Abstract
Importance of the field
Age-related macular degeneration (AMD) and diabetic retinopathy (DR) are two major causes of blindness. In these disorders, growth factors such as vascular endothelial growth factor (VEGF) are upregulated leading to either enhanced vascular permeability or proliferation of endothelium. While currently available corticosteroid therapies suffer from side effects including cataracts and elevated intraocular pressure, anti-VEGF antibody therapies require frequent intravitreal injections, a procedure that can potentially lead to retinal detachment or endophthalmitis. Thus, there is currently a need to develop safe, sustained release therapeutic approaches for treating AMD and DR.
Areas covered in this review
This review discusses the pharmacological basis for using celecoxib, an anti-inflammatory drug capable of selectively inhibiting cycloxygenase 2, in treating AMD and DR. In addition, this article discusses the safety, delivery advantage, and efficacy of celecoxib by transscleral retinal delivery, a periocular delivery approach that is less invasive to the globe compared to intravitreal injections.
What the reader will gain
The reader will gain insights into the development of a pharmacological agent and a sustained release delivery system for treating DR and AMD. Further, the reader will gain insights into the role of eye physiology including pigmentation and disease states such as DR on retinal drug delivery.
Take home message
Transscleral sustained delivery of anti-inflammatory agents is a viable option for treating retinal disorders.
Keywords: celecoxib, age related macular degeneration, diabetic retinopathy, periocular drug delivery, microparticles, nanoparticles
1. Introduction
Celecoxib, commercially available as Celebrex®, is an anti-inflammatory agent approved for the treatment of rheumatoid arthritis, osteoarthritis, juvenile rheumatoid arthritis, ankylosing spondylitis, acute pain, primary dysmenorrhea, and familial adenomatous polyposis1. It is a selective cyclooxygenase-2 inhibitor that has been investigated for various cancer therapies and is currently being investigated for its anti-proliferative and anti-VEGF effects in several cancers and in ocular disorders such as AMD 2 and DR 3.
1.1. DR and AMD
DR and AMD are two key prevailing causes of vision impairment today. The two disorders combined account for over 60% of cases of blindness in the US4. DR is a microvascular pathology of the retina, wherein vascular leakiness and proliferation are implicated in vision impairment 5. Inflammation is thought to play a critical role in the pathophysiology of DR 6-7. Several key signs of micro-inflammation such as vessel dilation, exudation of fluids from blood vessels, altered blood flow to the tissues, leakage of proteins, and accumulation of leucocytes have been shown to be involved in DR progression 6, 8-10. Administration of aspirin, a non steroidal anti-inflammatory drug, which is an inhibitor of cyclooxygenase-1 and cyclooxygenase-2 has been shown to reduce vascular leakage abnormalities in diabetic rats11 and dogs12, further suggesting that inhibition of inflammation could be a treatment modality for DR, however a Growth factors are involved at several stages in the progression of DR. Among several growth factors like basic fibroblast growth factor (bFGF), VEGF, pigment epithelium derived growth factor (PEDF), lens epithelium derived growth factor (LEDGF) and others, which are thought to be present and active in the retina, VEGF is the one which has been most widely investigated and is thought to have a key role in DR.
There are two forms of AMD, the wet (∼20% of the cases) and the dry form (∼80% of the cases), with more severe vision loss associated with the wet form of AMD13. Vascular proliferation is involved in the progression of wet form of the disease and is termed as choroidal neovascularization (CNV). In the pathophysiology of CNV, there is proliferation of the endothelial cells of the choroidal vasculature. The endothelium proliferates and the cells migrate through the Bruch's membrane and into the RPE and the neural retina as the neovascularization progresses. This new blood vessel invasion of the RPE and neural retina is subsequently associated with retinal detachment, retinal atrophy and central vision loss14.
Currently therapeutic approaches to treat these disorders are limited, although there is a significant interest and research initiative in discovering therapies to combat the progression of these disorders. During the last few years, therapeutic agents have been introduced into the market, to treat DR and AMD. Ozurdex, an injectable implant containing dexamethasone, an anti-inflammatory corticosteroid, was approved in 2009 for treating DR. Pegatinib, an anti-VEGF aptamer, and Lucentis, an anti-VEGF antibody fragment were approved for treating the wet form of AMD in 2004 and 2005, respectively. Thus, it is evident that anti-inflammatory agents and VEGF-inhibition are viable therapeutic options for treating DR and AMD. However, all the above therapeutic agents are injected intravitreally. Since intravitreal injections by themselves can cause retinal detachment, endophthalmitis, and cataracts15, there is a need to develop alternative strategies for drug delivery. Currently, investigations are underway to develop a delivery system that is less -invasive and has limited side effects. In addition to novel delivery approaches, there is a need for alternative therapeutic agents. For instance, corticosteroids, although effective in reducing macular edema associated with DR, may cause cataracts and elevation of intraocular pressure16. As described in this paper, transscleral delivery and use of celecoxib, a cox-2 selective anti-inflammatory agent might serve as a safer alternative to currently available therapies.
1.2. Role of VEGF in DR and AMD
Impairments to the retinas of DR and AMD patients are largely caused by the over-secretion of various growth factors. Although many growth factors including basic bFGF, PEDF, and VEGF have been shown to play a role in retinal angiogenesis, VEGF due to its potency is probably the most significant. VEGF has been shown to be up-regulated in the retina early in the course of diabetes17-18. VEGF is a major contributor to vascular leakage and plays an important role in the pathophysiology of DR – at early as well as more advanced stages. In the early stages VEGF modulates retinal vascular permeability19-24, whereas in the later stages VEGF plays a critical role in retinal neovascularization25. The up regulation of VEGF in diabetes can be related to several factors including oxidative stress, inflammation, and hyperglycemia, which are all interlinked. The induction of angiogenesis as well as fluid accumulation in macular edema caused by subsequent blood vessel formation can lead to harmful blood retinal barrier leakage or retinal detachment.
Several studies demonstrate an up regulation of VEGF in the fibro-vascular membranes associated with AMD and also in RPE of the patients with AMD26-27. In addition, animal models for investigating CNV utilize the strategy of overexpression of VEGF in the retina28-31. The putative mechanism that leads to choroidal neovascularization probably involves the increased secretion of VEGF from the RPE, which acts on the choroidal endothelial cells to cause proliferation and migration of the endothelium, leading to neovascularization.
Thus VEGF is a common factor involved in the pathophysiology of both DR as well as AMD. Anti-VEGF strategies can be effective therapeutic modalities for the treatment of these disorders.
1.3. Cell Proliferation in DR and AMD
The proliferation of the endothelial cells in the retinal as well as the choroidal vasculature leads to the sight threatening complications associated with DR and wet AMD. There is also RPE proliferation involved in the progression of AMD as well as proliferative DR32. Under normal circumstances, the endothelium in these vascular beds is at rest. However, under the influence of the pathologic growth factors, these cells begin to proliferate32. This proliferation can be actively targeted so that subsequent stages like formation of new blood vessels can be effectively blocked. Anti-proliferative drugs which act on specific phase of the cell cycle, and target the proliferating cells could be a beneficial approach for the treatment of both these disorders.
1.4. Upregulation of COX-2 in Diabetic Retinas
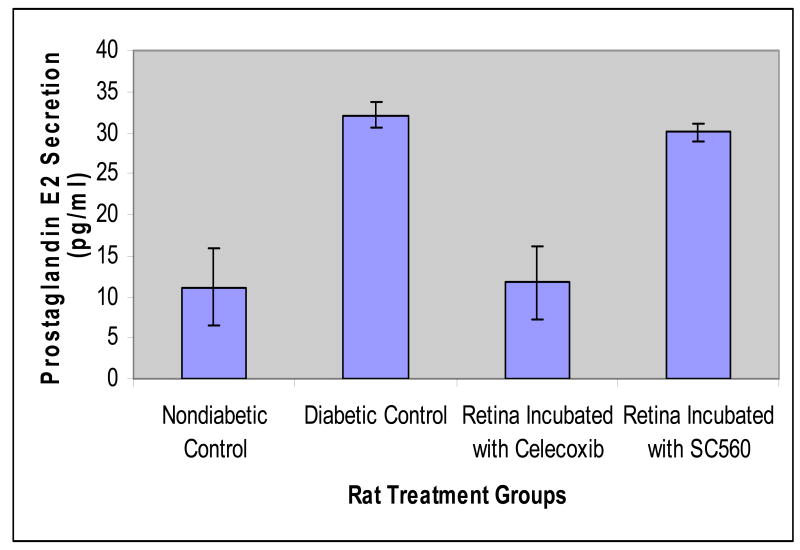
Among several factors which lead to the upregulation of VEGF in the retinal vasculature, upregulation of VEGF by cyclooxygenases is speculated to be an important factor17, 33-34. Four isoforms of the Cox enzyme are currently known to exist with differing roles in various normal and pathological conditions and two of the isoforms, Cox-1 and Cox-2, are predominant and widely studied35. Ayalasomayajula et al.34 demonstrated that cyclooxygenase-2 and not cyclooxygenase-1 has a direct effect on the diabetes induced secretion of prostaglandin E2 in the retina36. The authors incubated retinas ex vivo from rats with diabetes of 2-week duration and age-matched control rats with either celecoxib which is a selective Cox-2 inhibitor, or with SC560, which is a selective Cox-1 inhibitor. Their results demonstrate that retinal PGE2 secretion was 2.9 folds higher in the diabetic retinas as compared to the controls (Figure 1). When incubated with celecoxib the PGE2 secretion from diabetic retinas was reduced by approximately 65%, whereas the reduction with SC560 was only 9%. This indicates that the increased secretion of prostaglandins in the retina during diabetes is a Cox-2 mediated phenomenon and not a Cox-1 mediated phenomenon. This increase could be due to increase in the production of the Cox-2 enzyme or increased enzymatic activity. Indeed Sennlaub et al.37 have demonstrated that Cox-2 is induced in the retina of diabetic rat and mice.
