Abstract
Importance of this field
The efficacy of microneedles in the area of transdermal drug delivery is well-documented. Multiple studies have shown that enhancement of skin permeation via creation of microscopic pores in the stratum corneum can greatly improve the delivery rates of drugs. However, skin pretreatment with microneedles is not the only factor affecting drug transport rates. Other factors including drug formulation and rate of micropore closure are also important for optimizing delivery via this route.
Areas covered in this review
This review aims at highlighting work that has been done in these areas, with an emphasis on drug formulation parameters that affect transdermal flux.
What the reader will gain
This review creates an appreciation for the many factors affecting microneedle-enhanced delivery. Most results clearly indicate that microneedle skin pretreatment by itself may have different effects on drug transport depending on the formulation used, and formulation characteristics have different effects on the transport through untreated skin and microneedle-treated skin. Several formulation approaches are reported to optimize microneedle-enhanced drug delivery, including cosolvent use, vesicular, nanoparticulate and gel systems.
Take home message
In addition to well-established factors that affect microneedle-assisted delivery (geometry, type of microneedles, etc), formulation and pore viability are also critical factors that must be considered.
Keywords: Diffusion, Formulation, Microneedle, Micropores, Transdermal
1. Background/Introduction
Oral drug delivery is not optimal in many situations for reasons that include gastrointestinal side effects, extensive first-pass metabolism, enzymatic degradation, and poor bioavailability. A common alternative is to deliver the drug via injection with a hypodermic needle, which is painful, invasive, and less convenient for the patient. Transdermal drug delivery, by way of patches that adhere to the skin and deliver a consistent amount of drug over an extended period of time, would be a more appealing option for most patients. There are nearly 20 different transdermal delivery systems available in the United States, delivering a wide variety of drugs including fentanyl, nicotine, lidocaine, and various hormones (e.g. estradiol, testosterone).1 Despite its advantages, the clinical utility of transdermal drug delivery is greatly limited by the fact that the majority of drugs are not able to cross the stratum corneum (SC), the outermost layer and primary permeation barrier of the skin.2 With currently available passive transdermal systems, only drug molecules up to a few hundred Daltons can be delivered to achieve therapeutically relevant concentrations, and administration of highly hydrophilic compounds is difficult at best.1,3 Multiple approaches have been employed in attempt to enhance the delivery of drugs via the transdermal route, including (but not limited to) iontophoresis, ultrasound, chemical permeation enhancers, and microneedle arrays.1 3–9
Microneedles (MN) are a minimally invasive means of assisting the transport of drug molecules across the skin, and several reports have demonstrated that MN treatment is painless and well-tolerated by most patients.1,10–13 There are various ways that microneedles can assist in the transdermal delivery of drugs across the SC, and currently 4 major types of microneedle systems have been described.3 Solid microneedles pierce the skin and create pores (also referred to as microchannels) in the SC, which can be utilized to increase delivery of a variety of compounds by applying a gel formulation or transdermal patch over the MN-treated skin; this is called the “poke and patch” method.1,4 Microneedles can be coated with various drug formulations, which has been used to deliver DNA, proteins, and virus particles; this is referred to as the “coat and poke” approach.1,4 Polymeric microneedles have been prepared with encapsulated drug to be inserted, detached, and left in the skin, which can be used for either rapid or controlled release into the skin.3 Lastly, hollow microneedles have been utilized to deliver drug compounds (insulin, vaccines) by diffusion or pressure-driven flow through the bore of the needle.1,3,4 All of these applications are inherently different and it can be expected that each technique requires separate optimization.
There are many factors that affect drug delivery and permeability of the skin with respect to MN application. These include parameters such as mechanical properties of the MN arrays, formulation of drugs to be delivered, and lifetime of the pores following MN application. To date, the mechanical properties of microneedle arrays have been well described in the literature (many of these papers have been published only in the last few years), including parameters such as length, bore, sharpness, MN material, geometry, force of application, and number of microneedles.10,14–18 Complex optimization algorithms have been developed to improve skin permeability and determine the best MN geometry for both solid and hollow MN systems by examining such properties as number of microneedles, MN radius, MN length, MN patterns (square, triangular, diamond, etc.), aspect ratio, and skin thickness.17,19 Additionally, parametric analyses have been performed to examine the delivery of high molecular weight molecules from a MN system by performing quantitative analyses of various parameters, including blood concentrations of drug.18 The optimization of these mechanical factors affects the strength of the microneedles, the amount of pain associated with application, and the overall permeability of the skin (and therefore efficiency of drug delivery).3,17,18 An extensive discussion of the mechanical properties of microneedles is beyond the scope of this review article; however, these properties deserve note, as they do significantly affect the amount of drug delivered with a MN-assisted system and have received a great deal of attention in the recent literature. In large contrast to the mechanical properties of the MN arrays, the other factors affecting drug delivery via this route remain largely unexplored, specifically drug formulation and pore lifetime following MN treatment. This review will describe the current literature available addressing these topics and how that information may be applied to improving drug delivery associated with MN arrays, with special emphasis on solid microneedles.
2. Effects of Ionization, Co-solvents, and Concentration on Drug Delivery
The charge of a drug can affect drug physicochemical properties in a variety of ways, which may ultimately affect drug permeation through the skin. Thus, pH of the drug formulation may have a substantial effect on rates of transdermal delivery. Banks et al. assessed the effects of drug ionization on delivery rates of naltrexone and its active metabolite, naltrexol, through MN-treated and untreated guinea pig skin by comparing different pH values of aqueous formulations 20 Both naltrexone and naltrexol possess 2 ionizable groups – an aliphatic nitrogen and a phenolic group. The pKa values for naltrexone are 8.2 and 9.6 and for naltrexol are 7.4 and 9.4.20,21 The results demonstrated that the ionized form of naltrexol (at pH 4.5) was more than 2-orders of magnitude more soluble compared to the predominantly unionized form in the donor solution (at pH 8.5). Similarly, naltrexone also showed significant solubility improvement due to shifting the formulation pH value from 8.5 to 4.5.
The comparison of transdermal flux of charged and predominantly uncharged drugs through either MN-treated vs. untreated skin is also important. Naltrexol in its poorly soluble unionized form displayed only modest flux enhancement after MN-treatment. However, in the highly-soluble charged state, naltrexol not only permeated across untreated skin much faster, but also showed a greater flux improvement post MN-treatment. This suggests that, in the diffusion through untreated skin, the high concentration of drug in the donor solution (with corresponding non-rate-limiting dissolution rate) overrides lower apparent permeability coefficient of the charged drug. Also, the greater average MN flux enhancement for the ionized form of naltrexol as compared to the unionized form indicates that the selectivity of charged molecules to diffuse through microchannels is greater than through the intact skin around the microchannels. This is not surprising as it is expected that more lipophilic, uncharged molecules would still be able to permeate through intact skin around the microchannels, but it may prove much more difficult for the their charged counterparts to partition into and diffuse across the SC. On the other hand, the charge of naltrexone didn’t seem to have much influence on the transport through MN-treated skin (flux values were not statistically different). Rather, it is likely that the drug solubility in the donor solution plays a critical role in augmenting transport through MN-treated skin by providing a larger concentration gradient across the skin. It is noteworthy that this study demonstrated the same direction but not the same magnitude of the effect of pH formulation on the transport through intact skin and MN-treated skin, despite the different routes of penetration involved.
