Abstract
Background
It is generally believed that the hepatoprotective effect of interleukin-6 (IL-6) is mediated via activation of signal transducer and activator of transcription 3 (STAT3) in hepatocytes. IL-6-deficient mice are more susceptible to alcohol-induced hepatocyte apoptosis and steatosis and elevation of serum alanine transaminase (ALT); however, whereas hepatocyte-specific STAT3 knockout mice are more susceptible to alcohol-induced hepatic steatosis, they have similar hepatocyte apoptosis and serum ALT after alcohol feeding compared to wild-type mice. This suggests that the hepatoprotective effect of IL-6 in alcoholic liver injury may be mediated via activation of STAT3-independent signals in hepatocytes, activation of STAT3 in nonparenchymal cells, or both. We have previously shown that IL-6 also activates STAT3 in sinusoidal endothelial cells. Thus, the purpose of the present study was to investigate whether STAT3 in endothelial cells also plays a protective role in alcoholic liver injury.
Methods
Wild-type and endothelial cell-specific STAT3 knockout (STAT3E−/−) mice were pair-fed and fed ethanol containing diet for 4 weeks. Liver injury and inflammation were determined.
Results
Feeding mice with ethanol containing diet for 4 weeks induced greater hepatic injury (elevation of serum ALT) and liver weight in STAT3E−/− mice than wild-type control groups. In addition, ethanol-fed STAT3E−/− mice displayed greater hepatic inflammation and substantially elevated serum and hepatic levels of IL-6 and TNF-α compared to wild-type mice. Furthermore, ethanol-fed STAT3E−/− mice displayed a greater abundance of apoptotic sinusoidal endothelial cells and higher levels of serum hyaluronic acid than wild-type controls.
Conclusions
These data suggest that endothelial cell STAT3 plays important dual functions of attenuating hepatic inflammation and sinusoidal endothelial cell death during alcoholic liver injury.
Keywords: STAT3, alcoholic liver injury, endothelial cells, IL-6
Introduction
Chronic alcohol drinking is a leading cause of chronic liver disease worldwide. The spectrum of alcoholic liver disease includes fatty liver, alcoholic hepatitis, fibrosis, cirrhosis, and/or hepatocellular carcinoma (Robert S. O'Shea, 2009; Zakhari and Li, 2007). Emerging evidence suggests that alcoholic liver injury is caused by multiple mechanisms including alcohol-induced hepatotoxicity, oxidative stress, as well as complex interactions between alcohol metabolism, various hepatic cells, multiple cytokines, and the innate immune system (Arteel, 2003; Dey and Cederbaum, 2006; Gramenzi et al., 2006; Hoek and Pastorino, 2002; Nagy, 2003; Tsukamoto and Lu, 2001). We have previously provided several lines of evidence suggesting that interleukin-6 (IL-6) plays an important role in attenuating alcoholic liver injury. First, IL-6-deficient mice are more susceptible to alcohol-induced hepatic steatosis and apoptosis (El-Assal et al., 2004; Hong et al., 2002). Second, in vivo treatment with IL-6 ameliorates alcoholic fatty liver and injury (Hong et al., 2004). Third, ex vivo treatment with IL-6 reduces the mortality resulting from alcoholic fatty liver transplants (Sun et al., 2003). These findings suggest that the elevated levels of IL-6, in patients with alcoholic liver disease may play a compensatory role in preventing liver injury. The hepatoprotective actions of IL-6 have also been observed in various experimental models of liver injury and are believed to be largely due to activation of hepatic signal transducer and activator of transcription 3 (STAT3), an important survival signal in many cell types including hepatocytes (Gao, 2005; Haga et al., 2009; Haga et al., 2003). We have recently demonstrated that although mice with hepatocyte-specific deletion of STAT3 displayed higher levels of hepatic steatosis after alcohol feeding compared to wild-type mice, serum ALT levels were similar between these two groups (Horiguchi et al., 2008). This suggests that the exacerbated liver damage in IL-6 knockout mice after alcohol feeding may be due to STAT3-independent signaling of IL-6 in hepatocytes, IL-6 activation of STAT3 in nonparenchymal cells, or both.
STAT3 signaling in endothelial cells has been linked to anti-inflammatory action and protection against LPS-induced inflammation (Carrithers et al., 2005; Kano et al., 2003; Wang et al., 2007). In addition, endotoxin has been shown to stimulate the production of IL-6 by sinusoidal endothelial cells and Kupffer cells (Knolle et al., 1998), and IL-6 can activate STAT3 in sinusoidal endothelial cells (Gao, 2004; Sun et al., 2003). We therefore hypothesize that IL-6 activation of STAT3 may also help protect against ethanol-induced endothelial damage. To test this hypothesis, we investigated the functions of endothelial cell STAT3 in alcoholic liver injury through the use of mice with endothelial cell-specific deletion of STAT3 (STAT3E−/− mice). Our findings suggest that STAT3 in endothelial cells inhibits inflammation and sinusoidal endothelial cell apoptosis during chronic alcoholic liver injury.