Figure 1.
Secretion of Prostaglandin E2 in control, diabetic control, celecoxib and SC-560 incubated rat retinas. Data are presented as mean ± s.d for n = 4. Based on data from Ayalasomayajula et al., 2004; Eur J Pharmacol 498(1-3):275-278.
2. Therapeutic value of celecoxib in DR and AMD
2.1. VEGF Inhibition by Celecoxib
Celecoxib has VEGF inhibitory effects as demonstrated in several anticancer studies using different cell types. This inhibitory effect of celecoxib on VEGF secretion or expression could be most likely through the inhibition of Cox-2 enzyme.
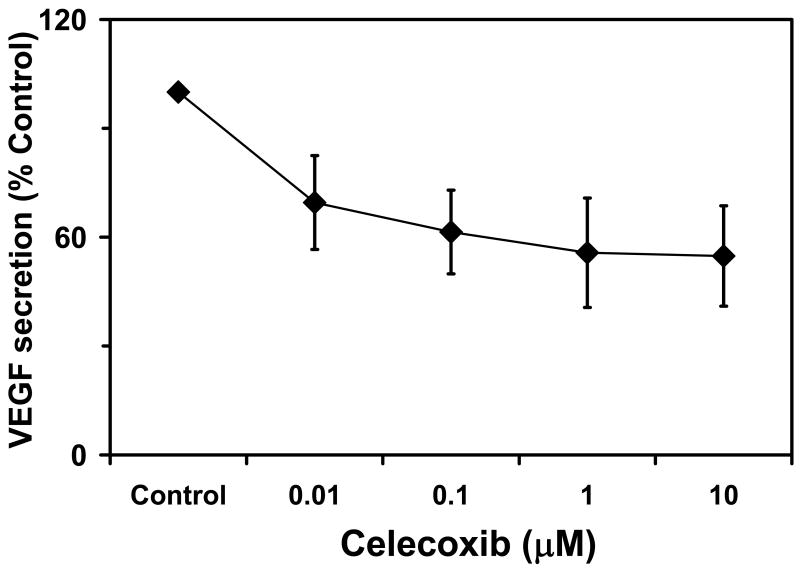
Celecoxib has been shown to have anti-VEGF effects even in the retinal cells. In a previous study, treatment with celecoxib resulted in a dose-dependent decrease in VEGF expression in the retinal pigment epithelium 2. ARPE-19 cells were incubated with celecoxib (0-10 μM) and VEGF secretion from the cells with and without celecoxib was analyzed after 12 hours of treatment 2. There was a dose dependent decrease in VEGF mRNA expression as well as VEGF protein secretion with increasing concentrations of celecoxib (Figure 2). The effect on the mRNA expression leveled off at about 50% inhibition whereas there was about 45 % inhibition in the VEGF secreted from these cell types. The VEGF inhibition in the RPE cells by celecoxib was at concentrations similar to the median IC50 of celecoxib for inhibition of the Cox -2 enzyme (40-90 nM)38, indicating that the inhibition of VEGF could be through a Cox-2 inhibitory mechanism of celecoxib. The inhibition of VEGF was not due to cytotoxicity as the concentrations of celecoxib having cytotoxic effects on the cells are much higher33. This VEGF inhibitory effect of celecoxib has potential value in treating DR and AMD and other VEGF induced neovascular conditions of the eye. The authors, however, have examined VEGF secretion from un-stimulated ARPE-19 cells. It is likely that celecoxib might be even superior in inhibiting VEGF secretion that is induced by other stimuli.
Figure 2.
Inhibition of VEGF secretion in ARPE-19 cells with increased concentration of celecoxib. Data are presented as mean ± s.d for n = 4. Based Amrite et al, 2006; Invest Ophthalmol Vis Sci 47(3): 1149-1160.
2.2. Inhibition of proliferation of RPE and endothelial cells by celecoxib
Another measure of the therapeutic effects of celecoxib on DR and AMD is at the level of cell proliferation. Celecoxib has been demonstrated to have anti-proliferative effects on several cell types including endothelial cells39-41. In addition, celecoxib exerts antiangiogenic and antiproliferative effects on cancer cells in vivo including in humans42-43. Furthermore celecoxib has been effective chemotherapeutic agent for colon polyps in several clinical trials44-47.
Cellular proliferation particularly proliferation of the endothelium and/or the RPE is involved in the proliferative stages of DR and in CNV associated with AMD. Amrite et al.48, demonstrated the antiproliferative effects of celecoxib on the retinal pigment epithelial cells (ARPE-19 cells) and the choroidal endothelial cells (RF/6A cells). Celecoxib inhibited the proliferation of ARPE-19 cells and RF/6A cells in a dose dependent manner with IC50 values of 23 μM and 13 μM, respectively. Celecoxib also inhibited VEGF induced proliferation of RF/6A cells with an IC50 of 20 μM. The anti-proliferative effect of celecoxib on these cell types was probably through a Cox-2 independent mechanism as rofecoxib (another more potent cox-2 inhibitor) had no anti-proliferative effects on the cell types at concentrations up to 100 μM, whereas flurbiprofen (a Cox-1 and Cox-2 inhibitor) had weak anti-proliferative effects on the choroidal endothelial cells with IC50 over 100 μM. In the same study the authors also demonstrated that celecoxib causes a G2-M phase cell cycle arrest in the RPE and choroidal endothelial cells. The concentrations of celecoxib required to have anti-proliferative effects were lower than the concentrations at which celecoxib had cytotoxic effect on these cell types. This anti-proliferative effect of celecoxib could be beneficial in the treatment of the proliferative stages of DR and AMD. Thus, the VEGF inhibitory activity of celecoxib as well as the anti-proliferative effects on proliferating retinal cells provides a strong rationale for the use of celecoxib in the proliferative disorders of the retina.
3. Retinal Delivery of Celecoxib
3.1. Oral Administration
Oral administration is the most widely used route for delivering drugs. Ayalasomayajula and Kompella17 have demonstrated that oral administration of celecoxib can reduce diabetes induced elevations in VEGF and vascular leakage in a rat model. However, the dose required for these effective levels is very high (50 mg/kg bid). This is because the oral pathway leads to circulation of the drug systemically in other parts of the body. Therefore, large amounts of the drug must be dosed in order to have an effective amount reaching the retina. This is also true for drugs other than celecoxib. Since cyclooxygenase-2 is expressed in other areas of the body, such as the heart, large doses of celecoxib and its Cox-2-inhibiting mechanisms could cause a number of side effects including cardiovascular problems. Similarly, topical approaches (e.g. eye drops) are inefficient because high doses are needed to compensate for drug loss during administration, which can lead to systemic toxicity. Therefore, in order to avoid systemic effects, periocular approach of administering the drug by injection closer to the retina for celecoxib delivery was studied 49. While intravitreal injections and surgical implants avoid these systemic effects, they are associated with complications such as retinal detachment and cataracts. Since the sclera is shown to be more permeable than the cornea and has a large surface area for sustained drug delivery, transscleral routes are emerging as alternatives to topical and intravitreal modes of administration for the treatment of retinal disorders49. In this route, drugs are administered adjacent to the sclera and reach the retina by passing the sclera and underlying tissues including the choroid-bruch layer and RPE 49.
