Abstract
Hepatic encephalopathy most commonly occurs in patients with cirrhosis and end-stage liver disease, however, the disorder can also occur in the presence of intra or extrahepatic shunts when the intrahepatic circulation is effectively bypassed. The majority of extrahepatic shunts described to date develop between a mesenteric vein and inferior vena cava. Herein we report a novel case of a superior mesenteric vein to left internal iliac vein shunt that led to hepatic encephalopathy in a 57 year old woman with no apparent underlying liver disorder. The patient presented with confusion, disorientation and hyperammonemia. Work-up for parenchymal liver disease was negative and liver biopsy findings did not show significant liver disease. Magnetic resonance imaging revealed a serpiginous 1 cm wide shunt that diverted superior mesenteric vein blood from the portal confluence to the left internal iliac vein. Surgical closure of the shunt led to marked improvement of the patient with resolution of hepatic encephalopathy. This report is the first description of a portosystemic shunt, likely congenital, linking these two vessels resulting in clinically significant hepatic encephalopathy. The findings emphasize that abdominal and pelvic imaging should be considered in patients with signs of hepatic encephalopathy that have none to minimal hepatic disease.
Keywords: Hepatic Encephalopathy, Portosystemic shunt, Non-cirrhotic liver
Case Report
A 57-year-old female with a past medical history of insulin dependent diabetes mellitus presented with a two week history of confusion and new onset melena. The patient had a prior history of temperate alcohol use; however, she denied alcohol consumption for the last 10 years. On presentation, the patient was confused, disorientated, and lethargic with a West Haven (Conn) score of 2. With the exception of holosystolic cardiac murmur, the physical examination was unremarkable and there was no hepatosplenomegaly. Liver panel showed normal Aspartate Aminotransferase (AST), Alkaline Phosphatase (AP) and γ-Glutamyltranspeptidase (GGTP) activities. There was mildly elevated Alanine Aminotransferase (ALT) of 50 (normal < 20 (IU/L)); hemoglobin was 8.2 g/DL) and plasma ammonia level was 140 uM/L. Upper endoscopy showed a bleeding duodenal ulcer which was cauterized and treated with hemoclips endoscopically. There were no esophageal or gastric varices or signs of portal hypertension. A complete hepatitis panel was obtained, including serum markers for autoimmune and viral hepatitis all of which were negative. Further serum tests ruled out iron or copper overload. An ultrasound of the liver with Dopplers showed normal liver and spleen sonographic characteristics without hepatosplenomegaly. However, there was decreased flow in the portal vein indicating possible portal vein thrombosis. A liver biopsy was performed and histologic findings included mild sinusoidal dilatation and hemosiderosis with no inflammation, necrosis, or fibrosis.
To further evaluate the possibility of portal vein thrombosis and rule out thromboembolic disease in the portal system, Magnetic Resonance Imaging and Angiography (MRI/A) of the abdomen was obtained. The MRA showed no portal vein thrombosis, however, a shunt 1–2 cm in diameter was revealed between the superior mesenteric vein and the left internal iliac vein. Subsequently, the patient underwent laparotomy. Intraoperatively, there were no signs of portal hypertension in the viscera or associated vasculature. There was an anomalous vein arising from the SMV, without collaterals, which communicated directly with the left internal iliac vein (Fig. 1). Intraoperative Dopplers were performed demonstrating that increased flow in the portal vein could be achieved with clamping of the shunt, without evidence for bowel ischemia. Consequently, the shunt was ligated resulting in restoration of flow in the portal vein. At follow-up 2 months later the patient’s ammonia level had returned to normal (12 uM/L) and mentally she was asymptomatic.