Also looking at the effects of formulation on the delivery of naltrexone hydrochloride (NTX• HCl) through untreated and MN-treated Yucatan miniature pig skin, Milewski and Stinchcomb investigated the influence of propylene glycol (PG)-water binary mixture donor solutions on the rates of drug transport through microchannels (data submitted for publication). Several donors containing a constant concentration of NTX•HCl in binary mixtures of PG and water were prepared. In MN-treated skin, transdermal transport proved to be a function of the donor solution composition, with the lowest flux obtained from a pure PG solution and the highest flux obtained from a pure aqueous solution, with the extent of flux difference obtained from MN-treated skin reaching as much as ≈40 fold. With the increasing PG content in the donor solution, flux gradually dropped in a non-linear fashion. For intact, non-MN treated skin, the flux values were much lower for all donor solution compositions. Despite a qualitative trend seen in untreated skin showing that water-rich donor solutions provide higher flux over PG-rich donor solutions, this trend doesn’t mimic that seen with MN-treated skin. The effect of donor solution composition is different and had a much more pronounced effect on MN-treated skin as compared to the untreated skin. The data was also analyzed using a model to take into account two parallel and independent permeation pathways: one through intact skin around the microchannels and another through the microchannels. The relative importance of each pathway was shown to be dependent on the formulation used, and the non-linear change in flux as a function of donor formulation can be rationalized on the basis of varying donor solutions’ viscosities. Microchannel flux values were found to be inversely related to the donor solution viscosity. This work emphasizes the ability of co-solvents/excipients to influence percutaneous transport rates through MN-treated skin in a fashion much different from that of untreated skin, which may be helpful in optimization of future microneedle-patch systems.
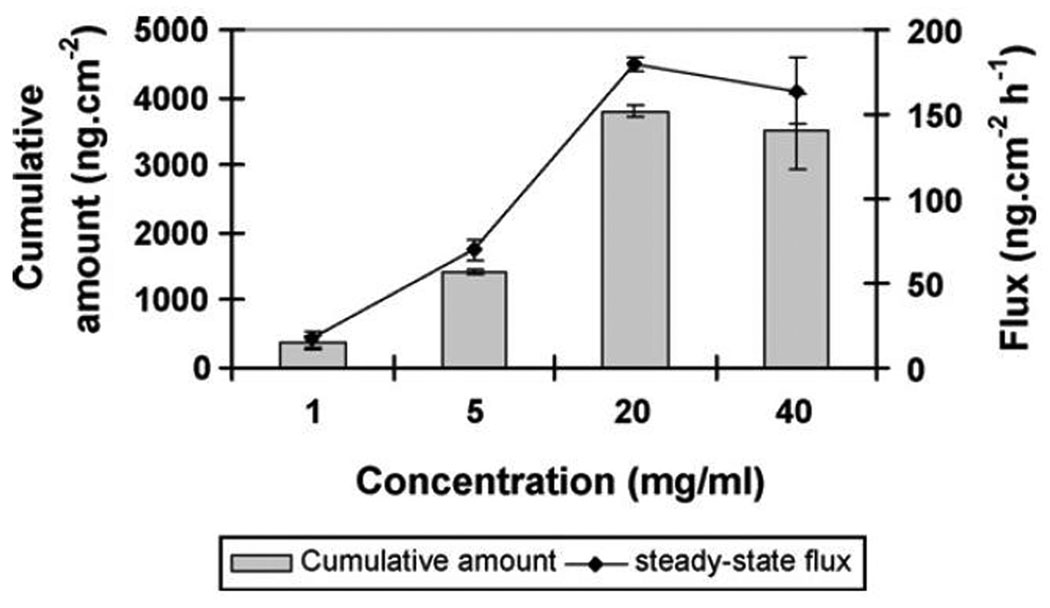
Guohua Li et al. investigated the transport of a model protein, IgG, using dissolvable maltose microneedles through hairless rat skin.22 A large, 150 kDa macromolecule cannot be delivered via the percutaneous route without the use of an enhancement method. This study evaluated the importance of three factors on transport of IgG through MN-enhanced skin: MN length, MN number, and IgG concentration in the donor solution (here we will only discuss the effects of IgG concentration on transport). Donor solutions comprised of human IgG solutions at 4 different concentrations (in phosphate buffer pH 7.4) were evaluated. As shown in Figure 1, a rise in the IgG donor solution concentration caused an increase in the MN-treated skin flux in a concentration-dependent manner. While the initial increase in the concentration from 1 to 5 mg/ml produced a proportional rise in flux (≈5-fold), further change in concentration to 20 mg/ml caused flux to increase only ≈12-fold as compared to the 1mg/ml donor. Even more pronounced non-linearity was evident when 40 mg/ml donor solution was compared to the 1 mg/ml donor – only an ≈11-fold difference in transdermal flux is observed. Clearly the gain in flux obtained by increasing diffusant donor concentration becomes less and less pronounced with augmenting concentration.. This may imply that using very concentrated (or saturated) solutions will not provide substantial improvement (in terms of flux) over their lower-concentration counterparts.
Figure 1.
Cumulative amounts of human IgG permeated and steady state flux across hairless rat skin pretreated with two-layered microneedles (54 needles, 500µm long) during 24 h of transdermal delivery with 200µl of donor at 40, 20, 5 or 1 mg/ml of human IgG concentrations (mean ± S.E.) (n = 3). Reproduced with permission from Li G, Badkar A, Nema S, Kolli CS, Banga AK. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm 2009 Feb 23;368(1–2):109-15., Copyright (2009) Elsevier.
The above study used steady-state flux to compare the in vitro performance of different donor concentrations of the diffusant. On the other hand, Galit Levin et al. investigated transdermal delivery of human growth hormone (hGH, 22 kDa) through microchannels in vivo by using radiofrequency ablation to form microchannels in rat and guinea pig skin.23 Although this method is not directly related to MN use, the resulting microchannels possess characteristics similar to those obtained after MN-treatement – hydrophilic nature, extending over epidermal thickness, circular geometry and very small fractional area in the experimental set-up (< 1%). The focus of this publication was to examine the bioavailability (relative to subcutaneous injection) and bioactivity of hGH by using different dose patches in vivo. The patches containing hGH in a thin, dry layer were placed on the microchannel-affected area of animal skin. The resulting plasma profiles of hGH resembled those obtained after subcutaneous injection rather than steady-state conditions, suggesting that the SC was no longer the rate-limiting step in transport. This in vivo study demonstrated that, in both rat and guinea pig, the total exposure to the hGH (as measured by AUC) was linearly dose-dependent in the lower concentration range (50–300 µg/patch), but further increasing the dose to 400–450 µg/patch did not translate into proportional exposure. This is also expressed in terms of bioavailability which remains at a steady level over the 50–300 µg/patch range but drops at higher doses. Therefore, this trend mimics in vitro findings of the previous publication, although the underlying reasons may be different. The authors evoked factors such as dissolution rate of hGH from the patches, diffusion rate through the channels, microchannel closure process and metabolism to tentatively explain the decrease in bioavailability at high doses. Overall, however, the bioavailability of hGH was found to be very high relative to subcutaneous injection - around 75% in rat and around 33% in guinea pigs. High bioavailability and retention of the hGH bioactivity during the patch manufacturing process indicate that this delivery route may become of practical clinical importance.
3. Liposomes and Nanoparticles
The pores created by MN pretreatment allow enhanced percutaneous transport of molecules that permeate slowly through intact skin, and also enable the transport of marcomolecules and nanoparticlulate systems normally deemed too large to permeate at all. The micrometer-scale diameter of the pore opening allows nanometer-scale particles to diffuse into the microchannel. The publications reviewed below demonstrate some formulation efforts to evaluate the influence of certain factors such as the size, composition and charge of particles on the transport rates across skin. The nature of the diffusing particle may have a significant effect on the results, and therefore data analysis should be cautious. For example, if the main focus is placed on the drug encapsulated in the particle alone rather than both the drug and the particle it may be very difficult to gain mechanistic understanding of the transport phenomena. Liposomal formulations are complex systems and one of the unanswered questions is whether liposomes can diffuse into and through the viable epidermis/dermis in intact form, or whether they break down and release encapsulated drug.24 Only detailed analysis of both drug and liposome constituents in skin could clarify the mechanism of drug-bearing liposome’s transport through MN-treated skin. On the other hand, the data interpretation coming from the use of solid nanoparticles where the nanoparticles themselves are quantified is more straight-forward.
