Abstract
After bone injury, developmental processes such as endochondral and intramembranous ossification are recapitulated as the skeleton regenerates. In contrast to development, skeletal healing involves inflammation. During the early stages of healing a variety of inflammatory cells infiltrate the injured site, debride the wound, and stimulate the repair process. Little is known about the inflammatory process during bone repair. In this work, we examined the effect of a pro-inflammatory cytokine, Interleukin 1beta (IL-1β), on osteoblast and stem cell differentiation and on intramembranous and endochondral ossification, because IL-1β exerts effects on skeletal homeostasis and is upregulated in response to fracture. We determined that IL-1β stimulated proliferation of osteoblasts and production of mineralized bone matrix, but suppressed proliferation and inhibited differentiation of bone marrow derived MSCs. We next performed loss- and gain-of-function experiments to determine if altering IL-1β signaling affects fracture healing. We did not detect any differences in callus, cartilage, and bone matrix production during healing of non-stabilized or stabilized fractures in mice that lacked the IL-1β receptor compared to wild type animals. We observed subtle alterations in the healing process after administering IL-1β during the early phases of repair. At day 10 after injury, the ratio of cartilage to callus was increased, and by day 14, the proportion of cartilage to total callus and to bone was reduced. These changes could reflect a slight acceleration of endochondral ossification, or direct effects on cartilage and bone formation.
Keywords: Fracture healing, inflammation, interleukin 1 beta, endochondral ossification, intramembranous ossification
Introduction
Fracture healing occurs in four distinct phases: (1) inflammation, (2) soft callus, (3) hard callus, and (4) remodeling. While the soft callus, hard callus and remodeling phases have been investigated intensively, little is known about the exact nature of the inflammatory response during skeletal repair. Inflammation is a key regulator of the repair process in other tissues, and a variety of inflammatory molecules may be important for efficient repair. Therefore, understanding the role of inflammation and the contribution of individual cytokines to the regenerative process will enhance our knowledge of fracture healing. In this work we have focused on the role of Interleukin-1β (IL-1β), because this is a potent inflammatory cytokine that is upregulated during inflammation 1, 2.
Several studies have focused on the effects of inflammatory molecules on skeletal cells in vitro and in vivo. In general, key inflammatory molecules such as TNF- α, IL-6, and IL-1 modulate the proliferation and differentiation of skeletal cells. For instance, TNF-α has been shown to promote DNA synthesis in rat osteoblast-like cells, to inhibit osteocalcin and alkaline phosphatase expression in rat and human osteoblastic cell lines, to attenuate alkaline phosphatase expression in pre-osteoblasts (MC3T3-E1), and to play a necessary role in intramembraneous and endochondral bone repair in mice 3-8. IL-6 has been shown to be associated with cartilage destruction in human rheumatoid arthritis, to regulate differentiation and apoptosis in pre-osteoblasts, and to affect the mineralization and maturation of the fracture callus in mice 9-11. IL-1β is active during the fracture repair process in vivo 12, 13, affects growth of skeletal cell types in vitro 5, 6, 8, 14, 15, and participates in interactions between skeletal cell types 16. The expression pattern of IL-1β during endochondral fracture repair is bimodal, peaking during the initial inflammatory phase and again during the later remodeling phase 12, 13. Although in vitro work suggests that the effect of IL-1β on proliferation and differentiation of osteoblasts is cell type-dependent 5, 6, 8, 17, the relatively high level of IL-1β expression during osteogenic phases of fracture repair 18 correlates with results indicating that IL-1β promotes osteoblast proliferation during acute bone repair 17. Furthermore, IL-1β inhibits proliferation and differentiation of chondrocytes through a SOX-9-mediated mechanism 15. These data indicate that IL-1β inhibits chondrogenesis and can promote osteogenesis in mice.
We hypothesized that IL-1β may regulate stem cell differentiation and bone and cartilage formation during fracture repair. We compared the effects of IL-1β on proliferation and differentiation of an established osteoblast cell line and primary bone marrow-derived murine cells that contain mesenchymal stem cells (MSCs). Then, we compared expression of IL-1β in stabilized and non-stabilized fractures, because they heal by intramembranous and endochondral ossification respectively. Finally, we assessed the role of IL-1β during healing in these fracture models.
