Abstract
Background:
Driving while under the influence of alcohol is a major public health problem whose neural basis is not well understood. In a recently published fMRI study (Meda et al, 2009), our group identified five, independent critical driving-associated brain circuits whose inter-regional connectivity was disrupted by alcohol intoxication. However, the functional connectivity between these circuits has not yet been explored in order to determine how these networks communicate with each other during sober and alcohol-intoxicated states.
Methods:
In the current study, we explored such differences in connections between the above brain circuits and driving behavior, under the influence of alcohol versus placebo. Forty social drinkers who drove regularly underwent fMRI scans during virtual reality driving simulations following two alcohol doses, placebo and an individualized dose producing blood alcohol concentrations (BACs) of 0.10%.
Results:
At the active dose, we found specific disruptions of functional network connectivity between the frontal-temporal-basal ganglia and the cerebellar circuits. The temporal connectivity between these two circuits was found to be less correlated (p <0.05) when driving under the influence of alcohol. This disconnection was also associated with an abnormal driving behavior (unstable motor vehicle steering).
Conclusions:
Connections between frontal-temporal-basal ganglia and cerebellum have recently been explored; these may be responsible in part for maintaining normal motor behavior by integrating their overlapping motor control functions. These connections appear to be disrupted by alcohol intoxication, in turn associated with an explicit type of impaired driving behavior.
Keywords: Motor, Fronto-Striatal, Virtual Reality, Driving while intoxicated, Cerebellum
Introduction
Driving while intoxicated is a major public health problem in the United States, resulting in nearly 13,000 alcohol-impaired driving fatalities (National Highway Traffic Safety Administration, 2008) representing an average of one alcohol-impaired driving fatality every 40 minutes in 2007. It has been estimated from published studies internationally that driving under the influence of alcohol with a blood alcohol concentration (BAC) above 100mg/100mL (BAC=0.1%) increases the risk of involvement in an accident by 13 to 18 times and the risk of a fatal crash by 50 to 90 times (Miller and Blewden, 2001). Prior research has characterized driving as a complex task requiring simultaneous auditory, kinesthetic, and visual information processing, mental alertness, manual dexterity, and hand-eye coordination (Weiler et al, 2000), thus involving the concurrent recruitment of multiple cognitive functions (Meda et al, 2009; Calhoun et al, 2004; Groeger et al, 2001; Spiers and Maguire, 2006). However, the pertinent neural circuits most affected by alcohol intoxication within these functional domains and especially their interactions remain understudied. The general effects of alcohol consumption on behavioral and cognitive functions necessary for driving are well documented (see review of Mitchell, 1985). Alcohol consumption has been found to have immediate effects on multiple cognitive-motor processing domains (Mongrain and Standing, 1989). Specifically sustained and divided attention tasks (Moskowitz and Sharma, 1974) and decision-making, information processing, and judgment (Hindmarch et al, 1991) are most significantly impacted. Alcohol ingestion producing blood alcohol concentrations (BAC) within legal limits in the United States (BAC 80 mg/ 100mL legal limit in many states) can induce significant impairments of multiple attentional abilities (Neill et al, 1991). Simulated driving environments using virtual reality or simulations have recently been used to evaluate brain function and ecologically valid behavioral impairment in conjunction with various pharmacologic challenges (McGinty et al, 2001; Calhoun et al, 2004; Arnedt et al, 2001; Linnoila et al, 1973; Allen et al, 2009; Carvalho et al, 2006; Calhoun et al, 2005). Based on our earlier study (Calhoun et al, 2004), our group implemented a specially designed virtual driving environment in combination with simultaneous functional magnetic resonance imaging (fMRI) to probe temporal brain dynamics while driving under the influence of alcohol (Meda et al, 2009). This approach identified five, independent critical driving-associated brain circuits whose representative regions include the superior, middle and orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Fig.1 A), primary/supplementary motor areas (Fig. 1 B), frontal-temporal-basal ganglia (Fig. 1 C), cerebellum (Fig. 1 D), and medial prefrontal, inferior parietal, and lateral temporal cortices (the default mode network; Fox et al, 2007) (Fig. 1 E). The Meda study (2009) documents that these five circuits are significantly affected by relatively high levels (BAC=0.1%) of alcohol, resulting in both impaired driving behavior and brain function related to motor planning and control (Groeger, 2001; Tarter and Jones, 1971), goal directedness, error monitoring, and short term memory (Meda et al, 2009; Rosen and Lee, 1976). However, the effective connectivity among these circuits has not yet been explored in order to determine how they communicate with each other in both the sober and in alcohol-intoxicated states.
Fig. 1.
