Abstract
Background
Acute ethanol administration increases plasma and brain levels of progesterone and deoxycorticosterone-derived neuroactive steroids (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP) and (3α,5α)-3,21-dihydroxypregnan-20-one (3α,5α-THDOC) in rats. However, little is known about ethanol effects on GABAergic neuroactive steroids in mice, non-human primates or humans. We investigated the effects of ethanol on plasma levels of 3α,5α- and 3α,5β-reduced GABAergic neuroactive steroids derived from progesterone, deoxycorticosterone, dehydroepiandrosterone and testosterone using gas chromatography-mass spectrometry.
Methods
Serum levels of GABAergic neuroactive steroids and pregnenolone were measured in male rats, C57BL/6J and DBA/2J mice, cynomolgus monkeys and humans following ethanol administration. Rats and mice were injected with ethanol (0.8 – 2.0 g/kg), cynomolgus monkeys received ethanol (1.5 g/kg) intragastrically and healthy men consumed a beverage containing 0.8 g/kg ethanol. Steroids were measured after 60 minutes in all species and also after 120 minutes in monkeys and humans.
Results
Ethanol administration to rats increased levels of 3α,5α-THP, 3α,5α-THDOC and pregnenolone at the doses of 1.5 g/kg (+228, +134 and +860%, respectively, p<0.001) and 2.0 g/kg (+399, +174 and +1125%, respectively, p<0.001), but not at the dose of 0.8 g/kg. Ethanol did not alter levels of the other neuroactive steroids. In contrast, C57BL/6J mice exhibited a 27% decrease in serum 3α,5α-THP levels (p<0.01), while DBA/2J mice showed no significant effect of ethanol, although both mouse strains exhibited substantial increases in precursor steroids. Ethanol did not alter any of the neuroactive steroids in cynomolgus monkeys at doses comparable to those studied in rats. Finally, no effect of ethanol (0.8 g/kg) was observed in men.
Conclusions
These studies show clear species differences among rats, mice and cynomolgus monkeys in the effects of ethanol administration on circulating neuroactive steroids. Rats are unique in their pronounced elevation of GABAergic neuroactive steroids, while this effect was not observed in mice or cynomolgus monkeys at comparable ethanol doses.
Keywords: GABAergic Neuroactive Steroids, Ethanol, C57BL/6J and DBA/2J Mice, Non-Human Primates, Humans
Introduction
Neuroactive steroids are endogenous neuromodulators, synthesized de novo in the brain as well as in the adrenals and gonads. They have potent effects on neurotransmission mediated by γ-aminobutyric acid type A (GABAA) receptors (Paul and Purdy, 1992) on which they act through specific binding sites on the α subunits (Hosie et al., 2006). The 3α,5α- and 3α,5β-reduced metabolites of progesterone, deoxycorticosterone, dehydroepiandrosterone (DHEA) and testosterone (Frye et al., 1996; Kaminski et al., 2005; Majewska et al., 1986) induce GABAergic actions that result in anxiolytic, anticonvulsant, sedative/hypnotic and cognitive effects (Biggio and Purdy, 2001; Morrow, 2007).
GABAergic neuroactive steroids play a crucial role in physiological states like stress (Purdy et al., 1991), pregnancy (Concas et al., 1998), ovarian cycling (Genazzani et al., 1998; Maguire et al., 2005), puberty (Grobin and Morrow, 2001; Shen et al., 2007) and aging (Schumacher et al., 2003). GABAergic neuroactive steroid levels are altered in several mood and emotional disorders, including anxiety, depression, premenstrual dysphoric disorder, schizophrenia, epilepsy and drug addiction (Girdler et al., 2001; Kaminski et al., 2005; Marx et al., 2006b; Morrow et al., 2006; Uzunova et al., 1998). Furthermore, neuroactive steroids possess neuroprotective and neurotrophic effects (Djebaili et al., 2005; Griffin et al., 2004; Wang et al., 2005) and their levels are altered in neurodegenerative diseases (Marx et al., 2006d). The neuroactive steroid 3α,5α-THP is increased in rat plasma and brain by administration of various psychoactive drugs, including ethanol (Morrow et al., 1998), caffeine (Concas et al., 2000), nicotine (Porcu et al., 2003), tetrahydrocannabinol (Grobin et al., 2005), morphine (Concas et al., 2006; Grobin et al., 2005), antidepressants (Pisu and Serra, 2004; Uzunov et al., 1996; Uzunova et al., 1998) and certain antipsychotics like clozapine and olanzapine (Barbaccia et al., 2001; Marx et al., 2000; Marx et al., 2006a; Marx et al., 2003).
Specifically, systemic administration of moderate doses of ethanol (1–2.5 g/kg) increases brain and plasma levels of (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP), (3α,5α)-3,21-dihydroxypregnan-20-one (3α,5α-THDOC) and their precursors in rodents (Barbaccia et al., 1999; Finn et al., 2004c; Gabriel et al., 2004; Khisti et al., 2005; Korneyev et al., 1993; Morrow et al., 1999; Morrow et al., 1998; O'Dell et al., 2004; Serra et al., 2003; VanDoren et al., 2000). The ethanol-induced increase in neuroactive steroids is mediated by the hypothalamic-pituitary-adrenal (HPA) axis, since it is no longer observed following adrenalectomy (Khisti et al., 2003; O'Dell et al., 2004; Porcu et al., 2004) or hypophysectomy (Boyd et al., 2009). However, ethanol can increase neuroactive steroids in hippocampal slices from both intact (Sanna et al., 2004) and adrenalectomized/gonadectomized rats (Follesa et al., 2006).
Ethanol-induced elevations in neuroactive steroids reach physiologically relevant concentrations that are capable of enhancing GABAergic transmission. A large body of evidence from multiple laboratories suggests that ethanol-induced elevations of GABAergic neuroactive steroids contribute to several behavioral effects of ethanol in rodents. Neuroactive steroids have been shown to modulate ethanol’s anticonvulsant effects (VanDoren et al., 2000), sedation (Khisti et al., 2003), impairment of spatial memory (Matthews et al., 2002; Morrow et al., 2001), anxiolytic-like (Hirani et al., 2005), antidepressant-like (Hirani et al., 2002) and proaggressive actions (Fish et al., 2001). These behavioral responses are modulated by pretreatment with finasteride and/or by prior adrenalectomy. The hypnotic effect of ethanol is partially blocked by adrenalectomy. Importantly, administration of 5α-dihydroprogesterone, the immediate precursor of 3α,5α-THP, to adrenalectomized rats restores effects of ethanol, implying that brain synthesis of neuroactive steroids also contributes to the effects of ethanol in vivo (Khisti et al., 2003). However, neuroactive steroids do not appear to influence the motor incoordinating effects of ethanol, since neither finasteride administration nor adrenalectomy diminish these actions (Khisti et al., 2004). Taken together, these studies suggest that elevations in neuroactive steroids influence many of the GABAergic effects of ethanol in vivo and contribute to sensitivity to behavioral effects of ethanol.
Studies in mice have mainly focused on two inbred strains, C57BL/6J and DBA/2J mice. While orally consumed ethanol increases brain 3α,5α-THP concentrations in male C57BL/6J mice (Finn et al., 2004c), injection of 2 g/kg ethanol increases brain 3α,5α-THP levels in DBA/2J male mice (Gabriel et al., 2004) but not C57BL/6J male mice (Finn et al., 2004c). However, serum levels of neuroactive steroids have not been reported in these studies. Indeed 3α,5α-THP modulates ethanol intake (Ford et al., 2007; Ford et al., 2005) and reinstates ethanol seeking behavior in C57BL/6J mice (Finn et al., 2008). Furthermore, the GABAergic neuroactive steroids are thought to modulate ethanol withdrawal severity across mouse strains (Finn et al., 2004b; Gililland and Finn, 2007).