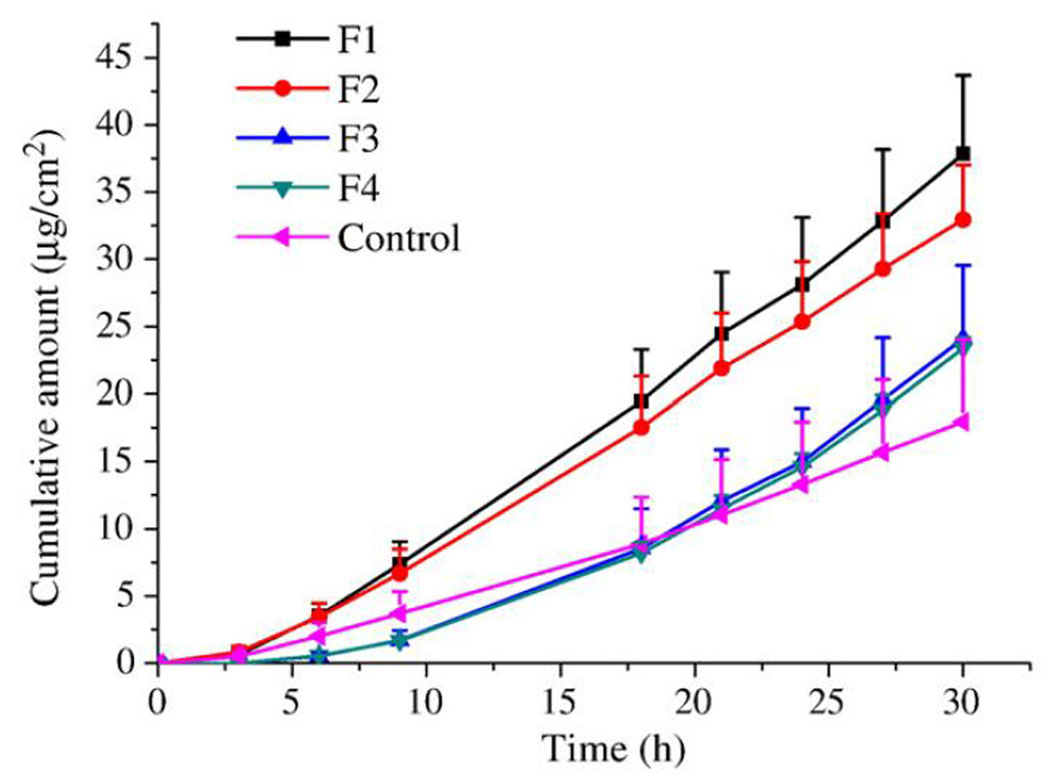
In order to evaluate the performance of different liposomal formulations across untreated and MN-treated skin, Yuquin Qiu et al. studied the permeation of docetaxel in various elastic liposomal formulations, with the goal of increasing skin permeation of a poorly water soluble (6–7 µg/ml), lipophilic (logP = 4.1) and fairly large molecule (MW=807.9 Da).25 The authors used 4 different liposomal formulations prepared by a thin film method: 2 formulations (F1 and F2) were elastic liposomes, while the remaining 2 (F3 and F4) were conventional liposomes. The entrapment efficiency for all four formulations was around 80–88% and the particle sizes were 43 ± 2 (F1), 197 ± 1.5 (F2), 173 ± 3.1 (F3) and 5697 ± 168 nm (F4) with the polydispersity index 0.08 ± 0.01, 0.35 ± 0.01, 0.41 ± 0.01 and 1.00 ± 0.00, respectively. Prepared donor solutions were compared in an in vitro diffusion experiment with untreated or 150 µm MN-treated porcine skin. An experiment employing untreated skin revealed that the elastic liposomes provided greater transdermal flux of docetaxel when compared to the conventional liposomal formulations, which performed poorly. On the other hand, an experiment with MN-treated porcine skin revealed different behavior of all formulations tested, as presented in Figure 2. First, MN-treatment by itself allowed the control solution of docetaxel to permeate the skin, demonstrating the effect of MN-treatment alone. Second, all liposomal formulations further increased the percutaneous flux, irrespective of the liposomal formulation, and produced similar steady-state flux. Additionally, the size of the liposomes varied greatly from formulation to formulation and yet this didn’t affect the outcome. One significant difference that was noted is the lag time. Elastic liposome formulations (F1 and F2) had lag times on the order of 4 hours while conventional liposomes (F3 and F4) had lag times around 14 hours. The authors set forth a hypothesis to explain the difference in terms of the ability of elastic and inability of conventional liposomes to rapidly release the drug in the microchannels into viable tissue. This cannot be concluded with certainty as the fate of conventional and elastic liposomes was not explicitly followed in the skin. Nevertheless, from a practical point of view, this investigation clearly demonstrated how the performance of 4 formulations and a control solution was changed by MN-treatment of the skin. Not only did the flux increase substantially, but the changes were different for each class of donors tested: elastic liposomes, conventional liposomes and drug solution.
Figure 2.
Penetration of DTX from liposomal formulations and control saturated 20% w/w ethanolic solution through porcine skin treated with microneedles (n=4). Reproduced with permission from Qiu Y, Gao Y, Hu K, Li F. Enhancement of skin permeation of docetaxel: a novel approach combining microneedle and elastic liposomes. J Control Release 2008 Jul 14;129(2):144-50. Copyright (2008) Elsevier.