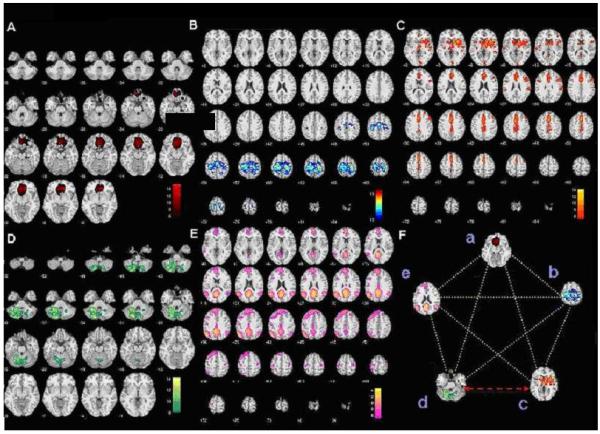
(A) Axial slices of anterior cingulate, middle and orbitofrontal gyri circuit. (B) Axial slices of primary/secondary motor cortex circuit. (C) Axial slices of frontal-temporal-basal ganglia circuit. (D) Axial slices of cerebellum circuit (E) Axial slices of default mode network circuit: medial prefrontal cortex, inferior parietal, inferior cingulate, and lateral temporal cortex. (F) Image of all five independent critical driving-associated brain circuits (a) anterior cingulate, middle and orbitofrontal gyri, (b) primary/secondary motor cortex, (c) frontal-temporal-basal ganglia, (d) cerebellum, and (e) default mode network. Yellow dotted lines show network connections which do not differ significantly between baseline sober and alcohol intoxication conditions; the red arrow shows the network connection between the frontal-temporal-basal ganglia and cerebellar circuits which differs significantly between baseline sober and alcohol intoxication conditions. Image 1.F labeled with lower case letters a-e corresponds to the axial component slices labeled A-E.
As outlined in several recent reviews, in regard to complex behaviors, interactions among functional circuits (e.g. those subserving attention and working memory) are likely as important as functional relationships within such circuits (e.g. Stevens MC, Pearlson GD, Calhoun VD. Hum Brain Mapp. 2009 (8):2356-66. In the previous (Meda et al, 2009) study we identified individual functionally integrated networks but we did not test their interconnections. Previous primate lesion and human movement disorder fMRI studies have reported connections between regions within the five independent circuits identified by Meda et al, (2009) including the frontal-temporal-basal ganglia, OFC, ACC (Price et al, 2009; Haber and Brucker, 2009; Stathis and Panourias, 2007), and cerebellum (Schmahmann and Pandya, 2008; Middleton and Strick, 1994).
Additional support comes from previous fMRI studies which report activation in primary and supplementary motor areas, cerebellum, basal-ganglia, and anterior cingulate during bimanual hand coordination and hand grip exercises during sober condition (Debaere et al, 2000; Wenderoth et al, 2005; Wong et al, 2007). Specifically, Price et al (2009) proposed in a study of patients with fronto-striatal dysfunction due to Parkinson's disease (PD), individuals with PD had deficits in rule-based learning tasks which may be attributed to decreased communication between neural regions associated with rule-based category learning such as the OFC-ACC circuit (Fig. 1 A) and the frontal-temporal-basal ganglia circuit (Fig. 1 C). The fronto-striatal brain regions (Meck and Benson, 2002; Ferrandez et al, 2003; Hinton and Meck, 2004) and the cerebellum (Gibbon et al, 1997; Irvy and Spencer 2004) are also involved in mental timekeeping, an area of research which focuses on the cognitive ability that allows one to internally track the passage of time.
In the current study, we used a functional network connectivity (FNC) approach (Jafri et al, 2008), to explore temporal relationships between five previously identified neural networks (Meda et al, 2009), and their relationships to driving behavior, under the influence of alcohol versus placebo. Specifically the FNC approach will be used on the sam data set (Meda et al, 2009) to characterize the weaker temporal dependency among components estimated using independent component analysis (ICA), rather than the strong temporal relationships shared by brain regions within a given component. As results from Meda et al, (2009) and previous studies (Calhoun et al, 2005; Linnoila et al, 1973; Neill et al, 1991; Zhu et al, 2004), have shown alcohol to have marked effects on higher order cognitive functions and motor coordination, it was reasonable to predict that specific interactions among the discrete neural circuits associated with motor planning and coordination, goal directedness, rule-based learning, intentionality, error monitoring and memory would be affected by alcohol and that in turn this would correlate with changes in particular driving behaviors. More specifically based on the above literature, we proposed two hypotheses; first we would expect differences in connectivity during sober and alcohol conditions between the anterior cingulate, middle frontal and orbitofrontal gyri (Circuit. A) and the frontal-temporal-basal ganglia (Circuit. C) circuits, due to the involvement of these components in rule-based learning, an important aspect of driving (Price et al, 2009; Buckley et al, 2009). Secondly, we hypothesized that connectivity among frontal-temporal-basal ganglia (Circuit C) (Meda et al, 2009), and cerebellar (Circuit D) circuits would be altered while driving under the influence of alcohol (i.e. not a general phenomenon affecting all connectivity equally). These circuits act together as a timing mechanism, evidenced both from a neuropsychology driving perspective (Groeger, 2001) and neural connectivity studies (Shadmehr and Krakauer, 2008) and are also known to be linked to alcohol intoxication-induced driving decrements found in our previous reports (Calhoun et al, 2004, Meda et al, 2009). Thus, investigating the connectivity among these circuits during an alcohol intoxicated state would be important in understanding how alcohol acts to impair driving abilities.
