Abstract
Tocopherols and tocotrienols, collectively known as vitamin E, are essential antioxidant nutrients. The biological fates and metabolite profiles of the different forms are not clearly understood. The objective of this study is to simultaneously analyze the metabolites of different tocopherols and tocotrienols in mouse and human samples. Using HPLC/electrochemical detection and mass spectrometry, 18 tocopherol-derived and 24 tocotrienol-derived side-chain degradation metabolites were identified in fecal samples. Short-chain degradation metabolites, in particular γ- and δ- carboxyethyl hydroxychromans (CEHCs) and carboxymethylbutyl hydroxychromans (CMBHCs) were detected in urine, serum and liver samples, with tocopherols additionally detected in serum and liver samples. The metabolite profiles of tocotrienols and tocopherols were similar, but new tocotrienol metabolites with double bonds were identified. This is the first comprehensive report describing simultaneous analysis of different side-chain metabolites of tocopherols and tocotrienols in mice and humans. Urinary metabolites may serve as useful biomarkers for nutritional assessment of vitamin E.
Keywords: tocopherols, tocotrienols, metabolites, HPLC, MS
INTRODUCTION
Vitamin E comprises a family of four tocopherols and four tocotrienols (1). They are phenolic compounds, and each contains a chromanol ring and a 16-carbon chain. Depending on the number and position of methyl groups on the chromanol ring, they exist as α-, β-, γ-, and δ-tocopherols with a saturated side chain and α-, β-, γ-, and δ-tocotrienols with three unsaturated bonds in the side chain. The structures, using γ-tocopherol and γ-tocotrienol as examples, are illustrated in Figure 1. Vegetable oils and nuts are the major sources of tocopherols in the diet of the U. S. residents (1). Although γ-T is more abundant than α-T in the diet, the latter is the major tocopherol present in blood and tissues. α-T has been traditionally considered as the classical vitamin E because of its superior biological activity over other tocopherols in the classical fertility-restoration assay (1).
Figure 1.
Structures of tocopherols/tocotrienols and the proposed metabolic pathways. The structures are illustrated with γ-tocopherol and γ-tocotrienol, which are both dimethylated at the 7- and 8- positions. The chromanol ring is trimethylated at 5-, 7-, and 8-positions in α-tocopherol, dimethylated at 5- and 8-positions in β-tocopherol, and methylated at 8-position in δ-tocopherol. The arrows (from right to left) indicate the sequential side chain degradation and the resulting metabolites.
Due to the antioxidant function of vitamin E, many previous studies have concentrated on the metabolites resulting from oxidation of the chromanol moiety. An oxidative product, tocopheryl quinone, has been reported to form from the reaction of the tocopheroxyl radical with a peroxyl radical (2). In addition, the unsubstituted positions (C-5 and C-7) on the aromatic ring make γ-T and δ-T more active in trapping electrophiles , especially reactive nitrogen species (3). The nitration reaction yields 5-NO2-γ-T (3) and 5-NO2-, 7-NO2-, or 5,7-di-NO2-δ-T (3) as metabolites.
The major metabolites of tocopherols, however, are the side-chain degradation products with an intact chromanol ring. Using γ-tocopherol as an example, this pathway is illustrated in Figure 1. The degradation is initiated by ω-hydroxylation, catalyzed by cytochrome P450 4F (CYP4F) (4, 5) and perhaps also CYP3A (6), and then oxidized to carboxylic acid. This is followed by five cycles of β-oxidation to cleave two carbon moieties from the main side chain in each cycle (5). The final products are carboxyethyl hydroxychromans (CEHCs) (5) and their precursors, carboxymethylbutyl hydroxychromans (CMBHCs) (5) (Figure 1); these products are then subjected to glucuronidation and sulfation followed by urinary excretion (1). This pathway is responsible for the generation of the major urinary metabolites detected in healthy individuals, and the excretion of α-CEHC has been proposed to be an indicator of adequate or excess tocopherol intake (7). Some studies have reported the formation of α- and γ-CEHCs in humans under different experimental conditions (7-9). However, these studies have measured only a few tocopherol metabolites, mainly CEHCs. The formation of different tocopherol or tocotrienol metabolites through the side-chain degradation pathway has been observed in HepG2 cells (4, 10). Complete metabolite profiles of tocopherols or tocotrienols in animals or humans have not been elucidated.
Recent results suggest that γ-tocopherol may be more effective than α-tocopherol in the prevention of cancer, cardiovascular diseases, and neurodegenerative disease, reviewed in (11-13), and tocotrienols may also have anticancer and cholesterol-lowering activities (14). Therefore, it is important to understand the metabolic fates of different tocopherols and tocotrienols in experimental animals and humans. In our previous study on the inhibition of colon carcinogenesis by γ-tocopherol-rich mixture of tocopherols, we observed several tocopherol metabolites in mouse colon and fecal samples (15). In this study, we used a sensitive HPLC/electrochemical (EC) detection and LC–ESI–MS to characterize the different tocopherol side-chain metabolites in mouse fecal, urine, liver, and serum samples, as well as in human fecal, urine, and serum samples. The metabolites of different tocotrienols were also studied. This report describes the simultaneous measurement of all these metabolites to provide a more comprehensive understanding of the metabolites of vitamin E compounds in mice and humans.
MATERIALS AND METHODS
Chemicals and reagents
Standards of α- and δ-tocopherols were purchased from Sigma-Aldrich Co. (St. Louis, MO). Standards of α-, γ-, and δ-CEHCs, and α-CMBHC were purchased from Cayman Co. (Ann Arbor, MI). Standards of α-, γ-, and δ-tocotrienols were obtained from Carotech Co. (Perak, Malaysia). The purity of these standards was >90%. A preparation of γ-tocopherol-rich mixture of tocopherols (m-T, Covi®-ox T-90 low d-α mixed tocopherols) was a gift from Cognis Co. (Kankakee, IL). A gram of m-T contains 900 mg total tocopherols with 13.0% α-tocopherol, 1.5% β-tocopherol, 56.8% γ-tocopherol, and 24.3% δ-tocopherol. A preparation of tocotrienol in a 65% oil suspension (TOCOMIN® 65%) was from Carotech Co. (Perak, Malaysia). It contains 20.2% α-tocotrienol, 4.0% β-tocotrienol, 16.1% γ-tocotrienol, 9.9% δ-tocotrienol, 14.8% α-tocopherol, and 3.1% γ-tocopherol by our analysis. γ-tocopherol (purity > 98.2%) was purified from m-T by silica gel column eluted with a mixture of hexane: ethyl acetate (40:1, v:v). 5-NO2-γ-tocopherol was synthesized following the published method (3). For human studies, a γ-tocopherol-rich tocopherol dietary supplement (High Gamma Vitamin E) was from Nature’s Bounty, Inc. (Bohemia, NY). A softgel contains 200 mg γ-tocopherol, 78 mg δ-tocopherol, 133 mg α-tocopherol, and 2 mg tocotrienols in a softgel) Stock solutions of CEHCs, α-CMBHC, and tocopherols in 75% aqueous ethanol were stable for at least six months at −20 °C. Type X-A β-glucuronidase from Escherichia coli, type H-1 sulfatase (contains 24190 U/g sulfatase activity and 300,000 U/g β-glucuronidase activity), and type VI sulfatase from Aerobacter aerogenes, were purchased from Sigma (St. Louis, MO). Acetonitrile (CH3CN), ethanol (EtOH), and other reagents were of HPLC-grade or the highest purity available commercially.