Only few studies are available on the effects of acute ethanol on GABAergic neuroactive steroids in other species such as non-human primates or humans. The effects of ethanol on GABAergic neuroactive steroids in non-human primates have never been reported to the best of our knowledge. However, acute ethanol administration did not alter serum levels of the steroids pregnenolone and deoxycorticosterone in cynomolgus monkeys (Porcu et al., 2006a; Porcu et al., 2006b). In humans, results are controversial. Male and female adolescents seen in the emergency room for alcohol intoxication had elevated 3α,5α-THP plasma levels (Torres and Ortega, 2003; Torres and Ortega, 2004). In contrast, laboratory administration of low or moderate doses of ethanol had no effect on plasma 3α,5α-THP levels (Holdstock et al., 2006) or decreased 3α,5α-THP levels (Nyberg et al., 2005; Pierucci-Lagha et al., 2006). However, some of the subjective effects of ethanol are diminished by prior administration of the neuroactive steroid inhibitor finasteride (Pierucci-Lagha et al., 2005), suggesting a role for neuroactive steroids in mediating ethanol sensitivity in humans.
Here we describe the effects of acute ethanol administration to male Sprague-Dawley rats, male C57BL/6J and DBA/2J mice, male cynomolgus monkeys and healthy men on serum levels of eight GABAergic neuroactive steroids. 3α,5α-THP, (3α,5β)-3-hydroxypregnan-20-one (3α,5β-THP), 3α,5α-THDOC, (3α,5β)-3,21-dihydroxypregnan-20-one (3α,5β-THDOC), (3α,5α)-3-hydroxyandrostan-17-one (3α,5α-androsterone), (3α,5β)-3-hydroxyandrostan-17-one (3α,5β-androsterone), (3α,5α,17β)-androstane-3,17-diol (3α,5α-androstandiol), (3α,5β,17β)-androstane-3,17-diol (3α,5β-androstandiol) and their precursor pregnenolone were measured by a gas chromatography-mass spectrometry (GC-MS) assay.
Materials and Methods
Animals: rats and mice
Male Sprague-Dawley rats (200–250 g) were purchased from Harlan (Indianapolis, IN, USA). After arrival at the animal facility, rats were allowed to acclimate for one week. They were housed four per cage under 12h light, 12h dark cycle (light on from 0700 to 1900 h) and at a constant temperature of 22 ± 2°C and relative humidity of 65%. They had free access to water and standard laboratory food at all times. Rats were habituated to handling and intraperitoneal (i.p.) injection every day for five days before the experiment.
Male C57BL/6J and DBA/2J mice (8 weeks old) were purchased from The Jackson Laboratories (Bar Harbor, ME, USA). After arrival at the animal facility, mice were allowed to acclimate for one week. They were housed six per cage under 12h light, 12h dark cycle (light on from 0700 to 1900 h) and at a constant temperature of 22 ± 2°C and relative humidity of 65%. They had free access to water and standard laboratory food at all times.
Ethanol (0.8, 1.5 or 2.0 g/kg, 20% v/v in saline) or saline were administered to rats or mice by intraperitoneal injection (i.p.) 60 minutes before sacrifice. Experiments were conducted in the morning from 0900 to 1100 h to avoid the circadian fluctuation in neuroactive steroid levels (Corpéchot et al., 1997). Blood was collected from the trunk immediately after decapitation into red cap Vacutainer tubes for rats and into lithium-heparin microtainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) for mice. It was centrifuged (3000 rpm for 15 min at 4°C) and serum samples were stored in plastic minivials at −80°C until use. A 6 µl aliquot of serum was analyzed for blood ethanol levels using an SRI 8610c gas chromatograph (SRI Instruments, Torrance, CA, USA) as described by Boyd et al., (2008). Adequate measures were taken to minimize pain or discomfort of the animals. Animal care and handling throughout the experimental procedures followed National Institutes of Health Guidelines under University of North Carolina School of Medicine Institutional Animal Care and Use Committee approved protocols.
Animals: cynomolgus monkeys
Eleven adult (4–5 year old) male cynomolgus monkeys (Macaca fascicularis) were individually housed in 76 × 60 × 70 cm stainless steel cages in an environment maintained at 21 ± 1°C, with 30–50% humidity and a 11:13h light:dark cycle. The housing, feeding and history of these monkeys are extensively described in Grant et al., (2008b). The study was conducted in accordance with the Wake Forest University Animal Care and Use Committee and the guidelines for the care and use of laboratory animal resources (Commission on Life Sciences, National Research Council, 1996).
To administer the ethanol challenge to the monkeys and collect the necessary blood samples following the challenge under non-stressful conditions, the monkeys were trained to sit in a primate-restraining chair. The animals were then trained to accept an infant nasal-gastric feeding tube for ethanol administration. The ethanol challenge was conducted as part of an extensive series of assessments of the HPA axis previously described (Porcu et al., 2006a; Porcu et al., 2006b). Ethanol (95%) (Warner-Graham, Cockeysville, MD, USA) was diluted in tap water to a concentration of 30% (w/v) for the 1.5 g/kg dose. Blood draws (3 ml) were obtained via the femoral vein with a 22g × 1 inch Vacutainer needle and a 3 ml Vacutainer hematology tube (Becton Dickinson, Franklin Lakes, NJ, USA). All blood samples were stored on ice until centrifuged (approximately 5 minutes). Samples were spun at 3000 rpm for 15 minutes at 4°C. The plasma was pipetted into 2 ml microtubes and samples for neuroactive steroid analysis were frozen at −80°C and stored until processing. Blood samples were drawn before and 15, 60, 90 and 120 minutes following intragastric administration of ethanol. Only the samples obtained before and 60 and 120 minutes following ethanol administration were assayed for neuroactive steroids. Blood ethanol concentrations (BEC) were determined in whole blood (20 µl) samples obtained from the Vacutainer tube prior to centrifugation. Blood samples were sealed in air-tight vials containing 500 µl of distilled water and 20 µl of isopropanol (10% internal standard) and stored at −4°C until assay using gas chromatography (Hewlett Packard 5890 Series II, Avondale, PA, USA).
Human Subjects
Human blood samples were obtained from healthy men as previously described (Holdstock et al., 2006). The study was conducted in compliance with the Declaration of Helsinki (http://www.wma.net/e/policy/b3.htm) and was approved by the University of Chicago Institutional Review Board. Briefly, nine healthy volunteers aged 21–35, participated in the study. They were recruited from the university and surrounding community and screened by telephone and in-person interviews. Screening included a physical examination and psychiatric interview. Candidates were excluded from the study if they (i) had an Axis I psychiatric disorder other than nicotine dependence or adjustment disorder in the last year, (ii) had significant medical problems, (iii) had a body mass index (BMI) <19 or >26, or (iv) had any history of drug-or ethanol-related problems (e.g., any legal, family or health problems possibly related to drugs or ethanol) or a MAST score >5. Before participating, participants read and signed a consent form which stated that the purpose of the study was to investigate individual differences in the subjective responses to commonly used drugs. Participants were informed that they might receive a tranquilizer, stimulant, ethanol or placebo, and the possible side effects were listed. Participants were instructed to abstain from drug use for 12 h before and after sessions, and compliance was verified at each session with breathalyzer and urine drug screens for stimulants, barbiturates, opioids and phencyclidine. Participants were instructed not to eat for 2 hours before the session. After completing the study, participants were debriefed and paid for their participation.