Participants
We tested 40, healthy, right-handed men (N = 20) and women, with a mean age of 24.75 ± 4.7 years, who were non-smokers, had good visual acuity, valid driver's licenses, drove 3 or more times weekly and were light or moderate users of alcohol (1-4 alcohol consumption sessions/week and 1-8 drinks/session; average consumption/month, 9 times with an average of 4 drinks per occasion). Subjects were screened using the Structured Clinical Interview for DSM IV, (SCID; First et al, 2002) to exclude individuals with Axis 1 psychiatric disorders on DSM IV-TR (American Psychiatric Association, 2000) including a history of substance abuse. This dataset is the same as used in our previous publication (Meda et al, 2009).
Treatment Conditions
During each of two visits, participants underwent a urine drug and baseline blood alcohol screen. Prior to beginning the task individuals were trained for 10 minutes on the simulated driving paradigm. Participants were instructed to eat only a light meal on the morning before the study and to avoid alcohol consumption for 24 hours prior to participation. All drinks, including placebo, were served in identical beverage containers wrapped in an alcohol soaked cloth with an additional small amount of alcohol on the drinks' surface to help disguise the contents (Hammersley et al, 1992). Drinks were made with either orange or cranberry juice to a constant volume of 350 ml. At each visit participants received administration of either placebo alcohol or an individualized dose of alcohol calculated based on body weight and sex following a published algorithm (Kapur, 1989), in a blinded, randomized trial, under identical circumstances. Participants were given doses designed to produce a measured blood alcohol concentration (BAC) of 0.10% or 0.0% (placebo). The BAC's of participants were measured prior at baseline and again at regular intervals after each scan session using a calibrated hand-held breathalyzer (Intoximeter Inc. St. Louis, MO). All participants gave written informed consent prior to participation in the study approved by the Hartford Hospital Institutional Review Board.
Procedures
Participants were asked to pace their beverage consumption to complete ingestion over a 10 min period and their BAC's were tested 5 and 10 minutes after consumption. They were placed in the MRI scanner 15 minutes after completing consumption; all participants successfully completed alcohol consumption without experiencing nausea. When entering the MRI scanner, the BAC for the alcohol dose was 0.071 ± 0.017 and when exiting the scanner 0.087 ± 0.013. Following the MRI scan, a study physician determined when participants were allowed to leave via cab or to be driven by a friend when their BAC reached ≤ 0.05. The order of dosage (placebo, alcohol) was counterbalanced for participants and the mean time between session 1 and 2 was 7.8 days.
Equipment Design and Setup
The driving simulator used custom built in-house software designed to mimic a realistic driving experience and capture real time aspects of driving behavior, with a modified videogame steering wheel, accelerator and brake pedals (St Germain et al, 2005; Allen et al, 2009; Carvalho et al, 2006; Meda et al, 2009). All ferromagnetic components within the driving setup were replaced with non-ferromagnetic (plastic, rubber or copper-beryllium) parts. The steering-wheel controller was connected to a computer outside the scanner room through a waveguide in the wall and an LCD projector (SHARP XG-P25X) outside the scanner room projected images onto a translucent screen subtended approximately a 25° field of view that provided participants a straight line of sight using a second waveguide. Participants viewed the projected images via a mirror attached to the head coil of the 3T MRI scanner (Allegra; Siemens, Erlangen, Germany).
We used a blocked fMRI design with each run consisting of three blocks and each block had three conditions: fixation (30 s), drive (90 s), and observe (60 s) (Meda et al, 2009). At the end of each block, an additional (30 s) fixation was completed totaling in 9.5 min/block. During the fixation phase, the subject was instructed to focus on a crosshair and during the driving block, the participant was asked to “drive” the car normally and safely and to abide by all conventional traffic rules. During the observe phase, the subject passively viewed a simulated driving scene and each run was repeated three times (separately) to increase the signal to noise ratio (SNR). Each participant completed 3 runs for a total of 28.5 minutes.
Data Acquisition
Driving Behavior
Continuous behavioral variables including, median/yellow line crossings, passenger side white line crossings, opposite side white line crossings, speed, collisions, and steering weave were recorded during the driving phase of the experiment. At the end of each run, an overall summary score for each behavior was created for every subject and performance was averaged across the three scans. Details of the data acquisition and analysis for these behavioral measures are described in detail elsewhere (Calhoun et al, 2005; Carvalho et al, 2006).
Imaging
Functional data were acquired on a 3T Siemens Allegra scanner using an echoplanar sequence with the following imaging parameters; repeat time (TR) = 1,500 ms, echo time (TE) = 27 ms, field of view (FOV) = 22cm, flip angle = 70°, acquisition matrix = 64 × 64, voxel size = 3.44 × 3.44, slice thickness = 5 mm, number of slices = 29, ascending acquisition (sequential) (Meda et al, 2009)
Data Processing
Driving behavior
All behavioral variables were investigated for BAC-related responses and screened for any major outliers before conducting statistical analyses. In order to relate specific functional connectivity differences to changes in driving behavior with alcohol we statistically compared correlations (using a Fishers-Z transform) between the change scores (alcohol – sober) of each fMRI component and driving behavior.
