Abstract
Background:
The neuropeptide Y (NPY) system of the central nucleus of amygdala (CeA) has been shown to be involved in anxiety and alcoholism. In this study, we investigated the molecular mechanisms by which NPY in the CeA regulates anxiety and alcohol drinking behaviors using alcohol-preferring (P) rats as an animal model.
Methods:
P rats were bilaterally cannulated targeting the CeA and infused with aCSF or NPY. Alcohol drinking and anxiety-like behaviors were assessed by the two-bottle free-choice paradigm and light/dark box (LDB) exploration test, respectively. The levels of NPY and related signaling proteins were determined by the gold immunolabeling procedure. The mRNA levels of NPY were measured by in situ RT-PCR. Double-immunofluorescence labeling was performed to observe the colocalization of NPY and Ca2+/calmodulin dependent protein kinase IV (CaMK IV).
Results:
We found that NPY infusion into the CeA produced anxiolytic effects, as measured by the LDB exploration test, and also decreased alcohol intake in P rats. NPY infusion into the CeA significantly increased protein levels of CaMK IV and phosphorylated CREB (pCREB), and increased mRNA and protein levels of NPY, but produced no changes in protein levels of CREB or the catalytic α-subunit of protein kinase A (PKA-Cα) in the CeA. We also observed that alcohol intake produced anxiolytic effects in P rats in the LDB test and also increased NPY expression and protein levels of pCREB and PKA-Cα without modulating protein levels of CREB or CaMK IV, in both the CeA and medial nucleus of amygdala (MeA). In addition, we found that CaMK IV-positive cells were co-localized with NPY in amygdaloid structures of P rats.
Conclusions:
These results suggest that NPY infusion may increase the expression of endogenous NPY in the CeA, which is most likely attributable to an increase in CaMK IV-dependent CREB phosphorylation and this molecular mechanism may be involved in regulating anxiety and alcohol-drinking behaviors of P rats.
Keywords: Neuropeptide Y, CREB, Amygdala, Anxiety, Alcohol Preference, P rat
Genetic factors play a critical role in the predisposition to alcoholism, leading to a rate of heritability of 50-60% among both male and female populations (Kendler et al., 1992; Prescott and Kendler, 1999; Radel and Goldman, 2001; Enoch, 2003). Inherent anxiety also contributes to the initiation and maintenance of alcohol drinking behavior (Koob, 2003; Novak et al., 2003; Pandey, 2003). Several animal lines have been developed to investigate the genetic basis of alcoholism (McBride and Li, 1998). One such animal line includes alcohol preferring (P) and non-preferring (NP) rats that are selectively bred for high and low alcohol preference respectively (Li et al., 1993; Murphy et al., 2002). In addition to higher alcohol intake, both male and female P rats display more anxiety-like behaviors compared to NP rats (Stewart et al., 1993; Salimov et al., 1996; Pandey et al., 2005). These studies suggest that P rats are suitable animal model to investigate the molecular mechanisms in the brain that may be involved in the co-morbidity of anxiety and alcoholism.
Neuropeptide Y (NPY), a 36-amino acid peptide, is highly expressed in various brain structures including cortex, hippocampus, and amygdala (Allen et al., 1983; Heilig and Widerlov, 1990; Wettstein et al., 1995), and has been implicated in several behaviors such as feeding, anxiety, and alcohol drinking (Clark et al., 1984; Heilig et al., 1993; Heilig and Widerlov, 1995; Thorsell and Heilig, 2002; Pandey, 2003; Pandey et al., 2003a). It has been shown that NPY mutant mice had a higher alcohol preference, whereas mice over-expressing NPY had a lower alcohol preference (Thiele et al., 1998). In addition, NPY mutant mice were more susceptible to the development of anxiety-like behaviors during ethanol withdrawal (Sparta et al., 2007). Interestingly, it has also been shown that the protein and mRNA levels of NPY were lower in several brain structures, including the amygdala, of P rats compared to NP rats and that intra-brain infusion of NPY attenuated alcohol intake of P rats (Hwang et al., 1999; Badia-Elder et al., 2001; 2003; Suzuki et al., 2004; Pandey et al., 2005). Additionally, intra-amygdalar overexpression of NPY was shown to suppress alcohol intake in rats with high but not low anxiety-like and alcohol drinking behaviors (Primeaux et al., 2006).