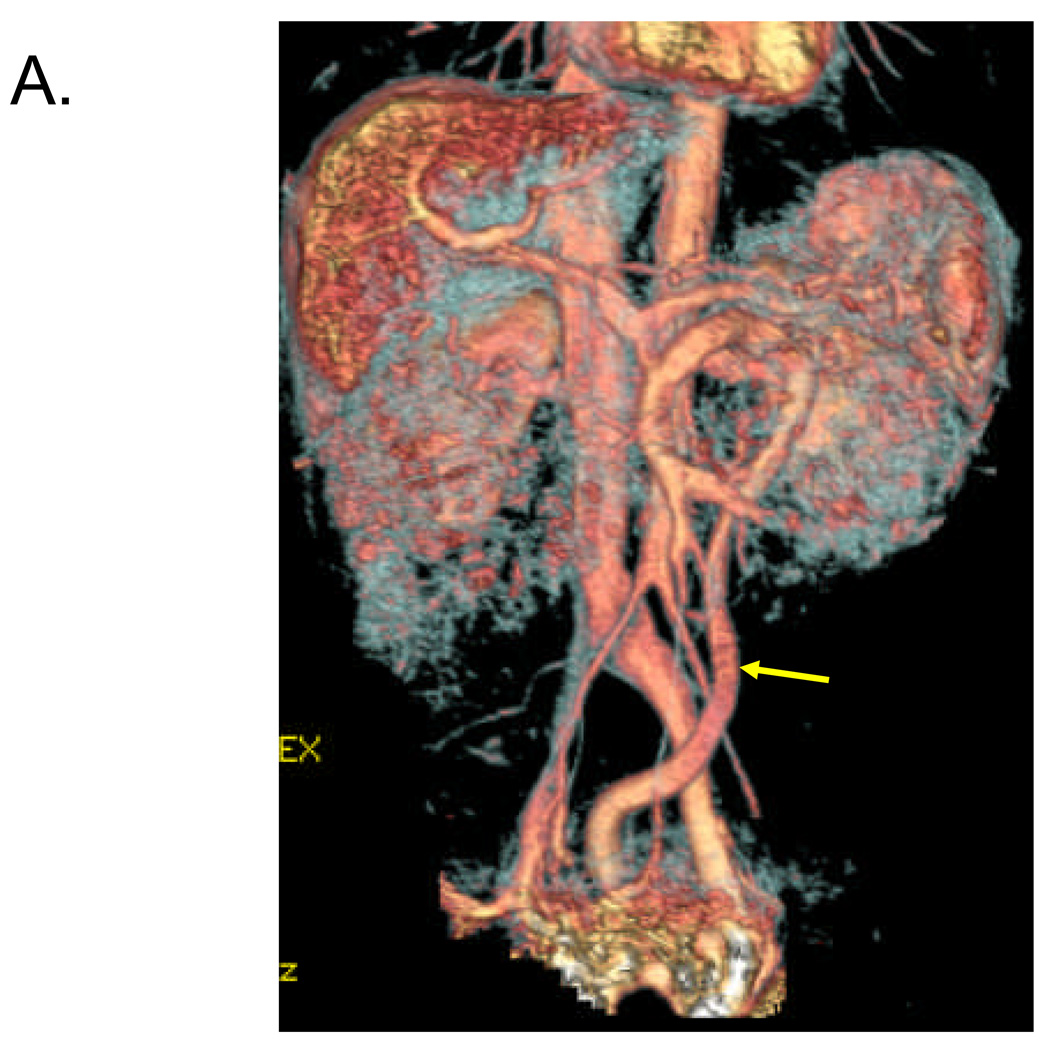
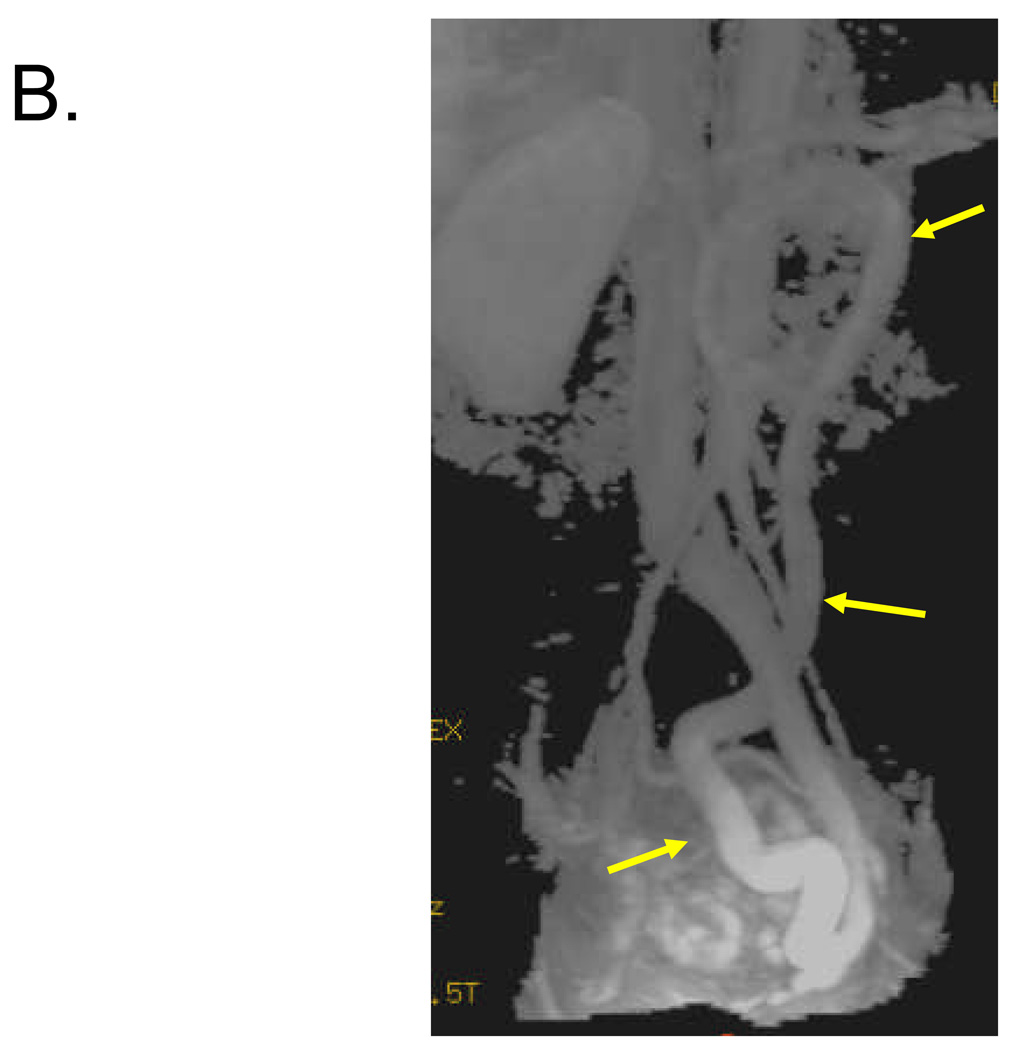
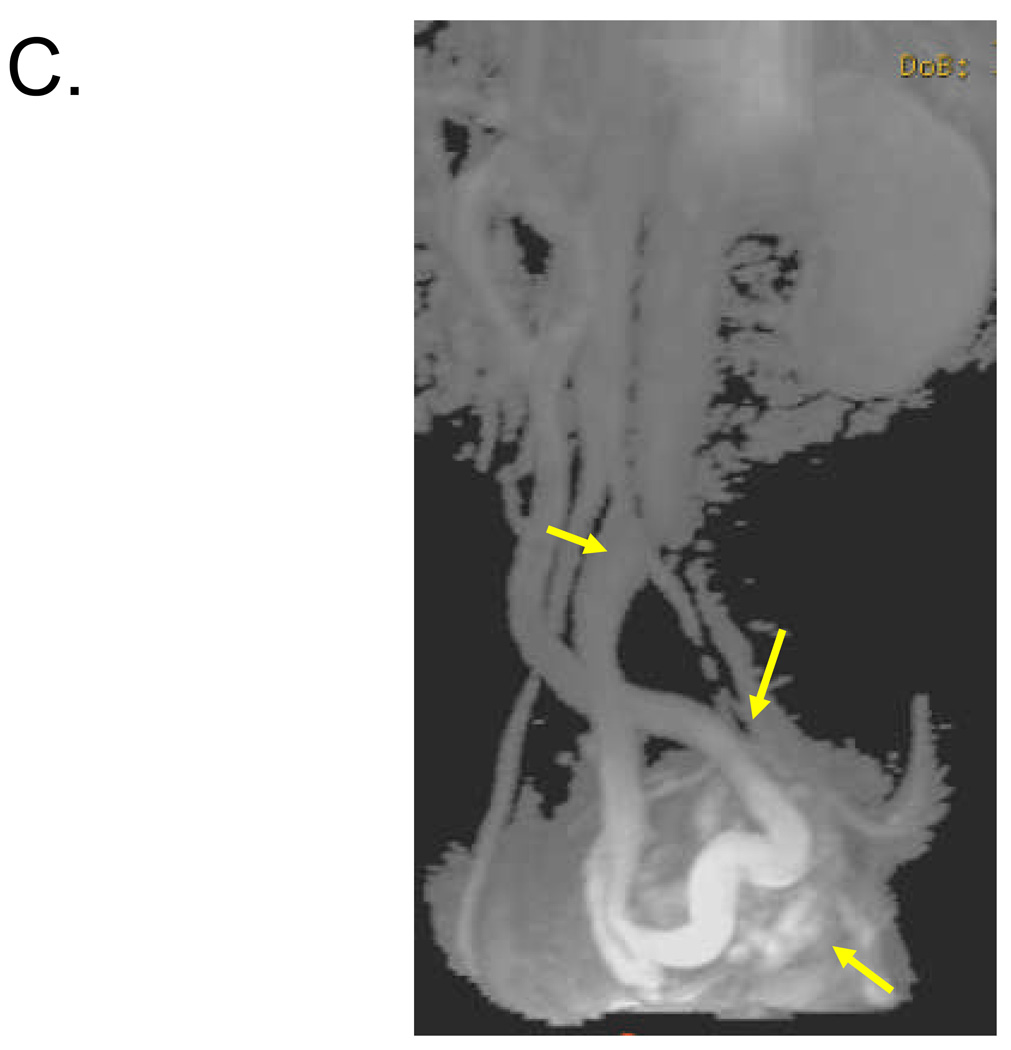
Figure 1. Identification of 1–2 cm shunt between SMV and left internal iliac vein in 57 year old female.