Materials and Methods
Mice
IL-6−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). STAT3Hep−/− (AlbCre+/−STAT3flox/flox) mice were generated by 2 steps of crossing STAT3flox/flox mice with transgenic mice expressing the bacterial Cre recombinase driven by the albumin promoter, obtained from the Jackson Laboratory (Horiguchi et al., 2008). AlbCrE−/−STAT3flox/flox wild-type littermates were used as controls. STAT3E−/− (Tie2Cre+/−STAT3flox/flox) mice were obtained via 2 steps of crossing STAT3flox/flox mice with Tie2-promoter Cre transgenic mice as described previously (Kano et al., 2003). Tie2Cre−/−STAT3flox/flox wild-type littermates were used as controls. All mice were used in accordance with Institutional guidelines for animal experimentation. The protocol for this study was approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
Mouse models of chronic ethanol consumption
Eight to ten week old male IL-6−/− mice, STAT3Hep−/− mice, STAT3E−/− mice and their wild-type littermates, weighing 20–25 g, were fed a nutritionally adequate Liber-Decari liquid diet containing 5% ethanol or a control diet in which ethanol was substituted isocalorically with dextrin maltose (BioServ, Frenchtown, NJ). Both diets were dispensed in glass liquid-diet feeding tubes (BioServ). Ethanol was introduced by gradually increasing the content by 1% (vol/vol) every day until the mice were consuming diets containing 5% (vol/vol) ethanol for 4 weeks.
Blood chemistry and hepatic triglyceride contents
Serum alanine transaminase (ALT) and triglycerides levels were determined using a clinical chemistry analyzer system (PROCHEM-V; Drew Scientific, Barrow-in-Furness, UK). Hepatic triglyceride content was measured as previously described (Horiguchi et al., 2008). Briefly, liver tissue (100 mg) was homogenized in lysis buffer. Lipids form total liver homogenate were extracted using chloroform/methanol (2:1), evaporated, and dissolved in 2-propanol.
Hematoxylin-eosin staining of liver sections
After fixation of the livers with 10% formalin/PBS, livers were sliced and stained with hematoxylin-eosin.
Serum TNF-α and IL-6 levels
Serum TNF-alpha and IL-6 levels were measured using cytometry bead array (BD Bioscience) according to the manufacturer’s protocol.
Terminal Uridine Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) assay
Staining was performed with an in situ apoptosis detection kit according to the manufacturer's instructions (Chemicon International, Temecula, CA) and examined by a light microscopy. TUNEL-positive hepatocytes were counted randomly in 5 fields (×100) of each slide. TUNEL-positive sinusoidal lining cells were counted randomly in 5 fields (×400) of each slide.
Hyaluronic acid levels
Liver sinusoidal endothelial cell (SEC) functionality was determined by measuring serum hyaluronic acid (HA) levels using an HA ELISA kit (Corgenix, Westminster, CO) according to manufacturer’s protocol.
Real Time PCR
Hepatic expression of proinflammatory cytokines and chemokines was measured by real-time quantitative PCR, using a model Chromo 4 real-time PCR detection system (Bio-Rad, Hercules, CA). Primers used in real-time PCR include TNF-α: 5’-AAG CCT GTA GCC CAC GTC GTA-3’ (forward); 5’-AGG TAC AAC CCA TCG GCT GG-3’ (reverse). IL-6: 5’-TCC ATC CAG TTG CCT TCT TG-3’ (forward); 5’-TTC CAC GAT TTC CCA GAG AAC-3’ (reverse). CCR2: 5’- ATG CAA GTT CAG CTG CCT GC-3’ (forward); 5’- ATG CCG TGG ATG AAC TGA GG-3’ (reverse). F4/80: 5’- GGA AAG CAC CAT GTT AGC TGC-3’ (forward); 5’- CCT CTG GCT GCC AAG TTA ATG-3’ (reverse).