Image preprocessing
All images were preprocessed using Statistical Parametric Mapping Software, SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2). Motion correction was achieved using INRIAlign (Freire and Mangin, 2001; Freire et al, 2002) to compensate for movement in the fMRI time series images. Movement parameters for each subject were visually screened to eliminate excessive head motion (greater than 3 mm of translational motion and 2 degrees of rotation). Motion corrected images were then spatially normalized to Montreal Neurological Institute (MNI) space by matching them to the standardized EPI template image in SPM2. Following spatial normalization, images were spatially smoothed with an 8 mm isotropic Gaussian kernel. The preprocessed images were used as the input for the ICA algorithm.
Prior ICA analysis (Meda et. al, 2009)
Independent component analysis is a fairly well established method that has been widely applied to fMRI datasets, which recovers underlying signals from linear mixtures of these signals and draws upon higher-order signal statistics to determine a set of “components” that are maximally spatially distant and functionally independent of one another (Calhoun and Adali, 2006; Jafri et al, 2008; Calhoun et al, 2008). In our previous study (Meda et al, 2009) on this dataset, ICA was used to find and characterize fourteen networks in the fMRI data collected during the driving task. Once the networks were identified, the time courses and spatial maps were back reconstructed for each participant as part of the group ICA algorithm thereby preserving the spatial and temporal characteristics of each individual subject. Each network's time course was then parameterized using multiple regression to provide association estimates (beta weights) between time courses and the cognitive phases of the paradigm (i.e., drive and observe) for each subject. These beta weights represent the degree of synchrony or modulation between component time courses and the canonical hemodynamic response model (in this study, estimates were derived for the observe and drive phase(s) during both alcohol dose conditions). Upon statistical comparison of the above beta weights the final results of our previous study indicated that five out of the above fourteen networks were significantly affected by alcohol dose (Meda et al, 2009).
Current FNC analysis
Following the ICA analysis and identification of five driving-associated alcohol influenced networks, in the present study we used a recently published algorithm (Jafri et. al, 2008) to further explore the effect of alcohol on the temporal connections in functional time courses or functional network connectivity (FNC) between the five identified networks. Analysis was performed using the FNC toolbox available at (http://www.ece.unm.edu/~vcalhoun/mia/software). Even though individual temporally-defined components derived through ICA are spatially independent, they can still be significantly temporally correlated with each other. The FNC approach examines this specific temporal correlation in a pairwise manner using a maximal lagged correlation approach. The time courses for all five components were initially interpolated to detect finer and sub TR hemodynamic differences across the two conditions. Following this, using the five components taken two at a time, the 10 pairwise combinations were computed within the FNC toolbox. Correlations values were calculated within subject and tested between and within sober and alcohol doses. First, in order to determine which circuits were significantly connected/correlated in the sober and alcohol conditions separately (i.e. within dose), we used a random effects one-sample t-test to assess group level significance for each of the 10 pairwise combinations. Accounting for multiple comparisons using the Bonferroni correction, the revised p level for significance was estimated to be p<0.02. We then used a paired sample t-test to determine significant statistical differences in correlation between groups. The FNC algorithm and equations are described in more detail in (Jafri et al, 2008).
Once significant temporal anomalies were identified between a set of networks, supplemental analyses were performed to assess the degree of correlation of each of these networks to the drive and observe phases of the task. This was achieved by computing a series of one-sample t-tests on the difference in association estimates (i.e. drive-observe) for each of the above identified networks to determine their dosage (sober, high) based relationships with the drive-observe condition.
Supplementary Analyses
To add to the interpretability of these results, we also conducted supplemental analyses to determine the degree of task-relatedness of each network's time course with fMRI driving task conditions, as well as post hoc tests linking altered driving behavior to network activity.
Results
Imaging/FNC
Figure 2 indicates that all 10 correlation/connectivity combinations between the five driving related circuits were preserved when tested for a significant non-zero correlation under both the sober and alcohol conditions. However as shown in figure 3, of the five spatially independent alcohol-influenced driving networks characterized in our recent study (Meda et al, 2009), we found a single, highly specific interaction between the frontal-temporal-basal ganglia and cerebellar networks that was significantly disrupted by alcohol administration. In other words, even though the connections were preserved in both the sober and alcohol states, we found a single specific connection to be significantly lowered upon alcohol intoxication. Fig. 1A through E shows the axial slices of the five spatially independent alcohol-influenced driving networks used in the FNC analysis. Fig. 1F is a representation of the FNC which depicts a change in connectivity between frontal-temporal-basal ganglia (circuit 3) and cerebellum (circuit 4) during the sober and alcohol intoxication conditions.
Fig. 2.
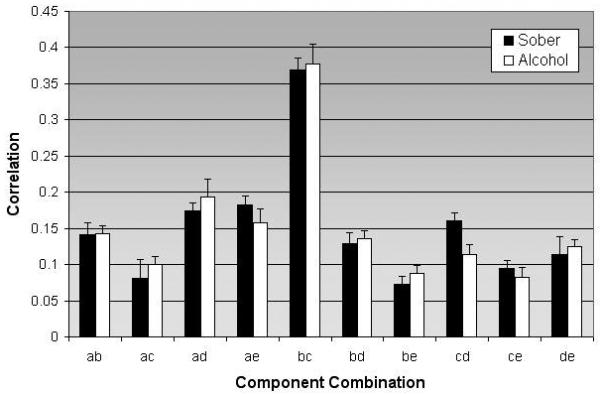
Correlation of circuit combinations for participants during sober and alcohol condition X-axis represents the 10 possible combinations, while the y-axis represents the degree of correlation for the particular combination (along with the standard error of mean). All circuit combinations were significant (p < 0.05)
Fig. 3.