3.2. Periocular VS. Systemic Administration
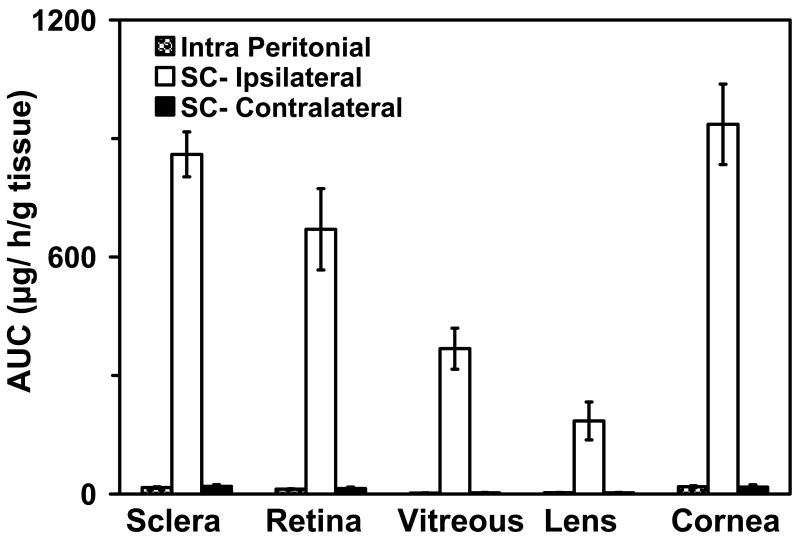
Significantly higher levels of the drugs in the retina can be achieved by the transscleral route as compared to the systemic mode of administration50. Ayalasomayajula and Kompella49 demonstrated that celecoxib delivery to the retina is 54 folds higher following subconjunctival administration as compared to systemic administration (Figure 3). The authors also demonstrate that the delivery of the drug to the contralateral eye is similar by the intraperitoneal and subconjunctival routes of administration. Based on their assessments more than 95 % of the celecoxib is available to the ipsilateral retina by local routes, probably direct penetrtation of celecoxib through the sclera and choroid-RPE and into the retina by the transscleral pathway.
Figure 3.
Extent of celecoxib delivery (AUC0-∞, μg.hr/g tissue) to the ocular tissues following intraperitoneal and periocular injection (3 mg to one eye) in normal Sprague Dawley rats. Data are presented as mean ± s.d for n = 4. Based on the data from Ayalasomayajula and Kompella, 2003; Pharm Res 21(10): 1797-1804.
3.3. Pharmacokinetics of Celecoxib in the Retina Following Periocular Administration
Celecoxib is a low molecular weight hydrophobic drug. Ayalasomayajula and Kompella49 describe the pharmacokinetics of celecoxib in the retina after periocular administration following administration in non-pigmented rats whereas Cheruvu et al.51 describe the pharmacokinetics of celecoxib after periocular administration in pigmented and non-pigmented rats. The retinal half-life of celecoxib is about 6 hours and is similar in both the pigmented and non pigmented rats51. Using deterministic compartmental modeling Amrite et al52., have demonstrated that celecoxib PK in the retina following periocular administration can be best described by a model incorporating primary elimination pathyways from the retina, choroid-RPE, and the periocular tissues. The major elimination pathway for celecoxib (loss to the systemic circulation) after periocular administration is through the periocular circulation and lymphatics, which is about an order of magnitude higher than loss to the systemic circulation by choroidal circulation52. Their modeling studies further indicate that suspensions are better than solutions for increased duration of delivery to the retina following periocular administration, which is applicable for celecoxib as well as other drugs. In addition the authors also demonstrate using simulations that the celecoxib delivery to the retina can be sustained for prolonged periods by designing systems which can slowly release celecoxib after administration into the periocular tissue52. Simulations using celecoxib as a model drug have demonstrated that slow release particulate systems with low clearance by the periocular blood and lymphatic circulation are important for better sustainment of celecoxib delivery to the retina following periocular administration48, 53.
3.4. Effect of Diabetes
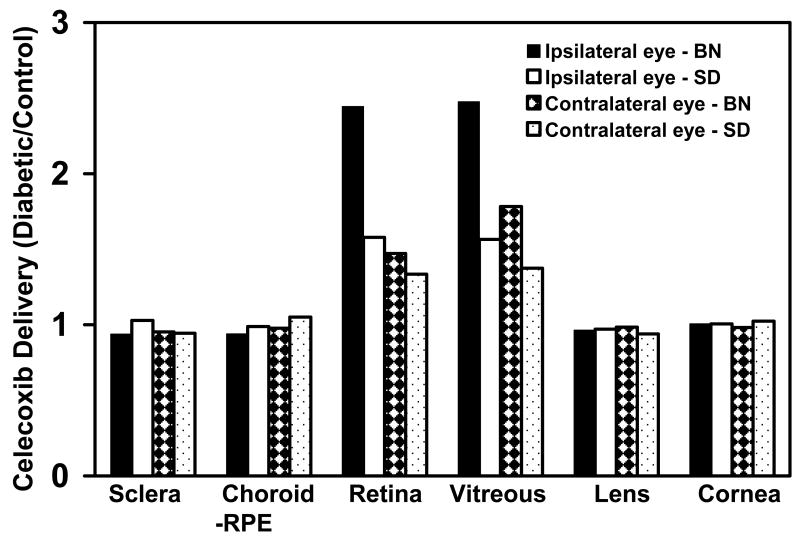
The transscleral delivery of small molecules is dependent on the physicochemical factors of the drug molecule/drug molecule delivery system combination as well as on the physiological or pathophysiological parameters affecting the surrounding tissues. In ocular drug delivery, certain disease states have been shown to affect drug delivery to particular ocular tissues. Ozturk and group have demonstrated that ocular infection and associated inflammation leads to a significantly higher level of antibiotics in the aqueous humor and vitreous humor concentrations of antibiotics administered by the topical ocular or systemic modes of administration54-57. Cheruvu et al.58 demonstrated that the delivery of celecoxib to the retina by the transscleral route is significantly enhanced in the diabetic state (Figure 4). In rats with diabetes duration of 2-months, the retinal total exposure (AUC) of celecoxib was 1.5-fold higher in non pigmented diabetic rats and 2.4 folds higher in the pigmented diabetic rats as compared to the respective normal rats without diabetes. This increased delivery could be a result of the breakdown of the blood-retinal barrier as a result of diabetes progression. The authors also demonstrate by using the FITC-dextran leakage assay that there is a 2.4 fold increased permeability of 4 kD dextran in nonpigmented diabetic rats and 3.5 folds increased permeability4 kD dextran in pigmented diabetic rats as compared to the normal rats. This breakdown could occur at the level of the RPE (outer BRB) or at the level of the endothelium (inner BRB). There are conflicting reports in the literature regarding the anatomical location of the blood-retinal barrier breakdown as a result of diabetes. It is also expected to be dependent on the type of tracer used for evaluating the blood retinal barrier breakdown.
Figure 4.
Relative delivery of celecoxib in diabetic rats compared to control rats following periocular injection of celecoxib Data are presented as the ratio of the mean AUCs or concentrations for n = 4. With kind permission from Springer Science+Business Media: Cheruvu NP, Amrite AC, Kompella UB. Effect of diabetes on transscleral delivery of celecoxib. Pharm Res 2009 Feb;26(2):404-14.
Thus, disease processes, particularly diabetes can influence retinal drug delivery by the transscleral route. These observations have clinical relevance as blood-retinal barrier breakdown as a result of diabetes progression has also been demonstrated in humans and this could mean higher delivery of celecoxib to the retina by the transscleral route, which is safer than the intravitreal mode of administration15.