Statistical Analysis
Data are expressed as means ± SE. To compare values obtained from two groups, the Student's t-test was performed. To compare values obtained from three or more groups, one-way ANOVA was performed followed by Tukey’s post hoc test. A value of P < 0.05 was considered significant.
Results
Increased susceptibility of IL-6 −/− mice to ethanol-induced liver injury and apoptosis is independent of STAT3 in hepatocytes
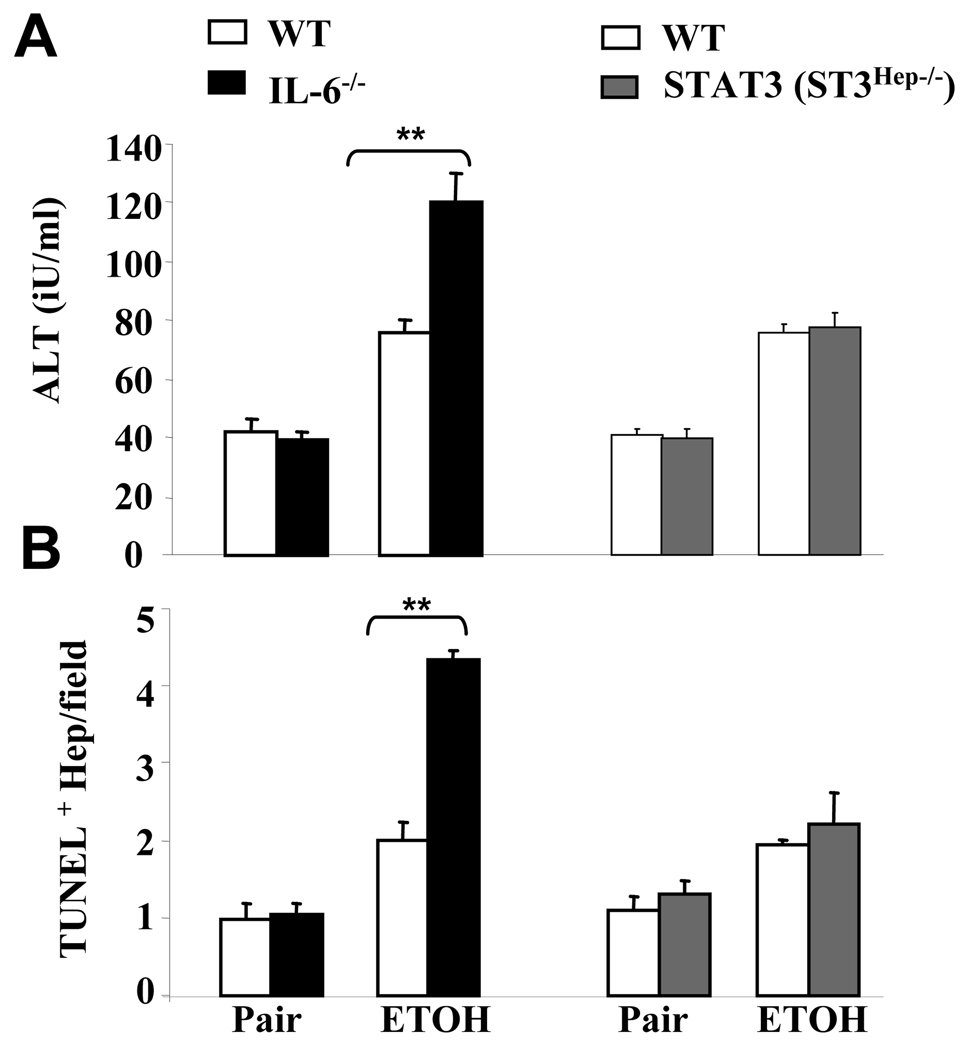
Fig. 1 shows that serum ALT levels and the number of apoptotic hepatocytes were greater in ethanol-fed IL-6−/− mice compared with ethanol-fed wild-type mice. In contrast, there was no significant difference in serum ALT levels and in the abundance of apoptotic hepatocytes between ethanol-fed STAT3Hep−/− mice and ethanol-fed wild-type mice. This suggests that IL-6 protection from ethanol-induced hepatocyte apoptosis is independent of STAT3 signaling in hepatocytes in this model of mild alcoholic liver injury.
Fig. 1. Increased susceptibility of IL-6−/− mice to ethanol-induced liver injury and apoptosis is independent of hepatocyte specific STAT3.
IL-6−/− mice, STAT3Hep−/− mice, and their corresponding wild-type controls were pair-fed or fed ethanol diet for 4 weeks. (A) Serum ALT levels. (B) Liver tissues were collected for TUNEL staining and the number of TUNEL+ hepatocytes nuclei was counted from randomly selected 5 fields (×100). The values represent means ± SE (n=4–5). ** P<0.01 in comparison with corresponding ethanol-fed WT groups.
STAT3E−/− mice are more susceptible to ethanol-induced liver injury
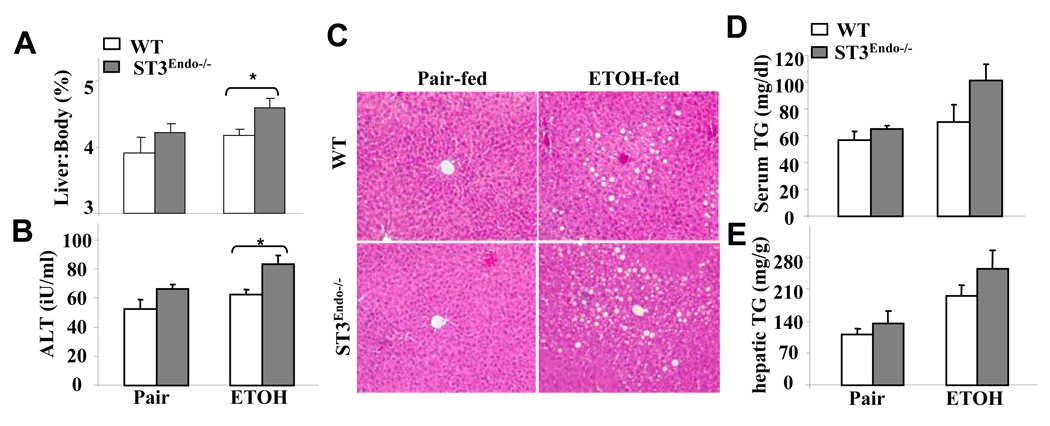
As alcohol-induced liver injury is unaffected by the absence of STAT3 from hepatocytes (see above) and IL-6 activates STAT3 in sinusoidal endothelial cells (Gao, 2004), we hypothesized that hepatoprotection by IL-6 in alcoholic liver injury is mediated via activation of STAT3 in endothelial cells. We tested this hypothesis in STAT3E−/− mice, in which the STAT3 gene is deleted in endothelial cells including sinusoidal endothelial cells (Kano et al., 2003). In response to 4-week ethanol feeding, the liver weight to body weight ratio and serum ALT levels were higher in STAT3E−/− vs. wild-type littermate control mice (Figs. 2A–B). However, no significant difference was detected in serum ALT levels between ethanol and pair-fed wild-type littermate control mice. The reason for the lack of hepatic injury in response to ethanol feeding in this strain of wild-type littermate control mice is likely attributed to the genetic background of this strain (Tie2Cre−/− STAT3flox/flox) that is not on a pure C57BL/6 background. Ethanol feeding induced similar levels of hepatic steatosis in STAT3E−/− and wild-type mice as documented by H&E staining (Fig. 2C). Also, serum and hepatic triglyceride levels were elevated in the ethanol-fed compared to pair-fed groups (Figs. 2D–E). Despite an apparent trend, there were no significant differences in serum and hepatic triglyceride levels between ethanol-fed STAT3E−/− and wild-type mice (Figs. 2D–E). In addition, more inflammatory cells were observed in ethanol-fed STAT3E−/− mice compared to ethanol-fed controls.