Subjects participated in an orientation session followed by two experimental sessions (one ethanol, one placebo), conducted 1 week apart. For the measurement of the GABAergic neuroactive steroids, only samples from the ethanol session (before and after ethanol consumption) were used. Sessions were conducted from 4:30 p.m. to 7:00 a.m. on the following day. Subjects were tested individually at the University of Chicago Clinical Research Center. After confirming negative breath and urine tests, an intravenous catheter was inserted for blood draws, and participants consumed a light snack. Forty-five minutes later, at 5:30 p.m., participants completed baseline mood questionnaires and a performance task, vital signs were taken, and blood was drawn to determine baseline steroid levels. Between 5:45 and 6:00 p.m., participants consumed two beverages (0.8 g/kg ethanol total), each over a 5-min period, with a 5-min rest in between. Beverages consisted of water, sugar-free grape Kool-aid sweetened with NutraSweet, and the appropriate concentration of ethanol to make up a 16% ethanol solution by volume. The volume of beverage was 450 ml for a 70-kg person, and adjustments were made for deviations in body weight by adjusting the volume. Beverages were served cold in a Styrofoam cup with a lid and consumed through a straw. Subjects were discharged at 7:05 a.m. During the sessions, when participants were not completing dependent measures, they were free to watch television or read. For GABAergic neuroactive steroids measures, only samples obtained at baseline, 60 and 120 minutes after the start of ethanol consumption were evaluated.
Neuroactive Steroid Analysis
Neuroactive steroids were measured in serum by GC-MS following purification by solid phase extraction, as previously described (Porcu et al., 2009). Serum samples (300 µl) were spiked with 400 pg/ml of each deuterated internal standard and applied to C18 solid phase extraction columns (RPN1910, 500 mg, GE Healthcare, UK) that had been preconditioned with 4 ml methanol and 4 ml distilled water. The column containing the sample was washed with 4 ml distilled water in order to remove high polar impurities. Columns were dried under vacuum for 30 minutes and neuroactive steroids were then eluted with 2 ml methanol. The extracts were evaporated in a speed vacuum concentrator (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The dry residue was resuspended in 2 ml of ethyl acetate/methanol (80/20, v/v) and the sample was filtered through a NH2 column (Supelclean LC-NH2, 500 mg, Supelco, Bellefonte, PA, USA) previously preconditioned with 4 ml of ethyl acetate and 4 ml of ethyl acetate/methanol (80/20, v/v). The neuroactive steroids passed unretained through the sorbent, and the eluate was collected. The NH2 column was further rinsed with 2 ml of the solvent mixture and the combined eluates were evaporated in the speed vacuum concentrator. Dried samples after purification were resuspended in 800 µl methanol and transferred to derivatization vials (Wheaton, Millville, NJ, USA). Methanol was evaporated and samples were derivatized in 450 µl of ethyl acetate and 50 µl of heptafluorobutyric acid anhydride, followed by vortex mixing. Samples were allowed to react for two hours at room temperature and were dried under a gentle stream of nitrogen. Derivatized samples were resuspended in 10 µl of heptane and 2 µl of each sample was injected in duplicate into the GC-MS. Analysis was carried out on an Agilent 6890 gas chromatograph coupled to a 5973 mass selective detector (Agilent Technologies, Inc., Santa Clara, CA, USA) operated in negative chemical ionization mode. A capillary column (30 m × 0.25 mm, 0.25 µm film thickness, 5%-phenyl-methylpolysiloxane, J&W Scientific, Agilent Technologies, Inc., Santa Clara, CA, USA) was used to separate the derivatives of each neuroactive steroid. Samples were injected into the GC in splitless mode at 12 psi and at 250°C using a 7683 series injector (Agilent Technologies, Inc., Santa Clara, CA, USA). The carrier gas was ultrapure helium (99.9995%, Airgas National Welders, Durham, NC, USA) set at constant flow of 1.0 ml/min. Methane (99.999% Airgas National Welders, Durham, NC, USA) was the reagent gas. The initial GC oven temperature was 75°C (0.86 min hold), followed by an increase to 210°C at 35° increments (3 min hold), an increase to 235°C at 2.5° increments (9 min hold) and finally to 310°C at 25° increments (2 min hold). The transfer line temperature was maintained at 280°C. Neuroactive steroids were analyzed by single ion monitoring. The data acquisition was broken into retention windows corresponding to the elution of the different neuroactive steroid groups. The temperatures of mass spectrometer source and quadrupole were 150°C. Neuroactive steroids were quantified by interpolation of linear regression standard curves. Calibration curves were made in 300 µl distilled water spiked with 5 µl human charcoal-stripped serum (Gemini Bio-Products, Woodland, CA, USA), with 400 pg/ml of each deuterated internal standard and with the appropriate known concentration of neuroactive steroids (2, 10, 20, 50, 100, 200, 500, 1000, 2000 and 3000 pg/ml). A blank standard (5 µl human charcoal-stripped serum/300 µl distilled water) was also included. Calibration curves underwent the same extraction procedure as the samples.
Steroid standards (>99% purity) for 3α,5α-androsterone, 3α,5β-androsterone, 3α,5α-androstandiol, 3α,5β-androstandiol, 3α,5β-THP, 3α,5β-THDOC and pregnenolone were purchased from Steraloids, Inc. (Newport, RI, USA). Deuterium-labeled standards (>95% purity) (d4–17,21,21,21)-3α,5α-THP and (d3–17,21,21)-3α,5α-THDOC were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). (d4-2,2,4,4)-3α,5α-androsterone was purchased from Cerilliant (Round Rock, TX, USA). 3α,5α-THP, 3α,5α-THDOC and (d4–17,21,21,21)-pregnenolone (98% purity) were synthesized by Dr. R.H. Purdy (Veterans Medical Research Foundation, San Diego, CA, USA). Derivatization reagent heptafluorobutyric acid anhydride was purchased from Pierce (Rockford, IL, USA). Organic solvents were pesticide grade from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Deoxycorticosterone was measured in mouse serum by radioimmunoassay as previously described (Porcu et al., 2006a), with minor modifications (only 100 µl of serum was used for deoxycorticosterone extraction). Progesterone levels were measured in mouse serum using a commercially available radioimmunoassay (MP Biomedicals, Indianapolis, IN, USA).
Statistical analysis
Statistical analysis was performed using a commercially available statistical program (GraphPad Prism 4.0, GraphPad Software, San Diego, CA, USA). One-way ANOVA or unpaired t-test was used to compare the effects of ethanol administration on serum neuroactive steroid levels.