3.5. Effect of Pigmentation
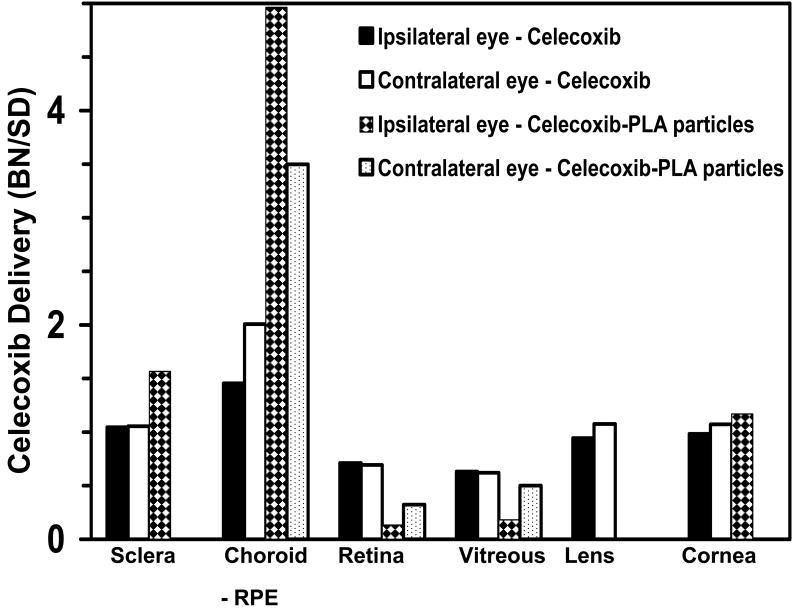
Several drugs are known to bind non-specifically to biomolecules within the body. It has been shown that melanin a pigment found in the skin and other parts of the body has an ability to bind to drugs. It is an important consideration for ocular drug delivery as the uveal tract is pigmented and can affect delivery to the inner tissues. Cheruvu et al.51 compared the relative availability of celecoxib to the retina following periocular administration in pigmented and non-pigmented rats (Figure 5). The total celecoxib exposure (AUC) in the retina was 1.5 folds higher in the non-pigmented rats as compared to the pigmented animals. This is most likely due to binding of celecoxib to the melanin pigment found in the choroid as celecoxib has been shown to bind to melanin pigment in vitro. The authors found that celecoxib could bind to both natural and synthetic melanin in vitro with binding affinity of 0.08 × 10-6 M which is less than that of chloroquine, a drug which strongly binds to melanin but greater than that reported for drugs like timolol and norfloxacin, which have also been shown to bind to melanin59-61. When celecoxib is delivered using a sustained release system (microparticles) injected in the periocular tissue, the retinal levels are 7.5 fold lower in the pigmented rats at 8 days post administration as compared to non-pigmented rats, possibly due to incomplete saturation of pigment binding sites due to slow release and low levels of drug delivery. Unlike retinal levels, the celecoxib concentrations are significantly higher in the choroid-RPE of the pigmented rats as compared to the non-pigmented rats. Since over 95% of celecoxib is delivered locally to the retina following periocular administration, the binding of celecoxib to melanin can have a significant impact on the amount of drug delivered17. Such differences in delivery between pigmented and non-pigmented animals have been demonstrated for other drugs including chloroquine and bromonidine albeit after topical administration62-65.
Figure 5.
Ratio of ocular tissue AUCs (for celecoxib suspension) or tissue concentrations (for celecoxib-PLA particles) between pigmented BN rats and albino SD rats. Data are presented as the ratio of the mean AUCs or concentrations for n = 4. In the celecoxib-PLA microparticle group, drug levels could not be detected in the contralateral sclera, lens, and cornea in albino SD rats and in contralateral lens and cornea of BN rats. reproduced with permission from Cheruvu NP, Amrite AC, Kompella UB. Effect of eye pigmentation on transscleral drug delivery. Invest OphthalmolVisSci 2008;49(1):333-41. Copyright (2008) ARVO.
3.6. Relevance of Pigmentation with Sustained Release Dosage Forms
Due to the chronic nature of diseases afflicting the posterior segment of eye and due to the short-half life of several drugs within the vitreous humor66, majority of the dosage forms currently developed are sustained release dosage forms including implants67, scleral plugs68, fibrin sealant69, microparticles33, 49, 70-71 and nanoparticles72. To compare and contrast the effect of pigmentation on sustained delivery versus bolus injection, Cheruvu et al51. studied the relative delivery of celecoxib between pigmented (BN, Brown Norway) and albino (SD, Sprague Dawley) rats following periocular injection of celecoxib suspension and celecoxib encapsulated in PLA microparticles. The BN:SD rat ratio of either tissue AUCs (celecoxib suspension study) or concentrations on day 8 (celecoxib-PLA particle study) were estimated. Drug distribution was considered the same in a given tissue between the two strains of rats, if this ratio is equal to 1. If the ratio is more than one, there is greater accumulation/delivery in BN rat. If it is less than 1, the delivery is lower in BN rat. The authors found that BN:SD rat tissue ratios were the highest in choroid-RPE among all the tissues and the lowest in the retina and vitreous (Figure 5). Greater reduction in BN:SD ratio for retinal and vitreal levels following periocular injection of celecoxib-PLA microparticles (Figure 5) clearly showed the limitation imposed by pigmentation in sustained drug delivery. With a slow release system, drug levels are maintained at low concentrations which may not be sufficient to saturate the pigment binding sites. For unpigmented tissues like cornea and lens and less pigmented sclera BN:SD ratio for celecoxib AUCs in the plain celecoxib dosing study were close to 1.
3.7. Effect of the Choroid-Bruch Layer, Lipophilicity, and Charge
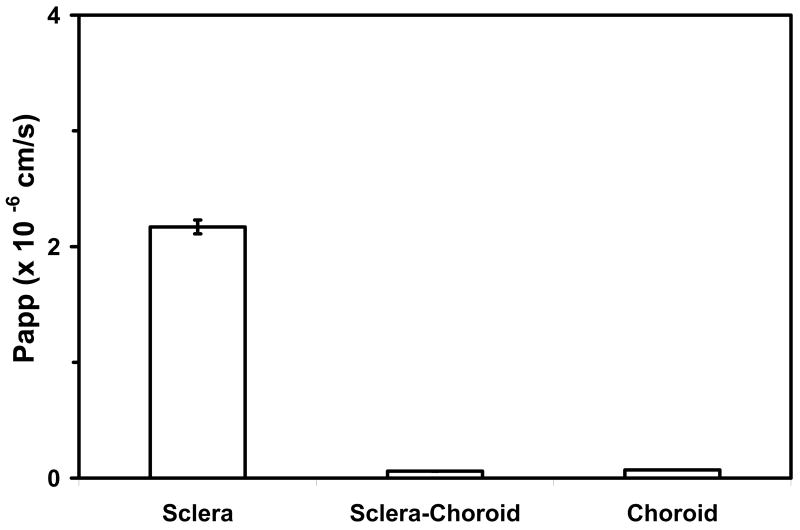
Retinal delivery of celecoxib following periocular administration involves the transscleral pathway. The drug diffuses through the sclera and the choroid-RPE to reach the retina. It is essential to study the permeability and other transport characteristics of these tissues to design better delivery approaches. In one study, the transport permeability of celecoxib and other small molecules such as budesonide, 3H-mannitol, sodium fluorescein, and rhodamine were determined across bovine and porcine sclera with or without the choroid-Bruch's layer 73. The order of permeability coefficients were 3H-mannitol > fluorescein > budesonide > celecoxib > rhodamine 6G 73. This order showed that hydrophilic molecules are more permeable than lipophilic ones. Also, the presence of the choroid-Bruch's layer reduced the permeabilities 73. The choroid-Bruch's layer proved to be a more significant barrier than the sclera, hindering the transport of lipophilic cationic solutes more than hydrophilic solutes 73. Investigation of permeabilities of celecoxib across sclera, sclera-choroid, and choroid layers indicated the following.
The transport of celecoxib across the sclera was several fold higher when compared to the choroid layer (Figure 6). This is likely associated with the binding of drug to melanin tissue present in the choroid-Bruch's layer, resulting in less drug delivered 73. This study also showed that size of the drug molecule alone does not necessarily determine permeability, as some of these solutes had similar molecular radii but very different permeabilities 73.
Figure 6.
Apparent permeability (Papp) of Celecoxib across bovine sclera, sclera-choroid and choroid. Data are presented as mean ± s.d. for n = 12. Based on data from Cheruvu and Kompella, 2006; Invest Ophthalmol Vis Sci 47(10): 4513-4522.
On the other hand, charge of the solute also could be important in determining the retinal availability of drugs following periocular administration. Cheruvu et al. have demonstrated that the sclera is more permeable to negatively charged solutes as compared to positive ones with the molecules used in that study 73. This is because the sclera is made up of collagen fibers and proteoglycans that are negatively charged under normal physiological conditions 73. Thus, positively charged molecules will likely bind better to this layer, resulting in poor transport 73.
Thus delivery of drugs across the sclera-choroid is better for solute molecules that are neutral or negatively charged as compared to positively charged molecules and also it is better for hydrophilic drugs as compared to the lipophilic ones. Transscleral delivery of celecoxib, a neutral hydrophobic drug can probably be enhanced by making it more hydrophilic while maintaining its Cox-2 inhibitory activity.