Fig. 2. STAT3E−/− mice are more susceptible to ethanol-induced liver injury.
Wild-type and STAT3E−/− mice were pair-fed or fed ethanol diet for 4 weeks. (A) Liver weight to body weight ratio. (B) Serum ALT. (C) Liver tissues were collected for H&E staining (×200). (D) Serum triglyceride (TG). (E) Hepatic TG levels. The values represent means ± SE (n=4–9). *P<0.05, ** P<0.01 in comparison with corresponding ethanol-fed WT groups.
STAT3E−/− mice are more susceptible to ethanol-induced liver inflammation
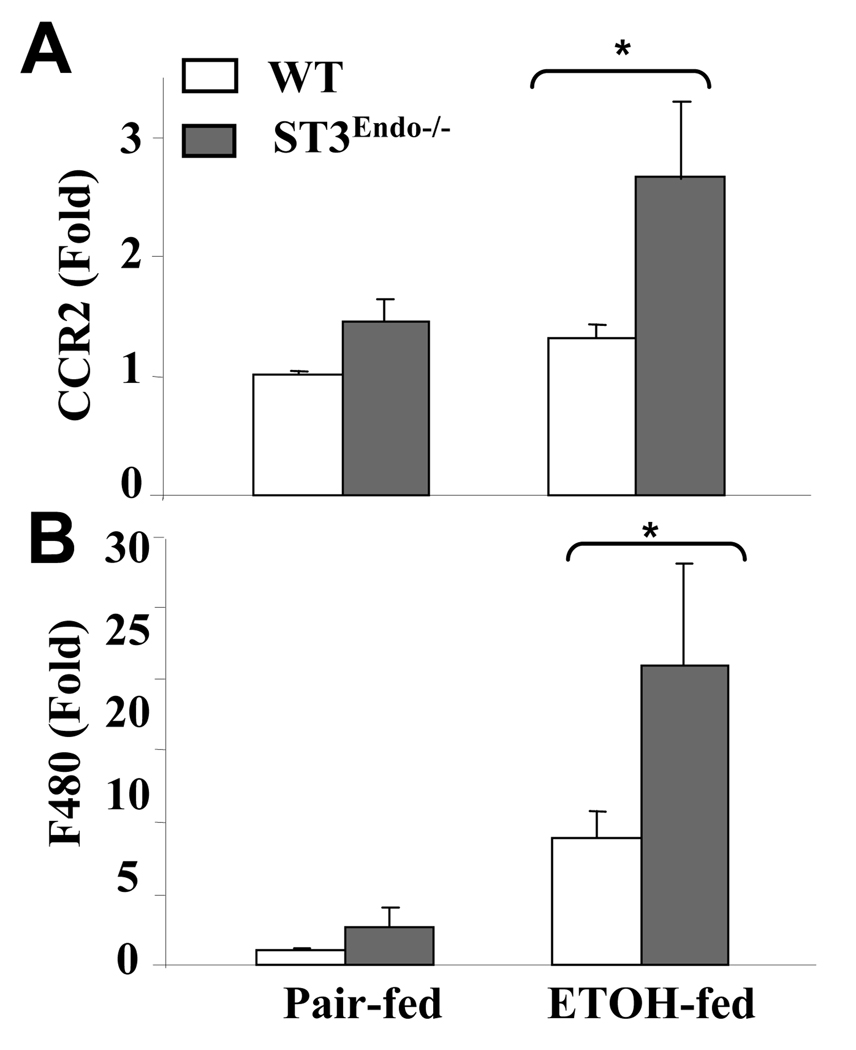
The effect of endothelial cell-specific STAT3 depletion on the hepatic infiltration of monocytes and macrophages in response to chronic alcohol feeding was examined by measuring the hepatic expression of monocyte (CC chemokine receptor 2, CCR2) and macrophage (F4/80) markers. As shown in Figs. 3A and 3B, ethanol feeding elevated the expression of CCR2 and F4/80 mRNAs significantly more in the livers of STAT3E−/− mice than in wild-type controls.
Fig. 3. Increased expressions of monocyte and macrophage markers in ethanol-fed-STAT3E−/− mice.
Wild-type and STAT3E−/− mice were pair-fed or fed ethanol diet for 4 weeks. The liver tissues were collected and used to analyze the expression of the monocyte marker CCR2 (A) and macrophage marker F4/80 (B) by real-time PCR analyses. Values from pair fed WT mice were normalized to one-fold, and all values represent means ± SE (n=4–8 mice). * P<0.05 in comparison with ethanol-fed wild-type groups.