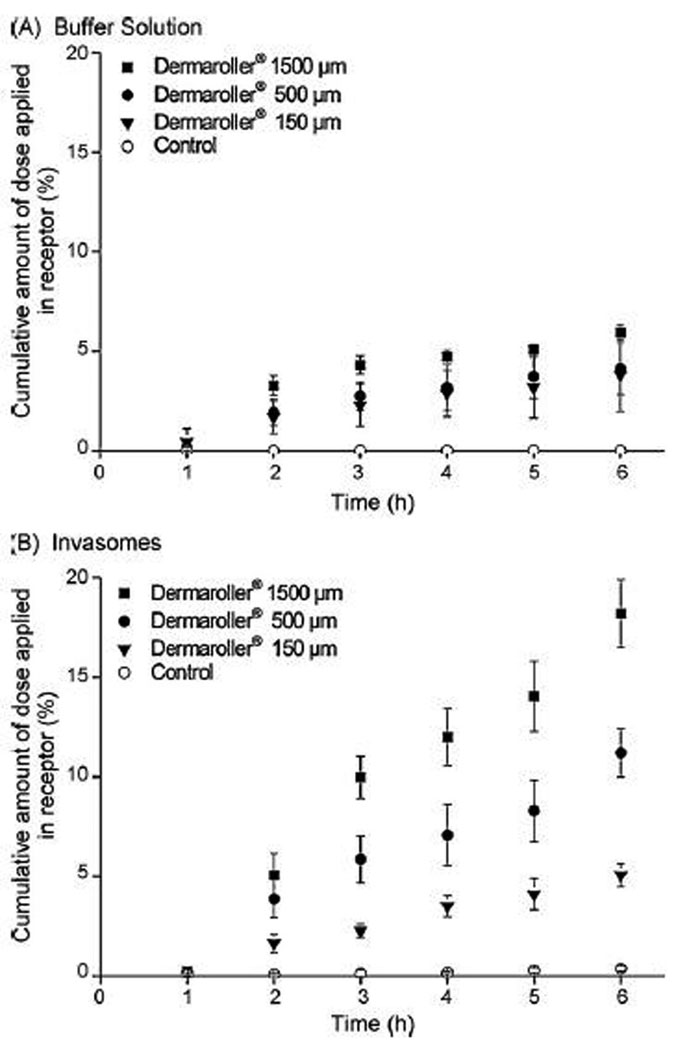
Badran et al. investigated the effect of microneedle size and applied formulation on the in vitro permeation of the model hydrophilic drug mannitol.26 In this study, microneedle lengths of 150, 500 and 1500 µm were evaluated; additionally, the effect of a colloidal drug carrier system – invasomes – was tested. Invasomes are highly flexible vesicles that contain permeation enhancers such as ethanol and terpenes besides phospholipids. This study evaluated the performance of invasomes in combination with MN skin perforation, hypothesizing that MN treatment may further facilitate transport of the invasomes across skin. Mannitol-loaded invasomes obtained by extrusion procedure were 123.6 ± 0.3 nm in diameter, with a polydispersity index of 0.08 ± 0.01. The results from 6 hour long in vitro diffusion experiments presented in terms of the percent of the applied dose that was found in the receiver solution are shown in Figure 3. First, without MN treatment of the skin, invasomes were able to deliver a small quantity of mannitol (0.4%) across the skin. Secondly, diffusion of invasomes through microchannels further enhanced the transport. The results also demonstrated that the magnitude of enhancement differed depending on the MN length used. Longer microneedles correlated with greater amount of diffusant found in the receiver solution after 6 hours, ranging from 18% (1500 µm MN) to 11% (500 µm MN) and 5% (150 µm MN). In other words, the length of microchannels formed after MN treatment was not of critical importance when combined with a buffer formulation, but the effect was much more pronounced with the invasome formulation. It should be noted, however, that these authors used invasome formulations without separation of the drug contained in the outer water phase. This further confounds the data analysis as at least two different species – drug in invasomes and free drug - are present in the donor solution. Authors suggest that the flexible vesicles can enhance the penetration of the drug dissolved in the outer water phase in addition to invasomes fusing or aggregating with epidermal cells, and hence augmenting their flow toward the epidermis from the donor solution. It seems that an alternative explanation could involve the difference in the diffusion coefficients of free drug and invasomes between dermis and solution in the microchannels. For free drugs the diffusivity in the viable tissue is usually up to 10 times lower as compared to aqueous milieu.2 On the other hand, if the diffusivity of invasomes drops more substantially while in dermis, and assuming they can diffuse intact throughout it, a greater sensibility of the vesicular flux to the microchannel length would be expected as shown in Figure 3. Despite the difficulties in the interpretation of the data, the experiments showed a pronounced effect of the formulation on the percent of the applied dose detected in the receiver solution, with invasomes being superior in this respect.
Figure 3.
Cumulative amount of radiolabeled mannitol in the receptor compartment on the formulation ((A) buffer solution; (B) invasomes) and Dermaroller® treatment (n = 3). The controls present the skin samples without Dermaroller® treatment. Reproduced with permission from Badran MM, Kuntsche J, Fahr A. Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: dependency on needle size and applied formulation. Eur J Pharm Sci 2009 Mar 2;36(4–5):511-23. Copyright (2009) Elsevier.
Coulman et al. studied the microneedle-mediated delivery of nanoparticles into human skin and through porous membranes.27 The purpose of the study was to evaluate the effect of artificial porous membrane surface charge and pore size on the permeation and to study the transport across MN-treated skin. Fluorescent polystyrene nanospheres served as model nanoparticles (NP) and were characterized by hydrodynamic diameter, zeta potential and surface morphology. The mean NP diameter measured by photon correlation spectroscopy was found to be 138 ± 25.1 nm. The pH of the NP formulation is important at least for two major reasons. First is the change of the surface charge with a variation in pH. It was shown that particles that don’t possess surface charge (zeta potential) high enough to provide electrostatic repulsion are prone to aggregation and hence instable. The second is a potential for charge-dependent NP-membrane/skin interactions that may affect the transport. Therefore, the authors decided to use pH as a means to control the surface charge of the NP, and showed that two units below the isoelectric point at pH 5 they were positively charged (≈40mV) while two units above pH 5.0 they were negatively charged (≈ −40mV). In order to eliminate biological variability, the first set of experiments employed the Isopore® membranes, which are considered to be surrogates for the MN-treated skin rather than the MN-treated skin itself. Membrane characteristics include cylindrical pore shape, three different pore sizes: 100nm, 1.2µm and 10µm, and low negative surface potential. Additionally, pH values of 7.4 and 3 were chosen to investigate the influence of NP charge on the transport through pores as they result in the zeta potential of −40mV and 40mV, respectively. At physiological pH 7.4 NP diffused rapidly through 10µm pores but the rate of transport through 1.2µm pores was substantially reduced, and the NPs were not able to permeate through 100 nm pores at all. The authors explained the observed phenomenon by evoking a greater hindrance the NP may experience diffusing through the pores of 1.2µm diameter as compared to 10µm. Alternatively, a change in the charge density on the internal size of individual microchannels was hypothesized to have an effect on the rate of permeation. The complete lack of detectable NP in the receiver solution after using a membrane with 100nm pores was believed to be due to a simple size exclusion principle. Next, the same experiments were repeated at pH 3.0. The reverse in the zeta potential caused a marked change in the outcome. For 10µm pores, the NP permeation was diminished as compared to pH 7.4; moreover, for pores sized 1.2µm and 100nm, no NP were detected in the receiver solution. The authors suggested that these differences in transport were largely due to the surface-charge dependent NP-membrane interactions. For 10µm pores, the pore diameter was sufficiently large to diminish their importance, however, for 1.2 µm it would be responsible for “immediate absorption of nanoparticles to the membrane and rapid accumulation, resulting in occlusion of microchannels”. Mass balance showed that as much as 56% of the applied formulation at pH 3.0 was not detected in either donor or receiver solution, which contrasts sharply with only 10% for pH 7.4. Based on this observation, as well as the increased membrane fluorescence detected at pH 3.0, the authors put forward a hypothesis that the electrostatic interactions between positively charged NP and the negatively charged membrane surface are responsible for altered transport of NP at this pH. Finally, a diffusion of 100nm NP through MN-treated human epidermis was investigated. Both MN-treatment and hypodermic needle-treatment of the epidermis resulted in elevated transport of NP as compared to the untreated epidermis. Scanning electron micrographs revealed that, in this experimental set-up, the microchannel diameters created by either MN or hypodermic needles were of approximately the same size of 50–100 µm. However, the number of such microchannels was greater for MN-treated skin. In this context, it is not surprising that MN-treatment afforded a greater percentage of the applied dose to permeate to the receiver solution as the fractional skin area corresponding to channels was also higher. Interestingly, this study indicates that whole NP can diffuse through MN-treated epidermis. However, it is not clear if epidermal sheets were fully perforated by the use of MN. Such a case would practically result in direct contact of donor solution with receiver solution with no viable tissue in between them. Therefore, the appearance of the NP formulation in the receiver would imply the ability of NP to diffuse through the channels rather than through the combination of channels and viable tissue underneath. Also, as the authors admit, the error associated with measurements is large, thus further complicating the analysis of the data. Nevertheless, experiments carried out with Isopore® membranes point out that even in a relatively simple system devoid of biological variability the electrostatic interactions between charged NP and porous membrane may play a substantial role in the permeation characteristics of a particulate formulation. Further studies are needed to elucidate its importance in the experiments with MN-treated skin.