Animal studies
All animal experiments were conducted under the protocol no. 02-027 approved by Rutgers University Institutional Animal Care and Use Committee. CF-1 mice (22–26 g) at 5 weeks of age were purchased from Charles River (Chicago, IL) and housed in our Animal Facility for a week for adaptation. The female mice (10/group) were maintained on the basal AIN-93M diet (75 IU/kg vitamin E, control) or the same diet supplemented with 0.3% m-T for 4 weeks. During the last four days of the experiment, pooled fecal or urine samples from 10 mice in each group were collected and stored at −20 °C. Urine samples were collected into a 50-mL tube on ice when each mouse was picked up and received a slight pressure to induce urination. For terminating the experiment, the mice (non-fasted) were sacrificed by CO2 asphyxiation. Blood was taken by cardiac puncture to prepare serum. Livers were removed and rinsed in ice-cold saline. Serum and liver samples were stored at −80 °C until use. We also used pooled fecal, urine, serum, liver, and colon samples from a previous chemoprevention study, in which male CF-1 mice were treated with azoxymethane (AOM, 5 mg/kg body weight, i.p., twice at four-day intervals, at 6 weeks of age) and dextran sulfate sodium (DSS, 1.5% in drinking water for a week at 8 weeks of age) to induce colon carcinogenesis. Different groups of mice were maintained on the AIN-93M diet or the same basal diet supplemented with 0.3% m-T, or 0.17% of a tocotrienol preparation, γ-tocopherol or δ-tocopherol for 21 weeks, starting 1 week before AOM injection until the termination of the experiment (15).
Human studies
Two healthy male adults, ages 60 and 67, participated in this study under the protocol no. 92-034 approved by Rutgers University Institutional Review Board. Written informed consent was obtained from both subjects. Their body mass indices were 22 – 25 kg/m2 and serum total cholesterols were 116 – 151 mg/dL. Exclusion criteria for the study included the use of vitamin E supplements within three months of the study, smoking, medical conditions, and medications known to affect xenobiotic metabolism. During the experiment period, the subjects consumed their regular diet. On the experiment day, each subject took a total of 12 softgels of γ-tocopherol-rich tocopherol dietary supplement in one day (six softgels at 0 h and again at 10 h). Fecal and urine samples collected at 0, 12, 24, and 48 h and blood at 0 and 12 h post-dose were used for metabolite profiling and identification.
Sample preparation
Urine and serum samples (20 μL each) were mixed with 130 μL of 1% ascorbic acid (in deionized water) and 150 μL of EtOH. Fecal samples (10 mg, wet mass) were homogenized in a mixture of 140 μL of 1% ascorbic acid and 150 μL of EtOH. Pooled liver or colon samples (100 mg, wet mass) were homogenized in a mixture of 50 μL of 1% ascorbic acid and 150 μL of EtOH. The above mixtures or homogenates were extracted with 1 mL of hexane twice. The dried hexane extracts were reconstituted in 50 μL of EtOH and termed as fraction (A), which contained tocopherols and long-chain metabolites. The aqueous residues were dried under vacuum, mixed with 50 μL of 1% ascorbic acid and 100 μL of enzyme solution containing 40 U of sulfatase and 500 U of β-glucuronidase in 0.4 mol/L sodium phosphate buffer at final pH 5.0, and then incubated at 37 °C for 90 min. After cooling on ice, the samples were extracted with 1 mL of ethyl acetate twice. The dried ethyl acetate extracts were reconstituted in 50 μL of 40% aqueous EtOH and termed as fraction (B), which contained medium-chain and short-chain tocopherol metabolites. For the analysis of tocopherols and all metabolites, fraction (A) and (B) were combined to form a final solution in 70% aqueous EtOH. The injection volumes of the final solution were 50 μL for HPLC/EC and 10 μL for LC/MS. Under these conditions, the analytes were stable with over 90% recoveries.
HPLC/EC
Samples were analyzed on a HPLC/EC system comprised of an ESA 5600A coulochem electrode array system (CEAS), two ESA Model 582 dual piston pumps, and an ESA Model 542 autosampler (set at 6 °C). A SUPELCOSIL C18 reversed-phase column (150 mm × 4.6 mm I.D., 5 μm-particle size) was used. The mobile phases consisted of solution A (CH3CN:EtOH:water, 165:135:700, v:v:v, containing 20 mmol/L ammonium acetate, pH: 4.4) and solution B (CH3CN:EtOH:water, 539:441:20, v:v:v, containing 20 mmol/L ammonium acetate, pH: 4.4). A gradient elution consisted of an 8-min initial linear increase of B from 18 to 77% at a flow rate of 0.6 mL/min, followed by maintaining B at 77% from 8 to 16 min. Then, B was changed from 77% to 100% from 16 min to 35 min at a flow rate of 0.8 mL/min. At 35.1 min, the flow rate was increased to 1.1 mL/min until 50 min. At 50.1 min, B was returned to 18% at a flow rate of 1 mL/min for 10 min to re-equilibrate the column. The eluent was monitored by the CEAS detector with potential settings at 200, 400, 500, and 700 mV. Data for the figures were from the channel set at 400 mV of the CEAS.
