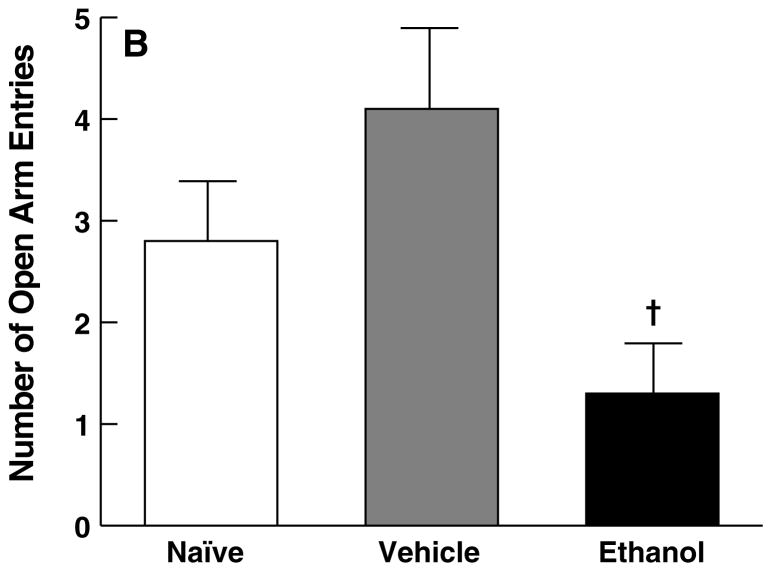
One-way ANOVA confirmed a significant main effect of treatment for open arm time (F2,29 = 3.8, p < 0.05) and open arm entries (F2,29 = 4.5, p < 0.05) on the EPM. Subsequent post hoc analyses verified that rats that had undergone the six-day ethanol intubation procedure showed significant anxiety-like behavior compared with vehicle controls, reflected by reduced open arm time (p < 0.05; Fig. 5A) and open arm entries (p < 0.05; Fig. 5B). Additionally, ethanol-treated rats showed decreased open arm time compared with experimentally naive rats (p < 0.05; Fig. 5A). However, no differences were observed between vehicle-intubated rats and experimentally naive controls with respect to open arm time (p > 0.05; Fig. 5A) and open arm entries (p > 0.05; Fig. 5B).
Figure 5.
Effect of ethanol withdrawal measured five days after completion of the ethanol dependence procedure on behavioral anxiety in the elevated plus-maze, reflected in mean (± SEM) for (A) the percentage of total arm time spent in the open arms and (B) open arm entries. *p < 0.05, different from naive group; †p < 0.05, different from vehicle group.
Discussion
The findings show that intragastric ethanol administration, under the conditions employed here, effectively induces and maintains reliable ethanol intoxication, evident by significant BAL elevations that ranged consistently between 200–250 mg% after the second and third intubation throughout this daily treatment period and across the six-day treatment regimen. Termination of ethanol exposure produced overt acute withdrawal signs across treatment days, confirming the utility of the procedure as a tool to rapidly induce physical dependence on ethanol. As previously shown with other ethanol dependence-induction methods (Lal et al., 1993; Valdez et al., 2002), ethanol dependence established via the present procedure was associated with elevated anxiety-like behavior measured following one week of withdrawal (i.e., during protracted withdrawal) on the elevated plus-maze. Additionally, comparison of anxiety-like behavior on the elevated plus-maze between experimentally naive and vehicle-intubated rats suggests that the intragastric intubation procedure per se, while possibly not devoid of acute stress effects, does not produce lasting behavioral changes that would complicate interpretation of the effects of chronic ethanol on subsequent stress sensitivity.
Relevant for the utility of this procedure to reliably induce ethanol dependence was the sustained elevation of BALs across treatment days at levels ranging between 200–250 mg% (see Fig. 2), comparable to BALs produced by the ethanol vapor inhalation procedure, one of the most widely employed dependence induction methods (O’Dell et al., 2004). Ethanol-intubated rats had significantly increased BALs 1 h after the second and third ethanol administrations on days 2, 4, and 6 of the procedure. During the subsequent 12-h non-intubation periods, BALs progressively decreased across treatment days to values below 50 mg%, levels at which somatic signs of withdrawal were consistently observed, and increased over the course of the six-day treatment period (Fig. 3). Thus, the consistency of the BAL values and over days 2–6 confirms that this procedure permits maintenance of significant and sustained BALs throughout daily intoxication periods of approximately 10 h across each of the treatment days, sufficient to establish significant physical dependence within only six days. Indeed, withdrawal measures, including VLR, vocalization, tail rigidity, and body tremor, measured 10–12 h following the final ethanol administration (see Fig. 3), were similar in magnitude to those observed with the ethanol vapor inhalation and ethanol liquid-diet procedures (e.g., Baldwin et al., 1991; Macey et al., 1996; Merlo Pich et al., 1995; Schulteis et al., 1996).