4. Polymeric Microparticles for Sustained Celecoxib Delivery to the Retina
DR and AMD are chronic disorders which require a prolonged duration of therapy. Sustained release delivery systems like implants as well as particulate systems can be designed that can provide prolonged delivery of the drug to the retina. It is essential to understand the disposition of the drug as well as the delivery system to design systems which can effectively deliver the drugs to the target tissue. Amrite and Kompella53 investigated the disposition of particulate systems after periocular administration. Their investigations reveal that particulate systems greater than 200 nm in size (diameter) are retained in the periocular tissue at least for 2 months. Small nanoparticles (20 nm) are rapidly cleared from the site of administration after periocular administration and are not suitable for sustained delivery to the retina. Using modeling and simulation, Amrite et al.74 have demonstrated that celecoxib can be delivered to the retina in a sustained manner using particulate systems. Microparticles because of their lower surface:volume ratio and much lower clearance from the periocular site of administration, probably best sustain the retinal delivery of celecoxib74. Ayalasomayajula and Kompella70 have determined that celecoxib delivery to the retina can be sustained for a period of at least 2-weeks following periocular administration of celecoxib-PLGA microparticles. The solution of celecoxib is unable to sustain retinal celecoxib delivery. Amrite and Kompella33 have shown that therapeutically effective levels of celecoxib in the retina can be achieved at 2-months post periocular administration of celecoxib-PLGA microparticles. Thus, particulate systems can effectively deliver celecoxib to the retina and can sustain the delivery for prolonged periods ranging in months.
5. In Vivo Effects of Celecoxib
Celecoxib effectiveness in diabetic rat models has been tested in a few studies17, 33, 70. Following oral administration of 50 mg/kg celecoxib b.i.d, celecoxib effectively inhibited diabetes induced increase in the expression of VEGF in the retina. Sprague-Dawley rats were injected with streptozotocin (60 mg/kg) in order to induce diabetes 17. After 8 days of treatment, the rats were sacrificed and retinas were collected and analyzed 17. Results showed that after induction of diabetes, the VEGF expression in rats was elevated (2.3 ± 0.8-fold) compared to control rats, but celecoxib effectively lowered the VEGF expression to control levels 17 This change in expression shows that the inhibitory effects of celecoxib on cyclooxgenase-2 are associated with a reduction in VEGF secretion which can have benefits in the treatment of DR.
Oxidative stress occurs in the retina as a result of diabetes and is associated with an increase in thiobarbituric acid and 4-hydroxynoneal levels and a decrease in GSH levels. The oxidative stress in turn is associated with increased secretion of VEGF. During synthesis of prostaglandins the Cox enzymes generate oxygen free radicals which together with other oxidative species resulting because of the increased glucose levels in diabetes can produce oxidative stress. Thus, inhibition of Cox activity in the retina can be helpful in reducing the associated oxidative stress. In a previous study, a single dose of subconjunctivally administered celecoxib was effective in reducing the oxidative stress in a diabetic model 75. Fourteen days after treatment, the thiobarbituric acid and 4-hydroxyneal levels were significantly decreased while the GSH levels were increased, reducing the overall oxidative stress and subsequent negative effects contributing to DR 75.
As described in the previous sections, VEGF is an important contributor to the pathology of DR and AMD. VEGF levels in the retina increase as diabetes progresses. These increased VEGF levels lead to increased vascular leakage in the retina. In addition, prostaglandins themselves, especially PGE2 has been associated with increased vascular permeability. Amrite et al33. have demonstrated that periocular administration of celecoxib-PLGA microparticles can effectively inhibit diabetes induced elevations in retinal PGE2, VEGF and vascular leakage (Figure 7). With 2 months of diabetes in rats, there was a 3-, 1.7- and 2.7 folds increase in the retinal PGE2, VEGF and vascular leakage. In the animals which were treated with a single dose periocular administration of celecoxib-PLGA microparticles, there was a significant reduction in the elevated levels of PGE2, VEGF and vascular leakage with 40%, 50% and 50% inhibition, respectively, with celecoxib treatment. Thus celecoxib had beneficial effects in a rat model of early DR. In addition, the authors demonstrated that the sustained release particulate system was safe and did not lead to any histopathological damage in the retina.
Figure 7.
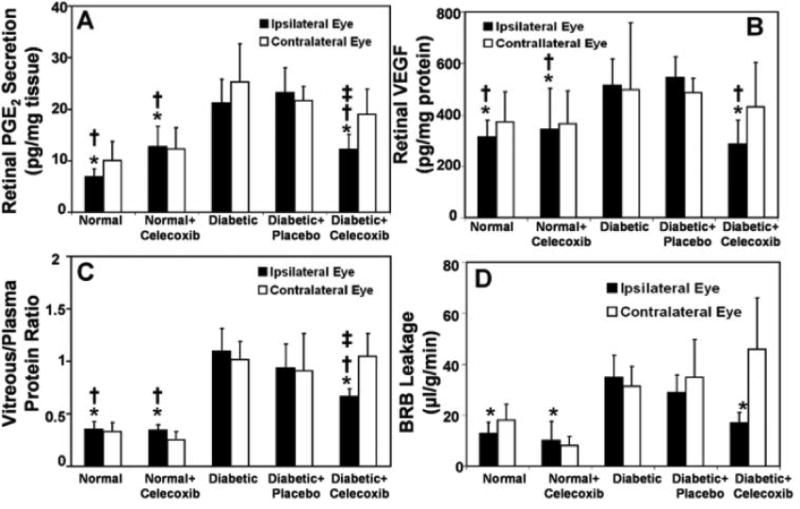
Inhibitory effects of celecoxib-PLGA microparticles on diabetes induced elevations in (A) retinal PGE2 (n = 4); (B) retinal VEGF (n =8–9); (C) vitreous-plasma protein ratio (n= 4); and (D) blood–retinal barrier leakage (n= 4). The parameters were estimated 60 days after periocular administration of the placebo or celecoxib-PLGA microparticles to rats. Data are expressed as the mean ± SD. Significantly different from the *diabetic group, the †diabetic + placebo group, or the ‡contralateral eye. Based on data from Amrite et al., 2006; Invest Ophthalmol Vis Sci 47(3): 1149-1160.
6. Conclusion
Celecoxib, a small molecule cyclooxygenase-2 inhibitor capable of inhibiting prostaglandin secretion, VEGF expression, and oxidative stress in retinal cells is a potential new treatment for DR and AMD (Figure 8). Celecoxib has been demonstrated to be effective in alleviating the biochemical changes in the retina associated with diabetes. It is effective orally, although large doses are required. Periocular administration of celecoxib can lead to retinal delivery of the drug through the transscleral pathway. The periocular delivery of celecoxib to the retina is several folds higher as compared to systemic administration. The retinal delivery of celecoxib following periocular administration can be sustained for a few months using sustained release microaprticulate systems. Celecoxib inhibits VEGF mRNA and VEGF secretion from RPE cells in vitro at nanomolar concentrations and has anti-proliferative effects on choroidal endothelial cells as well as RPE. In vivo periocular administration of celecoxib can reduced diabetes induced oxidative stress in the retina as well as can effectively inhibit diabetes induced elevations in PGE2, VEGF, and vascular leakage. Thus, celecoxib has the potential to treat DR as well as other proliferative and neovascular diseases of the eye.
Figure 8.
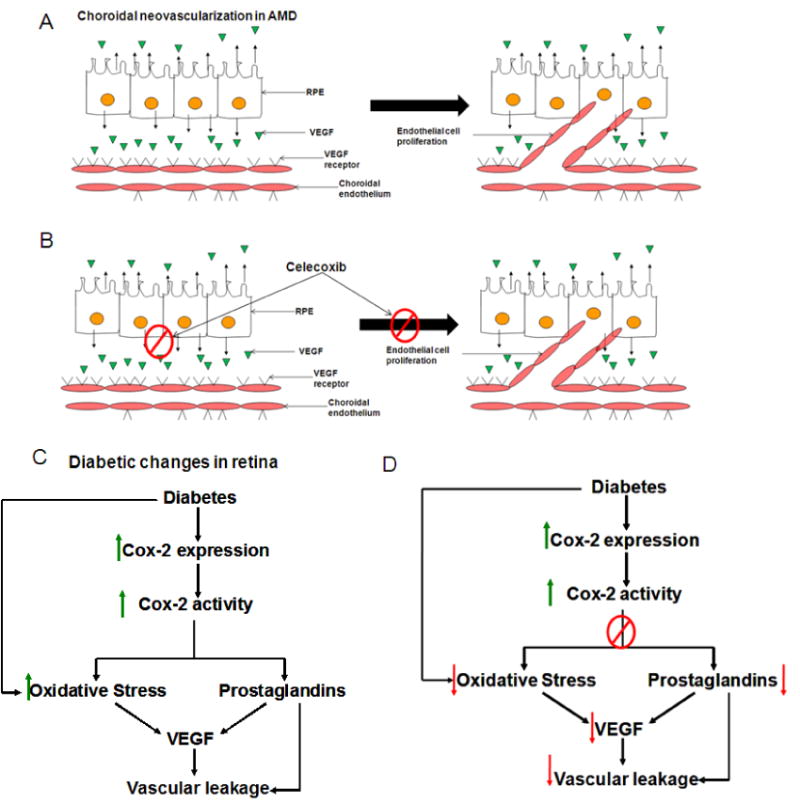
Probable mechanisms for celecoxib effectiveness in AMD and DR. Panel A: mechanism of choroidal neovascularization in AMD. Panel B: Celecoxib inhibits choroidal neovascularization in AMD by inhibiting VEGF secretion as well inhibiting endothelial cell proliferation. Panel C: Changes in retina as a result of diabetes. Panel D: Celecoxib inhibits Cox-2 leading to decreased oxidative stress, prostaglandins and decreased retinal VEGF leading to a decrease in vascular leakage in the retina.