Methods
Collection of Primary Bone Marrow Cells
Bone marrow was collected from 10-14 week old male mice (C57BL/6J) with DMEM. Red blood cells were lysed, nucleated cells were washed twice in PBS, resuspended in DMEM, and plated on culture dishes. Non-adherent cells were removed from culture by passaging cells four times before experimentation. While this procedure does not produce a pure population of MSCs, it does enrich the population of MSCs in culture.
In vitro Proliferation of Pre-Osteoblasts and Mesenchymal Stem Cells
Pre-osteoblasts (MC3T3-E1, ATCC, Manassus, VA) or MSCs were transferred to Falcon Culture Slides (75,000 cells/well, BD Biosciences, Bedford, MA) containing media with or without IL-1β (10 ng/mL in DMEM). At 26 hours, 200nM BrdU (Zymed, So. San Francisco, CA) was added, cells were fixed four hours later (70% ethanol), and stained as described by the manufacturer (Zymed). The percentage of proliferating cells in each well was determined by uniform random sampling using the Olympus CAST system (Olympus America, Center Valley, PA) and Visiopharm 2.0 (Denmark).
In vitro Differentiation of Pre-Osteoblasts and Mesenchymal Stem Cells
Pre-osteoblasts and MSCs were cultured until confluent, and differentiation was induced with the In Vitro Osteogeneis Kit (Millipore, Billerica, MA). At 13 days, cells were fixed with 70% ethanol and stained with Alizarin Red.
Animals and Fracture Protocol
All experimental procedures were approved by the UCSF IACUC. 10-14 week old male mice with targeted inactivation of the Interleukin 1 Receptor 1 (B6.129S7-Il1r1tm1Imx/J Jackson Laboratories, Bar Harbor, ME) and their controls (C57BL/6J) were anesthetized with 2% Avertin. Mid-diaphysial tibia fractures were created by 3 point bending as described 19. Buprenex (0.1 mg/kg) was given as an anagelsic at 4-6 hours and at 24 hours after fracture, and then at 24 hour intervals as needed. In one group of animals the fractures were stabilized using an external fixator as described 20. All animals were allowed to ambulate freely. Euthanasia was performed by cervical dislocation after intraperitoneal injection of 2% Avertin at several times after injury (n >= 4/time point).
Injection of IL-1β into the Fracture Site
The fracture site was injected (30μl) with a single dose of IL-1β in PBS (40ng) or PBS (30μl), at 24 hours after fracture using a method that delivers a repeatable dose of molecules to the fracture site 21. In a second experiment, IL-1β, or control PBS, was administered at 24 (40ng), 48 (10ng), and 72 hours (10ng) after injury because these doses are effective 22, 23. Fracture calluses were analyzed at several time points during the repair phase (n >= 5 per time point). Rectal temperature was monitored at 24 hours after fracture using a rectal thermometer for small animals; no fevers were detected.
Quantitative RT-PCR
Stabilized and non-stabilized tibia fractures were created as described above. Animals were sacrificed at 2 (n=4/group) and 7 (n=4/group) days after injury and fractured tibiae were collected. Briefly, we removed the skin from all specimens and then we dissected the fractured bone and all adjacent tissues located 0.5cm distal and proximal to the edge of the callus/hematoma. A similar region of the non-fractured tibia was used as control (n=4). RNA was extracted from these tissues and the quality of RNA was confirmed using RNA 6000 Nano Chip Kit (Agilent, Santa Clara, CA). Primers for mouse IL-1β were purchased from Realtimeprimers.com. cDNA synthesis and qPCR were performed following standard protocols. GAPDH was used as the internal control.
Histology and Histomorphometry
Tissues were fixed in 4% paraformaldehyde overnight, decalcified in 19% EDTA, dehydrated, cleared, and embedded in paraffin. Sagittal sections (10μm) through the entire callus were collected. In situ hybridization was performed using radio-labeled riboprobes as described 19. Cartilage was visualized using Safranin-O/Fast Green (SO/FG), and bone was visualized using modified Milligan's Trichrome (TC19). Volume of callus, new bone, and cartilage was estimated by histomorphometry 19. For each sample the first section was chosen by unbiased random sampling, and histomorphometry was performed on every 10th slide throughout the entire callus. Approximately 8-12 slides were analyzed for each sample, but this varied due to differences in callus size. A two-tailed t-test was used for statistical comparison.