NPY is one of the cAMP responsive element-binding (CREB) protein target genes (Higuchi et al., 1988; Akabayashi et al., 1994; Pandey et al., 2004). The function of CREB protein is regulated by phosphorylation at serine-133 by several protein kinases such as cAMP dependent protein kinase A (PKA), Ca2+/calmodulin dependent protein kinases II and IV (CaMK II & IV), and mitogen activated protein (MAP) kinases (Impey et al., 1999; Soderling, 1999; Lonze and Ginty, 2002; Pandey, 2004). Previous studies have shown that NPY infusion into the hypothalamus increased CREB phosphorylation in an unselected stock of rats and activated CaM kinases in cultured cells (Sheriff et al., 1997; 1998; 2002). Although inconclusive, these results suggest the possibility that exogenous NPY may increase CREB phosphorylation by a CaM kinase-dependent mechanism. We have previously reported that protein levels of CREB, phosphorylated CREB (pCREB), and mRNA and protein levels of NPY were lower in the central nucleus of amygdala (CeA) and medial nucleus of amygdala (MeA), but not in the basolateral amygdala (BLA) of P rats compared with NP rats. We also observed that NPY infused directly into the CeA attenuated anxiety-like behaviors as measured by the elevated plus maze (EPM) test and alcohol intake of P rats (Pandey et al., 2005). Taken together, these results indicate that a deficiency in both CREB and NPY in the CeA may be involved in anxiety-like and alcohol drinking behaviors. However, the molecular mechanism by which NPY in the CeA may ameliorate anxiety and alcohol drinking behaviors in P rats is currently unknown. Therefore, in the present investigation, we examined if a) NPY infusion into the CeA can attenuate both anxiety-like behaviors, as measured by the light/dark box (LDB) exploration test, and alcohol intake; and b) if NPY infusion can increase pCREB levels by increasing levels of either CaMK IV or the catalytic α-subunit of PKA (PKA-Cα), thereby increasing the expression of endogenous NPY in the CeA of P rats.
MATERIALS AND METHODS
Alcohol-Preferring Rats and Implantation of Cannulas
All experiments were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Alcohol-naïve adult male P rats were received from Indiana University (Alcohol Research Center) and were housed in a temperature and light-controlled environment. P rats were anesthetized using sodium pentobarbital [50 mg/kg, intraperitoneal(I.P.)], placed in a stereotaxic apparatus, and implanted bilaterally with CMA/11 guide cannulas (CMA Microdialysis, North Chelmsford, MA, USA) targeted 3 mm above the CeA as reported previously (Pandey et al., 2003b; 2005; 2006). The coordinates for the CeA were: 2.5 mm posterior and ± 4.2 mm lateral to the bregma, and 5.1 mm ventral from the point of entry at the skull surface. After a recovery period of one week, P rats were used to measure alcohol preference as described below.
Alcohol Intake in P Rats with and without NPY Manipulations
Central amygdaloid cannulated P rats were placed in individual cages and alcohol preference was measured using the two-bottle free-choice paradigm (Pandey et al., 2003b; 2005; 2006). Rats were habituated to drink water equally from two bottles over a period of approximately two weeks. One group of P rats was given ethanol in one bottle and water in the other bottle and referred to as Water/Ethanol (WE) group. The second group of P rats was given water in both bottles and referred to as Water/Water (WW) group. The WE group received 7% ethanol for 3 days followed by 9% ethanol for 6 days and these rats were bilaterally infused into the CeA once (within 2 minutes) daily (between 5:00-6:00 PM) with either 0.5 μl of aCSF (n=7) or 0.5 μl of 100 pM NPY (n=7) using microdialysis probes (CMA/11 14/03, CMA microdialysis AB) attached to an automatic infusion pump during the last 3 consecutive days of 9% ethanol intake. Studies from our lab and other labs have used the microdialysis probes for NPY infusion into different parts of the brain (Hastings et al., 1997; Pandey et al., 2003b; Meurs et al., 2007). The WW group was also bilaterally infused (between 5:00-6:00 PM) with 0.5 μl of aCSF (n=7) or 0.5 μl of 100 pM NPY (n=7) during the last 3 consecutive days of water intake. The microdialysis probes extended 3 mm beyond the guide cannulas into the CeA. The consumption of ethanol (ml) and water (ml) was measured daily (between 5:00- 6:00 pm) and fresh water and ethanol solutions were also provided at this time. The ethanol intake was calculated as g/kg/day. The dose of NPY was based on our previous studies showing that 0.5 μl of 100 pM NPY infused into CeA was able to suppress the alcohol intake in both P rats and an unselected stock of rats (Misra and Pandey, 2006; Pandey et al., 2003b; 2005). After 3 days of 7% ethanol intake and 6 days of 9% ethanol intake, P rats were used for anxiety measurements as described below.