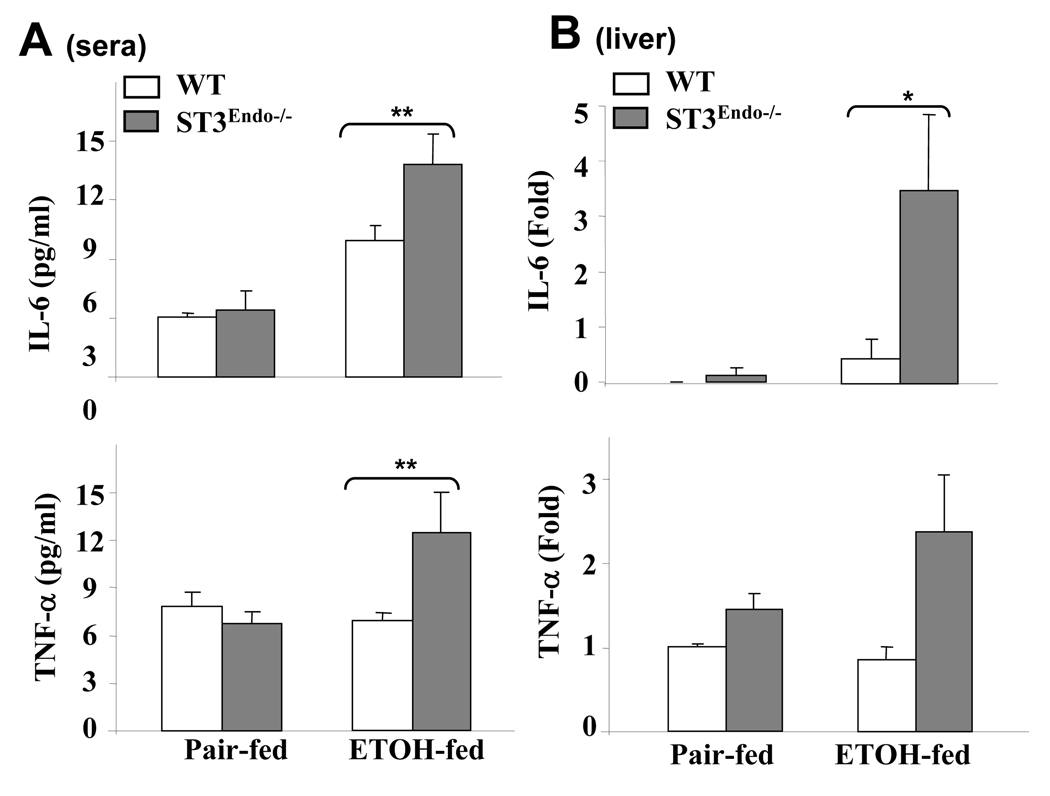
We also analyzed the expression of two proinflammatory cytokines, IL-6 and TNF-α, in the serum and livers from pair-fed and ethanol-fed mice. As illustrated in Fig. 4A, serum levels of both IL-6 and TNF-α were greater in ethanol-fed STAT3E−/− mice compared to wild-type controls. The hepatic expression of IL-6 and TNF-α was also greater in ethanol-fed STAT3E−/− mice vs. wild-type controls (Fig. 4B).
Fig. 4. STAT3E−/− mice are more susceptible to ethanol-induced liver inflammation.
Wild-type and STAT3E−/− mice were pair-fed or fed ethanol diet for 4 weeks. (A) Serum levels of IL-6 and TNF-α. (B) Liver tissue were collected and used to analyze the expression of IL-6, and TNF-α by real-time PCR analyses. Values from pair-fed WT mice in B were normalized to one-fold, and all values represent means ± SE (n=4–8 mice). *P<0.05, **P<0.01 in comparison with ethanol-fed wild-type groups.
STAT3E−/− mice are more susceptible to ethanol-induced sinusoidal endothelial cell apoptosis and dysfunction
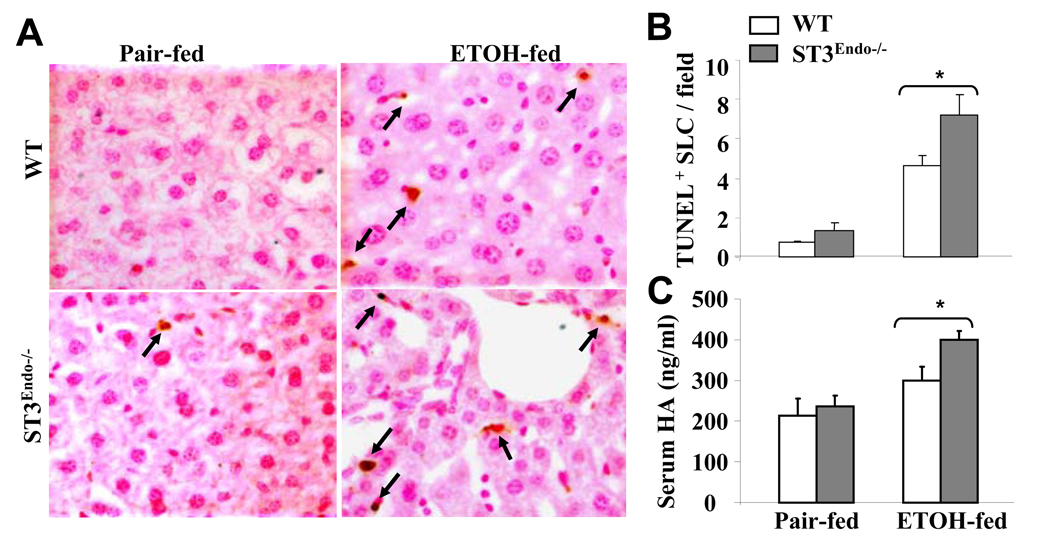
To assess the role of endothelial STAT3 in ethanol-induced cellular damage, we performed TUNEL assays on the livers from pair-fed and ethanol-fed mice. As shown in Figs. 5A–B, ethanol feeding significantly increased the number of TUNEL+ sinusoidal lining cells (SLC) and the increase was more profound in STAT3E−/− mice than in wild-type controls. Similarly, the ethanol-induced increase in serum hyaluronic acid (HA) levels, a marker of sinusoidal endothelial cell dysfunction (Eriksson et al., 1983; Nanji et al., 1996), was greater in STAT3E−/− than in wild-type mice (Fig. 5C).
Fig. 5. STAT3E−/− mice are more susceptible to ethanol-induced sinusoidal lining cell (SLC) apoptosis and dysfunction.