Volume rendered (A) and maximum intensity projection (B, C) reconstructed images from venous phase of contrast-enhanced 3D MRA demonstrate a long serpiginous shunt (arrows in A, B and C) which arises from the SMV near the portal confluence, descends into the pelvis, and empties into the left internal iliac vein.
Discussion
Most patients who develop hepatic encephalopathy (HE) have cirrhotic liver disease with established portal hypertension (1). However, a small percentage of patients develop HE, without liver disease, from other causes such as spontaneous portal vein thrombosis or portosystemic shunts (2). Portosystemic shunts arising without concomitant liver disease or portal hypertension can occur due to abdominal trauma, prior surgery, or postnatal massive necrosis secondary to either viral or hepatotoxic injury. Raskin et al (3) have proposed that non-cirrhotic shunts retaining the omphalomesenteric venous system are probably of congenital origin.
A non-cirrhotic intrahepatic shunt was first noted by Raskin et al (3) which was followed somewhat later by a report from Kerlan et al (4) who described an extrahepatic shunt. Since these earlier observations, a number of reports have verified the presence of non-cirrhotic portosystemic shunts which are likely congenital (4–13).
Similar to our case here, encephalopathy may not appear until older adulthood with non-cirrhotic congenital shunts (2). The immediate causes of HE in these patients are likely the usual triggers of high protein diet, intestinal bleeding, and constipation (2;13). Additionally, older adults may be more susceptible to hyperammonemia because of aging effects on the central nervous system and senescent hepatic function (2). On the other hand, HE can also develop in younger patients with congenital shunts as Voorhes et al (14) reported 12 cases of non-cirrhotic HE in children and young adults ages 8 to 29. Although liver disease was not present in our patient, congenital non-cirrhotic shunts can eventually lead to significant liver disease in older individuals. Uchino et al (15) suggested that this may be due to decreased blood flow to the liver over time thus leading to nutritional deficits and fatty degeneration of the hepatocytes ultimately causing hepatic dysfunction and liver atrophy.
Most extrahepatic systemic shunts involve a mesenteric vein and the vena cava. Other collateral connections that have been reported include gastroesophageal, gastrorenal, splenorenal, and inferior mesenteric-hemorrhoidal (16). To date, there are no case reports describing a shunt between the superior mesenteric vein and the left internal iliac vein as we observed here.
In addition to immediate medical therapy for treatment of HE secondary to the shunt, surgical or other interventional correction should be considered. Contraindications for surgical repair include congenital absence of the portal vein or the presence of a microshunt between the portal vein and hepatic artery, as systemic venous circulation in these patients depends on the shunt. Patients who have surgery for congenital shunt usually do well postoperatively provided they have high initial shunt ratios and portal flow is restored post surgery (2;15)
In summary, we have described an unusual spontaneous venous shunt that arose from the superior mesenteric vein and emptied into the left internal iliac vein. The shunt likely increased the patient’s susceptibility to HE which was exacerbated with an upper GI bleeding ulcer. Our patient's presentation was notable for 2 reasons: it occurred in a host with no evidence of serious liver disease, and it involved a combination of vessels that has not been previously reported.
Key Points
Though extrahepatic shunts appear to be rare they should be considered in patient’s presenting with altered consciousness and an elevated ammonia level.
Surgical correction of shunts can be curative and should be considered.
Intervention can alleviate HE and enrich patient quality of life.
Acknowledgments
Supported in part by grant from the National Institutes of Health R21 DK068453-01 (WNS).
List of abbreviations
- HE
Hepatic encephalopathy
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- AP
Alkaline Phosphatase
- GGTP
γ-Glutamyltranspeptidase
- MRI/A
Magnetic Resonance Imaging/Angiography
- SMV
superior mesenteric vein
Footnotes
Conflict of Interest.
None of the authors who participated in this study have commercial or other associations that might pose a conflict of interest.
Contributor Information
Sobia Ali, Email: Sobia-ali@uiowa.edu.