Results
Effects of acute ethanol administration on serum neuroactive steroids in rats
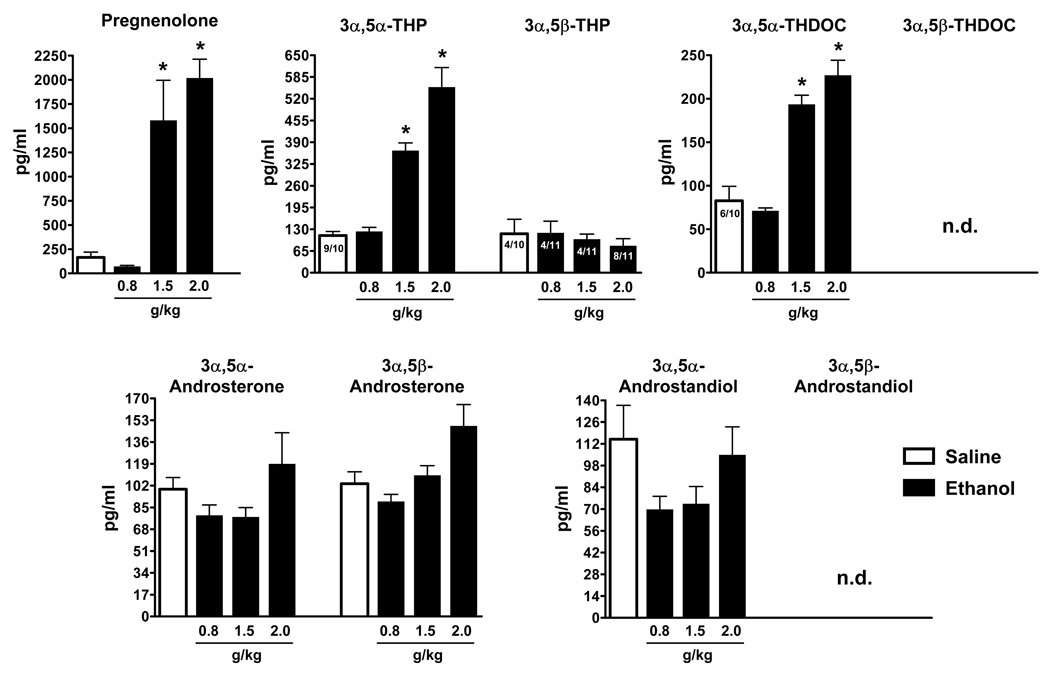
Basal levels of serum GABAergic neuroactive steroids in rats are presented in Table 1 and are comparable to those previously reported using this GC-MS assay (Porcu et al., 2009). Acute ethanol administration selectively increased serum levels of GABAergic neuroactive steroids and their precursor pregnenolone (Figure 1). The doses of 1.5 and 2.0 g/kg ethanol significantly elevated pregnenolone (+ 860 and + 1125%, respectively, p<0.001), 3α,5α-THP (+ 228 and + 399%, respectively, p<0.001) and 3α,5α-THDOC (+ 134 and + 174%, respectively, p<0.001). In contrast, the dose of 0.8 g/kg ethanol did not alter serum levels of these steroids. 3α,5α-androsterone, 3α,5β-androsterone and 3α,5α-androstandiol were not altered by ethanol administration at any dose examined. 3α,5β-THP was detected only in 4/10 control rats, 4/11 rats treated with ethanol 0.8 and 1.5 g/kg and 8/11 rats treated with ethanol 2.0 g/kg. Its levels were not altered by any dose of ethanol administered. 3α,5β-THDOC and 3α,5β-androstandiol were not detected in rat serum samples even following ethanol administration. Blood ethanol levels in these rats were 81 ± 2.0, 212 ± 7.2 and 257 ± 6.0 mg/dl (average ± SEM) at 0.8, 1.5 and 2.0 g/kg, respectively.
Table 1.
Basal serum levels of GABAergic neuroactive steroids across species.
| Rats | C57BL/6J Mice | DBA/2J Mice | Monkeys | Humans | |
|---|---|---|---|---|---|
| Pregnenolone | 164.9 ± 55.3 (8/9) | 48.8 ± 6.0 (7/7) | 27.6 ± 8.3 (6/7) | 944.8 ± 287.1 (10/11) | 296.9 ± 28.9 (8/8) |
| 3α,5α-THP | 111.3 ± 12.2 (9/10) | 1043 ± 91.3 (14/14) | 455.2 ± 63.4 (8/8) | 59.8 ± 14.7 (11/11) | 124.8 ± 12.2 (8/8) |
| 3α,5β-THP | 116.5 ± 43.4 (4/10) | n.d. | n.d. | 19.3 ± 7.4 (11/11) | 109.2 ± 4.5 (8/8) |
| 3α,5α-THDOC | 82.8 ± 16.5 (6/10) | 589.4 ± 76.0 (14/14) | 393.6 ± 64.5 (7/8) | 27.5 ± 2.4 (10/10) | 63.0 ± 21.6 (8/8) |
| 3α,5β-THDOC | n.d. | n.d. | n.d. | n.d. | n.d. |
| 3α,5α-Androsterone | 99.2 ± 9.1 (10/10) | 61.3 ± 7.6 (14/14) | 38.7 ± 8.9 (7/8) | 103.6 ± 14.3 (11/11) | 97.3 ± 12.0 (8/8) |
| 3α,5β-Androsterone | 103.5 ± 9.3 (10/10) | n.d. | n.d. | n.d. | 177.5 ± 88.7 (8/8) |
| 3α,5α-Androstandiol | 115.0 ± 21.8 (10/10) | 72.2 ± 2.8 (14/14) | 72.5 ± 8.6 (8/8) | 4.7 ± 1.9 (8/11) | 34.9 ± 4.3 (8/8) |
| 3α,5β-Androstandiol | n.d. | 50.5 ± 1.9 (11/14) | 50.1 ± 4.6 (5/8) | n.d. | 23.4 ± 1.4 (8/8) |
Serum GABAergic neuroactive steroids were measured by GC-MS as previously described (Porcu et al., 2009). Levels are expressed as pg/ml and are average ± SEM. The n values for each group are reported in parentheses. The limit of detection for the GC-MS assay was 2 pg/ml for 3α,5β-THP, 3α,5α-THDOC, 3α,5β-THDOC, 3α,5β-androsterone, 3α,5α-androstandiol, 3α,5β-androstandiol, and 10 pg/ml for 3α,5α-THP, 3α,5α-androsterone and pregnenolone.
Figure 1.
Effect of ethanol administration to male rats on serum levels of GABAergic neuroactive steroids. Ethanol (0.8, 1.5 and 2 g/kg, 20% v/v in saline) or saline were administered i.p. 60 minutes before sacrifice. Levels are expressed as pg/ml and are average ± SEM of ten rats in the saline-treated group and eleven rats in the ethanol-treated groups. 3α,5β-THP was detected only in 4/10 rats in the saline-treated group and 4/11, 4/11 and 8/11 rats in the ethanol-treated groups at 0.8, 1.5 and 2.0 g/kg, respectively. 3α,5β-THDOC and 3α,5β-androstandiol were undetectable (n.d.). *p<0.001 vs the respective saline-treated control values; one-way ANOVA followed by Newman-Keuls post hoc test.
Effects of acute ethanol administration on serum neuroactive steroids in C57BL/6J and DBA/2J mice
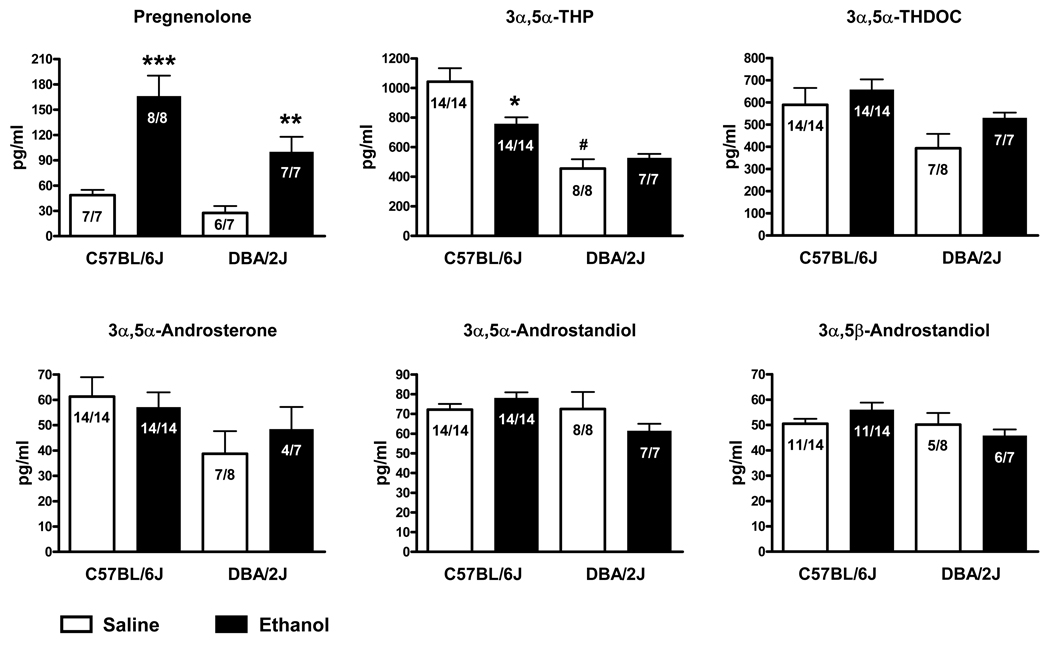
Basal levels of serum GABAergic neuroactive steroids in C57BL/6J mice and DBA/2J mice are reported in Table 1. Basal levels of 3α,5α-THP are significantly lower (−56%, p<0.0005) in DBA/2J mice compared to C57BL/6J mice (Figure 2 and Table 1). A trend for lower basal levels was also observed for pregnenolone (−43%, p=0.06), 3α,5α-androsterone (−37%, p=0.09) and 3α,5α-THDOC (−33%, p=0.11).