Wild-type and STAT3E−/− mice were pair-fed or fed ethanol diet for 4 weeks. (A) Liver tissues were collected for TUNEL staining. Arrows are indicating TUNEL+ SLC. (B) The number of TUNEL+ SLC nuclei per field were counted (×400) (C) Sera were collected for the measurement of hyaluronic acid (HA) levels as an index of SEC dysfunction. The values represent means ± SE (n=4–9). *P<0.05 in comparison with ethanol-fed wild-type groups.
Discussion
Our recent findings in cell-type specific STAT3 knockout mice suggest that STAT3 in hepatocytes has a predominant role in inhibiting fatty acid synthesis, whereas STAT3 in Kupffer cells and macrophages inhibits inflammation during alcoholic liver injury (Horiguchi et al., 2008). Here we show that activation of endothelial cell STAT3 helps to prevent sinusoidal endothelial cell damage and dysfunction and also inhibits inflammation induced by chronic alcohol intake. We have integrated these findings into our recently proposed model that depicts the interplay of hepatic, myeloid cell, and endothelial cell STAT3 in preventing alcoholic liver injury (Fig. 8).
STAT3 in endothelial cells functions as an anti-apoptotic signal during alcoholic liver injury
Sinusoidal endothelial cells are early targets in both alcohol- (Deaciuc et al., 2001a; Deaciuc et al., 2001b; Sarphie et al., 1997) and ischemia/reperfusion-induced liver injury (Gao et al., 1998; Huet et al., 2004; Natori et al., 2003). In animal models as well as in clinical studies of alcoholic liver injury, plasma hyaluronic acid levels are markedly increased, which is indicative of its decreased hepatic clearance by sinusoidal endothelial cells (Engstrom-Laurent et al., 1985; Gibson et al., 1992; Sarphie et al., 1997). Ethanol treatment also induces the apoptosis of hepatic sinusoidal endothelial cells (Zheng et al., 2006). Here we showed that feeding mice with an ethanol diet for 4 weeks significantly increased sinusoidal endothelial cell apoptosis and the serum level of HA in wild-type mice, which is consistent with previous reports (Deaciuc et al., 2001a; Deaciuc et al., 2001b; Sarphie et al., 1997). Disruption of STAT3 in endothelial cells resulted in a marked increase in these ethanol-induced effects, strongly suggesting that STAT3 in endothelial cells protects these cells from ethanol-induced damage. Previously, we have demonstrated that IL-6 can activate STAT3 in sinusoidal endothelial cells and such activation likely contributes to the hepatoprotective effect of IL-6 in fatty liver transplantation (Sun et al., 2003). In addition, Zheng et al (2006) have demonstrated that sphingosine 1-phosphate (S1P), a serum-derived lipid originating from activated platelets, protects against ethanol-induced sinusoidal endothelial cell injury and apoptosis, in part, via activation of Ca2+ dependent eNOS activation. It is known that S1P can activate STAT3 in cardiomyocytes, and S1P has been proposed to mediate the cardioprotective effect of rHDL (Frias et al., 2009). It would be interesting to find out whether S1P also activates STAT3 in sinusoidal endothelial cells and whether S1P participates in hepatoprotection in alcoholic liver disease.
STAT3 in endothelial cells acts as an anti-inflammatory signal during alcoholic liver injury
We have previously found that STAT3 in myeloid cells inhibits liver inflammation during alcoholic liver injury (Horiguchi et al., 2008). Here we showed that ethanol feeding induced significantly higher levels of inflammatory cell infiltration and proinflammatory cytokine production in the liver of STAT3E−/− mice vs. wild-type controls, suggesting that endothelial STAT3 also functions as an anti-inflammatory signal during alcoholic liver injury. The inhibitory effect of endothelial cell STAT3 on inflammation has also been reported in other organ injury models such as heart ischemia/reperfusion injury and oxidant-induced lung injury (Wang et al., 2007; Zhang et al., 2006). However, the underlying mechanisms remain largely unknown. SECs are exposed to high concentrations of ethanol in sinusoidal blood flow in response to alcohol consumption, which induces SEC death and subsequently disrupts the integrity of the endothelium allowing for increased infiltration of immune cells, including macrophages and monocytes, from the blood into hepatic parenchyma. In addition, Kano et al (2003) have shown that endothelial cell STAT3 protects against endotoxin-induced inflammation via stimulating these cells to produce a soluble factor that suppresses inflammation. Moreover, mice deficient in CD31, a cell adhesion molecule that mediates homophilic and heterophilic binding in hematopoietic and endothelial cells, are more susceptible to LPS-induced septic shock that coincides with impaired endothelial cell STAT3 signaling and vascular integrity (Carrithers et al., 2005). All of these mechanisms may also apply to the anti-inflammatory effect of endothelial cell STAT3 in alcoholic liver injury, because endotoxin is believed to contribute to the pathogenesis of alcoholic liver injury (Arteel, 2003; Dey and Cederbaum, 2006; Gramenzi et al., 2006; Hoek and Pastorino, 2002; Nagy, 2003; Tsukamoto and Lu, 2001).