Alan H. Stolpen, Email: alan-stolpen@uiowa.edu.
Warren N. Schmidt, Email: warren-schmidt@uiowa.edu.
Reference List
- 1.Stewart CA, Cerhan J. Hepatic encephalopathy: A dynamic or static condition. Metab Brain Dis. 2005;20:193–204. doi: 10.1007/s11011-005-7207-x. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: Classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15:969–979. doi: 10.1046/j.1440-1746.2000.02283.x. [DOI] [PubMed] [Google Scholar]
- 3.Raskin NH, Fishman RA, Price JB. Portal-Systemic Encephalopathy Due to Congenital Intrahepatic Shunts. N Eng J Med. 1964;270 doi: 10.1056/NEJM196401302700503. 225-&. [DOI] [PubMed] [Google Scholar]
- 4.Kerlan RK, Sollenberger RD, Palubinskas AJ, et al. Portal-Systemic Encephalopathy Due to A Congenital Portacaval-Shunt. Am J Roentgenol. 1982;139:1013–1015. doi: 10.2214/ajr.139.5.1013. [DOI] [PubMed] [Google Scholar]
- 5.Akahoshi T, Nishizaki T, Wakasugi K, et al. Portal-systemic encephalopathy due to a congenital extrahepatic portosystemic shunt: Three cases and literature review. Hepatogastroenterology. 2000;47:1113–1116. [PubMed] [Google Scholar]
- 6.Chagnon SF, Vallee CA, Barge J, et al. Aneurysmal Portahepatic Venous Fistula - Report of 2 Cases. Radiology. 1986;159:693–695. doi: 10.1148/radiology.159.3.3517953. [DOI] [PubMed] [Google Scholar]
- 7.Honda Y, Ueda M, Kyoi M, et al. Unusual Case of Portasystemic Encephalopathy Caused by Splenic Vein Occlusion Following Gastrectomy. Am J Gastroenterol. 1978;69:590–593. [PubMed] [Google Scholar]
- 8.Komatsu S, Nagino M, Hayakawa N, et al. Congenital Absence of Portal Venous System Associated with A Large Inferior Mesenteric-Caval Shunt - A Case-Report. Hepatogastroenterology. 1995;42:286–290. [PubMed] [Google Scholar]
- 9.Mori H, Hayashi K, Fukuda T, et al. Intrahepatic Portosystemic Venous Shunt-Occurrence in Patients with and Without Liver-Cirrhosis. Am J Roentgenol. 1987;149:711–714. doi: 10.2214/ajr.149.4.711. [DOI] [PubMed] [Google Scholar]
- 10.Ohtomo K, Furui S, Saito M, et al. Case-Report - Enormous Intrahepatic Communication Between the Portal-Vein and the Hepatic Vein. Clin Radiol. 1986;37:513–514. doi: 10.1016/s0009-9260(86)80085-x. [DOI] [PubMed] [Google Scholar]
- 11.Otake M, Kobayashi Y, Hashimoto D, et al. An inferior mesenteric-caval shunt via the internal iliac vein with portosystemic encephalopathy. Internal Medicine. 2001;40:887–890. doi: 10.2169/internalmedicine.40.887. [DOI] [PubMed] [Google Scholar]
- 12.Raskin NH, Bredesen D, Ehrenfeld WK, et al. Periodic Confusion Caused by Congenital Extrahepatic Portacaval-Shunt. Neurology. 1984;34:666–669. doi: 10.1212/wnl.34.5.666. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Takatama M, Okamoto K, et al. Extrahepatic Portal-Systemic Encephalopathy Without Portal-Hypertension. Tohoku J Exp Med. 1995;175:77–90. doi: 10.1620/tjem.175.77. [DOI] [PubMed] [Google Scholar]
- 14.Voorhees AB, Chaitman E, Schneide S, et al. Portal-Systemic Encephalopathy in Noncirrhotic Patient - Effect of Portal-Systemic Shunting. Arch Surg. 1973;107:659–663. doi: 10.1001/archsurg.1973.01350230017005. [DOI] [PubMed] [Google Scholar]
- 15.Uchino T, Matsuda I, Endo F. The long-term prognosis of congenital portosystemic venous shunt. J Pediatr. 1999;135:254–256. doi: 10.1016/s0022-3476(99)70031-4. [DOI] [PubMed] [Google Scholar]
- 16.Nunez D, Russell E, Yrizarry J, et al. Portosystemic Communications Studies by Trans-Hepatic Portography. Radiology. 1978;127:75–79. doi: 10.1148/127.1.75. [DOI] [PubMed] [Google Scholar]