HPLC assay was validated with specificity, precision (coefficients of variation ≤ 10%) and accuracy (≥ 87%), linearity (10 nmol/L – 200 μmol/L). The limits of detection were 0.1 pmol for CEHCs, 0.05 pmol for tocopherols, and the limits of quantitation were 0.5 pmol for CEHCs, 0.2 pmol for tocopherols. The extraction recovery was over 90%. In our EC detection system, α-, γ-, and δ-CEHCs showed the same peak areas. α-, γ-, and δ-tocopherols also showed same responses. The signals of equimolar tocopherols were around two-fold lower than those of CEHCs. Because authentic standards of many tocopherol metabolites were not available, we used γ-CEHC as surrogate standard for short-chain (2 or 4 carbons in the primary aliphatic chain) and mediumchain (6 or 8 carbons) metabolites, including δ- and γ-CMBHCs, CMHHCs, CDMOHCs, and γ-tocopherol for long-chain (greater or equal to 10 carbons) metabolites, including CDMDHCs and carboxyl tocopherols. Therefore, the reported levels can be only considered as estimated values.
LC-ESI-MS
LC/MS was conducted on a Thermo LTQ linear ion trap mass detector (ThermoFisher Scientific, San Jose, CA) interfaced by an ESI probe, with a Surveyor MS pump and a Surveyor autosampler (set at 6 °C). Chromatographic separation was performed on a column identical to the one used for HPLC/EC analysis. The LC mobile phases (delivered at 0.3 mL/min) had the same composition as those used in the HPLC/EC analysis, only without ammonium acetate salt. The linear gradient was increased from 35% to 100% of B from 0 to 15 min, maintained at 100% of B from 15 min to 42 min, and then re-equilibrated to 35% of B for 18 min. Optimized MS parameters in the negative-ion ESI mode were tuned for tocopherols or tocotrienols. LC/MS assay was validated with specificity, precision (coefficients of variation ≤ 15%) and accuracy (≥ 85%), linearity (20 nmol/L – 200 μmol/L). The limits of detection were 0.2 pmol for CEHCs, 1 pmol for tocopherols, and the limits of quantitation were 1 pmol for CEHCs, 4 pmol for tocopherols. Data acquisition was performed in full-scan MS and MSn modes. The MS product-ion spectra were displayed by CID of the deprotonated molecules [M–H]− of all analytes at their respective LC retention times.
RESULTS
Metabolite profiles of tocopherols or tocotrienols in mouse fecal samples
In this study, we used HPLC/EC detection and ESI/MS to analyze the major metabolites of different tocopherols or tocotrienols in the samples. Representative HPLC chromatograms of the metabolites detected in mouse fecal samples collected after administration of tocopherol or tocotrienol supplemented diet are shown in Figure 2A. In the 0.3% m-T supplemented mice, there were three groups of metabolites from δ-, γ-, and α-tocopherols according to their retention times (Rt) on HPLC/EC detection: a) short-chain metabolites – CEHCs (5.0-8.0 min) and CMBHCs (10.0-13.0 min); b) medium-chain metabolites – carboxymethylhexyl hydroxychromans (CMHHCs) and carboxydimethyloctyl hydroxychromans (CDMOHCs) (15.0-21.0 min); and c) long-chain metabolites – carboxydimethyldecyl hydroxychromans (CDMDHCs) and carboxyl tocopherols (23.0-29.0 min), in addition to their parent compounds, δ-, γ-, and α-tocopherols (36.0-39.0 min). The six peaks at Rt of 5.3, 9.3, 15.4, 18.9, 23.8, and 24.8 min were identified as δ-tocopherol-derived metabolites; and the six peaks with Rt of 6.2, 11.5, 17.0, 20.0, 25.8, and 26.7 min were identified as γ-tocopherol-derived metabolites. These δ- or γ-derived metabolites were enhanced, respectively, when δ- or γ-tocopherol enriched diet was given in the AOM/DSS-treated mice (data not shown). Peaks appearing in the m-T supplemented groups, but not in the pure δ- and γ-tocopherol supplemented groups, were identified as α-tocopherol-derived metabolites, of which the most prominent was the peak of α-carboxyl tocopherol with Rt of 28.5 min. The estimated concentrations of metabolites in fecal samples from 0.3% m-T group are shown in Table 3. The peak heights of γ-tocopherol- and δ-tocopherol-derived metabolites were higher than α-tocopherol-derived metabolites and short-chain tocopherol-derived metabolites were higher than long-chain metabolites. Incubation of the fecal sample extracts with glucuronidase and sulfatase did not change the peak areas for all metabolites (data not shown), suggesting that these metabolites existed in un-conjugated forms.
Figure 2.
Identification of multiple tocopherol/tocotrienol metabolites in mouse fecal samples. A: HPLC chromatogram of tocopherol/tocotrienol metabolites. The peaks eluted between 13.5 – 30 min for the tocotrienol group were not assigned. They could be derived from tocotrienols or other compounds. B: Representative MS2 spectra and structural elucidation of deprotonated ions from γ-derived metabolites.
Table 3.
Levels of Tocopherol Metabolites and Tocopherols in Fecal, Urine, Serum, and Liver Samples Collected from 0.3% m-T Supplemented Micea
| Compound | Feces (μmol/kg b) |
Urine (μM) |
Serum (μM) |
Liver (nmol/kg b) |
|---|---|---|---|---|
| δ-CEHC | 28 | 104 | 0.36 | 0.17 |
| γ-CEHC | 32 | 171 | 0.63 | 0.03 |
| α-CEHC | 11 | 19 | 0.12 | ND |
| δ-CMBHC | 54 | 79 | 1.1 | 1.6 |
| γ-CMBHC | 109 | 110 | 2.4 | 0.54 |
| α-CMBHC | 23 | 5 | 0.03 | ND |
| δ-CMHHC | 9 | ND | 0.17 | Trace |
| γ-CMHHC | 34 | ND. | 0.06 | Trace |
| δ-CDMOHC | 14 | ND | Trace | Trace |
| γ-CDMOHC | 27 | ND | Trace | Trace |
| δ-CDMDHC | 53 | ND | 0.8 | Trace |
| γ-CDMDHC | 86 | ND | 7.2 | Trace |
| δ-tocopherol | 831 | ND | 0.5 | 0.4 |
| γ-tocopherol | 2505 | ND | 1.0 | 4.4 |
| α-tocopherol | 418 | ND | 10 | 24 |
Values obtained from HPLC/EC analysis are given as mean of duplicate analysis. ND, nondetectable; Trace, less than 10 nM or 10 nmol/kg. The mice were maintained on the 0.3% m-T supplemented diet for 4 weeks. Fecal, urine, and serum samples were pooled samples collected from 10 mice in each group. Liver samples were pooled from 4 mice. The results corresponded to the chromatograms for 0.3% m-T group in Figures 2, 4, and 5, respectively.
wet weight.