Morning BALs progressively decreased over days 2, 4, and 6 of the treatment procedure (see Fig. 2). This observation may reflect either ethanol tolerance or increased ethanol clearance. Tolerance to ethanol following a period of intoxication is known to persist into withdrawal (Rimondini et al., 2008) and is associated with increased ethanol consumption, as observed, for example, during acute withdrawal (8 h post-withdrawal) from chronic intermittent ethanol vapor inhalation (O’Dell et al., 2004) or following chronic ethanol liquid-diet exposure (Schulteis et al., 1996). With the present procedure, peak BALs remained within a stable range during the daily treatment windows and across treatment days without the need for increasing daily ethanol doses (see Fig. 1). Thus, daily peak BALs were not lowered despite the gradual decrease in residual morning BALs, an observation that points toward the development of increased ethanol clearance rates rather than metabolic tolerance. Importantly, however, the progressive increase in overall withdrawal ratings over the six-day treatment period (see Fig. 3) showed a mild inverse relationship with the gradual decrease in morning BALs, such that the reductions in morning BALs provide an explanation for the concomitant increases in ethanol withdrawal severity.
The body weights of ethanol-treated rats showed a significant decrease after 4 days of intragastric ethanol administration compared to vehicle-intubated rats (Fig. 4). Weight loss, however, did not continue beyond day 4 of ethanol treatment, such that the overall decrease in body weight at completion of the ethanol dependence induction procedure was less than 10% compared to vehicle-intubated rats. This degree of body weight loss is comparable to that associated with dependence induction by the ethanol vapor inhalation method (Macey et al., 1996), and is generally not considered to be a major concern with respect to the health of rats.
A prominent ethanol withdrawal symptom in humans is anxiety (Driessen et al., 2001). In rodents, anxiety-like behavior measured in several animal models of anxiety is a commonly reported sign of ethanol withdrawal (Baldwin et al., 1991; File et al., 1993; Gatch et al., 1999; Knapp et al., 2004; Overstreet et al., 2004; Valdez et al., 2002; Zhang et al., 2007). With the present procedure, six days of intragastric ethanol intubation were sufficient to produce lasting elevations in spontaneous anxiety-like behavior measured five days post-withdrawal on the elevated plus-maze, one of the most widely used rodent models for measuring anxiety-like behavior (Pellow et al., 1985; Treit et al., 1993). This observation replicates earlier findings in which spontaneous anxiety-like behavior measured on the elevated plus-maze increased in rats during acute withdrawal and various stages of protracted abstinence, including the present five-day abstinence period (Lal et al., 1993; Valdez et al., 2002). Thus, the consistency in anxiogenic-like effects between this and other studies using different means of ethanol dependence-induction lends further support for the utility and reliability of intragastric ethanol intubation as a dependence induction procedure. A particularly relevant finding was that the observation of anxiogenic-like effects was specifically linked to ethanol withdrawal and not to possible long-term anxiogenic consequences of intragastric intubation, as suggested by the statistically indistinguishable elevated plus-maze performance of experimentally naive and vehicle-intubated rats (Fig. 5). This finding provides confidence that the intragastric ethanol intubation procedure employed here is associated with few intrinsic stress effects that would otherwise confound behavioral or neurobiological studies of ethanol withdrawal.