Results
Up-regulation of IL-1β after tibial fractures
As a first step in evaluating the role of IL-1β in fracture healing we examined its expression profile using qPCR (Fig. 1). We observed that expression of IL-1β was upregulated in both stabilized and non-stabilized fractures at day 2 after fracture compared to non-fractured limbs. At day 7, IL1-β remained high in both types of fractures, but to a lesser extent compared to day 2. Compared to non-stabilized fractures, stabilized fractures appeared to have higher levels of IL-1β at the fracture site.
Figure 1. Expression of IL-1β in fractured legs after injury.
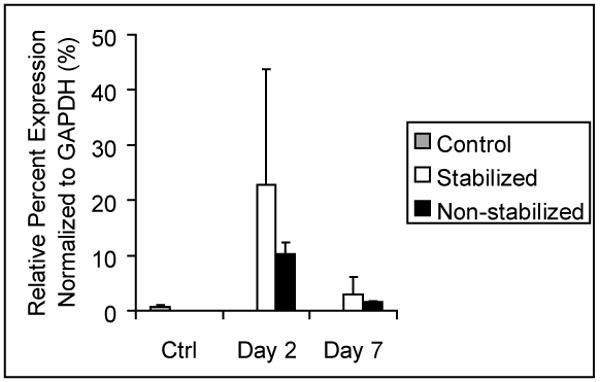
Gene expression was determined with qPCR. The level of IL-1β is presented as the relative percent expression normalized to GAPDH. Data are mean +/- standard deviation.
Effect of IL-1β on proliferation and differentiation of pre-osteoblasts and MSCs
We next assessed the effect of this molecule on proliferation of preosteoblasts and MSCs in vitro, because we observed upregulation during the early stages of cell differentiation and in stable fractures, which heal primarily by intramembranous ossification. After 30 hours, the proliferation rate of pre-osteoblasts exposed to IL-1β was significantly higher than in the absence IL-1β (Fig. 2A-C). A similar approach was used to assess the effect of IL-1β on MSCs, but the results were different. IL-1β significantly reduced proliferation of MSCs at 48 hours compared to controls (Fig. 2E-G).
Figure 2. Effect of IL-1β on osteoblasts and stem cells in vitro.
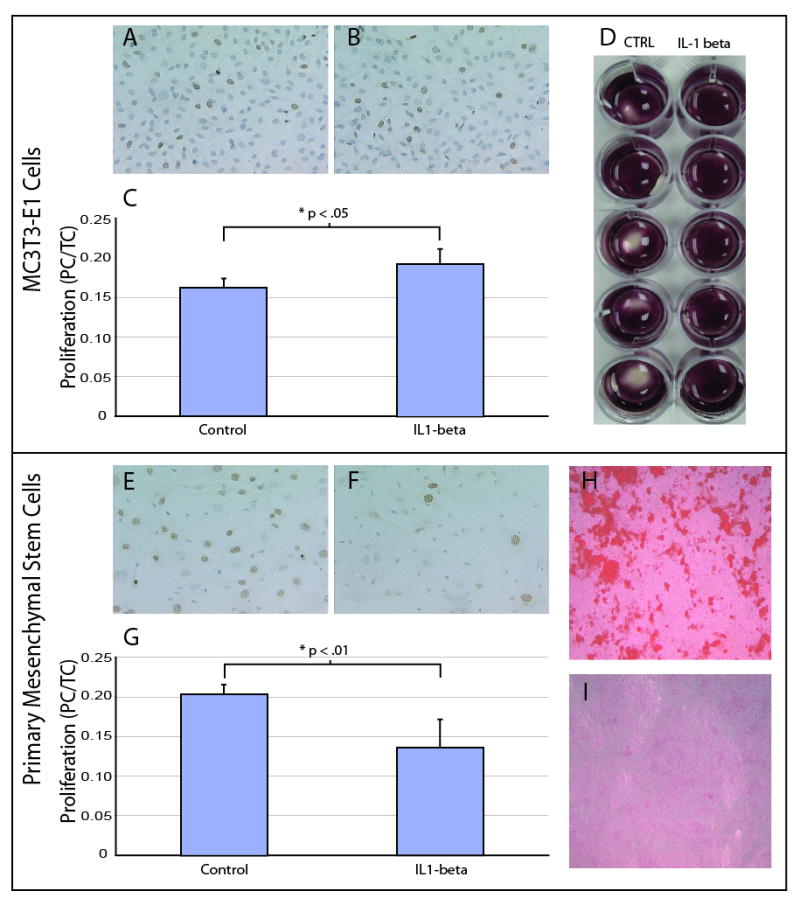
(A,C) Proliferation of control pre-osteoblasts (n=5), and (B,C) pre-osteoblasts cultured with IL-1β (n=5, PC/TC=proliferating cells to total cells). Proliferating cells are stained with BrdU staining. (D) Differentiation of preosteoblasts in the presence (n=5) and absence (n=5) of IL-1β. Cells are stained with Alizarin red. (E,G) Proliferation of control MSCs (n=4) and (F,G) MSCs cultured with IL-1 β (n=5). Proliferating cells are stained with BrdU staining. (H) Differentiation of control MSCs (n=5), and (I) MSCs in the presence of IL-1 β (n=5). Cells are stained with Alizarin red. Data are mean +/-standard deviation.