In fecal samples from the mice supplemented with the tocotrienol preparation, γ- and α-tocotrienols, δ-, γ-, and α-CEHCs and CMBHCs, as well as a new metabolite eluted at 10.8 min were observed in the HPLC/EC chromatogram (Figure 2A). The new metabolite was subsequently identified as γ-carboxymethylbutenyl hydroxychroman (CMBenHC) by LC–MS/MS (Table 2). Most of the γ- and δ-CEHCs and CMBHCs were likely to be derived from the more abundant tocotrienols in the diet (10), even though they could also be metabolites of 3.1% γ-tocopherol and trace amounts of δ-tocopherol present in the diet. More short-chain tocotrienol-derived metabolites than long-chain metabolites were present in mouse fecal samples.
Table 2.
Structural Information of the Metabolites of δ-, γ-, α-Tocotrienols Obtained by LC-MSn in Negative ESI Sourcea
| Compound | Rt1 (min) | MSn fragments (relative intensity, %) |
|---|---|---|
| δ-CEHC | 6.1 |
MS2/249: 205 (100), 157 (10); MS3/205: 190 (30), 135 (100), 122 (15). |
| γ-CEHC | 7.4 |
MS2/263: 245 (5), 219 (100), 171 (15); MS3/219: 204 (30), 149 (100), 136 (10). |
| α-CEHC | 8.1 |
MS2/277: 259 (30), 233 (100), 185 (50); MS3/233: 218 (40), 176 (10), 163 (100), 150 (15). |
| δ-CMBenHC | 10.8 | MS2/289: 271 (70), 245 (100), 135 (20). |
| γ-CMBenHC | 11.8 | MS2/303: 285 (40), 259 (100), 205 (40), 149 (20). |
| α-CMBenHC | 12.7 | MS2/317: 281 (15), 273 (100), 258 (10), 163 (10). |
| δ-CMBHC | 11.0 |
MS2/291: 273 (10), 247 (100), 135 (5), 122 (20); MS3/247: 229 (18), 148 (80), 135 (100). |
| γ-CMBHC | 12.6 |
MS2/305: 287 (15), 261 (100), 149 (5), 136 (45); MS3/261: 191 (40), 162 (80), 149 (100). |
| α-CMBHC | 13.9 |
MS2/319: 301 (40), 275 (100), 163 (10), 150 (10); MS3/275: 176 (50), 163 (100), 150 (20). |
| δ-CMHenHC | 12.9 | MS2/317: 299 (10), 273 (100), 258 (15), 135(10). |
| γ-CMHenHC | 14.4 | MS2/331: 313 (20), 287 (100), 272 (40), 149 (25). |
| α-CMHenHC | 16.0 | MS2/345: 327 (20), 309 (15), 301 (100), 163 (25). |
| δ-CDMO(en)2HC | 16.6 | MS2/357: 339 (40), 313 (100), 297 (25), 135 (10). |
| γ-CDMO(en)2HC | 18.0 | MS2/371: 353(70), 327 (100), 149 (15). |
| α-CDMO(en)2HC | 18.8 |
MS2/385: 367 (40), 341 (100), 293 (10), 269 (15), 221 (10), 163 (10). |
| δ-CDMOenHC | 17.1 | MS2/359: 341 (20), 315 (100), 135 (30). |
| γ-CDMOenHC | 18.4 | MS2/373: 355 (30), 329 (100), 149 (30). |
| α-CDMOenHC | 19.3 | MS2/387: 369 (70), 343 (100), 163 (15), 150 (40). |
| δ-CDMD(en)2HC | 19.7 |
MS2/385: 367 (20), 341 (100), 326 (25), 249 (5), 135 (15). |
| γ-CDMD(en)2HC | 20.8 |
MS2/399: 381 (10), 355 (100), 340 (40), 249 (10), 149 (20). |
| α-CDMD(en)2HC | 21.6 |
MS2/413: 395 (35), 369 (100), 354 (15), 249 (5), 163 (70), 150 (10). |
| Carboxyl δ-tocotrienol | 23.1 |
MS2/425: 407 (5), 389 (10), 381 (100), 366 (25), 289 (5), 135 (8). |
| Carboxyl γ-tocotrienol | 24.0 |
MS2/439: 421 (35), 395 (100), 380 (40), 289 (5), 149 (18). |
| Carboxyl α-tocotrienol | 24.9 | MS2/453: 435 (5), 417 (20), 409 (100), 289 (5), 163 (40). |
| δ-tocotrienol | 27.0 | MS2/395: 380 (100), 135 (20). |
| γ-tocotrienol | 28.1 | MS2/409: 394 (100), 149 (20). |
| α-tocotrienol | 29.7 | MS2/423: 408 (30), 163 (100) |
The retention times on LC–MS/MS (Rt1) are shown.
Identification of metabolites by LC–MS
To identify metabolites by LC–MS, the characterization of chromatograms and mass spectrometric properties of candidate compounds were compared to those of the parent compounds and other likely metabolites. In this study, the negative-ion ESI mode was more sensitive for analysis of tocopherols than the positive-ion ESI mode and the peaks due to the deprotonated molecule [M–H]−, m/z 429, 415, and 401 for α-, γ-, and δ-tocopherols were predominant in the spectra. Their fragmentation pathways were analyzed based on the MSn fragmentation of the major product ions. The MS2 spectrum of m/z 429 of α-tocopherol displayed major product ions of 414 ([M–H–CH3]−) and 163. The MS2 spectrum of m/z 415 of γ-tocopherol displayed major ions of 400 ([M–H–CH3]−) and 149. The MS2 spectrum of m/z 401 of δ-tocopherol displayed major ions of 386 ([M–H–CH3]−), and 135. One common fragmentation pattern observed in this study is the retro-Diels-Alder fragmentation of the break in the bond between O-1 and C-2 position and another bond between C-3 and C-4 position in the chromanol structure (Figure 1), making respective product ions at m/z 163, 149, and 135 for α-, γ-, and δ-tocopherols; this observation was similar to that demonstrated previously (16, 17). The same CID pathway was observed for tocotrienols. Therefore, the characteristic product ions at m/z 163, 149, and 135 were used as selective marker fragment ions to identify those metabolites whose structural modification does not occur at the chromanol moiety. Moreover, the MS spectra obtained from the tested samples were compared with the control samples so that the possible metabolites could be identified.