Interplay of hepatic, endothelial, and myeloid cell STAT3 in controlling alcoholic liver injury
We have shown that the enhanced alcohol-induced hepatocellular damage caused by the absence of IL-6 can be replicated by the selective deletion of the downstream signaling molecule STAT3 from endothelial cells but not from hepatocytes. These findings suggest that the hepatoprotective effect of IL-6 is mediated via activation of STAT3 in endothelial cells. However, in the case of other hepatotoxins, such as Fas (Haga et al., 2003), LPS (Sakamori et al., 2007), or CCl4 (Moh et al., 2007), selective deletion of STAT3 from hepatocytes was found to increase hepatocellular damage. It is not clear why STAT3 in hepatocytes does not contribute to the liver damage caused by ethanol. It is plausible that in the model used here, ethanol feeding only caused very mild liver damage as compared to the significantly greater liver injury induced by other hepatotoxins. Hepatic STAT3 may still play a role in protecting against more severe ethanol-induced liver damage. In addition, activation of STAT3 in both endothelial cells and myeloid cells inhibits liver inflammation during alcoholic liver injury. Thus, interplay of STAT3 in myeloid cells, sinusoidal endothelial cells, and hepatocytes may be required for protecting against ethanol-induced hepatocellular damage and inflammation. A therapeutic strategy to increase STAT3 activity in these cells may be beneficial for the treatment of alcoholic liver disease.
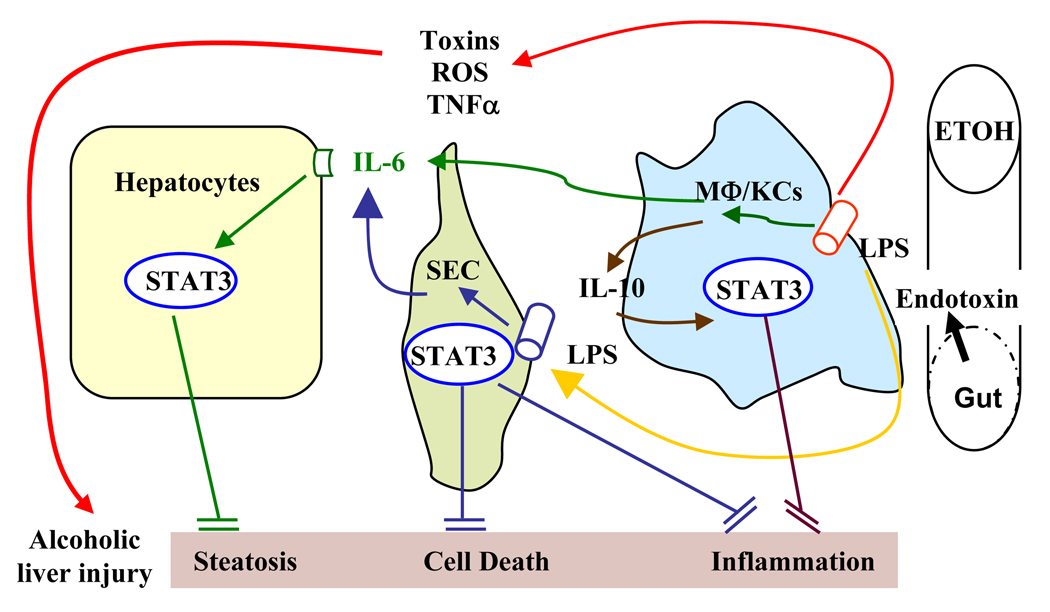
Fig. 6. Interplay of hepatic, myeloid cell, and endothelial cell STAT3 in preventing alcoholic liver injury.
Chronic alcohol intake increases the permeability of the gut leading to endotoxin accumulation in the liver and subsequent activation of macrophages/Kupffer cells. These activated cells produce TNFα, ROS, and toxic mediators leading to hepatic steatosis and inflammation. Knolle et al (1997) initially demonstrated that endotoxin stimulates the production of IL-6 from endothelial cells and stimulates IL-6 and IL-10 production from Kupffer cells. Subsequently, IL-6 activates STAT3 in hepatocytes and attenuates steatosis via inhibition of SREBP1; IL-6 activates STAT3 in endothelial cells and subsequently inhibits endothelial cell apoptosis and inflammation. In addition to inhibiting IL-6 produced by SEC in response to stimulation with endotoxin, IL-10 produced from Kupffer cells also activates STAT3 in macrophages/Kupffer cells in an autocrine/paracrine manner, serving as an anti-inflammatory signal to inhibit inflammation during alcoholic liver injury.
Acknowledgments
This work was supported by the intramural program of NIAAA, NIH. No conflicts of interest exist for all authors.