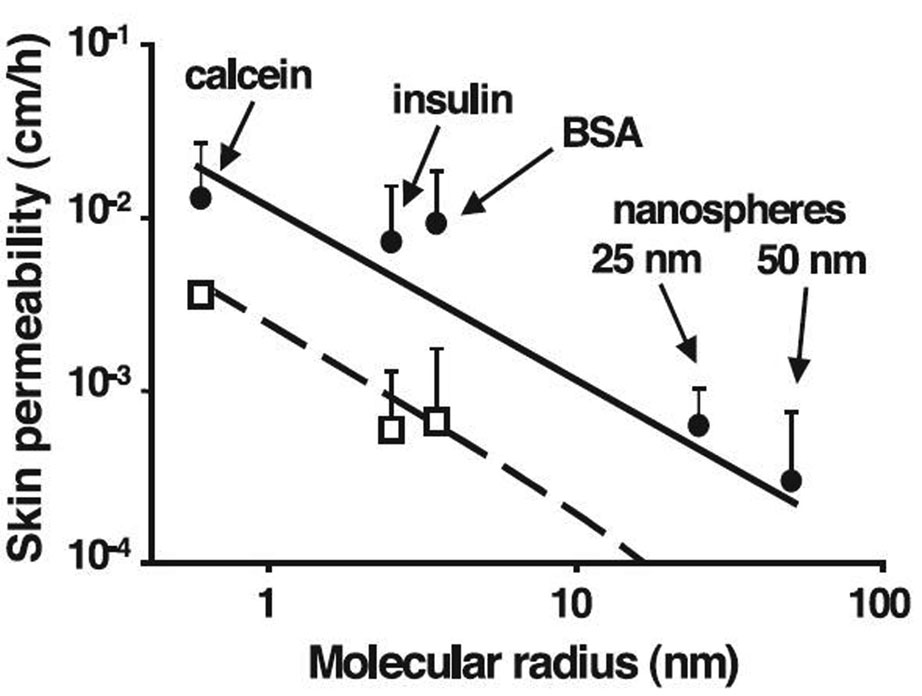
McAllister et al. studied the delivery of macromolecules and nanoparticles through MN-treated skin.28 The hypothesis under scrutiny was that MN can create transport pathways that allow small and large drugs, and nanoparticles to be delivered transdermally without pain. Apart from the engineering aspects of MN fabrication and characteristics, the authors also described in vitro diffusion experiments in which a variety of model compounds of different molecular size were used. Calcein, insulin, bovine serum albumin (BSA) and polystyrene latex nanospheres of 25 and 50 nm were chosen to provide a wide spectrum of molecular/particular radius. A model was used which described the apparent permeability coefficients in terms of fractional area of the microchannel pathway, molecular diffusivity in the microchannels, and length of the microchannels in combination with the Einstein-Stokes equation, and recognizing possible hindrance to diffusion of nanoparticles within annular gaps. This allowed correlation of molecular size (radius) with skin permeability. The intact skin was assumed to be effectively impermeable to diffusants. Additionally, two cases were compared – MN inserted and left in the skin, and MN inserted and then removed from the skin. The first case resulted in the creation of annular gaps inbetween MN and surrounding skin, while the second case produced residual microchannels in the skin. Electron microscopic measurements afforded calculation of the fractional area available for diffusion in each case. The results of in vitro experiments and calculated permeability coefficients are summarized in Figure 4. Experimental data agreed well with the predicted permeability values over the molecular radius span of about 2 orders of magnitude. This supports the idea that transport indeed occurs across aqueous microchannels created with the help of MN in accordance with a simple model proposed. In this study authors used fully perforated human epidermal membranes and the length of the channels was taken to be 50 µm. Hence, the skin permeability reported corresponded to the permeability of model compounds in the microchannels alone. This clearly demonstrates the ability of diffusants as large as nanoparticles to permeate along microchannels; however, their ability to diffuse in the viable tissue was not studied at this time. Moreover this work suggests that the drug diffusivity in the microchannels can be successfully predicted using the Einstein-Stokes equation, much like in the bulk solution.
Figure 4.
Skin permeability to molecules and particles of different sizes after treatment with microneedles. The permeability of human cadaver epidermis was increased by orders of magnitude with a 400-needle array inserted (□) and after the array was removed (●) for calcein, insulin, BSA, and latex nanospheres of 25 nm and 50 nm radius. Reproduced with permission from McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A 2003 Nov 25;100(24):13755-60. Copyright 2003 National Academy of Sciences, U.S.A.
4. Microneedle Coating and Polymer Microneedles
One alternative method of MN application involves coating the MN with drug solution. This mode of MN use is much different from other MN applications in many respects. The drug is coated directly onto the MN and does not require any type of additional reservoir or accompanying patch. However, the overall amount of drug delivered is much lower and the release profile is also expected to differ significantly from the combined MN-patch approach. The complete process involves coating of a drug solution on solid MN, insertion into skin and drug dissolution within the skin.
From a practical standpoint, it is crucial to be able to consistently apply uniform coatings with reproducible and high amount of drug. Gill et al. investigated coating formulations for microneedles in order to develop a rational and systematic approach for optimizing the coating processes, in terms of amount of drug deposited, uniformity and thickness of coating for a variety of compounds of different physicochemical properties.29 The authors used a micro-dip-coating process to cover the surface of solid MN with drug formulation and to fill into specially designed “pockets” within the MN. The important physical properties affecting the thermodynamics and hydrodynamics of dip-coating are surface tension and viscosity of the formulation. Given complicated kinetics of wetting and de-wetting processes involved in dip-coating, theoretical model development is difficult and the authors decided to experimentally study the effect of surface tension and viscosity effects instead. The coating solution consisting of the model drug sulforhodamine (characterized by low viscosity and high surface tension) did not produce any coating on the microneedles. Experiments indicated that reducing the surface tension of the coating formulations by the use of surfactants such as Lutrol F-68 or Tween 20 produced uniform but thin coating sheets deposited on MN. However, a thicker coating is required in order to increase the drug loading. An augmented viscosity can provide such improvement by increasing the hydrodynamic drag on the liquid during the dipping withdrawal phase. Excipients that were successfully used for this purpose included carboxymethylcellulose (CMC) and sucrose. Overall, the combination of reduced surface tension and increased viscosity resulted in homogenously spread thick coatings. Further investigation focused on the optimization of the excipient choice for specific application, for example, to avoid disadvantageous drug-excipient interactions. Additional viscosity-enhancers were tested in combination with Lutrol F-68, including hyaluronic acid, xanthan gum, sodium alginate, polyvinylpyrollidone and sucrose. All except sucrose produced thick uniform coatings. A formulation containing sucrose didn’t coat the MN tips, which was attributed to the relatively high surface tension of that solution. Other strategies facilitating the coating process included the use of organic solvents (rather than water), and modification of MN surface properties. When selectively filling “pockets” in special MN design, both high viscosity and high surface tension proved to be advantageous and prevented the formulation from wetting the MN surface. Drugs of different physicochemical properties may require aqueous, organic or molten coating solutions for optimal coating results. Regio-specific coating and the use of multiple layers are also possible, thus affording better control over release profiles of drugs. Authors demonstrated wide potential for application of the drug-coated MN and it can be expected that the identification of the surface tension and viscosity as key parameters may greatly facilitate future work in this area.
Yet another mode of MN application involves drug-containing biodegradable MN left in the skin for prolonged, controlled release. Similarly to the previous case, the total amount of drug is limited by the microscopic dimensions of the MN. However, this approach lends itself to the possibility of better control of the release, which can be modified by the use of different encapsulation techniques.