Figure 2.
Effect of ethanol administration to male C57BL/6J and DBA/2J mice on serum levels of GABAergic neuroactive steroids. Ethanol (2 g/kg, 20% v/v in saline) or saline were administered i.p. 60 minutes before sacrifice. Levels are expressed as pg/ml and are average ± SEM of 4–14 mice per group (exact number is indicated in each bar). 3α,5β-THP, 3α,5β-THDOC and 3α,5β-androstandiol were undetectable. *p<0.01, **p<0.005 and ***p<0.001 vs the respective saline-treated group in each strain; unpaired t-test comparing the saline- and ethanol-treated groups for each strain. #p<0.0005 vs the saline-treated C57BL/6J group; unpaired t-test comparing only the saline-treated groups.
Acute ethanol administration (2.0 g/kg, i.p.) significantly increased serum pregnenolone levels in both C57BL/6J and DBA/2J mice (+241%, p<0.001 and +263%, p<0.005, respectively; Figure 2). However, there was no increase in its neuroactive metabolites. In fact, 3α,5α-THP serum levels were significantly decreased in C57BL/6J mice (−27%, p<0.01) and were not altered in DBA/2J mice (Figure 2). 3α,5α-THDOC, 3α,5α-androsterone, 3α,5α-androstandiol and 3α,5β-androstandiol were also not altered in either strain of mice following ethanol administration (Figure 2). 3α,5β-THP, 3α,5β-THDOC and 3α,5β-androsterone were not detected in either C57BL/6J or DBA/2J mice following saline or ethanol administration.
Given that acute ethanol increases pregnenolone levels in these mice but has differential effects on its neuroactive metabolites, we decided to measure plasma levels of progesterone and deoxycorticosterone, the immediate precursors of the GABAergic neuroactive steroids 3α,5α-THP and 3α,5α-THDOC, respectively. Basal progesterone and deoxycorticosterone levels in C57BL/6J and DBA/2J mice are reported in Table 2. DBA/2J mice had significantly lower basal deoxycorticosterone levels compared to C57BL/6J mice (−54%, p<0.005). Acute ethanol administration significantly increased progesterone and deoxycorticosterone serum levels in both C57BL/6J and DBA/2J mice. Progesterone levels were increased by 515% (p<0.005) in C57BL/6J and by 803% (p<0.005) in DBA/2J mice. Deoxycorticosterone levels were increased by 185% (p<0.05) in C57BL/6J mice and by 308% (p<0.0001) in DBA/2J mice (Table 2).
Table 2.
Effects of acute ethanol administration on serum levels of the neuroactive steroid precursors progesterone and deoxycorticosterone in male C57BL/6J and DBA/2J mice.
| Progesterone | Deoxycorticosterone | |
|---|---|---|
| C57BL/6J | ||
| Saline | 2.3 ± 0.6 | 7.7 ± 1.1 |
| Ethanol | 14.4 ± 3.6b | 21.8 ± 5.3a |
| DBA/2J | ||
| Saline | 1.3 ± 0.1 | 3.5 ± 0.6d |
| Ethanol | 11.4 ± 2.2b | 14.3 ± 1.7c |
Ethanol (2 g/kg, 20% v/v in saline) or saline were administered i.p. 60 minutes before sacrifice. Levels are expressed as ng/ml and are average ± SEM of 7–8 mice per group.
p<0.05
p<0.005 and
p<0.0001 vs the respective saline-treated group; unpaired t-test.
p<0.005 vs the respective saline-treated C57BL/6J mice group; unpaired t-test.
Blood ethanol levels were 196 ± 4.1 and 196 ± 5.0 mg/dl (average ± SEM) for C57BL/6J and DBA/2J mice, respectively.
Effects of acute ethanol administration on serum neuroactive steroids in cynomolgus monkeys
Basal levels of serum GABAergic neuroactive steroids in cynomolgus monkeys are reported in Table 1. Acute ethanol administration (1.5 g/kg, intragastrically) did not alter serum levels of any of the neuroactive steroids measured (Figure 3). Average ± SEM blood ethanol levels were 125 ± 24 mg/dl at 60 minutes and 139 ± 19 mg/dl at 120 minutes.
Figure 3.
Effect of ethanol administration to male cynomolgus monkeys on serum levels of GABAergic neuroactive steroids. Ethanol (1.5 g/kg, 30% w/v in tap water) was administered intragastrically and blood samples were collected before, 60 and 120 minutes from ethanol administration. Levels are expressed as pg/ml and are average ± SEM of twelve subjects. 3α,5β-androstandiol was detected only in 9/12 subjects before ethanol administration and in 10/12 subjetcs after ethanol administration. 3α,5β-THDOC, 3α,5β-androsterone and 3α,5β-androstandiol were undetectable (n.d.).
Effects of acute ethanol consumption on serum neuroactive steroids in healthy men
Basal levels of serum GABAergic neuroactive steroids in healthy men are reported in Table 1 and Table 3. Consumption of ethanol did not alter serum levels of any of the neuroactive steroids measured (Table 3). Blood ethanol levels were 75 ± 10 mg/dl (average ± SEM).
Table 3.
Effects of ethanol consumption on serum levels of GABAergic neuroactive steroids in healthy men.
| −15 min | 60 min | 120 min | |
|---|---|---|---|
| Pregnenolone | 296.9 ± 28.9 | 262.4 ± 10.5 | 239.1 ± 14.9 |
| 3α,5α-THP | 124.8 ± 12.2 | 144.6 ± 12.6 | 134.4 ± 10.2 |
| 3α,5β-THP | 109.2 ± 4.5 | 105.1 ± 1.7 | 105.2 ± 2.1 |
| 3α,5α-THDOC | 63.0 ± 21.6 | 65.3 ± 19.6 | 45.1 ± 7.5 |
| 3α,5β-THDOC | n.d. | n.d. | n.d. |
| 3α,5α-Androsterone | 97.3 ± 12.0 | 63.4 ± 6.4 | 101.1 ± 22.9 |
| 3α,5β-Androsterone | 177.5 ± 88.7 | 275.8 ± 105.6 | 245.2 ± 71.9 |
| 3α,5α-Androstandiol | 34.9 ± 4.3 | 31.8 ± 2.4 | 34.6 ± 3.0 |
| 3α,5β-Androstandiol | 23.4 ± 1.4 | 27.3 ± 2.6 | 22.4 ± 1.2 |
Ethanol (0.8 g/kg, 16% w/v beverage solution) was consumed at time 0 and blood samples were collected 15 minutes before and 60 and 120 minutes from the start of ethanol consumption. Levels are expressed as pg/ml and are average ± SEM of eight subjects. 3α,5β-androsterone was detected only in 6/8 subjects 60 minutes after ethanol consumption; 3α,5β-androstandiol was detected only in 7/8 subjects 120 minutes after ethanol consumption. 3α,5β-THDOC was undetectable (n.d.).