The present intubation procedure resembles in some aspects the Majchrowicz method (Majchrowicz, 1975) that was widely used several decades ago. The Majchrowicz method involves administration of very high ethanol doses (up to 15 g/kg/day) over only a four-day period to produce exceedingly high BALs of 400 mg% associated with a high incidence of convulsions and a mortality rate of 20% (Majchrowicz, 1975). This method remains effective for studying convulsive seizures associated with severe ethanol withdrawal (e.g., Feng et al., 2007). However, the procedures in the present report were designed to deliver smaller, yet pharmacologically relevant, doses of ethanol (10–11 g/kg/day) to produce BALs in the range of only 200–250 mg% and over a somewhat longer treatment period. Mortality rate with the present procedure was minimal (4.3%) and resulted from complications with the intubation technique per se rather than being a direct or indirect result of intoxication. Moreover, the use of a dietary liquid vehicle (as opposed to the water vehicle in the standard Majchrowicz procedure) possibly reduces the incidence of gastrointestinal irritation that could influence behavioral measures of dependence and withdrawal. Overall, the present procedure would appear to be compatible with applications in the context of behavioral research and studies of ethanol reinforcement and motivation in subjects with a history of ethanol dependence.
All major methods for inducing ethanol dependence in experimental animals employ some form of involuntary or forced ethanol intoxication. Each of the methods has its advantages and disadvantages. For example, dependence induced by the ethanol vapor inhalation method, similar to the present ethanol intubation procedure but in contrast to the ethanol liquid-diet method, provides effective control over ethanol dose as well as the duration and temporal pattern of daily exposure, thus permitting maintenance of stable target BALs across animals and treatment periods. Following ethanol dependence induction using ethanol vapor inhalation or ethanol liquid-diet procedures, animals can be maintained in the dependent state for extended periods of time to test the relationships between the duration of chronic ethanol exposure, acute or protracted withdrawal-related behaviors (Schulteis et al., 1996; Valdez et al., 2002), and associated neurobiological changes. Whether a state of dependence can be sustained over an extended time period via chronic intragastric ethanol intubation using the current methodology remains for future research. A prime advantage of the present procedure, however, is that physical dependence on ethanol is achieved within a short six-day period. Moreover, the procedure mimics the diurnal intake cycle in alcoholics characterized by high-dose intake during the day followed by abstinence at night and recurrent experience of early morning withdrawal. Lastly, the method is easy to implement, inexpensive, associated with minimal mortality rates, and importantly, devoid of apparent stress artifacts.
In conclusion, the results confirm that intragastric ethanol administration can be implemented as an effective tool for the induction of ethanol dependence in rats within a short period of time and without producing enduring nonspecific anxiety-like consequences that would interfere with measures of ethanol-induced changes in anxiety or stress sensitivity. This procedure, therefore, provides a useful alternative ethanol dependence induction method that is suitable for use in the context of behavioral and neurobiological studies of ethanol reward and motivation.
Acknowledgments
This is publication 20096 from The Scripps Research Institute. We thank M Arends for editorial assistance.
This study was supported by National Institute on Alcohol Abuse and Alcoholism grants AA014351 and AA010531 (FW).
References
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Eriksson K. Genetic selection for voluntary alcohol consumption in the albino rat. Science. 1968;159:739–741. doi: 10.1126/science.159.3816.739. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Yang L, Faingold CL. Role of the amygdala in ethanol withdrawal seizures. Brain Res. 2007;1141:65–73. doi: 10.1016/j.brainres.2007.01.017. [DOI] [PubMed] [Google Scholar]
- File SE, Andrews N, Al-Farhan M. Anxiogenic responses of rats on withdrawal from chronic ethanol treatment: effects of tianeptine. Alcohol Alcohol. 1993;28:281–286. [PubMed] [Google Scholar]
- Gatch MB, Wallis CJ, Lal H. Effects of NMDA antagonists on ethanol-withdrawal induced “anxiety” in the elevated plus maze. Alcohol. 1999;19:207–211. doi: 10.1016/s0741-8329(99)00045-2. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H, Prather PL, Rezadeh SM. Potential role of 5-HT1C and/or 5-HT2 receptors in the minaserin-induced prevention of anxiogenic behaviors occurring during ethanol withdrawal. Alcohol Clin Exp Res. 1993;17:411–417. doi: 10.1111/j.1530-0277.1993.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–96. [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang MT, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin B, File SE, Briley M. Validation of the open: closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Dall’Olio R, Heilig M. Long-lasting tolerance to alcohol following a history of dependence. Addict Biol. 2008;13:26–30. doi: 10.1111/j.1369-1600.2007.00079.x. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytiä P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alcohol Clin Exp Res. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav. 1993;44:463–469. doi: 10.1016/0091-3057(93)90492-c. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morse AC, Koob GF, Schulteis G. Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res. 2007;31:1811–1819. doi: 10.1111/j.1530-0277.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]