Jung-Hwan Park et al. investigated polymer microneedles for controlled-release drug delivery, using poly-lactide-co-glycolide (PLGA) microneedles with model drugs to study the effect of single and double encapsulation on the release profiles.30 Hypodermic needles are typically used in conjunction with polymeric particles to achieve controlled-release of drugs in the body. The authors hypothesized that polymeric microneedles made with a drug-containing biodegradable polymer introduced into the skin could be a viable alternative. Two model compounds were included in the studies: calcein and bovine serum albumin (BSA). The in vitro release studies were conducted using full-thickness cadaver skin. Single encapsulation formulation consisted of directly entrapped calcein or BSA in the PLGA matrix of the MN. Both model compounds displayed release profiles consistent with a diffusion-controlled release, suggesting that no significant degradation of polymer occurred during the experiment. This was further confirmed by microscopic observation of MN after the completion of the experiment. Also, a large difference in the rate of release between two compounds of substantially different molecular weights supports this conclusion. A modified Higuchi equation, in which amount of drug released is a function of square root of time, was used to obtain apparent diffusion coefficients of calcein and BSA in the PLGA matrix of MN. These coefficients were found to be as follows: 1.2×10−10 cm2s−1 and 3.0×10−12 cm2s−1 and contrasted sharply with much greater diffusivity of the above compounds in water being 5.0×10−6 cm2s−1 and 5.9×10−7 cm2s−1, respectively. Thus, the single encapsulation greatly slowed down diffusion, which is advantageous for prolonged and controlled release. The effect of double encapsulation was also studied. First, calcein was entrapped within CMC microparticles and then encapsulated into the PLGA microneedles. Alternatively, calcein was entrapped in the poly-L-lactide (PLA) microparticles and subsequently encapsulated within the PLGA microneedles. Each double-encapsulation caused a substantial delay of calcein release when compared to the single encapsulation formulation. The use of CMC afforded a decrease in the release kinetics by more than one order of magnitude with some burst effect attributed to calcein not bound to CMC present in the MN matrix. On the other hand, the use of PLA resulted in an initial burst effect followed by a very slow release (another two orders of magnitude apparent diffusivity decrease) of calcein later on. Overall, this work demonstrated the effect of single- and double-encapsulation on the release kinetics of drug from polymeric MN. It is interesting to note how well the release kinetics can be controlled by the use of techniques suggested by the authors. Additionally, unlike in the case of aqueous donor solutions used in most in vitro studies reported earlier for the “poke and patch” method, this investigation emphasizes the rate-limiting role of the MN composition itself for the delivery of the drug into systemic circulation. These findings can help design future controlled-release MN systems.
5. In vivo Pore Lifetime Following Microneedle Application
In addition to the formulation of drug to be combined with MN treatment, another important factor that can affect drug delivery is the lifetime of the pores that are created in the SC, as rapid closure of the pores would severely limit drug delivery. The lifetime of the pores created can be monitored by various methods, including transepidermal water loss (TEWL) and electrical resistance. Disruption in the barrier is accompanied by an increase in TEWL and a decrease in resistance.15,31
Various means of disrupting the barrier function of the SC have been extensively described (in addition to MN treatment), including tape-stripping and acetone treatment. Tape stripping removes the SC mechanically, while acetone treatment extracts lipids from the SC.32,33 Restoration of barrier function occurs in a similar amount of time following tape stripping and acetone treatment, with complete recovery achieved in approximately 3 days in most cases.34,35 In addition to the time required for barrier restoration, many laboratories have also studied the physiological mechanisms underlying the restoration of barrier function following these insults, which includes processes involving lamellar bodies, ions (calcium and potassium), and cytokines (IL-1, IL-6, tumor necrosis factor), among others.36–38
Similar to tape-stripping and acetone treatment, MN treatment also provides a means of disrupting the barrier function of the SC. However, the time frame in which restoration of barrier function occurs and the associated physiological processes are not well understood. This topic is receiving increasing attention in the literature, and a recent publication utilized confocal laser scanning microscopy to describe the kinetics of pore closure in 6 healthy human subjects that were treated with microneedles. This data demonstrated that the pores do, in fact, close very rapidly (approximately 15 minutes in most cases).39
One compound that is ideal for the study of MN-assisted transdermal delivery and micropore closure is naltrexone HCl (NTX), an opioid antagonist used for the treatment of opioid and alcohol dependence. NTX is an ideal candidate for the study of MN-assisted transdermal delivery because its physicochemical properties (specifically its hydrophilicity) make it difficult to deliver across intact skin, and it exhibits erratic bioavailability when given via oral administration. A recent study examined MN-assisted delivery of naltrexone HCl (NTX) in healthy human subjects and, as expected, NTX levels were undetectable in control subjects, confirming that NTX does not readily cross intact skin. In contrast, NTX levels in MN-treated subjects demonstrated that the pores do allow delivery of NTX across the SC. Additionally, results suggested that the pores remained viable for up to 48 hours (under occlusion) in most subjects (72 hours for 2 subjects), as plasma levels of NTX remained relatively constant during this time.40 The authors also demonstrated that the electrical resistance of the skin dropped after microneedle treatment, followed by a gradual increase that further supported the NTX pharmacokinetic data. Another study was performed by Banks et al, studying rates of pore closure in hairless guinea pigs (in press).41 Hairless guinea pigs were treated with MN arrays, followed by application of an occlusive naltrexol (NTXOL, active metabolite of NTX) transdermal patch. Pore lifetime was evaluated via NTXOL pharmacokinetic analysis and TEWL measurements. This data demonstrated that pore closure occurred after approximately 48 hours following MN treatment, correlating well with the human study mentioned previously. Additionally, this group has data demonstrating that pore lifetime can be extended up to 7 days with daily application of Solaraze® gel (3% diclofenac sodium, a non-specific cyclooxygenase (COX) inhibitor) (PharmDerm® Melville, NY), indicating a possible role of inflammatory mediators in the pore healing process.42
Additional factors that may also affect the repair of barrier function of the SC (and thus the utility of MN treatment) are hydration and occlusion. Prolonged occlusion of the skin leads to increased skin hydration, an effect that can be readily seen via TEWL and resistance measurements.43,44 This increase in hydration can have pronounced effects on the permeability of the SC, and often increases drug penetration through the skin.44–46 Additionally, water transit has been proposed to be a signal involved in stimulation of barrier repair following disruption, and may have an effect on the duration of pore lifetime.47,48 Recent data in a hairless rat model indicated that pores close in approximately 15 h when left unoccluded, but this time frame extends to 72 h when the pores are occluded by a plastic film or solution.48 As noted previously, treatment sites were occluded in the human study of MN-assisted permeation of NTX, and this may have positively affected the duration of pore lifetime by inhibiting the barrier recovery.40 This effect was also noted in the study with hairless guinea pigs, as unoccluded sites appeared to restore barrier recovery much more quickly than sites that were occluded. From a clinical standpoint, current transdermal delivery systems involve complete occlusion of the skin for hours to days at a time, and thus the effects cannot be ignored. In fact, the effects of hydration and occlusion have positive implications with MN treatment, as the “poke and patch” method of MN-assisted drug delivery involves occlusion of the newly formed pores with a transdermal drug patch. Ultimately, the combination of slowed restoration of barrier function and increased drug permeability could be useful in the clinical setting.
6. Conclusion
Formulation plays an important role affecting drug delivery rates through MN-treated skin. Either in the case when a maximum percutaneous flux is sought or the case when a slow, controlled release is required, the formulation is of great significance. The publications to date indicate that the influence of a given formulation on the transport through untreated and MN-enhanced skin varies. As reviewed in this article, most authors currently focus on the in vitro diffusion studies. In the in vitro experiments the diffusive path length can be controlled by the choice of skin of a certain thickness (epidermis vs. full thickness) and MN length. This path length corresponds to the distance a drug molecule has to traverse through the microchannel and viable tissue in order to reach receiver solution from the donor solution. Authors chose different experimental conditions that result in not only change in the rate of permeation but also in the shape of delivery profiles. While some authors report permeation profiles indicative of reaching steady-state (SS) conditions, others report permeation profiles showing non-steady-state (non-SS) conditions (Table 1). One factor that seems to affect the shape of the profiles significantly is the thickness of the viable tissue between the microchannel and the receiver solution (authors’ unpublished data). When a thick wedge of viable tissue is present the permeation profiles can be described well in terms of the steady-state flux and lag time. However, in the absence of the viable tissue under the microchannel (skin fully perforated) profiles show initial high permeation rates that taper off at a later time. This underlines the importance of experimental condition selection.