Discussion
With the GC-MS procedure described here and elsewhere (Porcu et al., 2009), we have extended previous investigations on the effects of ethanol on circulating neuroactive steroid concentrations to eight different GABAergic neuroactive steroids in four different species. This comprehensive examination revealed pronounced species differences in the ethanol-stimulated concentrations of neuroactive steroids. Ethanol produces elevations of pregnenolone and the 3α,5α-reduced metabolites of progesterone and deoxycorticosterone in rats at doses of 1.5 g/kg or greater. In contrast, C57BL/6J mice exhibit a 27% decrease in the progesterone metabolite 3α,5α-THP, while DBA/2J mice show no significant effect of ethanol (2 g/kg), although both mouse strains exhibit increases in pregnenolone (241 and 263%), progesterone (515 and 803%) and deoxycorticosterone (185 and 308%) precursors. Cynomolgus monkeys show no ethanol-induced (1.5 g/kg) changes in any steroids measured. Similarly, ethanol (0.8 g/kg) had no effect on any steroid measured in humans.
Numerous previous reports have shown that acute ethanol administration increases 3α,5α-THP and 3α,5α-THDOC levels in rat plasma and cerebral cortex (Barbaccia et al., 1999; Morrow et al., 1999; Morrow et al., 1998; Porcu et al., 2004; Serra et al., 2003; VanDoren et al., 2000), using chromatographic separation followed by radioimmunoassay to detect and quantify the steroids. The present study replicates and extends these findings using a highly sensitive and specific GC-MS assay (Porcu et al., 2009). Absolute values for basal 3α,5α-THP and 3α,5α-THDOC levels are much lower using the GC-MS assay (Marx et al., 2009; Marx et al., 2006b; Marx et al., 2006c; O'Dell et al., 2004; Porcu et al., 2009; Romeo et al., 1998; Strohle et al., 2000) compared to previous radioimmunoassay studies mentioned above, possibly indicating less specificity in the radioimmunoassay due to antibody cross-reactivity. However the percent change in steroid levels following ethanol administration is comparable to the previously published observations (vide supra).
Our results in rats replicate previous work on the effects of ethanol on 3α,5α-THP, 3α,5α-THDOC and pregnenolone levels (Barbaccia et al., 1999; Korneyev et al., 1993; Morrow et al., 1999; Morrow et al., 1998; O'Dell et al., 2004; Porcu et al., 2004; Serra et al., 2003; VanDoren et al., 2000) and extend the analysis to the other GABAergic neuroactive steroids. The results suggest that the effects of ethanol require doses of ethanol (1.5 – 2.0 g/kg) capable of inducing blood alcohol levels greater than 200 mg/dl. Ethanol has a selective effect on the progesterone and deoxycorticosterone metabolites and does not alter the DHEA and testosterone metabolites. Indeed, past studies on the effects of ethanol on these precursors are controversial. It has been reported that acute ethanol administration either decreases (Rivier, 1999) or increases testosterone levels in rats (Alomary et al., 2003); furthermore, it does not alter (Morrow et al., 2001) or it decreases (Robel and Baulieu, 1995) DHEA levels in several brain regions of rats.
In contrast, the same dose, route of administration and the time course of ethanol that increase the GABAergic neuroactive steroids in rats, decrease 3α,5α-THP levels in C57BL/6J mice and have no effect in DBA/2J mice. The differential effect of ethanol on serum 3α,5α-THP levels may relate to the differential behavioral sensitivity to ethanol in these mouse strains (Phillips and Crabbe, 1991). A differential effect of ethanol on brain 3α,5α-THP levels has also been observed in these mouse strains, where acute ethanol administration increased brain 3α,5α-THP levels in male DBA/2J mice (Gabriel et al., 2004), but not male C57BL/6J mice (Finn et al., 2004c). Our results may differ due to the use of serum vs. whole brain tissue. Indeed, the lack of effect of ethanol on serum levels of 3α,5α-THP coupled with the ethanol-induced increase in brain levels of 3α,5α-THP in DBA/2J mice may suggest that serum and brain levels of this neuroactive steroid could be differentially regulated.
Ethanol administration increases serum levels of pregnenolone and its metabolites progesterone and deoxycorticosterone in C57BL/6J and DBA/2J mice. However, this effect is not associated with an increase in the serum GABAergic neuroactive metabolites. These two strains of mice have the same blood alcohol levels; values are similar to those in rats that are capable of increasing 3α,5α-THP and 3α,5α-THDOC levels. Therefore, it is possible that these strains of mice are less sensitive than rats to the ethanol-induced increases in GABAergic neuroactive steroids, perhaps because of differences in the enzymatic activity that leads to the conversion of progesterone and deoxycorticosterone into 3α,5α-THP and 3α,5α-THDOC, respectively. Previous studies have shown that C57BL/6J and DBA/2J mice differ in the Srd5a-1 gene sequence that codes for the 5α-reductase type 1 enzyme, suggesting that C57BL/6J and DBA/2J mice may express functionally distinct 5α-reductase enzymes (Jenkins et al., 1991). Indeed, Finn et al., (2004a), showed that basal enzyme activity was greater in the hippocampus and cerebral cortex of C57BL/6J vs. DBA/2J mice. This difference in basal enzyme activity may explain the differences in basal levels of 3α,5α-THP that we observed in these strains. However, the effects of ethanol on enzyme expression or activity in the mouse have never been reported to the best of our knowledge. Our data may suggest that adrenal 5α-reductase and 3α-hydroxysteroid dehydrogenase activities in C57BL/6J and DBA/2J mice are impaired by ethanol, given that the increase in plasma precursor steroids does not result in increased levels of the plasma 3α,5α-THP or 3α,5α-THDOC.
We have also found that C57BL/6J and DBA/2J mice differ in basal 3α,5α-THP serum levels and show a trend for different basal levels of the other GABAergic neuroactive steroids. DBA/2J mice have lower basal 3α,5α-THP levels in serum compared to C57BL/6J mice. A similar finding has been reported by Finn et al., (2003) with respect to 3α,5α-THP levels in brain (but see also Finn et al., 1997). DBA/2J mice also show increased seizure susceptibility and increased ethanol withdrawal-induced severity compared to C57BL/6J mice (Finn et al., 2004a), suggesting that higher baseline neuroactive steroids (as found in C57BL/6J mice) may exert a protective role against ethanol withdrawal.
In contrast to the rodent data, acute ethanol administration did not alter the GABAergic neuroactive steroids in monkeys or humans. Ethanol was delivered to the stomachs of cynomolgus monkeys and consumed in a beverage by the human subjects. Indeed, the same dose of ethanol used in the human studies (0.8 g/kg), failed to alter neuroactive steroids in rats, suggesting that a minimum blood alcohol level is required to increase neuroactive steroid concentrations. Higher doses could not be tested in humans due to ethical constraints. In contrast, the dose of ethanol used in the monkey study (1.5 g/kg) significantly increased neuroactive steroids in rats, but had no effect in cynomolgus monkeys. However, the same dose of ethanol (1.5 g/kg) produced different blood alcohol levels in monkeys and rats (125 mg/dl vs. 212 mg/dl, respectively). Thus, it is possible that doses of ethanol which induce higher blood alcohol levels may also increase circulating neuroactive steroids in monkeys. Furthermore, given the possibility of local synthesis of neuroactive steroids in the brain (Follesa et al., 2006; Sanna et al., 2004), we cannot rule out the possibility that lower doses of ethanol increase brain levels of neuroactive steroids in all species.