Measurement of Anxiety-Like Behaviors by Light/Dark Box Exploration Test
The test procedure was the same as described previously by us and other investigators (Jonkman et al., 2005; Pandey et al., 2008; Slawecki, 2005). The light/dark box (LDB) was located in a dark room and consisted of both an unlit dark compartment and an illuminated light compartment. Both compartments were connected through an opening. Prior to testing, P rats were located in the room adjacent to the LDB behavioral testing room in order to avoid disturbing the animal during the transport between rooms. On the day of testing, P rats were removed from their home cages and individually taken to the testing room in a cage containing bedding. The cage was placed on the floor and each P rat was allowed a 5-min pretest habituation period in the room prior to testing. After the habituation period, the test P rat was placed gently in the dark box with its head facing away from the opening. The exploratory activity of rats was monitored by computer over a 5-minute test period. After testing each rat, the compartments were wiped with a wet paper towel and then dried. The light/ dark compartments were equipped with an infrared beam and rat movement was measured through beam breaks using a computer. The data were analyzed to determine the time (in seconds) spent in either the light or dark compartment by each rat. The general activity of rats was analyzed in terms of the total ambulations in each of the light and dark compartments.
Gold-Immunolabeling Procedure for CREB, p-CREB, CaMK IV, PKA-Cα and NPY
After behavioral testing, P rats were anesthetized (pentobarbitol 50 mg/kg, I.P.) and perfused intracardially with 200 ml of n-saline, followed by 400 ml of 4% ice-cold paraformaldehyde (PFA) fixative prepared in 0.1 M phosphate buffer (pH 7.4). Before perfusion of the animals, blood was collected to measure the alcohol levels using an Analox Alcohol Analyzer (Lunenburg, MA). After perfusion, brains were dissected out and post-fixed overnight in PFA at 4 °C. After post-fixation, brains were cryoprotected using a sucrose gradient (10%, 20% and 30%) prepared in 0.1 M phosphate buffer (pH 7.4). Brains were then frozen and 20 μm coronal sections were collected using a cryostat. Brain sections were used for gold-immunolabeling as described previously by us (Pandey et al., 2001; Roy and Pandey, 2002; Pandey et al., 2008) using antibodies against CREB, pCREB (Millipore, Billerica, MA), CaMK IV (BD Biosciences, San Jose, CA), PKA-Cα (Santa Cruz Biotechnology, Santa Cruz, CA), or NPY (Immunostar Inc., Hudson, WI). In brief, sections were washed with PBS (2 × 10 min) and then incubated with RPMI 1640 medium containing L-Glutamine (Life Technologies, Grand Island, NY) for 30 min, followed by incubation with 10% normal goat serum (NGS) diluted in PBS containing 0.25% Triton X-100 (PBST) for 30 min at room temperature. Then, sections were blocked with 1% BSA (prepared in PBST) for 30 min at room temperature. Sections were further incubated with antibodies against CREB (1:500), p-CREB (1:500), CaMK IV (1:200), PKA-Cα (1:200); or NPY (1:500) diluted in 1% BSA prepared in PBST for 18 h at room temperature. Following 2 × 10-min washes with PBS and 2 × 10-min washes with 1% BSA in PBS, sections were further incubated for 1h at room temperature with either gold particle (1.4 nm)-conjugated (Nanoprobes, Inc., Yaphank, NY) anti-rabbit secondary antibody for CREB, pCREB, PKA-Cα and NPY labeling or gold particle-conjugated anti-mouse secondary antibody for CaMK IV labeling (1:200 dilution in 1% BSA in PBS). Sections were then rinsed three times each in 1% BSA in PBS followed by three washes with double distilled water. The gold particles were then silver enhanced (Ted Pella Inc., Redding, CA) for 12 to 20 min and washed several times with tap water. For the negative control sections an identical protocol was used, except that the primary antibodies were substituted instead with 1% BSA in PBST. After washing with tap water, the sections were mounted on slides and examined under a light microscope. Sections from each brain were also processed to check for both the position of the cannulas and toxicity using Nissl staining.