Eighteen tocopherol-derived metabolites and the parent tocopherols were analyzed in the mouse fecal and urine samples using LC–MS. Their retention times, parent ions, and product ions are summarized in Table 1. To illustrate our structural identification procedure, a major metabolite found in mouse fecal samples with a Rt of 25.8 min from HPLC/EC detection (Figure 2A) was used as an example. From full-scan LC–MS using negative ESI source, its extracted ion chromatogram gave a precursor ion m/z 403, which was 28 Da higher than m/z 375 of γ-CDMOHC, suggesting this molecular structure may have an additional CH2CH2 moiety. Its CID product ion spectrum showed ions at m/z 385 ([M–H–H2O]−), 341, 253, and 149 (γ-marker product ion). The product ion at m/z 341 ([M–H–H2O–CO2]−) suggested the presence of a carboxyl group in the structure. The product ion at m/z 253 ([M–2H–149]−) was the remains after the loss of m/z 149. Based on these data, the metabolite was identified as γ-CDMDHC and its proposed fragmentation pathway is shown in Figure 2B (d). This approach enabled us to identify all the metabolites shown in Table 1. Figure 2B showed the MS2 spectra for structural identification of major γ-derived metabolites. The retro-Diels-Alder fragmentation, gave rise to product ions at m/z 163, 149, and 135 in MS2 or as base peaks in MS3 for α-, γ-, and δ-derived tocopherol/tocotrienol metabolites. The fragmentation pathway was consistent with previous results with ion trap (17). For better qualitative analysis, ion trap was used here.
Table 1.
Structural Information of the Metabolites of δ-, γ-, α-Tocopherols Obtained by LC-MSn in Negative ESI Sourcea
| Compound | Rt1 (min) | Rt2 (min) | MSn fragments (relative intensity, %) |
|---|---|---|---|
| δ-CEHC | 6.1 | 5.3 |
MS2/249: 205 (100), 157 (10); MS3/205: 190 (30), 135 (100), 122 (15). |
| γ-CEHC | 7.4 | 6.2 |
MS2/263: 245 (5), 219 (100), 171 (15); MS3/219: 204 (30), 149 (100), 136 (10). |
| α-CEHC | 8.1 | 8.0 |
MS2/277: 259 (30), 233 (100), 185 (50); MS3/233: 218 (40), 176 (10), 163 (100), 150 (15). |
| δ-CMBHC | 11.0 | 9.3 |
MS2/291: 273 (10), 247 (100), 135 (5), 122 (20); MS3/247: 229 (18), 148 (80), 135 (100). |
| γ-CMBHC | 12.6 | 11.5 |
MS2/305: 287 (15), 261 (100), 149 (5), 136 (45); MS3/261: 191 (40), 162 (80), 149 (100). |
| α-CMBHC | 13.9 | 13.0 |
MS2/319: 301 (40), 275 (100), 163 (10), 150 (10); MS3/275: 176 (50), 163 (100), 150 (20). |
| δ-CMHHC | 15.0 | 15.4 | MS2/319: 301 (100), 183 (15), 135(20), 122 (25). |
| γ-CMHHC | 16.5 | 17.0 | MS2/333: 315 (100), 183 (20), 149 (35), 136 (25). |
| α-CMHHC | 18.0 | 18.0 | MS2/347: 329 (100), 183 (20), 163 (20), 150 (15). |
| δ-CDMOHC | 19.1 | 18.9 |
MS2/361: 343 (100), 225 (35), 135 (35), 122 (35); MS3/343: 188 (60), 174 (60), 148 (20), 135 (100). |
| γ-CDMOHC | 20.1 | 20.0 | MS2/375: 357 (100), 225 (70), 149 (70), 136 (50). |
| α-CDMOHC | 20.9 | 20.7 | MS2/389: 371 (100%), 225 (10), 163 (10), 150 (10). |
| δ-CDMDHC | 22.2 | 23.8 |
MS2/389: 371 (100), 253 (15), 135 (15), 122 (10); MS3/371: 188 (40), 174 (40), 148 (20), 135 (100). |
| γ-CDMDHC | 23.0 | 25.8 |
MS2/403: 385 (100), 341 (20), 253 (25), 149 (25), 136 (15). |
| α-CDMDHC | 23.8 | 26.0 |
MS2/417: 399 (100), 253 (40), 163 (15), 150 (10); MS3/399: 202 (10), 163 (100). |
| Carboxyl δ-tocopherol | 23.8 | 24.8 |
MS2/431: 413 (100), 295 (49), 135 (15), 122 (10); MS3/413: 202 (20), 174 (80), 135 (100). |
| Carboxyl γ-tocopherol | 24.8 | 26.7 | MS2/445: 427 (100), 295 (75), 149 (35), 136 (35). |
| Carboxyl α-tocopherol | 26.0 | 28.5 |
MS2/459: 441 (100), 295 (70), 163 (90), 150 (90); MS3/441: 202 (5), 186 (5), 163 (100), 150 (10). |
| δ-tocopherol | 35.6 | 36.7 | MS2/401: 386 (100), 135 (20). |
| γ-tocopherol | 37.9 | 37.7 | MS2/415: 400 (100), 149 (30). |
| α-tocopherol | 40.6 | 38.9 | MS2/429: 414 (20), 163 (100). |
The retention times on LC–MS/MS (Rt1) and on HPLC/EC (Rt2) are shown. The retention times in these two separations were different, but the elution sequences were the same.
The retention times observed in the LC–MS were different from those shown in Figure 2 because the flow rates used in two systems and pump efficiencies were different. However, the elution sequences, based on hydrophobicity, were identical in these two methods. Therefore, this enabled us to obtain structural information on the peaks in HPLC/EC analysis.
It is worth noting that on LC–MS/MS analysis, three abundant peaks eluted at 26.9 min (m/z 445, MS2: 445→429), 26.0 min (m/z 447, MS2: 447→429), and 27.3 min (m/z 461, MS2: 461→443) were detected only in fecal samples collected from m-T supplemented mice with AOM/DSS treatment; these compounds could be α-tocopheryl quinone, γ- and α-tocopheryl-quinone-epoxides, respectively; the elimination of tocopheryl quinone was consistent with previous report (18). The structures of these metabolites need to be further verified. Using HPLC/EC detection, peaks for tocopheryl quinones, which lack the redox activity on the phenolic groups, were not detected by the EC detector under the current chromatographic conditions.