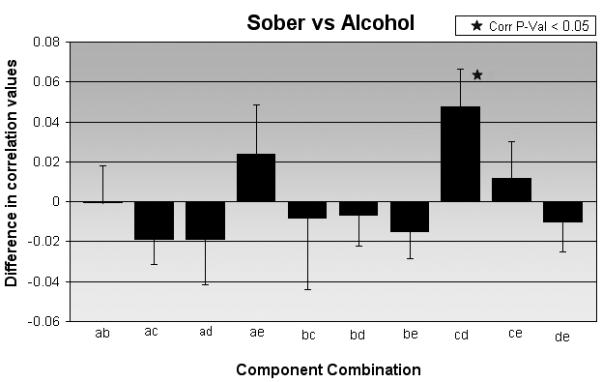
Correlation differences of circuit combinations for sober versus alcohol condition. X-axis represents the 10 possible combinations, while the y-axis represents the degree of correlation (along with the standard error of mean) for the particular combination. The plot also highlights the correlation between circuit c (frontal-temporal-basal ganglia) and circuit d (cerebellum) that was significantly different between the two conditions.
The correlations (mean ±standard deviation) for the sober and alcohol condition were 0.1612±0.1 and 0.1135±0.06 respectively. In this comparison between the baseline sober and alcohol intoxication conditions using FNC analysis (Fig. 2), the temporal connectivity between the frontal-temporal-basal ganglia and cerebellar circuits was found to be less correlated (p = 0.01 corrected for multiple comparisons) when driving under the influence of alcohol (Fig. 3). This represents a medium to large standardized effect size of Cohens d=0.53. The graph (Fig. 3) shows that the correlation between the frontal-temporal-basal ganglia circuit and the cerebellum was significantly reduced during alcohol administration. Interestingly, temporal interactions between activation alterations due to intoxication in the other three networks remained unaffected.
Supplemental analyses revealed that the cerebellar circuit was very strongly associated (p<0.0001) with the driving minus observe condition of the experiment during both dose conditions (albeit to a lesser extent at the active dose).
Behavior
Change scores of correlation between the frontal-temporal-basal ganglia and cerebellar circuits were significantly associated (r= −0.38, p=0.02 and r=0.20, p=0.02 respectively) with unstable motor vehicle steering. In addition, differences in correlation values were assessed to be significant (Z=2.45; p=0.007) using a Fisher's Z-transform accounting for correlated samples (Steel et al, 1997).
Discussion
A prior ICA analysis identified five critical driving-associated neural components in healthy participants during a simulated driving task. The current analysis used a functional network connectivity approach on this dataset to examine differences in connectivity among these components and found a specific, significant decrease in connectivity between the frontal-temporal-basal ganglia and cerebellar components during the alcohol condition, which was partly consistent with our hypotheses which also predicted that alcohol intoxication would alter connectivity between the anterior cingulate,-middle frontal- orbitofrontal gyrus circuit and the frontal-temporal-basal ganglia circuit due to the role of these regions in rule-based learning (Price et al, 2009). This connectivity pattern was not altered by alcohol. It has been proposed that the anterior cingulate and orbitofrontal cortex, prefrontal cortex and basal ganglia together create both excitatory and inhibitory pathways for rule based learning input (Buckley et al, 2009; Price et al, 2009).
However, our results do support our second hypothesis of specific impairments during the alcohol condition in comparison to baseline between frontal-temporal-basal ganglia and cerebellar circuits that together govern aspects of higher order cognitive function and motor planning, and coordination (Groeger, 2001; Herrero et al, 2002), intentionality (Dunnett et al, 2005), error monitoring, goal directedness (Hollerman et al, 2000) and short term memory (Dunnett et al, 2005; Rosen and Lee, 1976). This change in connectivity between the frontal-temporal-basal ganglia and cerebellar circuits during alcohol condition may be a vulnerable point where alcohol acts to impair one's ability to drive a motor vehicle. Researchers may gain more insight into the specific mechanism by which alcohol disrupts motor coordination by examining the neural anatomical connections between these circuits.
Connections between basal ganglia, prefrontal cortex and cerebellum have been explored, as reviewed in Schmahmann and Pandya, (2008) and Middleton and Strick, (1994). One hypothesis is that the basal ganglia and cerebellum are connected through loops that provide a means for linking widespread cortical regions, including the prefrontal cortex with motor output regions of the primary motor cortex (Middleton and Strick, 1994). It is thought that these loops funnel information into the motor system to produce commands for movement. Specifically, the frontal-basal ganglia loop is often associated with executive, attentional control and goal directed responses (Benke et al, 2003; Dunnett et al, 2005, Hollerman et al, 2000; Levy and Czernecki, 2006).