Expert Opinion
AMD and DR together constitute a majority of the cases of blindness in the aging population. There is an unmet need for safer and more effective therapies for the treatment of these disorders. These disorders have some common factors involved in their pathophysiological progression; these include involvement of growth factors like VEGF, neovascularization, and cell proliferation. Drugs having anti-inflammatory, anti-proliferative and/or anti-VEGF activities can be beneficial in the treatment of the above disorders. Celecoxib is a selective Cox-2 inhibitor which has anti-inflammatory, anti-proliferative, and anti-VEGF effects in the retina. Celecoxib reduces the secretion of VEGF from the RPE and has anti-proliferative effects on the RPE and choroid endothelium. The anti-proliferative effects of celecoxib are through a Cox-2 independent mechanism, thus all Cox-2 inhibitors may not be useful for treatment of these disorders. These two effects combined make celecoxib a unique candidate drug for the treatment of AMD and DR. A hurdle in the treatment of retinal disorders is the effective delivery of drugs to the retina. The topical route is ineffective and systemic route is associated with systemic toxicity because of the high doses required to achieve therapeutic levels in the retina. The intra-vitreal route can provide effective drug levels but is associated with risks particularly when multiple injections are required. The periocular route is a safer alternative to the intravitreal route and could be effective for potent drugs including drugs like celecoxib. Celecoxib can be delivered to the retina by the periocular route of administration and has been shown to be effective in treating diabetes induced elevations in retinal PGE2, VEGF, and vascular leakage. Celecoxib, when given by the periocular route, reaches the retina by the transscleral pathway. Further, it has been observed that diabetes increases the transscleral delivery of celecoxib to the retina probably because of the breakdown of the outer blood-retinal barrier. Further, due to the breakdown of inner blood-retinal-barrier in diabetic animals, celecoxib reaches the contralateral retina in the undosed eye to a greater extent, when compared to normal animals. Thus, the breakdown of the BRB as a result of the progression of diabetes can be utilized for enhanced delivery of drugs like celecoxib. Celecoxib binds to melanin with moderate affinity and its delivery to the retina is reduced in pigmented eyes as compared to non-pigmented eyes. Thus, pathophysiological factors like disease state and anatomical and physiologic factors like pigmentation of ocular tissues can significantly affect the delivery of drugs to the retina by the periocular route. Both AMD and DR require long-term therapy for their management because they are chronic disorders. Use of sustained drug delivery systems is essential for the chronic treatment of these disorders of the retina. The periocular route with sustained drug delivery systems can provide effective celecoxib levels in the retina for a period of months. Further animal studies followed with clinical studies need to be performed to conclusively determine if celecoxib could be safe and effective in treating the human DR and AMD either by itself or as an adjunct to existing therapies. Based on its mode of action celecoxib might also be effective in other proliferative and neovascular conditions of the eye including eye cancers and corneal neovascularization.
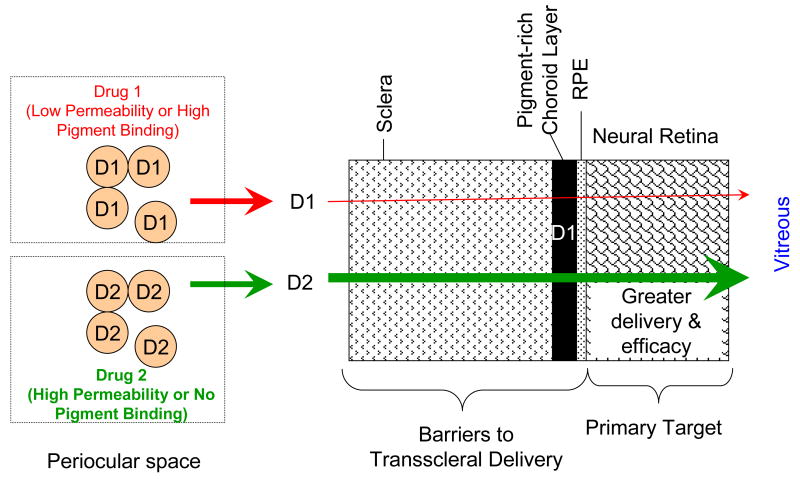
With respect to transscleral retinal drug delivery, the drug has to overcome clearance by the circulatory system and permeate across multiple barriers including sclera, choroid-Bruch's layer, and RPE to reach the neural retina and the vitreous. The target sites for DR and AMD therapies are either in the retina or the choroid layer. Pigmented choroid and RPE layers are expected to be significant barriers for drugs capable of binding to the eye pigment. Such binding reduces the solute gradients, and hence, drug transport to the neural retina. When low drug concentrations of drug are maintained in the tissue surroundings, as is the case with slow release delivery systems, the relative impedance of neural retinal drug delivery is further aggravated due to pigment binding. Drugs that bind less to the eye pigment or those exhibiting high permeability are expected to be delivered better to the retina via the transscleral pathways (Figure 9).
Figure 9.
Selection of drugs for enhanced transscleral retinal drug delivery. Drugs that bind to pigment exhibit low permeability across the choroid layer and potentially retinal pigment epithelium, with the drug delivery differences being more dramatic for slow release systems. By selecting more permeable drugs, transscleral retinal delivery and hence effects can be potentially enhanced. Arrows indicate solute release or overall permeability. Tissue layers are not drawn to scale. Key: D1 – drug 1; D2 – drug 2.
Article Highlights
DR and AMD are leading causes of blindness. Both these disorders are associated with an increase in VEGF in the retina. In addition, it has been demonstrated that inflammation plays a key role in the progression of both DR and AMD. Currently available therapies are not sufficient to halt the progression of these disorders.
Celecoxib, a selective inhibitor of cyclooxygenase 2 enzyme, has been demonstrated to have anti-VEGF activity in the retinal cells. In addition, celecoxib has anti-proliferative effects on retinal pigment epithelial and choroidal endothelial cells.
A problem with effective pharmacotherapy of retinal disorders is the poor delivery of drugs to the retina. Topical and systemic routes are generally ineffective or require high doses and the intravitreal route is associated with several complications that may compromise safety.
Periocular routes of drug delivery provide effective therapeutic levels of the drug to the retina and are less invasive to the globe as compared to the intravitreal mode of administration.
For lipophilic drugs like celecoxib, it has been demonstrated that eye pigmentation reduces transscleral retinal drug delivery, with the effects being more dramatic for slow release delivery systems. On the other hand, disease states such as diabetes increase retinal drug delivery after periocular administration.
Polymeric microparticles sustain retinal delivery of celecoxib better than nanoparticles.
Periocular celecoxib microparticles do not cause retinal atrophy or hypertrophy and reduce diabetes induced retinal oxidative stress, prostaglandin E2 secretion, VEGF expression, and vascular leakage.
Acknowledgments
The authors received funding from the NIH (grants EY017533 and DK064172).
This work was supported by NIH grant EY017533 and DK DK064172.
Footnotes
Declaration of Interest: The authors state no conflicts of interest and have received no payment in the preparation of this manuscript.
Contributor Information
Uday Kompella, University of Colorado Denver - Pharmaceutical Sciences, C238-P15, Research 2 12700 E. 19th Ave., Room 4004, Aurora, Colorado 80045, United States.
Aniruddha C Amrite, Quinitles Inc - Clinical Pharmacology, 6700 W 115th Street, Overland Park, Kansas 66211, United States.
Vidya Pugazhenthi, University of Colorado at Denver - Pharmaceutical Sciences, 12700 East 19th Avenue, Aurora, Colorado, United States.
Narayan PS Cheruvu, Covidien - Clinical Pharmacology, 675 Mcdonnell Blvd, Hazelwood, Missouri, United States.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Drug Therapy Guidelines: Pain/Arthritis Celebrex®(celecoxib) 2009 [cited 2009 November 3rd, 2009]; Available from: https://securews.bsneny.com/wps/wcm/connect/0cc10b804b0ebef28fea8f649fb47e57/neny_prov_guid_Celebrex.pdf?MOD=AJPERES.