Finally, we assessed the ability of the cells to differentiate and produce bone matrix in the presence of IL-1β. Both cell types were cultured in the presence or absence of IL-1β for 13 days. After this time the cultures were stained with Alizarin red to visualize the production of mineralized bone matrix as an indicator of differentiation. The pre-osteoblasts cultured in the presence of IL-1β produced matrix that covered the entire surface of each culture dish, while in the absence of IL-1β pre-osteoblasts produced mineralized matrix that did not cover the entire surface of each well (Fig. 2D). In contrast, MSCs that were cultured in osteogenic conditions in the absence of IL-1β for 21 days exhibited robust mineralization (Fig. 2H), but in the presence of IL-1β MSCs exhibited sparse mineralization (Fig. 2I). Thus, IL-1β stimulates proliferation and differentiation of pre-osteoblasts, but IL-1β has inhibitory effects on MSCs.
Effect of IL-1β signaling on fracture repair: Loss-of-Function
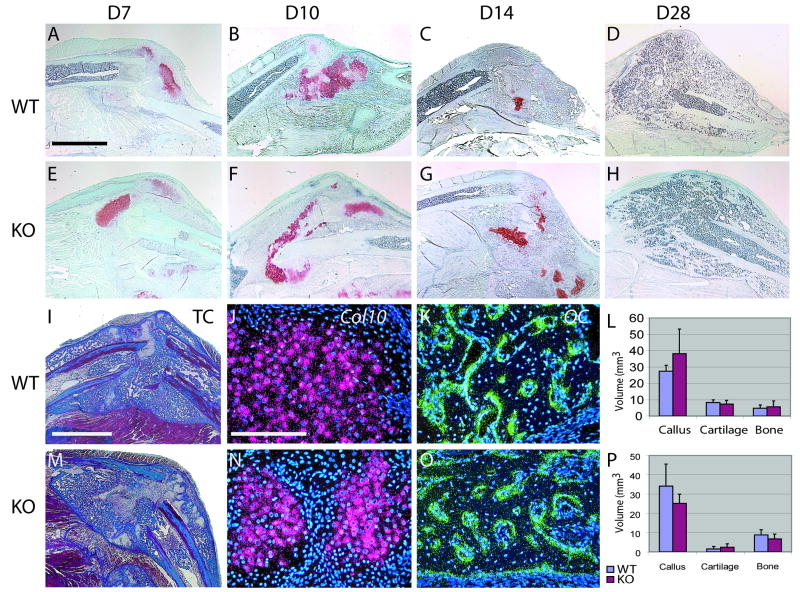
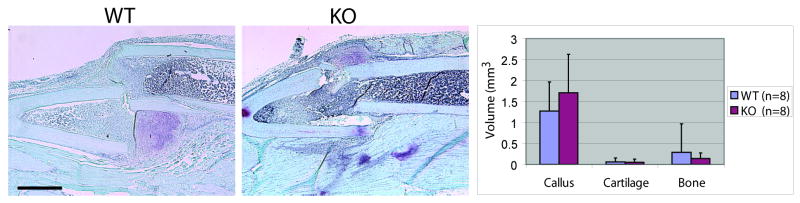
A number of studies indicate that IL-1β is an essential component of the inflammatory cascade that stimulates the repair process. Therefore, we examined fracture healing in mice that lack the IL-1β receptor (Ilr1-/-) non-stable and stable fracture models. At days 7, 10, 14, and 28 we observed normal progression of endochondral ossification (Fig. 3A-H). At day 14 we did not detect differences between Col10 (Fig. 3 J,N) or osteocalcin (Fig 3K,) between mutant and wild type mice. Further, we did not detect differences in callus size, cartilage volume, and bone volume at days 10 (Fig. 3L) and 14 (Fig. 3P). Although original expression data demonstrated a greater up-regulation of IL-1β in stabilized compared to non-stabilized fractures, we were unable to detect differences in callus volume, bone volume, or cartilage volume between the mutant and wild type animals during healing of stabilized fractures (Fig. 4).
Figure 3. Endochondral ossification in the absence of IL-1β signaling in a non-stabilized fracture model.