Table 1.
Comparison of different in vitro diffusion experimental conditions and resulting permeation profiles.
| Author | Diffusion cell | Skin type | MN length | Permeation profile |
|---|---|---|---|---|
| Banks et al. | Flow-through cell |
Full-thickness hairless guinea pig skin and full- thickness human abdominal skin |
750 µm | SS |
| Milewski and Stinchcomb |
Flow-through cell |
Full-thickness Yucatan miniature pig skin |
750 µm | SS |
| Li et al. | Franz cell | Full-thickness hairless rat skin |
200 and 500 µm | SS* |
| Qiu et al. | Franz cell | Full-thickness abdominal rat skin and 600 µm dermatomed porcine skin |
150 µm | SS |
| Badran et al. | Franz cell | Full-thickness abdominal human skin |
150, 500 and 1500 µm | Non-SS |
| Coulman et al. | Franz cell | Human breast epidermis |
≈300 µm | Non-SS |
some other profiles not shown in this article are non-SS
Overall, multiple strategies can be employed to achieve maximum flux through MN-enhanced skin by use of the “poke and patch” method. First, the formulation should not be rate-controlling. Simply increasing the drug donor concentration results in an elevated concentration gradient through the skin and hence augmented delivery rates are expected. For ionizable drugs, pH control or salt formation may be the simplest tool to achieve this goal. However, at high drug concentrations substantial deviations from ideal behavior are observed and the increase in drug concentration may not be paralleled by a proportional rise in flux. Also, cosolvent use could be potentially helpful, but propylene glycol has been shown to cause marked decrease in the flux, possibly by decreasing drug diffusivity in the barrier (Milewski and Stinchcomb, data submitted for publication). Vesicular and nanoparticulate systems could be useful for compounds that fail to be formulated using a simpler method and don’t permeate well through intact skin. Their performance showed a high dependence on the size of the microchannel and its length, although further studies are needed to improve understanding of the phenomena involved in their transport through skin.
Lastly, the kinetics of pore closure after MN insertion and removal play a critical role in the ability of drugs to cross the SC. This has already been demonstrated via correlation of TEWL and pharmacokinetic data in both humans and hairless guinea pigs.40 In both models there appears to be a relationship demonstrating increasingly unpredictable pharmacokinetic profiles as the pores begin to close. While MN pretreatment is certainly an effective means of increasing permeability of various drugs through the skin (especially when combined with other factors such as hydration and occlusion), maintaining the lifetime of the pores is crucial for maintaining the usefulness of this approach to drug delivery.
7. Expert opinion
In the diffusion experiments employing untreated skin, often the influence of formulation on the transport is analyzed in terms of ideal thermodynamic behavior. A drug saturated in different solvents exhibits the same maximum activity in those solvents. Hence, its partitioning into the rate-limiting SC lipid domain should result in the same concentration in the membrane, and transdermal flux values obtained from such donor solutions are expected to be the same. Deviations from this behavior are approached by evoking permeation enhancing properties of cosolvents by their ability to alter the barrier properties of the SC. In the case of MN-treated skin, the creation of microchannels allows drug molecules to bypass the SC barrier. The most relevant question seems to be what becomes the barrier in the absence of the SC? As reported previously, the formulation itself can become the rate limiting step in the delivery.30 Authors showed how using drug encapsulated in polymer MN could allow the delivery rates to be controlled by the drug release from MN. In this situation the skin release profiles are better described by a modified Higuchi equation, which linearly relates mass released to the square root of time, rather than the steady-state conditions. Next, when the drug diffusion coefficients in the formulation are high (as it is the case in the bulk aqueous solution) it could be expected that viable tissue will be the primary rate limiting step in the skin permeation process. This appears to be the case for most articles reviewed here. Additionally, it seems that in the situation when formulation excipients can diffuse out of the vehicle and into the viable tissue they can alter its diffusional properties as suggested by Milewski and Stinchcomb. It is likely that viscosity-increasing propylene glycol can change the microviscosity of the viable tissue and, therefore, decrease the mobility of drugs in this rate-limiting environment.
One aspect of the MN-assisted drug delivery that has been underrated and will be investigated more closely in the near future is the pharmacokinetics of drugs delivered with the help of a MN-and-patch method. So far, it has been typically assumed that steady-state plasma profiles will prevail after MN-pretreatment of the skin. In vivo studies in guinea pigs (data submitted for publication) and one study in humans have shown that the plasma drug concentrations are relatively constant.40,41 If, under in vivo conditions, some formulations controlled the release of the drug and were rate-limiting in transport, then the plasma drug concentrations should eventually decline with time. This seems to be the case in the study reported here where authors used a dry thin film of drug in the patch that resulted in plasma profiles similar to those obtained after subcutaneous injection.23
All of the above mentioned factors do not take into account the kinetics of pore closure in vivo and changes in the diffusional properties of the microchannel environment. To date, very limited data is available to provide clues about the lifetime of pores and mechanisms of restoring barrier function following MN treatment. Relatively rapid closure of the pores severely limits the clinical utility of MN application, and understanding the factors involved in healing the pores created by MN treatment is critical for optimizing the use of microneedles in clinical situations. Once weekly dosing of a transdermal patch would be ideal for clinical applications, requiring patients to change a patch only once every 7 days. Effects of hydration and occlusion on pore lifetime appear to be helpful in extending pore lifetime, and are a natural (and convenient) effect of the “poke and patch” method. In order to continue to push the MN field forward towards clinical practice, substantial efforts need to be put forth to develop safe and effective means of extending pore lifetime to this endpoint.
Article Highlights box
Microneedle application creates micropores in the skin, thus providing a minimally invasive means of bypassing the stratum corneum barrier and increasing the number of drug molecules that can be transdermally delivered.
Several parameters can ultimately affect the degree of drug delivery and skin permeability associated with MN-assisted transdermal delivery.
Highly-soluble, ionized species have been shown to substantially increase drug delivery rates as compared to their low-solubility, neutral counterparts.
At high drug concentrations, non-linearity in in vitro transport rates and in vivo exposure has been observed.
Optimization of drug-containing nanoparticulate systems currently presents many challenges and more thorough studies are needed to elucidate their behavior in the dermis.
Studies involving microneedle coating and polymer microneedles demonstrate the potential of formulation in tailoring drug release rates for immediate or controlled absorption.
Due to the low diffusional resistance of MN-treated skin, a shift in the transport rate limiting step from skin to transdermal device may occur.
Short lifetime of the pores created following MN application limits the clinical utility of this approach.
Continued optimization of several components related to MN-assisted transdermal delivery is necessary in order to develop drug delivery systems that can be applied for a clinically relevant period of time.
Acknowledgments
ALS is funded by NIH grants R01DA13425, R01DA18822, R41AA016499, R43DA26266, and RC2DA28984.
Commonly used abbreviations
- MN
Microneedle
- SC
Stratum corneum
Footnotes
Declaration of interest
ALS is a majority shareholder in AllTranz, Inc., a specialty pharmaceutical company developing COX inhibitor technology for use with MN systems.