Middleton (1994) first suggested that both the basal ganglia and cerebellum mediate cognitive processes in addition to motor functions. These nodes are involved in cognitive operations including planning future behavior (Herrero et al, 2002), motor sequence learning (Doyon, 2008), rule-based learning (Ashby et al, 2003) and working memory (Ben-Yehudah et al, 2007), all of which are important during driving. Evidence from more recent imaging studies (e.g. Zhu et al, 2004) supports cerebellar involvement in cognitive operations via neuroanatomical connections with the frontal cortex. Such connections are consistent with our FNC findings that indicate disrupted connectivity between frontal-temporal-basal ganglia and cerebellum during alcohol intoxication.
Driving is a complex and dynamic task which requires combining different sources of visual information with particular motor patterns. As hypothesized by Groeger (2001), following registration of a motivation to act, information is passed to the prefrontal cortex (PFC) to organize goals and to establish intentionality, planning, and general aims for the action. Regions within the PFC are believed to help prepare, formulate, and oversee the execution of action sequences and monitor the strategic aspects of success or failure and the consequences of actions (Bradshaw and Mattingley, 1995). The PFC enables the performance of different tasks in response to changing domains and goals (Heyder et al, 2004). Information from the PFC is hypothesized to be passed to premotor regions to effect selection of appropriate motor programs and then to primary motor cortex, basal ganglia, and cerebellum to help create accurate, coordinated and smoothly integrated motor actions to achieve the goal previously established by the PFC. (Groeger, 2001; Uchiyama et al, 2003). A recent study of movement disorders suggested that the basal ganglia help formulate the expected costs of motor commands and the expected rewards of the predicted sensory states (Shadmehr and Krakauer, 2008). While the basal ganglia are thought to help regulate higher cognitive planning and non-motor cognitive behaviors and the planning and execution of complex motor actions, the cerebellum appears to predict sensory consequences of motor commands and also operates as a timing mechanism (Shadmehr and Krakauer, 2008; Groeger, 2001). The cerebellum is also described as a integration and predictive control system for a variety of limbic, motor, and sensory systems and hypothesized to regulate coordination of multiple joints, posture and movement execution, especially visual-based aiming and tracking to ensure smooth movements (Groeger, 2001). However, because timing is an important aspect of movement, it is not surprising that control of movements inherently involves activating motor units sequentially at the correct times (Salman 2002).
Buhusi and Meck (2005), hypothesized that the brain tracks the passage of time by detecting coincidental activation of different neural populations and that organisms have developed multiple timing strategies with various degrees of precision. The three timing strategies; (circadian, interval, and millisecond) likely rely on different neural mechanisms (Buhusi and Meck, 2005). It is possible that alcohol's effects on brain function specifically disrupt mental timekeeping in either of these two proposed timekeeping systems. Recent (fMRI) and positron emission tomography (PET) studies that observe neural activity when time reproduction, estimation, or discrimination is required show activation in multiple brain regions including sensorimotor, lateral premotor, and supplementary motor, dorsolateral prefrontal, insula, anterior cingulate cortices, superior temporal gyrus, right parietal lobe, the cerebellum, basal ganglia structures, and the thalamus (Macar et al, 2002; Lewis and Miall, 2003; Rubia and Smith, 2004; Bushusi and Meck, 2005). Of these regions, the fronto-striatal brain regions (Meck and Benson, 2002; Ferrandez et al, 2003; Hinton and Meck, 2004) and the cerebellum (Gibbon et al, 1997; Irvy and Spencer 2004) have been proposed to be the most likely primary neural substrates of mental timekeeping. These two sets of brain regions have been shown to form independent neural networks involved in mental timekeeping (Stevens et al, 2007). Also, support for a possible role of disrupted timekeeping in alcohol intoxication emerges from a review of timing/sensorimotor processes that describes the cerebellum as a timing system that provides a near-infinite set of interval-type timers while controlling the execution of movement (Salman 2002). Additionally, the results of pharmacopsychological studies of healthy subjects (Rammsayer et al, 1993, 1997) suggest that humans have an internal clock consisting of a pacemaker and accumulator which work together to generate an internal representation of time passage and then record this information. The speed of the internal clock is thought to be modulated by dopaminergic activity in the basal ganglia and higher clock-rate are attributed to finer temporal resolution (Rammsayer 1999).Therefore, the frontal-temporal-basal ganglia to cerebellum connection highlighted in our study may be responsible in part for maintaining normal motor behavior by integrating these overlapping motor control (Neychev et al, 2008) and timing functions. The significantly decreased connectivity between the frontal-temporal-basal ganglia and the cerebellar components during alcohol intoxication is consistent with our second hypothesis. The connections between these motor regions have been identified as essential for the production of motor commands and maintenance of internal timing mechanisms and appear specifically disrupted by alcohol intoxication, in turn associated with an explicit type of impaired driving behavior. Literature describing the effects of alcohol intoxication on interval-timing mechanisms is currently lacking; therefore future studies should investigate this understudied relationship.
A possible limitation of the ICA and FNC approach used in our study is that our analyses were restricted to five driving-associated alcohol-influenced circuits. Future studies could investigate other circuits that may not have shown alcohol related intra-network spatial differences, but may potentially characterize differential temporal relationships following alcohol consumption. Participants in our study were all moderate-social drinkers; future studies including participants who are heavy-drinkers may show variant alcohol-moderated connectivity patterns.