In mouse fecal samples collected after supplementation of tocotrienol preparation, CEHCs, CMBHCs, carboxymethylhexenyl hydroxychroman (CMHenHCs), carboxydimethyloctenyl hydroxychromans (CDMOenHCs), and carboxydimethyldecadienyl hydroxychromans (CDMD(en)2HCs) were identified as tocotrienol metabolites on LC–MS/MS analysis (Table 2). These metabolites are consistent with those reported for tocotrienols in HepG2 cells (10). Three additional groups of tocotrienol metabolites were also identified: major peaks of CMBenHCs (10.0-13.0 min) with one double bond in the side chain, minor peaks of carboxydimethyloctadienyl hydroxychromans (CDMO(en)2HCs) (16.0-19.0 min) with two double bonds, and carboxyl tocotrienols (23.0-25.0 min) (Table 2). New metabolite of γ-CMBenHC with Rt of 11.8 min, and precursor ion of m/z 303 in full-scan LC–MS by using negative ESI source, was found both in mouse fecal and urine samples. Its CID product ion spectrum showed fragment ions at m/z 285 ([M–H–H2O]−), 259 ([M–H–CO2]−), and 149 (γ-marker product ion).
Metabolite profiles of tocopherols in human fecal samples
The tocopherol metabolite profiles in human fecal samples after oral dose of tocopherol supplement are shown in Figure 3. Compared with the control mouse fecal samples, more peaks in human fecal samples before supplementation were observed and some peaks may not be related to tocopherols. After taking tocopherol supplement, almost all the side-chain degradation products identified in mouse fecal samples were found in the human fecal sample collected at 24 h post-dose and the metabolite concentrations in human fecal samples increased over time. At 24 h after dose, γ-tocopherol-derived metabolites were more prominent than δ- and α-derived metabolites, due to the higher amounts of γ-tocopherol in the supplement and greater γ-tocopherol degradation.
Figure 3.
HPLC chromatogram of tocopherol metabolites in human fecal samples. Fecal samples were collected at 0, 12, 24, and 48 h after ingestion of γ-tocopherol-rich tocopherol softgels. The responses of tocopherols (right) and tocopherol metabolites (left) are shown in different scales.
Metabolite profiles of tocopherols and tocotrienols in mouse urine samples
Representative profiles of tocopherol and tocotrienol metabolites in mouse urine samples are shown in Figure 4A. In the 0.3% m-T supplemented group, the approximate concentrations of the major metabolites in urine samples after glucuronidase and sulfatase treatment (shown in Table 3) were six to ten-fold higher than those in the unhydrolyzed samples. Incubation of the urine with glucuronidase yielded more CEHCs and CMBHCs than incubation with sulfatase. This result indicates that CEHCs and CMBHCs are excreted in the mouse urine mainly as glucuronide conjugates. Parent tocopherols were not detected. In the urine samples from mice administrated with tocotrienol preparation, short-chain metabolites, CEHCs, CMBHCs, and CMHenHCs were detected.
Figure 4.
HPLC chromatograms of tocopherol/tocotrienol metabolites in urine samples. A: tocopherol/tocotrienol metabolites in mouse urine samples. B: tocopherol metabolites in enzyme-hydrolyzed and un-hydrolyzed human urine samples collected at 0, 12, 24, and 48 h. C: tocopherol metabolites in pre-dose human urine samples after and before enzymatic hydrolysis shown in a more sensitive scale.
Metabolite profiles of tocopherols in human urine samples
The tocopherol metabolite profiles in unhydrolyzed and hydrolyzed human urine samples are shown in Figure 4B. The major metabolites in human urine samples were CEHCs and CMBHCs. In urine samples collected before tocopherol supplementation (Figure 4C), the levels after hydrolysis with glucuronidase and sulfatase (shown in Table 3) were two to six-fold higher than the levels observed without enzymatic hydrolysis. Figure 4C illustrated that, with a more sensitive scale, these urinary metabolites from humans who were not taking vitamin E supplements could be sensitively detected. Enzymatic hydrolysis studies using β-glucuronidase or sulfatase indicated that CEHCs and CMBHCs were excreted in the human urine mainly as glucuronide conjugates. At all time points, CEHCs were present at higher levels than CMBHCs; whereas parent tocopherols were not detected. Fecal and urine samples for a second human subject after taking the supplements yielded similar metabolite profiles (data not shown).
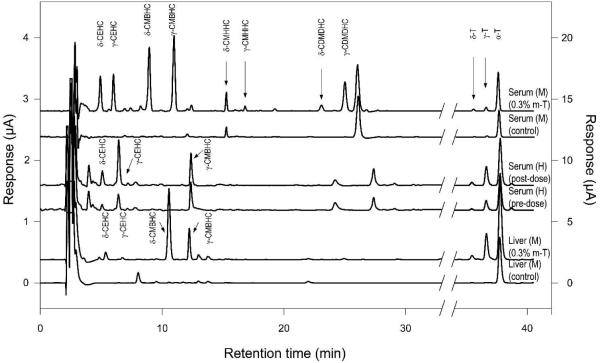
Metabolite profiles of tocopherols in mouse liver and serum samples and human serum samples
Representative HPLC chromatograms of mouse liver and serum samples, as well as human serum samples, are shown in Figure 5 and the concentration of tocopherols and their metabolites in mouse samples from 0.3% m-T supplemented group are shown in Table 3. In control mouse serum and liver samples, as well as human serum samples collected before tocopherol supplementation, all metabolites and tocopherols were at much lower levels. In human serum samples collected after tocopherol supplementation, the concentrations of α-, γ-, and δ-tocopherols were 21.1, 6.19, 0.5 μM, respectively, and the metabolite concentrations after enzymatic hydrolysis were: CEHCs (α-: 0.02, γ-: 0.35, δ-: 0.09 μM) and CMBHCs (α-: 0.03, γ-: 0.21, δ-: 0.08 μM).
Figure 5.
HPLC chromatogram of tocopherol metabolites in mouse (M) and human (H) serum samples as well as mouse liver samples. The responses of tocopherols (right) and tocopherol metabolites (left) are shown in different scales.
In AOM/DSS treated mice, 5-NO2-γ-tocopherol with Rt of 39.8 min in HPLC/EC was detected in fecal, urine, serum, liver, and colon samples, and the levels in the liver and colon samples from the m-T supplemented mice were three-fold higher than those in control group (Table 4). However, 5-NO2-γ-tocopherol was not detected in mouse samples which did not receive AOM/DSS treatment or in human samples.
Table 4.