References
- 1.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn GL. Cutaneous and Transdermal Delivery: Processes and Systems of Delivery. In: Banker CTR GS, editor. Modern Pharmaceutics. 2nd ed. New York: Marcel Dekker; 1990. pp. 239–298. [Google Scholar]
- 3.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharm. 2008;364(2):227–236. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Burkoth TL, Bellhouse BJ, Hewson G, Longridge DJ, Muddle AG, Sarphie DF. Transdermal and transmucosal powdered drug delivery. Crit Rev Ther Drug Carrier Syst. 1999;16(4):331–384. doi: 10.1615/critrevtherdrugcarriersyst.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- 6.Prausnitz MR, Bose VG, Langer R, Weaver JC. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci U S A. 1993;90(22):10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269(5225):850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 8.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22(2):192–197. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- 10.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, Morrissey A, Birchall JC. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11(1):35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 11.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92(2):502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 12.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 13.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8(4):415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 14.Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37(8):1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Banga AK. Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6(4):343–354. doi: 10.1517/17425240902841935. [DOI] [PubMed] [Google Scholar]
- 16.Verbaan FJ, Bal SM, van den Berg DJ, Dijksman JA, van Hecke M, Verpoorten H, van den Berg A, Luttge R, Bouwstra JA. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J Control Release. 2008;128(1):80–88. doi: 10.1016/j.jconrel.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Al-Qallaf B, Das DB. Optimizing microneedle arrays to increase skin permeability for transdermal drug delivery. Ann N Y Acad Sci. 2009;1161:83–94. doi: 10.1111/j.1749-6632.2009.04083.x. [DOI] [PubMed] [Google Scholar]
- 18.Al-Qallaf B, Das DB, Mori D, Cui Z. Modelling transdermal delivery of high molecular weight drugs from microneedle systems. Philos Transact A Math Phys Eng Sci. 2007;365(1861):2951–2967. doi: 10.1098/rsta.2007.0003. [DOI] [PubMed] [Google Scholar]
- 19.Al-Qallaf B, Das DB. Optimizing microneedle arrays for transdermal drug delivery: extension to non-square distribution of microneedles. J Drug Target. 2009;17(2):108–122. doi: 10.1080/10611860802472370. [DOI] [PubMed] [Google Scholar]
- 20.Banks SL, Pinninti RR, Gill HS, Crooks PA, Prausnitz MR, Stinchcomb AL. Flux across [corrected] microneedle-treated skin is increased by increasing charge of naltrexone and naltrexol in vitro. Pharm Res. 2008;25(7):1677–1685. doi: 10.1007/s11095-008-9578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann JJSM, Koski WS. Microelectrometric titration measurement of the pKa's and partition and drug distribution coefficients of narcotics and narcotic antagonists and their pH and temperature dependence. Journal of Medicinal Chemistry. 1975;18(7):647–655. doi: 10.1021/jm00241a001. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Badkar A, Nema S, Kolli CS, Banga AK. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm. 2009;368(1–2):109–115. doi: 10.1016/j.ijpharm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Levin G, Gershonowitz A, Sacks H, Stern M, Sherman A, Rudaev S, Zivin I, Phillip M. Transdermal delivery of human growth hormone through RF-microchannels. Pharmaceutical Research. 2005;22(4):550–555. doi: 10.1007/s11095-005-2498-6. [DOI] [PubMed] [Google Scholar]
- 24.EL Maghraby GMM, Williams AC, Barry BW. Can drug-bearing liposomes penetrate intact skin? Journal of Pharmacy and Pharmacology. 2006;58(4):415–429. doi: 10.1211/jpp.58.4.0001. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y, Gao Y, Hu K, Li F. Enhancement of skin permeation of docetaxel: a novel approach combining microneedle and elastic liposomes. J Control Release. 2008;129(2):144–150. doi: 10.1016/j.jconrel.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Badran MM, Kuntsche J, Fahr A. Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: dependency on needle size and applied formulation. Eur J Pharm Sci. 2009;36(4–5):511–523. doi: 10.1016/j.ejps.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, Birchall JC. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009;366(1–2):190–200. doi: 10.1016/j.ijpharm.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 28.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100(24):13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 30.Park JH, Allen MG, Prausnitz MR. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm Res. 2006 doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Yamamoto Y. Electrical properties of the epidermal stratum corneum. Med Biol Eng. 1976;14(2):151–158. doi: 10.1007/BF02478741. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Mao-Qiang M, Taljebini M, Elias PM, Feingold KR. Topical stratum corneum lipids accelerate barrier repair after tape stripping, solvent treatment and some but not all types of detergent treatment. Br J Dermatol. 1995;133(5):679–685. doi: 10.1111/j.1365-2133.1995.tb02738.x. [DOI] [PubMed] [Google Scholar]
- 33.Bashir SJ, Chew AL, Anigbogu A, Dreher F, Maibach HI. Physical and physiological effects of stratum corneum tape stripping. Skin Res Technol. 2001;7(1):40–48. doi: 10.1034/j.1600-0846.2001.007001040.x. [DOI] [PubMed] [Google Scholar]
- 34.Rissmann R, Oudshoorn MH, Hennink WE, Ponec M, Bouwstra JA. Skin barrier disruption by acetone: observations in a hairless mouse skin model. Arch Dermatol Res. 2009;301(8):609–613. doi: 10.1007/s00403-009-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oudshoorn MH, Rissmann R, van der Coelen D, Hennink WE, Ponec M, Bouwstra JA. Development of a murine model to evaluate the effect of vernix caseosa on skin barrier recovery. Exp Dermatol. 2009;18(2):178–184. doi: 10.1111/j.1600-0625.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- 36.Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127(7):1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 37.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90(2):482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias PMFK, Fluhr JW. In: Fitzpatrick's Dermatology in General Medicine. 6th ed. Freedberg IMEA, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. New York: Mc-Graw Hill; 2003. [Google Scholar]
- 39.Bal S, Kruithof A, Liebl H, Tomerius M, Bouwstra J, Lademann J, Meinke M. In vivo visualization of microneedle conduits in human skin using laser scanning microscopy. Laser Phys Let, ed. 2009 p In press. [Google Scholar]
- 40.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, Stinchcomb AL. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105(6):2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks S, Pinninti R, Gill H, Paudel K, Crooks P, Brogden N, Prausnitz M, Stinchcomb A. Transdermal delivery of naltrexol and skin permeability lifetime after microneedle treatment in hairless guinea pigs in vivo. Journal of Pharmaceutical Sciences. 2010 doi: 10.1002/jps.22083. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banks S, Paudel K, Stinchcomb A, Loftin C. COX enzyme inhibitor enables drug delivery through microneedle treated skin for seven days. AAPS Journal. 2008;10(S2) [Google Scholar]
- 43.Curdy C, Naik A, Kalia YN, Alberti I, Guy RH. Non-invasive assessment of the effect of formulation excipients on stratum corneum barrier function in vivo. Int J Pharm. 2004;271(1–2):251–256. doi: 10.1016/j.ijpharm.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Kennish L, Reidenberg B. A review of the effect of occlusive dressings on lamellar bodies in the stratum corneum and relevance to transdermal absorption. Dermatol Online J. 2005;11(3):7. [PubMed] [Google Scholar]
- 45.Zhai H, Ebel JP, Chatterjee R, Stone KJ, Gartstein V, Juhlin KD, Pelosi A, Maibach HI. Hydration vs. skin permeability to nicotinates in man. Skin Res Technol. 2002;8(1):13–18. doi: 10.1046/j.0909-752x.2001.10312.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhai H, Maibach HI. Occlusion vs. skin barrier function. Skin Res Technol. 2002;8(1):1–6. doi: 10.1046/j.0909-752x.2001.10311.x. [DOI] [PubMed] [Google Scholar]
- 47.Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989;30(3):323–333. [PubMed] [Google Scholar]
- 48.Kalluri H, Banga A. Microneedles and transdermal drug delivery. Journal of Drug Delivery Science and Technology. 2009;19(5):303–310. [Google Scholar]