(A) Sections stained with safranin-O and fast green illustrate that endochondral ossification in wild type animals at day 7, (B) day 10, (C) day 14, and (D) day 28 appears similar to (E) mutants at day 7, (F) day 10, (G) day 14, and (H) day 28. (I) Modified Milligans Trichrome stain of section derived from a wild type mouse at day 14. (J) At this time Col10 and (K) osteocalcin (OC) are robust in the fracture callus. (L) At day 10, no differences in bone, cartilage and callus volume were detected between wild type (n=4) and knock-out (n=4). (M) Modified Milligan's Trichrome stain of section derived from a mutant mouse at day 14. (N) At this time Col10 and (O) osteocalcin (OC) appeared similar to wild type animals. (P) Similarly, wild type (n=6) and mutants (n=6) did not exhibit significant differences at day 14. Scale bar: A-H=2mm, I,M=2mm, J,K,N,O=200μm. Data are mean +/- standard deviation.
Figure 4. Fracture healing in stabilized fractures in mutants at day 10.
(A) Safranin-O staining of stable fractures in wild type (n=4) and (B) mutant (n=4) animals. (C) Callus, cartilage, and bone measurements at day 10. Scale=1mm. Data are mean +/- standard deviation.
Effect of IL-1β signaling on fracture repair: Gain-of-Function
The inflammatory milieu at the fracture site is complex, and the absence of a single factor may not be sufficient to alter the progression of fracture healing. Therefore, we performed a gain-of-function experiment. One group of mice received a single injection of IL-1β into the fracture site at 24 hours after injury, and a second group of mice received injections at 24, 48, and 72 hours after fracture.
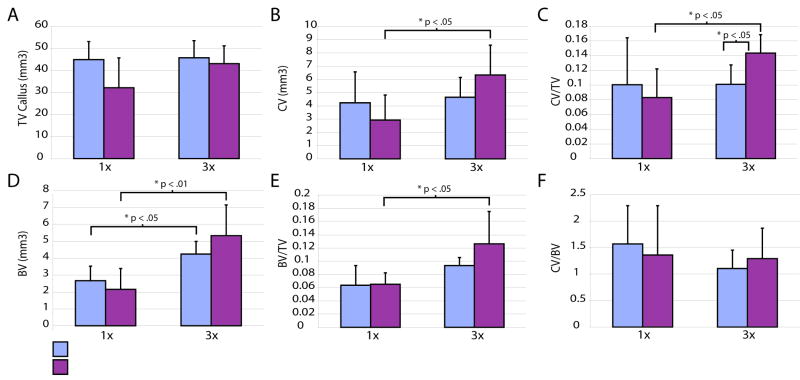
In animals receiving a single dose of IL-1β we saw no difference in callus size (Fig. 5A), cartilage volume (Fig. 5B), and bone volume (Fig. 5D), as well as the proportion of bone and cartilage in the callus (Fig. 5C, E, F) at day 10. In contrast, at this time we observed a significant increase in the ratio of cartilage volume to callus volume in animals receiving 3 daily injections of IL-1β compared to control animals receiving daily injections of PBS alone (Fig. 5C), but callus volume (Fig. 5A), total cartilage volume (Fig. 5B), total bone volume (Fig. 5D), bone to callus ratio (Fig. 5E), and cartilage to bone ratio (Fig. 5F) were not significantly affected. We also noted that some of these parameters were increased in all of the animals, treated and control, receiving three injections (Fig. 5B, C, D, E) suggesting that the additional handling may have stimulated tissue accretion.