Levels of 5-NO2-γ-Tocopherol in Fecal, Urine, Serum, Liver, and Colon Samples Collected from AIN-93M or m-T Supplemented Mice Treated with AOM/DSSa
| Feces (nmol/kg b) |
Urine (nM) |
Serum (nM) |
Liver (nmol/kg b) |
Colon (nmol/kg b) |
|
|---|---|---|---|---|---|
| Control | 173 | 93 | 87 | 110 | 430 |
| 0.3% m-T | 175 | 80 | 113 | 330 | 800 |
Values obtained from HPLC/EC analysis are given as mean of duplicate analysis. The AOM/DSS-treated mice were maintained on the AIN-93M diet or the 0.3% m-T supplemented diet for 21 weeks. Fecal, urine, and serum samples were pooled samples collected from 10 mice in each group. Liver and colon samples were pooled from 4 mice.
wet weight.
DISCUSSION
It has been proposed that vitamin E degradation pathway involves an initial ω-oxidation followed by cycles of β-oxidation. Although different metabolites from tocopherols or tocotrienols have been demonstrated in HepG2 cells (4, 10), the complete set of metabolites has never been shown in animals. In the present study, we developed sensitive and efficient HPLC/EC and MS assays that allow simultaneous analysis of different vitamin E forms and their metabolites. All the possible metabolites from α-, γ-, and δ-tocopherols as well as from tocotrienols were identified. To our knowledge, this study is the first comprehensive analysis of all the side-chain degradation metabolites of vitamin E in animals and humans. The various side-chain degradation products identified herein were similar to those observed in HepG2 cells (4, 10).
In our results, the abundance of tocopherols, tocotrienols, and their metabolites in fecal samples, suggests that fecal excretion is their major elimination route. The fecal vitamin E metabolites are in non-conjugated forms. In a preliminary experiment, incubation of tocopherols with fecal suspensions under anaerobic conditions failed to produce chain-degradation metabolites, which was consistent with a previous report (19). However, the degradation of tocopherols by intestinal microflora cannot be ruled out. In urine samples, only the short-chain metabolites, CEHCs and CMBHCs, were determined. The majority of vitamin E metabolites in mouse and human urine are excreted as glucuronide conjugates. This is different from the previous observations that CEHC was shown to be excreted as sulfate in rat urine (20, 21) and glucosidated-CEHC was detected in mouse urine (22). Our results on the basal (without vitamin E supplementation) urine levels of CEHCs in human show comparable levels of γ-CEHC as, but lower levels of α-CEHC as compared to previous reports (8, 9). The rather high basal levels of CMBHCs in serum and urine samples in humans and mice is a novel observation.
Our study showed that 5-NO2-γ-tocopherol can only be detected in samples from mice treated with AOM/DSS, which had inflammation and were under nitrosative stress, and the tissue level was increased two or three-fold in m-T supplemented mice. This is the first report of nitrated γ-tocopherol in AOM/DSS treated mouse model and it suggests the trapping of reactive nitrogen species by γ-tocopherol.
In addition to the tocotrienol metabolites that were previously observed in a cell culture system (10), we observed novel tocotrienol metabolites, CMBenHCs, CDMO(en)2HCs, and carboxyl tocotrienols (Table 2). The observation of CMBenHCs in fecal and urine samples suggests the formation of the precursors containing double bond before the formation of CMBHCs in the side-chain degradation pathway, revealing that the unsaturated side chain of tocotrienols can be metabolized in the same mechanism as tocopherols.
Consistent with previous reports (23, 24), we observed that γ- and δ-tocopherols were more extensively degraded and their side-chain degradation products were more abundant than those of α-tocopherol, even in the mice that did not receive γ-tocopherol supplementation (Figure 2-5). It is known that hepatic α-tocopherol transfer protein selectively facilitates the transfer of α-tocopherol from liver to the blood (1). The γ- and δ-tocopherols remaining in the liver are metabolized via side-chain degradation. The more lipophilic long-chain metabolites together with parent tocopherols as well as other fat soluble compounds are excreted through the bile into the intestine (25), whereas the more hydrophilic short-chain metabolites are conjugated, enter the blood, and excreted in the urine. In our results, some rather abundant peaks for long-chain degradation metabolites were observed in mouse serum, which were consistent with the reports of long-chain carboxychromanols detected in rat plasma (26). The human blood basal levels of tocopherols and CEHCs measured here are in the range of those reported in the literature (27, 28). Mouse blood basal levels of tocopherols in our results are of the same order, but higher than those measured by Traber et al. (29).
The side-chain degradation metabolites still maintain the intact chromanol moiety, can quench reactive oxygen and nitrogen species, and may have other biological activities that contribute to disease prevention (11, 13). It has also been reported recently that the long-chain metabolites of γ- and δ-tocopherols can inhibit COX-2 activity (30).
In the United States, it has been estimated that only a low percentage of the population consumes diets that meet the recommended daily allowance for vitamin E (1); therefore, nutritional monitoring is increasingly an important issue. There has been proposal of using α-CEHCs as a biomarker to assess vitamin E status (7). With the growing interest in the disease prevention activities of γ- and δ-tocopherols, we propose the use of urinary levels of α-, γ-, and δ-tocopherol-derived metabolites, in addition to blood levels of α-, γ-, and δ-tocopherols, as biomarkers for dietary intake and metabolism of these tocopherols. Urinary levels of tocopherol metabolites (CEHCs and CMBHCs) reflect the metabolized tocopherols. The presently developed method allows these tocopherol metabolites to be easily measured with on-column detection limits of 0.1 pmol. This sensitivity enables the measurement of tocopherol metabolites even in individuals who do not take vitamin E supplements (Figure 4). Once this concept has been verified in human feeding studies, these markers could be useful in epidemiological studies and nutritional assessment studies on vitamin E.
ACKNOWLEDGEMENT
This work was supported by NIH grant CA120915, John L. Colaizzi Chair Endowment fund, and Jewels of Charity award to the Cancer Institute of New Jersey and Facility Cores (supported by NIH grants ES05022 and CA72720).
ABBREVIATIONS USED
- LC-ESI-MS
Liquid chromatography–electrospray ionization–mass spectrometry
- CEHC
carboxyethyl hydroxychroman
- CMBHC
carboxymethylbutyl hydroxychroman
- CMHHC
carboxymethylhexyl hydroxychroman
- CDMOHC
carboxydimethyloctyl hydroxychroman
- CDMDHC
carboxydimethyldecyl hydroxychroman
- CMBenHC
carboxymethylbutenyl hydroxychroman
- CMHenHC
carboxymethylhexenyl hydroxychroman
- CDMO(en)2HC
carboxydimethyloctadienyl hydroxychroman
- CDMOenHC
carboxydimethyloctenyl hydroxychroman
- CDMD(en)2HC
carboxydimethyldecadienyl hydroxychroman
- m-T
γ-tocopherol-rich mixture of tocopherols
Footnotes
The authors have declared no conflict of interest.