In summary, the results of our study suggest that a highly specific connection between frontal-temporal-basal ganglia and cerebellar components is affected by alcohol intoxication during a simulated driving task. These connectivity changes were also linked with an impaired driving behavior, which suggests the involvement of the frontal-temporal-basal ganglia and cerebellar components in maintaining normal motor behavior while driving. Although alcohol's ability to impair motor behaviors is well documented, the results from this study may draw attention to a specific site where alcohol acts to cause such impairment.
Acknowledgments
This work was supported by the National Institutes of Health; grant numbers: R01 EB 000840 and R01 AA015615. We would like to thank the staff at the Olin Neuropsychiatry Research Center.
Footnotes
Disclosure/Conflicts of Interest
All authors have no conflicts of interest, financial or otherwise, to declare.
References
- Allen AJ, Meda SA, Skudlarski P, Calhoun VD, Astur R, Ruopp KC, et al. Effects of alcohol on performance on a distraction task during simulated driving. Alcohol Clin Exp Res. 2009;33:617–625. doi: 10.1111/j.1530-0277.2008.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Revised 4th ed. Author; Washington, DC: 2000. [Google Scholar]
- Arnedt JT, Wilde GJ, Munt PW, MacLean AW. How do prolonged wakefulness and alcohol compare in the decrements they produce on a simulated driving task? Accid Anal Prev. 2001;33:337–344. doi: 10.1016/s0001-4575(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Noble S, Filoteo JV, Waldron EM, Ell SW. Category learning deficits in Parkinson's disease. Neuropsychology. 2003;17:115–124. [PubMed] [Google Scholar]
- Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: beyond cognitive theory. Cerebellum. 2007;6:193–201. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- Benke T, Delazer M, Bartha L, Auer A. Basal ganglia lesions and the theory of fronto-subcortical loops: neuropsychological findings in two patients with left caudate lesions. Neurocase. 2003;9:70–85. doi: 10.1076/neur.9.1.70.14374. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Mattingley JB. Clinical neuropsychology : behavioral and brain science. Academic; San Diego: 1995. [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, et al. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, Pearlson GD. Alcohol intoxication effects on simulated driving: exploring alcohol-dose effects on brain activation using functional MRI. Neuropsychopharmacology. 2004;29:2097–2017. doi: 10.1038/sj.npp.1300543. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Carvalho K, Astur R, Pearlson GD. Using virtual reality to study alcohol intoxication effects on the neural correlates of simulated driving. Appl Psychophysiol Biofeedback. 2005;30:285–306. doi: 10.1007/s10484-005-6384-0. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Unmixing fMRI with independent component analysis. IEEE Eng Med Biol Mag. 2006;25:79–90. doi: 10.1109/memb.2006.1607672. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1 Suppl):S163–172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho KN, Pearlson GD, Astur RS, Calhoun VD. Simulated driving and brain imaging: combining behavior, brain activity, and virtual reality. CNS Spectr. 2006;11:52–62. doi: 10.1017/s1092852900024214. [DOI] [PubMed] [Google Scholar]
- Connor J, Norton R, Ameratunga S, Jackson R. The contribution of alcohol to serious car crash injuries. Epidemiology. 2004;15:337–344. doi: 10.1097/01.ede.0000120045.58295.86. [DOI] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Beatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. Neuroimage. 2001;14:947–958. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Meldrum A, Muir JL. Frontal-striatal disconnection disrupts cognitive performance of the frontal-type in the rat. Neuroscience. 2005;135:1055–1065. doi: 10.1016/j.neuroscience.2005.07.033. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. [Google Scholar]
- Ferrandez AM, Hugueville L, Lehericy S, Poline JB, Marsault C, Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: an fMRI study. Neuroimage. 2003;19:1532–1544. doi: 10.1016/s1053-8119(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel C. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Groeger JA. Understanding Driving: Applying Cognitive Psychology to a Complex Everyday Task. Psychology Press; New York: 2001. [Google Scholar]
- Haber SN, Brucker JL. Cognitive and limbic circuits that are affected by deep brain stimulation. Front Biosci. 2009;14:1823–1834. doi: 10.2741/3344. [DOI] [PubMed] [Google Scholar]
- Hammersley R, Finnigan F, Millar K. Alcohol placebos: you can only fool some of the people all of the time. Br J Addict. 1992;87:1477–1480. doi: 10.1111/j.1360-0443.1992.tb01926.x. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychol (Amst) 2004;115:271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Kerr JS, Sherwood N. The effects of alcohol and other drugs on psychomotor performance and cognitive function. Alcohol Alcohol. 1991;26:71–79. [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Res Cogn Brain Res. 2004;21:171–182. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur, BM. Computer Blood Alcohol Calculator v1.20 ARF Software. Addiction Research Foundation; Toronto, Canada: 1989. [Google Scholar]