Figure 5. The effect of increased of IL1-beta signaling on fracture at day 10.
(A) Total volume of the fracture callus in control animals compared to animals treated with one dose (1×) or three doses (3×) of IL-1β. (B) Total cartilage in control and treated animals. (C) Ratio of cartilage volume to callus volume. (D) Volume of bone in each group. (E) Ratio of bone to callus volume. (F) Ratio of cartilage volume to bone volume. 1×: IL1-beta (n=6), control (n=5); 3×: IL1-beta (n=5), control (n=5). Data are mean +/-standard deviation.
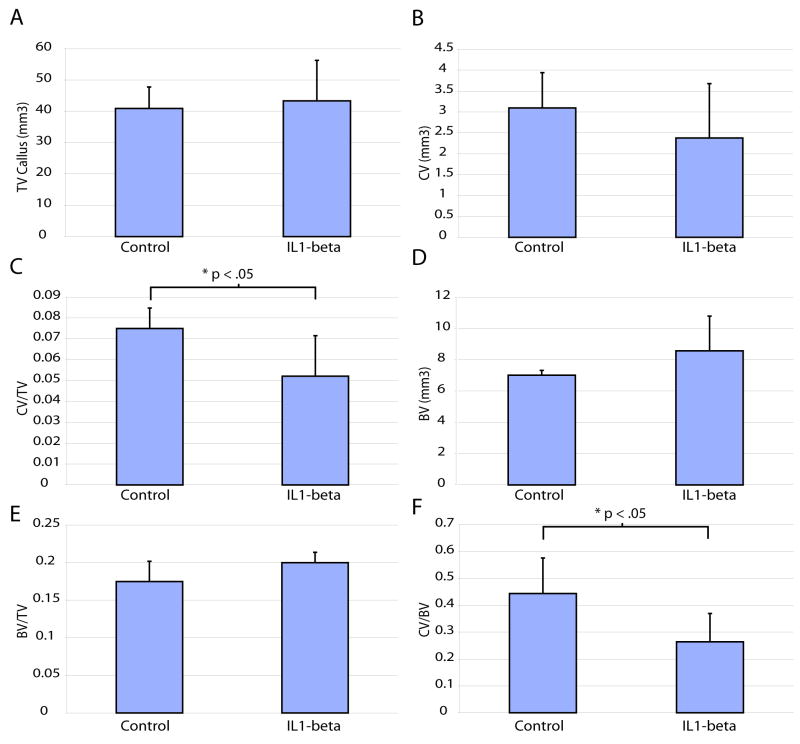
Since we only observed an effect after giving repeated doses of IL-1β we examined healing at day 14 in animals that received 3 doses of IL-1β. At this time point, total volume of callus (Fig. 6A), cartilage (Fig. 6B), bone (Fig. 6D), and the ratio of bone to total callus size (Fig. 6E) in those treated animals were not significantly different from controls that received daily PBS injection. Interestingly, the ratio of cartilage volume to callus volume (Fig. 6C) and the ratio of cartilage volume to bone volume (Fig. 6F) in animals that received 3 doses of IL-1β were significantly decreased from that in controls. These findings that repeated IL-1β treatments increased the ratio of cartilage volume to callus volume at day 10 (Fig. 5C) and decreased this ratio at day 14 (Fig. 6C) suggest that IL-1β may have subtle influence on fracture healing.
Figure 6. The effect of increased of IL1-beta signaling on fracture at day 14.