- 2.Amrite AC, Kompella UB. Celecoxib inhibits proliferation of retinal pigment epithelial and choroid-retinal endothelial cells by a cyclooxygenase-2-independent mechanism. J Pharmacol Exp Ther. 2008 Feb;324(2):749–58. doi: 10.1124/jpet.107.128918. [DOI] [PubMed] [Google Scholar]; • This article demonstrates that celecoxib but not rofecoxib has anti-proliferative effects in retinal cells.
- 3.Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006 Mar;47(3):1149–60. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first report demonstrating the safety and efficacy of celecoxib microparticles in treating diabetic vascular leakage.
- 4.Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004 Apr;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Shah CA. Diabetic retinopathy: A comprehensive review. Indian J Med Sci. 2008 Dec;62(12):500–19. [PubMed] [Google Scholar]
- 6.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002 Feb;160(2):501–9. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004 Sep;18(12):1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 8.Hata Y, Clermont A, Yamauchi T, Pierce EA, Suzuma I, Kagokawa H, et al. Retinal expression, regulation, and functional bioactivity of prostacyclin-stimulating factor. J Clin Invest. 2000 Aug;106(4):541–50. doi: 10.1172/JCI8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, Aiello LP, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000 Apr;41(5):1153–8. [PubMed] [Google Scholar]
- 10.Miyamoto K, Ogura Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin Ophthalmol. 1999;14(4):233–9. doi: 10.3109/08820539909069542. [DOI] [PubMed] [Google Scholar]
- 11.Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001 Aug 1;88(3):230–5. doi: 10.1016/s0002-9149(01)01631-9. [DOI] [PubMed] [Google Scholar]
- 12.Kern T, Engerman R. Pharmacological inhibition of diabetic retinopathy: aminoguanidine and aspirin. Diabetes. 2001 Jul;50(7):1636–42. doi: 10.2337/diabetes.50.7.1636. [DOI] [PubMed] [Google Scholar]
- 13.Ferris Fr, Fine S, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984 Nov;102(11):1640–2. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni A, Kuppermann B. Wet age-related macular degeneration. Adv Drug Deliv Rev. 2005 Dec;57(14):1994–2009. doi: 10.1016/j.addr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Ambati J, Adamis AP. Transscleral drug delivery to the retina and choroid. Prog Retin Eye Res. 2002 Mar;21(2):145–51. doi: 10.1016/s1350-9462(01)00018-0. [DOI] [PubMed] [Google Scholar]
- 16.Carnahan M, Goldstein D. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Current Opinion in Ophthalmology. 2000;11(6):478. doi: 10.1097/00055735-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003 Jan 5;458(3):283–9. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]; • This is the first report demonstrating the efficacy of orally administered celecoxib in reducing VEGF expression and vascular leakage in diabetic retinas.
- 18.Xu X, Zhu Q, Xia X, Zhang S, Gu Q, Luo D. Blood-retinal barrier breakdown induced by activation of protein kinase C via vascular endothelial growth factor in streptozotocin-induced diabetic rats. Curr Eye Res. 2004 Apr;28(4):251–6. doi: 10.1076/ceyr.28.4.251.27834. [DOI] [PubMed] [Google Scholar]
- 19.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997 Sep;46(9):1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 20.Aiello LP. Vascular endothelial growth factor. 20th-century mechanisms, 21st-century therapies. Invest Ophthalmol Vis Sci. 1997 Aug;38(9):1647–52. [PubMed] [Google Scholar]
- 21.Aiello LP. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997 Jun;8(3):19–31. doi: 10.1097/00055735-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Aiello LP. Vascular endothelial growth factor and the eye: biochemical mechanisms of action and implications for novel therapies. Ophthalmic Res. 1997;29(5):354–62. doi: 10.1159/000268033. [DOI] [PubMed] [Google Scholar]
- 23.Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The temporal and spatial vascular endothelial growth factor expression in retinal vasculogenesis of rat neonates. Lab Invest. 1996 Jan;74(1):68–77. [PubMed] [Google Scholar]
- 24.Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996 Apr;74(4):819–25. [PubMed] [Google Scholar]
- 25.Duh EJ, Yang HS, Haller JA, De Juan E, Humayun MS, Gehlbach P, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol. 2004 Apr;137(4):668–74. doi: 10.1016/j.ajo.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Kliffen M, van der Schaft TL, Mooy CM, de Jong PT. Morphologic changes in age-related maculopathy. Microsc Res Tech. 1997 Jan 15;36(2):106–22. doi: 10.1002/(SICI)1097-0029(19970115)36:2<106::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996 Aug;37(9):1929–34. [PubMed] [Google Scholar]
- 28.Baffi J, Byrnes G, Chan CC, Csaky KG. Choroidal neovascularization in the rat induced by adenovirus mediated expression of vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 2000 Oct;41(11):3582–9. [PubMed] [Google Scholar]
- 29.Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997 Jul;151(1):281–91. [PMC free article] [PubMed] [Google Scholar]
- 30.Schwesinger C, Yee C, Rohan RM, Joussen AM, Fernandez A, Meyer TN, et al. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol. 2001 Mar;158(3):1161–72. doi: 10.1016/S0002-9440(10)64063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000 Jul;157(1):135–44. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spaide . Etiology of late age related macular disease. In: Alfaro VD, L P, Mieler WF, Quiroz-Mercado H, Jager R, Tano Y, editors. Age Related Macular Degeneration. Philadelphia, PA: Lippincot Williams & Wilkins; 2006. pp. 23–39. [Google Scholar]
- 33.Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest OphthalmolVisSci. 2006;47(3):1149–60. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayalasomayajula SP, Amrite AC, Kompella UB. Inhibition of cyclooxygenase-2, but not cyclooxygenase-1, reduces prostaglandin E2 secretion from diabetic rat retinas. EurJPharmacol. 2004;498(1-3):275–8. doi: 10.1016/j.ejphar.2004.07.046. [DOI] [PubMed] [Google Scholar]; • This report demonstrates the superior ability of celecoxib, a selective Cox-2 inhibitor, to reduce prostaglandin E2 secretion from diabetic retinas when comapred to SC560, a selective Cox-1 inhibitor.
- 35.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004 Sep;56(3):387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 36.Ayalasomayajula SP, Amrite AC, Kompella UB. Inhibition of cyclooxygenase-2, but not cyclooxygenase-1, reduces prostaglandin E2 secretion from diabetic rat retinas. Eur J Pharmacol. 2004 Sep 13;498(1-3):275–8. doi: 10.1016/j.ejphar.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Sennlaub F, Valamanesh F, Vazquez-Tello A, El-Asrar A, Checchin D, Brault S, et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 2003;108(2):198. doi: 10.1161/01.CIR.0000080735.93327.00. [DOI] [PubMed] [Google Scholar]
- 38.Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13313–8. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazanov D, Dvory-Sobol H, Pick M, Liberman E, Strier L, Choen-Noyman E, et al. Celecoxib but not rofecoxib inhibits the growth of transformed cells in vitro. Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):267–71. doi: 10.1158/1078-0432.ccr-0412-3. [DOI] [PubMed] [Google Scholar]
- 40.Niederberger E, Manderscheid C, Grosch S, Schmidt H, Ehnert C, Geisslinger G. Effects of the selective COX-2 inhibitors celecoxib and rofecoxib on human vascular cells. Biochem Pharmacol. 2004 Jul 15;68(2):341–50. doi: 10.1016/j.bcp.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Lin HP, Kulp SK, Tseng PH, Yang YT, Yang CC, Chen CS. Growth inhibitory effects of celecoxib in human umbilical vein endothelial cells are mediated through G1 arrest via multiple signaling mechanisms. Mol Cancer Ther. 2004 Dec;3(12):1671–80. [PubMed] [Google Scholar]
- 42.Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P. Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res. 2005;7(4):R422–35. doi: 10.1186/bcr1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soo RA, Wu J, Aggarwal A, Tao Q, Hsieh W, Putti T, et al. Celecoxib reduces microvessel density in patients treated with nasopharyngeal carcinoma and induces changes in gene expression. Ann Oncol. 2006 Nov;17(11):1625–30. doi: 10.1093/annonc/mdl283. [DOI] [PubMed] [Google Scholar]
- 44.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006 Aug 31;355(9):885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 45.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006 Aug 31;355(9):873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 46.Gadgeel SM, Ruckdeschel JC, Heath EI, Heilbrun LK, Venkatramanamoorthy R, Wozniak A. Phase II study of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), and celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, in patients with platinum refractory non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007 Apr;2(4):299–305. doi: 10.1097/01.JTO.0000263712.61697.69. [DOI] [PubMed] [Google Scholar]
- 47.Heath EI, Canto MI, Piantadosi S, Montgomery E, Weinstein WM, Herman JG, et al. Secondary chemoprevention of Barrett's esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007 Apr 4;99(7):545–57. doi: 10.1093/jnci/djk112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amrite AC, Kompella UB. Celecoxib inhibits proliferation of retinal pigment epithelial and choroid-retinal endothelial cells by a cyclooxygenase-2-independent mechanism. JPharmacolExpTher. 2008;324(2):749–58. doi: 10.1124/jpet.107.128918. [DOI] [PubMed] [Google Scholar]
- 49.Ayalasomayajula SP, Kompella UB. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res. 2004 Oct;21(10):1797–804. doi: 10.1023/b:pham.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]; •• This article provides quantitative evidence for the superiority of transscleral retinal delivery of celecoxib when compared to systemic administration.