In drug discrimination studies with monkeys as subjects, 1.0 g/kg ethanol is a robust stimulus with substitution of GABAergic neuroactive steroids 3α,5α-THP, 3α,5β-THP and 3α,5α-androsterone, suggesting that the effects perceived by the animals are similar (Grant et al., 1996; Grant et al., 1997; Grant et al., 2008a; Green et al., 1999). In addition, circulating levels of progesterone, a direct precursor of 3α,5α-THP, influence the sensitivity of ethanol substitution, shifting the dose-response curve to the left and suggesting additive effects of circulating neuroactive steroids with administered ethanol (Grant et al., 1997). Hence, it is possible that ethanol (1.0 g/kg) increases brain neuroactive steroids in monkeys with no increase in plasma levels of these steroids. Further studies to measure neuroactive steroids in brain tissue are needed to address this possibility.
The lack of change in serum levels of all these GABAergic neuroactive steroids and their precursor pregnenolone in humans is consistent with previous studies. In fact, laboratory administration of low or moderate doses of ethanol in a non alcohol-abusing population, similar to the one reported here, had no effect on serum 3α,5α-THP levels (Holdstock et al., 2006) or decreased 3α,5α-THP levels (Nyberg et al., 2005; Pierucci-Lagha et al., 2006). However, others have reported that male and female adolescents seen in the emergency room for alcohol intoxication had elevated serum levels of 3α,5α-THP, presumably because of the elevated doses of ethanol ingested (although blood alcohol levels between 85–108 mg/dl were reported) (Torres and Ortega, 2003; Torres and Ortega, 2004). Interestingly, administration of the neuroactive steroid inhibitor finasteride attenuates the subjective effects of ethanol in individuals homozygous for the A allele at the GABAA receptor α2 subunit (GABRA2) gene polymorphism but not in individuals with the G allele (associated with alcohol dependence), suggesting a role for neuroactive steroids in mediating ethanol sensitivity in humans (Pierucci-Lagha et al., 2005). Therefore, it is possible that our results may not be generalized to the entire population and the genotype of the individuals in the present study was not determined. Thus, the effects of ethanol on circulating levels of GABAergic neuroactive steroids appear to be influenced by dose, metabolism and genotype as well as different analytic methods to measure neuroactive steroids or environmental factors that influence neuroactive steroid synthesis in humans. Alternatively, the effects of ethanol on neuroactive steroid levels may involve neurosteroidogenesis independent of circulating steroid levels. Nonetheless, we cannot rule out the possibility that higher intoxicating doses of ethanol may increase circulating neuroactive steroids in healthy human subjects. The potential ethanol-induced increases in GABAergic neuroactive steroids may relate to ethanol sensitivity and may play a role in preventing excessive alcohol consumption in healthy individuals (Morrow et al., 2006). In contrast, alcohol-dependent subjects show decreased neuroactive steroid responses in agreement with suppressed HPA axis function (Hill et al., 2005, Porcu et al., 2008, Romeo et al., 1996). The loss of neuroactive steroid responses may promote excessive alcohol consumption to achieve the desired effects of ethanol. Excessive alcohol consumption is a significant risk factor for alcohol dependence and alcoholism. Thus, neuroactive steroids may have therapeutic utility in restoring ethanol sensitivity in alcohol-dependent subjects.
In conclusion, these results show clear species differences between rats and mice at comparable ethanol doses and blood alcohol concentrations. Species differences between rats and monkeys are confounded by the different blood alcohol levels achieved using the same dose of ethanol. Differences between humans and these other species cannot be assessed due to ethanol dosing constraints. In fact, the same dose used in the human study failed to alter circulating neuroactive steroids in rats. Rats are unique in their pronounced elevation of GABAergic neuroactive steroids, while this effect was not observed in mice and cynomolgus monkeys at comparable ethanol doses, although comparable blood alcohol levels were not achieved. Translational studies on the role of neuroactive steroids in ethanol actions, including tolerance and risk for ethanol abuse or dependence should consider the similarities and differences in neuroactive steroids across species.
Acknowledgments
Source of Support: This study was supported by the National Institute of Health grants AA010564 and AA016677 to ALM, AA13510 to KAG and DA02812 to HdW.
References
- Alomary AA, Vallee M, O'Dell LE, Koob GF, Purdy RH, Fitzgerald RL. Acutely administered ethanol participates in testosterone synthesis and increases testosterone in rat brain. Alcohol Clin Exp Res. 2003;27(1):38–43. doi: 10.1097/01.ALC.0000047304.28550.4F. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25(4):489–497. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases "GABAergic" neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384(2–3):R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Biggio G, Purdy RH. Int Rev Neurobiol. Vol 46. New York: Academic Press; 2001. Neurosteroids and Brain Function. [Google Scholar]
- Boyd KN, Kumar S, O'Buckley TK, Morrow AL. Ethanol induction of steroidogenesis is dependent upon pituitary ACTH release and de novo adrenal StAR synthesis. 2009 doi: 10.1111/j.1471-4159.2009.06509.x. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KN, O'Buckley TK, Morrow AL. Role of acetaldehyde in ethanol-induced elevation of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in rats. Alcohol Clin Exp Res. 2008;32(10):1774–1781. doi: 10.1111/j.1530-0277.2008.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U.S.A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Porcu P, Sogliano C, Serra M, Purdy RH, Biggio G. Caffeine-induced increases in the brain and plasma concentrations of neuroactive steroids in the rat. Pharmacol Biochem Behav. 2000;66(1):39–45. doi: 10.1016/s0091-3057(00)00237-9. [DOI] [PubMed] [Google Scholar]
- Concas A, Sogliano C, Porcu P, Marra C, Brundu A, Biggio G. Neurosteroids in nicotine and morphine dependence. Psychopharmacology (Berl) 2006;186(3):281–292. doi: 10.1007/s00213-005-0111-7. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22(1):106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004a;101(2):91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: sex and strain differences. Pharmacol Biochem Behav. 2004b;78(3):435–443. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Finn DA, Mark GP, Fretwell AM, Gililland-Kaufman KR, Strong MN, Ford MM. Reinstatement of ethanol and sucrose seeking by the neurosteroid allopregnanolone in C57BL/6 mice. Psychopharmacology (Berl) 2008;201(3):423–433. doi: 10.1007/s00213-008-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Long S, Tanchuck M, Phillips TJ. Neurosteroid consumption has anxiolytic effects in mice. Pharmacol Biochem Behav. 2003;76(3–4):451–462. doi: 10.1016/j.pbb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Lotrich F, Gallaher EJ. Genetic differences in behavioral sensitivity to a neuroactive steroid. J Pharmacol Exp Ther. 1997;280:820–828. [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004c;123(4):813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, DeBold JF, Miczek KA. Alcohol, allopregnanolone and aggression in mice. Psychopharmacology. 2001;153:473–483. doi: 10.1007/s002130000587. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology. 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29(9):1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, Erskine MS. Behavioral effects of 3α-androstanediol 1. Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996;79:109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL, Finn DA. Allopregnanolone does not influence ethanol-induced conditioned place preference in DBA/2J mice. Psychopharmacology (Berl) 2004;176(1):50–56. doi: 10.1007/s00213-004-1862-2. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Gililland KR, Finn DA. The impact of gonadectomy and adrenalectomy on acute withdrawal severity in male and female C57BL/6J and DBA/2J mice following a single high dose of ethanol. Alcohol Clin Exp Res. 2007;31:1846–1857. doi: 10.1111/j.1530-0277.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology. 1996;124:340–346. doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3α-hydroxy-5α-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Helms CM, Rogers LS, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008a;326(1):354–361. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008b;32(10):1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KL, Azarov AV, Szeliga KT, Purdy RH, Grant KA. The influence of menstrual cycle phase on sensitivity to ethanol-like discriminative stimulus effects of GABAA-positive modulators. Pharmacol Biochem Behav. 1999;64(2):379–383. doi: 10.1016/s0091-3057(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10(7):704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Morrow AL. 3α-Hydroxy-5α-pregnan-20-one levels and GABAA receptor-mediated 36Cl flux across development in rat cerebral cortex. Brain Res Dev Brain Res. 2001;131(1–2):31–39. doi: 10.1016/s0165-3806(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Grobin AC, VanDoren MJ, Porrino LJ, Morrow AL. Cortical 3α-hydroxy-5α-pregnan-20-one levels after acute administration of Δ9-tetrahydrocannabinol, cocaine and morphine. Psychopharmacology (Berl) 2005;179(3):544–550. doi: 10.1007/s00213-004-2084-3. [DOI] [PubMed] [Google Scholar]