- Levy R, Czernecki V. Apathy and the basal ganglia. J Neurol. 2006;253(Suppl 7):VII54–61. doi: 10.1007/s00415-006-7012-5. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Mattila MJ. Interaction of alcohol and drugs on psychomotor skills as demonstrated by a driving simulator. Br J Pharmacol. 1973;47:671P–672P. [PMC free article] [PubMed] [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, et al. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp Brain Res. 2002;142:475–485. doi: 10.1007/s00221-001-0953-0. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Shih RA, Garrett E, Calhoun VD, Pearlson GD. Assessment of intoxicated driving with a simulator: a validation study with on-road driving; Proc. Human Centered Transportation Simulation Conf; Iowa City, IA: 2001. Paper presented at the. [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain's internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Meda SA, Calhoun VD, Astur RS, Turner BM, Ruopp K, Pearlson GD. Alcohol dose effects on brain circuits during simulated driving: an fMRI study. Hum Brain Mapp. 2009;30:1257–1270. doi: 10.1002/hbm.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Miller TR, Blewden M. Costs of alcohol-related crashes: New Zealand estimates and suggested measures for use internationally. Accid Anal Prev. 2001;33:783–791. doi: 10.1016/s0001-4575(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Mitchell MC. Alcohol-induced impairment of central nervous system function: behavioral skills involved in driving. J Stud Alcohol Suppl. 1985;10:109–116. doi: 10.15288/jsas.1985.s10.109. [DOI] [PubMed] [Google Scholar]
- Mongrain S, Standing L. Impairment of cognition, risk-taking, and self-perception by alcohol. Percept Mot Skills. 1989;69:199–210. doi: 10.2466/pms.1989.69.1.199. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Sharma S. Effects of alcohol on peripheral vision as a function of attention. Hum Factors. 1974;16:174–180. doi: 10.1177/001872087401600209. [DOI] [PubMed] [Google Scholar]
- Neill RA, Delahunty AM, Fenelon B. Discrimination of motion in depth trajectory following acute alcohol ingestion. Biol Psychol. 1991;31:1–22. doi: 10.1016/0301-0511(90)90075-8. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Filoteo JV, Maddox WT. Rule-based category learning in patients with Parkinson's disease. Neuropsychologia. 2009;47:1213–1226. doi: 10.1016/j.neuropsychologia.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammsayer TH. On dopaminergic modulation of temporal information processing. Biol Psychol. 1993;36:209–222. doi: 10.1016/0301-0511(93)90018-4. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Are there dissociable roles of the mesostriatal and mesolimbocortical dopamine systems on temporal information processing in humans? Neuropsychobiology. 1997;35:36–45. doi: 10.1159/000119328. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B. 1999;52:273–286. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- Rosen LJ, Lee CL. Acute and chronic effects of alcohol use on organizational processes in memory. J Abnorm Psychol. 1976;85:309–317. doi: 10.1037//0021-843x.85.3.309. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp (Wars) 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Salman MS. The cerebellum: it's about time! But timing is not everything--new insights into the role of the cerebellum in timing motor and cognitive tasks. J Child Neurol. 2002;17:1–9. doi: 10.1177/088307380201700101. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44:1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Germain SA, Kurtz MM, Pearlson GD, Astur RS. Driving simulator performance in schizophrenia. Schizophr Res. 2005;74:121–122. doi: 10.1016/j.schres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Stathis P, Panourias IG, Themistocleous MS, Sakas DE. Connections of the basal ganglia with the limbic system: implications for neuromodulation therapies of anxiety and affective disorders. Acta Neurochir Suppl. 2007;97:575–586. doi: 10.1007/978-3-211-33081-4_67. [DOI] [PubMed] [Google Scholar]
- Steel RD, Torrie JH, Dickey TA. Principles and Practice of Statistics: A Biomedical Approach. McGraw Hill, USA: 1997. [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Hum Brain Mapp. 2007;28:394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Jones BM. Absence of intellectual deterioration in chronic alcoholics. J Clin Psychol. 1971;27:453–455. [PubMed] [Google Scholar]
- Traffic Safety Facts – 2007 (DOT HS No. 810 016) U.S. Department of Transportation National Highway Traffic Safety Administration. 2008 [Google Scholar]
- Uchiyama Y, Ebe K, Kozato A, Okada T, Sadato N. The neural substrates of driving at a safe distance: a functional MRI study. Neurosci Lett. 2003;352:199–202. doi: 10.1016/j.neulet.2003.08.072. [DOI] [PubMed] [Google Scholar]
- Weiler JM, Bloomfield JR, Woodworth GG, Grant AR, Layton TA, Brown TL, et al. Effects of fexofenadine, diphenhydramine, and alcohol on driving performance. A randomized, placebo-controlled trial in the Iowa driving simulator. Ann Intern Med. 2000;132:354–363. doi: 10.7326/0003-4819-132-5-200003070-00004. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35:698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Zador PL, Krawchuk SA, Voas RB. Alcohol-related relative risk of driver fatalities and driver involvement in fatal crashes in relation to driver age and gender: an update using 1996 data. J Stud Alcohol. 2000;61:387–395. doi: 10.15288/jsa.2000.61.387. [DOI] [PubMed] [Google Scholar]
- Zhu W, Volkow ND, Ma Y, Fowler JS, Wang GJ. Relationship between ethanol-induced changes in brain regional metabolism and its motor, behavioural and cognitive effects. Alcohol. 2004;39:53–58. doi: 10.1093/alcalc/agh023. [DOI] [PubMed] [Google Scholar]