Abbreviations
- STAT
signal transducer and activator of transcription
- ALT
alanine transaminase
- IL-6
interleukin-6
- STAT3E−/−
endothelial cell-specific STAT3 knockout
- SEC
sinusoidal endothelial cell
- SLC
sinusoidal lining cell
- HA
hyaluronic acids
References
- Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124(3):778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166(1):185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaciuc II, D'Souza NB, Fortunato F, Hill DB, Sarphie TG, McClain CJ. Alcohol-induced sinusoidal endothelial cell dysfunction in the mouse is associated with exacerbated liver apoptosis and can be reversed by caspase inhibition. Hepatol Res. 2001a;19(1):85–97. doi: 10.1016/s1386-6346(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Deaciuc IV, Fortunato F, D'Souza NB, Hill DB, McClain CJ. Chronic alcohol exposure of rats exacerbates apoptosis in hepatocytes and sinusoidal endothelial cells. Hepatol Res. 2001b;19(3):306–324. doi: 10.1016/s1386-6346(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(2) Suppl 1:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol. 2004;1(3):205–211. [PubMed] [Google Scholar]
- Engstrom-Laurent A, Loof L, Nyberg A, Schroder T. Increased serum levels of hyaluronate in liver disease. Hepatology. 1985;5(4):638–642. doi: 10.1002/hep.1840050420. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Fraser JR, Laurent TC, Pertoft H, Smedsrod B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983;144(1):223–228. doi: 10.1016/0014-4827(83)90458-5. [DOI] [PubMed] [Google Scholar]
- Frias MA, James RW, Gerber-Wicht C, Lang U. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1-phosphate. Cardiovasc Res. 2009;82(2):313–323. doi: 10.1093/cvr/cvp024. [DOI] [PubMed] [Google Scholar]
- Gao B. Therapeutic potential of interleukin-6 in preventing obesity- and alcohol-associated fatty liver transplant failure. Alcohol. 2004;34(1):59–65. doi: 10.1016/j.alcohol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gao B. Cytokines, STATs and liver disease. Cell Mol Immunol. 2005;2(2):92–100. [PubMed] [Google Scholar]
- Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27(6):1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- Gibson PR, Fraser JR, Brown TJ, Finch CF, Jones PA, Colman JC, Dudley FJ. Hemodynamic and liver function predictors of serum hyaluronan in alcoholic liver disease. Hepatology. 1992;15(6):1054–1059. doi: 10.1002/hep.1840150614. [DOI] [PubMed] [Google Scholar]
- Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24(8):1151–1161. doi: 10.1111/j.1365-2036.2006.03110.x. [DOI] [PubMed] [Google Scholar]
- Haga S, Ozaki M, Inoue H, Okamoto Y, Ogawa W, Takeda K, Akira S, Todo S. The survival pathways phosphatidylinositol-3 kinase (PI3K)/phosphoinositide-dependent protein kinase 1 (PDK1)/Akt modulate liver regeneration through hepatocyte size rather than proliferation. Hepatology. 2009;49(1):204–214. doi: 10.1002/hep.22583. [DOI] [PubMed] [Google Scholar]
- Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, Okuyama T, Takeda K, Akira S, Ogino T, Irani K, Ozaki M. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112(7):989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27(1):63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21(1):32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40(4):933–941. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P, Kunos G, Gao B. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134(4):1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet PM, Nagaoka MR, Desbiens G, Tarrab E, Brault A, Bralet MP, Bilodeau M. Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver. Hepatology. 2004;39(4):1110–1119. doi: 10.1002/hep.20157. [DOI] [PubMed] [Google Scholar]
- Kano A, Wolfgang MJ, Gao Q, Jacoby J, Chai GX, Hansen W, Iwamoto Y, Pober JS, Flavell RA, Fu XY. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J Exp Med. 2003;198(10):1517–1525. doi: 10.1084/jem.20030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle PA, Loser E, Protzer U, Duchmann R, Schmitt E, zum Buschenfelde KH, Rose-John S, Gerken G. Regulation of endotoxin-induced IL-6 production in liver sinusoidal endothelial cells and Kupffer cells by IL-10. Clin Exp Immunol. 1997;107(3):555–561. doi: 10.1046/j.1365-2249.1997.d01-959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle PA, Uhrig A, Protzer U, Trippler M, Duchmann R, Meyer zum Buschenfelde KH, Gerken G. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology. 1998;27(1):93–99. doi: 10.1002/hep.510270116. [DOI] [PubMed] [Google Scholar]
- Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B, Flavell RA, Fu XY. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87(10):1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228(8):882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Tahan SR, Khwaja S, Yacoub LK, Sadrzadeh SM. Elevated plasma levels of hyaluronic acid indicate endothelial cell dysfunction in the initial stages of alcoholic liver disease in the rat. J Hepatol. 1996;24(3):368–374. doi: 10.1016/s0168-8278(96)80018-3. [DOI] [PubMed] [Google Scholar]
- Natori S, Higuchi H, Contreras P, Gores GJ. The caspase inhibitor IDN-6556 prevents caspase activation and apoptosis in sinusoidal endothelial cells during liver preservation injury. Liver Transpl. 2003;9(3):278–284. doi: 10.1053/jlts.2003.50019. [DOI] [PubMed] [Google Scholar]
- Robert S, O'Shea SD, Dasarathy S, McCullough A. Alcoholic liver disease Hepatology on line. 2009 [Google Scholar]
- Sakamori R, Takehara T, Ohnishi C, Tatsumi T, Ohkawa K, Takeda K, Akira S, Hayashi N. Signal transducer and activator of transcription 3 signaling within hepatocytes attenuates systemic inflammatory response and lethality in septic mice. Hepatology. 2007;46(5):1564–1573. doi: 10.1002/hep.21837. [DOI] [PubMed] [Google Scholar]
- Sarphie G, D'Souza NB, Van Thiel DH, Hill D, McClain CJ, Deaciuc IV. Dose-and time-dependent effects of ethanol on functional and structural aspects of the liver sinusoid in the mouse. Alcohol Clin Exp Res. 1997;21(6):1128–1136. [PubMed] [Google Scholar]
- Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan HN, Jaruga B, Batkai S, Hoshino S, Tian Z, Kunos G, Diehl AM, Gao B. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125(1):202–215. doi: 10.1016/s0016-5085(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15(8):1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. Endothelial STAT3 plays a critical role in generalized myocardial proinflammatory and proapoptotic signaling. Am J Physiol Heart Circ Physiol. 2007;293(4):H2101–H2108. doi: 10.1152/ajpheart.00125.2007. [DOI] [PubMed] [Google Scholar]
- Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang G, Zhang SS, Otterbein LE, Fu XY, Lee PJ. Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J. 2006;20(12):2156–2158. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]
- Zheng DM, Kitamura T, Ikejima K, Enomoto N, Yamashina S, Suzuki S, Takei Y, Sato N. Sphingosine 1-phosphate protects rat liver sinusoidal endothelial cells from ethanol-induced apoptosis: Role of intracellular calcium and nitric oxide. Hepatology. 2006;44(5):1278–1287. doi: 10.1002/hep.21384. [DOI] [PubMed] [Google Scholar]