- Hill M, Popov P, Havlikova H, Kancheva L, Vrbikova J, Kancheva R, Pouzar V, Cerny I, Starka L. Altered profiles of serum neuroactive steroids in premenopausal women treated for alcohol addiction. Steroids. 2005;70:515–524. doi: 10.1016/j.steroids.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt’s forced swim test: modulation by 3α-hydroxy-5α-pregnan-20-one. Neuropharmacology. 2002;43(8):1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3α-hydroxy-5α-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology. 2005;180(2):267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Holdstock L, Penland SN, Morrow AL, De Wit H. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3α-hydroxy-5α-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology. 2006;186:442–450. doi: 10.1007/s00213-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Jenkins EP, Hsieh CL, Milatovich A, Normington K, Berman DM, Francke U, Russell DW. Characterization and chromosomal mapping of a human steroid 5α-reductase gene and pseudogene and mapping of the mouse homologue. Genomics. 1991;11(4):1102–1112. doi: 10.1016/0888-7543(91)90038-g. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim W, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46(6):819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti RT, Boyd KN, Kumar S, Morrow AL. Systemic ethanol administration elevates deoxycorticosterone levels and chronic ethanol exposure attenuates this response. Brain Res. 2005;1049(1):104–111. doi: 10.1016/j.brainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, Matthews DB, Morrow AL. Ethanol-induced elevation of 3alpha-hydroxy-5alpha-pregnan-20-one does not modulate motor incoordination in rats. Alcohol Clin Exp Res. 2004;28(8):1249–1256. doi: 10.1097/01.alc.0000134232.44210.06. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O'Buckley TK, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–265. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- Korneyev AY, Costa E, Guidotti A. During anesthetic-induced activation of hypothalamic pituitary adrenal axis, blood borne steroids fail to contribute to the anesthetic effect. Neuroendocrinology. 1993;57:559–565. doi: 10.1159/000126405. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8(6):797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Marx CE, Duncan GE, Gilmore JH, Lieberman JA, Morrow AL. Olanzapine increases allopregnanolone in the rat cerebral cortex. Biol Psychiatry. 2000;47:1000–1004. doi: 10.1016/s0006-3223(99)00305-4. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-Concept Trial with the Neurosteroid Pregnenolone Targeting Cognitive and Negative Symptoms in Schizophrenia. Neuropsychopharmacology. 2009;34(8):1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Shampine LJ, Khisti RT, Trost WT, Bradford DW, Grobin AC, Massing MW, Madison RD, Butterfield MI, Lieberman JA, Morrow AL. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol Biochem Behav. 2006a;84(4):609–617. doi: 10.1016/j.pbb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006b;31(6):1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, Rose JE. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006c;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry. 2006d;60(12):1287–1294. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28(1):1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL, Tokunaga S, McDaniel JR. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res. 2002;26(11):1747–1751. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- Morrow AL, editor. Pharmacol Ther. 1. Vol. 116. 2007. Neurosteroids Special Issue; pp. 1–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: A new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23(12):1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Devaud LL. Effects of progesterone or neuroactive steroid? Nature. 1998;395:652–653. doi: 10.1038/27106. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Andersson A, Zingmark E, Wahlstrom G, Backstrom T, Sundstrom-Poromaa I. The effect of a low dose of alcohol on allopregnanolone serum concentrations across the menstrual cycle in women with severe premenstrual syndrome and controls. Psychoneuroendocrinology. 2005;30:892–901. doi: 10.1016/j.psyneuen.2005.04.016. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004;484(2–3):241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB Journal. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC. Behavioral studies of genetic differences in alcohol action. In: Crabbe JC, Harris RA, editors. The Genetic Basis of Alcohol and Drug Actions. New York: Plenum Press; 1991. pp. 25–104. [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30(6):1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Pisu MG, Serra M. Neurosteroids and neuroactive drugs in mental disorders. Life Sci. 2004;74:3181–3197. doi: 10.1016/j.lfs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Porcu P, Grant KA, Green HL, Rogers LS, Morrow AL. Hypothalamic-pituitary-adrenal axis and ethanol modulation of deoxycorticosterone levels in cynomolgus monkeys. Psychopharmacology. 2006a;186:293–301. doi: 10.1007/s00213-005-0132-2. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Morrow AL, Adinoff B. Differential hypothalamic-pituitary-adrenal activation of the neuroactive steroids pregnenolone sulfate and deoxycorticosterone in healthy controls and alcohol-dependent subjects. Psychoneuroendocrinology. 2008;33(2):214–226. doi: 10.1016/j.psyneuen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Rogers LSM, Morrow AL, Grant KA. Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic-pituitary-adrenal axis. Pharmacol Biochem Behav. 2006b;84:618–627. doi: 10.1016/j.pbb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74(3):683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Ibba C, Piredda M, Tocco S, Marra C, Purdy RH, Biggio G, Concas A. Failure of γ-hydroxybutyric acid both to increase neuroactive steroid concentrations in adrenalectomized-orchiectomized rats and to induce tolerance to its steroidogenic effect in intact animals. Brain Res. 2004;1012(1–2):160–168. doi: 10.1016/j.brainres.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U. S. A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Alcohol rapidly lowers plasma testosterone levels in the rat: evidence that a neural brain-gonadal pathway may be important for decreased testicular responsiveness to gonadotropin. Alcohol Clin Exp Res. 1999;23(1):38–45. doi: 10.1111/j.1530-0277.1999.tb04021.x. [DOI] [PubMed] [Google Scholar]
- Robel P, Baulieu EE. Dehydroepiandrosterone (DHEA) is a neuroactive neurosteroid. Ann NY Acad Sci. 1995;774:82–110. doi: 10.1111/j.1749-6632.1995.tb17374.x. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. J Pharmacol Exp Ther. 1996;247:309–322. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24(29):6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71(1):3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Cara V, Purdy RH, Biggio G. Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. J Neurochem. 2003;85(1):257–263. doi: 10.1046/j.1471-4159.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10(4):469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Pasini A, Romeo E, Hermann B, Spalletta G, Di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3α,5α-tetrahydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res. 2000;34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28(6):1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology. 2004;172(3):352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U.S.A. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U.S.A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20(5):1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25(19):4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]