Measurement of Immunogold Particles for CREB, p-CREB, CaMK IV, PKA-Cα and NPY
The gold-immunolabeled particles of CREB, p-CREB, CaMK IV, PKA-Cα, and NPY in the CeA, MeA, and BLA were counted using the grain counting program of the Image Analysis System (Loats Associates, Westminster, MD) connected to a light microscope. The threshold of each image was set up in such a way that areas without staining gave zero counts. Under this condition, gold particles in the defined areas of three adjacent brain sections for a total of nine fields in each rat were counted. Then values were averaged for each rat and presented as the number of immunogold particles/100 μm2 area of defined brain structures.
In situ RT-PCR for NPY mRNA Measurement
NPY mRNA levels were determined in brain sections using in situ reverse transcription (RT)-PCR as described by us previously (Pandey et al., 2003b; 2005; 2008). Briefly, brain sections (40 μm) were treated with proteinase K and then DNase digested. Sections were rinsed in PBS and transferred to PCR tubes containing 100 μl of PCR reaction mixture (Applied Biosystems, Foster City, CA) to reverse transcribe for 1 h at 42 °C using reverse transcriptase enzyme in the presence of oligo d(T)16. Negative control sections were subjected to RT without reverse transcriptase enzyme. PCR was performed (94°C for 3 min; 94°C for 45 sec; 60°C for 45 sec; 72°C for 45 sec; for a total of 25 cycles and then 72°C for 7 min) using Taq DNA polymerase enzyme and 100 pmol of each NPY primer (Primers 5′-TAGGTAACAAACGAATGGGG-3′ and 5′-AGGATGAGATGAGATGTGGG-3′) and 1 mM of each NTP except that dTTP was replaced by digoxigenin (DIG)-11-dUTP in 100 μl reaction mixture. After PCR amplification, sections were mounted on slides and NPY mRNA-positive cell bodies were detected by an alkaline phosphatase conjugated anti-DIG antibody using NBT/BCIP as the specific substrate (Roche Molecular Biochemical, Mannheim, Germany). The optical density (O.D.) of NPY-positive cell bodies in the CeA, MeA, and BLA of each rat was calculated using the Image Analysis System (Loats Associates, Westminster, MD) after substracting the O.D. of the negative brain sections (amygdaloid areas). The results are represented as the mean O.D. /100 pixels area.
Double Immunofluorescence Staining of NPY and CaMK IV
The brains collected from the P rats (n = 3) were used for double immunofluorescence labeling of NPY and CaMK IV in amygdaloid structures. The procedures for the anesthesia, perfusion, and fixation of the brains for immunofluorescence microscopy are the same as described above. Transverse sections of the frozen brains were cut at 20 μm in a cryostat and collected in PBS (0.01 M, pH 7.4). The free-floating sections were then washed with PBS for 30 min (10 min × 3), blocked with 10 % NGS diluted in PBS containing 0.25% Triton × 100 (PBST) for 30 min and further blocked with 1% BSA prepared in PBST for 30 min. The sections were then incubated in primary antibodies against NPY (1:500 dilution) and CaMK IV (1:200 dilution) diluted in PBST containing 2% NGS, at 4 °C overnight. For the negative control, some sections were incubated in 2% NGS diluted in PBST. Brain sections were washed with PBS followed by incubation with AlexaFluor-488 dye-conjugated goat anti-rabbit and AlexaFluor-568 dye-conjugated goat anti-mouse (Invitrogen, Molecular Probes, Inc. Eugene, OR) secondary antibodies (1:500 dilution with PBST containing 2%NGS) to localize the NPY and CaMK IV respectively, for 2 h at room temperature. Sections were washed with PBS for 30 min (10 min × 3), mounted on the glass slides using mounting medium and images were taken using a confocal microscope (LSM 510, Carl Zeiss Inc., Thornwood, NY).