(A) Histomorphometry to estimate total callus volume, (B) cartilage volume, (C) the ratio of cartilage to total callus volume, (D) bone volume, (E) the ratio of bone to callus size, and (F) the ratio of cartilage to bone in control (n=5) and treated animals (n=5). Data are mean +/- standard deviation.
Discussion
In this work we examined the effect of IL-1β on skeletal repair. Our data revealed that IL-1β is upregulated in response to fracture. We next performed studies in vitro to assess the effect of IL-1β on various cell types. Our data demonstrate that IL-1β inhibits proliferation and mineralizing potential of murine bone marrow stem cells, but promotes proliferation and mineralizing potential of preosteoblasts. Interestingly, the mitogenic and inhibitory effects of IL-1β on murine precursor cells in vitro were not apparent in our in vivo experiments. Despite the dramatic upregulation of IL-1β in the fracture environment of wild-type animals, the absence of this signaling pathway in Ilr1-/- mice has no apparent effect on the skeletogenic precursor cells at the fracture site. This observation supports the notion that the effect of IL-1β may be dependent on the wound environment. For example, IL-1β facilitates healing of oral wounds, but can be inconsequential and sometimes detrimental during dermal wound healing 24-26. Further, IL-1β may share functional redundancy with other cytokine signaling pathways. TNF-α might be a strong candidate for providing such a redundant signal since it plays an important role in both intramembranous and endochondral fracture repair 3, 4. Furthermore, TNF-α and IL-1β both converge on NFκB, a key transcription factor that promotes upregulation of COX2, which is essential for differentiation of osteoblasts in vivo 27-29.
Although IL-1β is not necessary for the fracture repair in vivo, we hypothesized that overexpression of IL-1β may alter healing. IL-1β promoted osteoblastogenesis in a bone defect model in rats 17, and differentiation of osteoblasts in our studies in vitro. The introduction of IL-1β into non-stabilized fractures did not appear to significantly diminish callus size or significantly enhance bone formation. These data imply that neither MSC nor osteoblast differentiation at the fracture site was significantly affected by exogenous IL-1β. Since the non-stabilized fracture environment is chondrogenic, the osteogenic potential of IL-1β that we observed in vitro may be overridden by factors inherent in the non-stabilized fracture environment that promote chondrogenesis. For example, compressive forces on mouse embryonic mesenchyme inhibit IL-1β expression and promote Sox9 and aggrecan expression 30. The mechanical stresses on the non-stabilized fracture environment may counteract IL-1β activity, but this needs to be followed-up in future research.
In summary, we have demonstrated that IL-1β inhibits the proliferation and differentiation of primary murine MSCs but promotes the proliferation and differentiation of murine pre-osteoblasts in vitro. In the fracture study, repeated IL-1β treatments exhibited subtle effect on the percentage of cartilage in callus without altering the absolute volume of cartilage. Despite these findings, we were unable to demonstrate any significant effect of IL-1β on fracture healing in a murine tibial fracture model in both loss- and gain-of-function experiments. TNF-α may confer a compensatory mechanism for the loss of IL-1β signaling and compressive forces may play a role in inhibiting IL-1β signaling in vivo. In all, this study illustrates the multifactorial nature of fracture repair and underscores the importance of both in vitro and in vivo studies to developing a complete understanding of the mechanisms underlying tissue regeneration.
Acknowledgments
This work was supported by grants from the NIH (R01-AR053645 to T.M). We are grateful for the support of all of our colleagues at the Orthopaedic Trauma Institute at UCSF especially the members of the Laboratory for Skeletal Regeneration.