LITERATURE CITED
- (1).Traber MG. In: Modern Nutrition in Health and Disease. Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Lippincott, Williams and Wilkins; Baltimore, MD: 2006. pp. 396–411. [Google Scholar]
- (2).Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit. Rev. Toxicol. 1993;23:147–169. doi: 10.3109/10408449309117115. [DOI] [PubMed] [Google Scholar]
- (3).Patel A, Liebner F, Netscher T, Mereiter K, Rosenau T. Vitamin E chemistry. Nitration of non-alpha-tocopherols: products and mechanistic considerations. J. Org. Chem. 2007;72:6504–6512. doi: 10.1021/jo0706832. [DOI] [PubMed] [Google Scholar]
- (4).Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- (5).Brigelius-Flohe R. Vitamin E and drug metabolism. Biochem. Biophys. Res. Commun. 2003;305:737–740. doi: 10.1016/s0006-291x(03)00811-8. [DOI] [PubMed] [Google Scholar]
- (6).Birringer M, Drogan D, Brigelius-Flohe R. Tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic. Biol. Med. 2001;31:226–232. doi: 10.1016/s0891-5849(01)00574-3. [DOI] [PubMed] [Google Scholar]
- (7).Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohe R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr. 1995;62:1527S–1534S. doi: 10.1093/ajcn/62.6.1527S. [DOI] [PubMed] [Google Scholar]
- (8).Morinobu T, Yoshikawa S, Hamamura K, Tamai H. Measurement of vitamin E metabolites by high-performance liquid chromatography during high-dose administration of alpha-tocopherol. Eur. J. Clin. Nutr. 2003;57:410–414. doi: 10.1038/sj.ejcn.1601570. [DOI] [PubMed] [Google Scholar]
- (9).Galli F, Lee R, Dunster C, Kelly FJ. Gas chromatography mass spectrometry analysis of carboxyethyl-hydroxychroman metabolites of alpha- and gamma-tocopherol in human plasma. Free Radic. Biol. Med. 2002;32:333–340. doi: 10.1016/s0891-5849(01)00800-0. [DOI] [PubMed] [Google Scholar]
- (10).Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 2002;132:3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- (11).Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- (12).Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol. Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS. Cancer preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp205. in press. DOI: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol. Aspects Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila Pa) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pereira AS, Siqueira DS, Elias VO, Simoneit BR, Cabral JA, Aquino Neto FR. Three series of high molecular weight alkanoates found in Amazonian plants. Phytochemistry. 2002;61:711–719. doi: 10.1016/s0031-9422(02)00348-5. [DOI] [PubMed] [Google Scholar]
- (17).Lienau A, Glaser T, Krucker M, Zeeb D, Ley F, Curro F, Albert K. Qualitative and quantitative analysis of tocopherols in toothpastes and gingival tissue employing HPLC NMR and HPLC MS coupling. Anal Chem. 2002;74:5192–5198. doi: 10.1021/ac020316k. [DOI] [PubMed] [Google Scholar]
- (18).Chow CK. Biological functions and metabolic fate of vitamin E revisited. J Biomed Sci. 2004;11:295–302. doi: 10.1007/BF02254433. [DOI] [PubMed] [Google Scholar]
- (19).Klatskin G, Tisdale WA. The effects of experimental hepatobiliary disease on certain aspects of tocopherol metabolism in the rat. J Clin Invest. 1957;36:1627–1637. doi: 10.1172/JCI103562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chiku S, Hamamura K, Nakamura T. Novel urinary metabolite of d-delta-tocopherol in rats. J. Lipid Res. 1984;25:40–48. [PubMed] [Google Scholar]
- (21).Li YJ, Luo SC, Lee YJ, Lin FJ, Cheng CC, Wein YS, Kuo YH, Huang CJ. Isolation and identification of alpha-CEHC sulfate in rat urine and an improved method for the determination of conjugated alpha-CEHC. J Agric Food Chem. 2008;56:11105–11113. doi: 10.1021/jf802459d. [DOI] [PubMed] [Google Scholar]
- (22).Cho JY, Kang DW, Ma X, Ahn SH, Krausz KW, Luecke H, Idle JR, Gonzalez FJ. Metabolomics reveals a novel vitamin E metabolite and attenuated vitamin E metabolism upon PXR activation. J Lipid Res. 2009;50:924–937. doi: 10.1194/jlr.M800647-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Radosavac D, Graf P, Polidori MC, Sies H, Stahl W. Tocopherol metabolites 2, 5, 7, 8-tetramethyl-2-(2′-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and 2, 7, 8-trimethyl-2-(2′-carboxyethyl)-6-hydroxychroman (gamma-CEHC) in human serum after a single dose of natural vitamin E. Eur. J. Nutr. 2002;41:119–124. doi: 10.1007/s00394-002-0365-3. [DOI] [PubMed] [Google Scholar]
- (24).Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic. Biol. Med. 2005;38:857–866. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- (25).Drevon CA. Absorption, transport and metabolism of vitamin E. Free Radic Res Commun. 1991;14:229–246. doi: 10.3109/10715769109088952. [DOI] [PubMed] [Google Scholar]
- (26).Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J Nutr. 2009;139:884–889. doi: 10.3945/jn.108.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Galli F, Lee R, Atkinson J, Floridi A, Kelly FJ. gamma-Tocopherol biokinetics and transformation in humans. Free Radic. Res. 2003;37:1225–1233. doi: 10.1080/10715760310001604125. [DOI] [PubMed] [Google Scholar]
- (28).Lee BL, New AL, Ong CN. Simultaneous determination of tocotrienols, tocopherols, retinol, and major carotenoids in human plasma. Clin Chem. 2003;49:2056–2066. doi: 10.1373/clinchem.2003.022681. [DOI] [PubMed] [Google Scholar]
- (29).Traber MG, Siddens LK, Leonard SW, Schock B, Gohil K, Krueger SK, Cross CE, Williams DE. Alpha-tocopherol modulates Cyp3a expression, increases gamma-CEHC production, and limits tissue gamma-tocopherol accumulation in mice fed high gamma-tocopherol diets. Free Radic. Biol. Med. 2005;38:773–785. doi: 10.1016/j.freeradbiomed.2004.11.027. [DOI] [PubMed] [Google Scholar]
- (30).Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci U S A. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]