- 50.Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004 Nov;1(1):99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PubMed] [Google Scholar]; •• This article provides a comprehensive review of various approaches for transscleral drug delivery to the back of the eye.
- 51.Cheruvu NP, Amrite AC, Kompella UB. Effect of eye pigmentation on transscleral drug delivery. Invest OphthalmolVisSci. 2008;49(1):333–41. doi: 10.1167/iovs.07-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article is the first to demonstrate that transscleral retinal drug delivery is impeded by eye pigmentation, with the hinderance being greater for slow release delivery systems.
- 52.Amrite AC, Edelhauser HF, Kompella UB. Modeling of corneal and retinal pharmacokinetics after periocular drug administration. Invest OphthalmolVisSci. 2008;49(1):320–32. doi: 10.1167/iovs.07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article presents pharmacokinetic modeling approaches to predict retinal and corneal delivery of drugs from periocular routes. Also, it demonstrates that similar models can explain the data in rat and rabbit models.
- 53.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005 Dec;57(12):1555–63. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]; •• This is the first report demonstrating that while 200 nm and larger particles are retained in the periocular space for at least 2 months, 20 nm particles disappear rapidly with a half-life in the order of a few hours.
- 54.Öztürk F, Kortunay S, Kurt E, Inan Ü, Ilker SS, Basci N, et al. The effect of long-term use and inflammation on the ocular penetration of topical ofloxacin. Current Eye Research. 1999;19(6):461–4. doi: 10.1076/ceyr.19.6.461.5277. [DOI] [PubMed] [Google Scholar]
- 55.Öztürk F, Kurt E, I?nan Ü, Kortunay S, I?lker SS, Basci NE, et al. Penetration of topical and oral ofloxacin into the aqueous and vitreous humor of inflamed rabbit eyes. International Journal of Pharmaceutics. 2000;204(1-2):91–5. doi: 10.1016/s0378-5173(00)00482-8. [DOI] [PubMed] [Google Scholar]
- 56.Ozturk F, Kortunay S, Kurt E, Ilker SS, Basci NE, Bozkurt A. Penetration of topical and oral ciprofloxacin into the aqueous and vitreous humor in inflamed eyes. Retina. 1999;19(3):218–22. doi: 10.1097/00006982-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Ozturk F, Kortunay S, Kurt E, Ilker SS, Inan UU, Basci NE, et al. Effects of trauma and infection on ciprofloxacin levels in the vitreous cavity. Retina. 1999;19(2):127–30. doi: 10.1097/00006982-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Cheruvu NP, Amrite AC, Kompella UB. Effect of diabetes on transscleral delivery of celecoxib. Pharm Res. 2009 Feb;26(2):404–14. doi: 10.1007/s11095-008-9757-2. [DOI] [PubMed] [Google Scholar]; •• This article provides a quantitative evidence for the differences in transscleral retinal drug delivery in albino as well as pigmented diabetic animal models.
- 59.Abrahamsson T, Bostrom S, Brautigam J, Lagerstrom PO, Regardh CG, Vauqelin G. Binding of the beta-blockers timolol and H 216/44 to ocular melanin. Exp Eye Res. 1988 Oct;47(4):565–77. doi: 10.1016/0014-4835(88)90095-4. [DOI] [PubMed] [Google Scholar]
- 60.Ono C, Tanaka M. Binding characteristics of fluoroquinolones to synthetic levodopa melanin. J Pharm Pharmacol. 2003 Aug;55(8):1127–33. doi: 10.1211/002235703322277168. [DOI] [PubMed] [Google Scholar]
- 61.Ono C, Yamada M, Tanaka M. Absorption, distribution and excretion of 14C-chloroquine after single oral administration in albino and pigmented rats: binding characteristics of chloroquine-related radioactivity to melanin in-vivo. J Pharm Pharmacol. 2003 Dec;55(12):1647–54. doi: 10.1211/0022357022340. [DOI] [PubMed] [Google Scholar]
- 62.Acheampong AA, Shackleton M, John B, Burke J, Wheeler L, Tang-Liu D. Distribution of brimonidine into anterior and posterior tissues of monkey, rabbit, and rat eyes. Drug Metab Dispos. 2002 Apr;30(4):421–9. doi: 10.1124/dmd.30.4.421. [DOI] [PubMed] [Google Scholar]
- 63.Acheampong AA, Small D, Baumgarten V, Welty D, Tang-Liu D. Formulation effects on ocular absorption of brimonidine in rabbit eyes. J Ocul Pharmacol Ther. 2002 Aug;18(4):325–37. doi: 10.1089/10807680260218498. [DOI] [PubMed] [Google Scholar]
- 64.Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959 Oct 3;2:478–80. doi: 10.1016/s0140-6736(59)90604-x. [DOI] [PubMed] [Google Scholar]
- 65.Salazar-Bookaman M, Wainer I, Patil P. Relevance of drug-melanin interactions to ocular pharmacology and toxicology. Journal of Ocular Pharmacology and Therapeutics. 1994;10(1):217–39. doi: 10.1089/jop.1994.10.217. [DOI] [PubMed] [Google Scholar]
- 66.Durairaj C, Shah J, Senapati S, Kompella U. Prediction of vitreal half-life based on drug physicochemical properties: quantitative structure–pharmacokinetic relationships (QSPKR) Pharmaceutical Research. 2009;26(5):1236–60. doi: 10.1007/s11095-008-9728-7. [DOI] [PubMed] [Google Scholar]; •• This is the first comprehensive report for predicting vitreal half-life of drugs based on drug physicochemical properties. The article presents a large dataset and predictive equations in the albino and pigmented rabbit models.
- 67.Aukunuru JV, Sunkara G, Ayalasomayajula SP, DeRuiter J, Clark RC, Kompella UB. A biodegradable injectable implant sustains systemic and ocular delivery of an aldose reductase inhibitor and ameliorates biochemical changes in a galactose-fed rat model for diabetic complications. PharmRes. 2002;19(3):278–85. doi: 10.1023/a:1014438800893. [DOI] [PubMed] [Google Scholar]; • This article demonstrates the usefulness of an injectable biodegradable implant for sustained retinal drug delivery.
- 68.Yasukawa T, Kimura H, Tabata Y, Ogura Y. Biodegradable scleral plugs for vitreoretinal drug delivery. Adv Drug Deliv Rev. 2001 Oct 31;52(1):25–36. doi: 10.1016/s0169-409x(01)00192-2. [DOI] [PubMed] [Google Scholar]
- 69.Tsui JY, Dalgard C, Van Quill KR, Lee L, Grossniklaus HE, Edelhauser HF, et al. Subconjunctival Topotecan in Fibrin Sealant in the Treatment of Transgenic Murine Retinoblastoma. Investigative Ophthalmology & Visual Science. 2008;49(2):490–6. doi: 10.1167/iovs.07-0653. [DOI] [PubMed] [Google Scholar]
- 70.Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. EurJPharmacol. 2005;511(2-3):191–8. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]; • This article demonstrates polymeric microparticles of celexocib inhibit diabetes-induced retinal oxidative damage.
- 71.Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest Ophthalmol Vis Sci. 2003 Mar;44(3):1192–201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]; •• This is the first report assessing polymeric nanoparticles for transscleral drug delivery.
- 72.Amrite A, Kompella UB. Ocular distribution of intact nano- and microparticles following subconjunctival and systemic routes of administration. Drug Delivery Tech. 2003;3:62–7. [Google Scholar]
- 73.Cheruvu NP, Kompella UB. Bovine and porcine transscleral solute transport: influence of lipophilicity and the Choroid-Bruch's layer. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4513–22. doi: 10.1167/iovs.06-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is the first report to demonstrate that choroid layer, independent of RPE, is a significant barrier for transscleral retinal drug delivery.
- 74.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Molecular Vision. 2008;14:150–60. [PMC free article] [PubMed] [Google Scholar]; • This is the first report demonstrating that small nanoparticles (20 nm diameter) rapidly escape into the systemic and lymphatic circulations from the periocular site, explaining their short half-life at the site of administration.
- 75.Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol. 2005 Mar 28;511(2-3):191–8. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]