References
- 1.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–65. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 2.Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30:1075–9. doi: 10.1016/s1357-2725(98)00081-8. [DOI] [PubMed] [Google Scholar]
- 3.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–94. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–92. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 5.Kuroki T, Shingu M, Koshihara Y, Nobunaga M. Effects of cytokines on alkaline phosphatase and osteocalcin production, calcification and calcium release by human osteoblastic cells. Br J Rheumatol. 1994;33:224–30. doi: 10.1093/rheumatology/33.3.224. [DOI] [PubMed] [Google Scholar]
- 6.Nakase T, Takaoka K, Masuhara K, et al. Interleukin-1 beta enhances and tumor necrosis factor-alpha inhibits bone morphogenetic protein-2-induced alkaline phosphatase activity in MC3T3-E1 osteoblastic cells. Bone. 1997;21:17–21. doi: 10.1016/s8756-3282(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 7.Sharrow AC, Li Y, Micsenyi A, et al. Modulation of osteoblast gap junction connectivity by serum, TNFalpha, and TRAIL. Exp Cell Res. 2008;314:297–308. doi: 10.1016/j.yexcr.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taichman RS, Hauschka PV. Effects of interleukin-1 beta and tumor necrosis factor-alpha on osteoblastic expression of osteocalcin and mineralized extracellular matrix in vitro. Inflammation. 1992;16:587–601. doi: 10.1007/BF00919342. [DOI] [PubMed] [Google Scholar]
- 9.Andreas K, Lubke C, Haupl T, et al. Key regulatory molecules of cartilage destruction in rheumatoid arthritis: an in vitro study. Arthritis Res Ther. 2008;10:R9. doi: 10.1186/ar2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Backesjo CM, Haldosen LA, Lindgren U. IL-6 receptor expression and IL-6 effects change during osteoblast differentiation. Cytokine. 2008;43:165–73. doi: 10.1016/j.cyto.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Ricciardi BF, Hernandez-Soria A, et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–36. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Einhorn TA, Majeska RJ, Rush EB, et al. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–81. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 13.Kon T, Cho TJ, Aizawa T, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–14. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 14.Gowen M, Wood DD, Russell RG. Stimulation of the proliferation of human bone cells in vitro by human monocyte products with interleukin-1 activity. J Clin Invest. 1985;75:1223–9. doi: 10.1172/JCI111819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem. 2000;275:3687–92. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- 16.Lambert C, Oury C, Dejardin E, et al. Further insights in the mechanisms of interleukin-1beta stimulation of osteoprotegerin in osteoblast-like cells. J Bone Miner Res. 2007;22:1350–61. doi: 10.1359/jbmr.070508. [DOI] [PubMed] [Google Scholar]
- 17.Olmedo ML, Landry PS, Sadasivan KK, et al. Regulation of osteoblast levels during bone healing. J Orthop Trauma. 1999;13:356–62. doi: 10.1097/00005131-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Miclau T, Lu C, Thompson Z, et al. Effects of delayed stabilization on fracture healing. J Orthop Res. 2007;25:1552–8. doi: 10.1002/jor.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C, Miclau T, Hu D, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 21.Lu C, Xing Z, Yu Y, et al. Recombinant Human Bone Morphogenetic Protein-7 Enhances Fracture Healing in an Ischemic Environment. J Orthop Res. 2009 doi: 10.1002/jor.21033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manni L, Aloe L. Role of IL-1 beta and TNF-alpha in the regulation of NGF in experimentally induced arthritis in mice. Rheumatol Int. 1998;18:97–102. doi: 10.1007/s002960050065. [DOI] [PubMed] [Google Scholar]
- 23.van de Loo AA, van Beuningen HM, van Lent PL, van den Berg WB. Direct effect of murine rIL-1 on cartilage metabolism in vivo. Agents Actions. 1989;26:153–5. doi: 10.1007/BF02126590. [DOI] [PubMed] [Google Scholar]
- 24.Graves DT, Nooh N, Gillen T, et al. IL-1 plays a critical role in oral, but not dermal, wound healing. J Immunol. 2001;167:5316–20. doi: 10.4049/jimmunol.167.9.5316. [DOI] [PubMed] [Google Scholar]
- 25.Ishida Y, Kondo T, Kimura A, et al. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J Immunol. 2006;176:5598–606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Ding I, Chen K, et al. Interleukin 1beta (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat Res. 2006;165:181–91. doi: 10.1667/rr3478.1. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 28.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–61. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Schwarz EM, Young DA, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi I, Nuckolls GH, Takahashi K, et al. Compressive force promotes sox9, type II collagen and aggrecan and inhibits IL-1beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci. 1998;111(Pt 14):2067–76. doi: 10.1242/jcs.111.14.2067. [DOI] [PubMed] [Google Scholar]