Statistics
The neurochemical and LDB test differences between various groups were analyzed by ANOVA and alcohol intake data were analyzed by repeated measures of two way ANOVA. The post hoc comparisons between the groups were performed using the Tukey test.
RESULTS
Effect of Central Amygdaloid Infusion of NPY on Anxiety-like Behavior and Alcohol Preference in P Rats
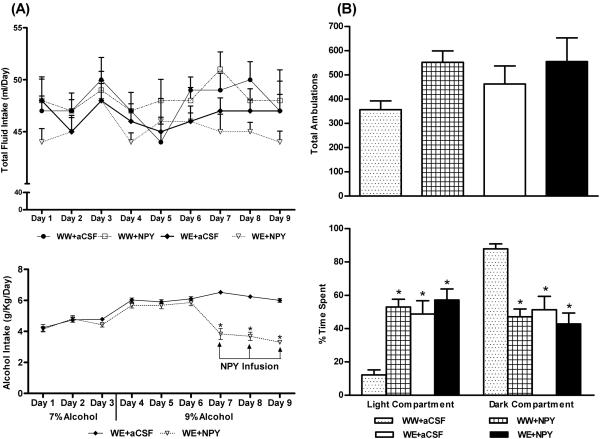
Anxiety-like behavior in P rats was measured by the LDB exploration test. We infused 0.5 μl of aCSF or 0.5 μl of 100 pM NPY into the CeA and examined the effects on alcohol intake, anxiety levels, and NPY signaling in various amygdaloid structures. The cannula positions and targeted areas of CeA of various groups of P rats are shown in representative photomicrographs of Figure 1. A 2 × 9 (group × day) repeated-measure ANOVA was performed on changes in alcohol intake that yielded a significant group effect (F (1,108) = 143.81; p<0.001), a significant day effect (F (8,108) = 25.09; p<0.001) and a significant interaction (F (8,108) = 23.51; p<0.001) of these variables in P rats. Post hoc comparisons revealed that infusion of NPY into the CeA significantly (p<0.001) decreased the 9 % alcohol intake of P rats during the last 3 days of alcohol drinking (Fig. 2A). The total fluid intake (ml/day) ranged from 44-51 ml/day with no significant differences between groups at each day of drinking (Fig. 2A). The mean body weights [mean ± S.E.M. (g); WW group + aCSF = 427± 11.2; WW group + NPY = 433 ± 7.7; WE + aCSF = 440 ± 0.5; WE + NPY= 446 ± 3.6] of the P rats at the end of the experiments showed no significant differences among groups.
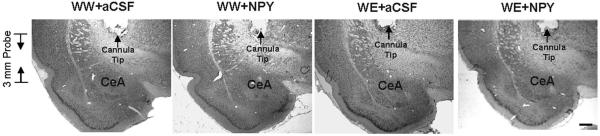
Figure 1.
Representative photomicrographs of low-magnification views of Nissl stained brain sections of P rats (with or without alcohol drinking) infused with aCSF or NPY showing the position of cannula tip, length of probe, and central amygdaloid target regions (WW+aCSF, Water/Water + aCSF; WW+NPY, Water/Water + NPY; WE+aCSF, Water/Ethanol + aCSF; WE+NPY, Water/Ethanol + NPY). Scale Bar = 500 μm.
Figure 2.
A) Alcohol drinking (g/kg/day) patterns (7% and 9% ethanol) and effect of NPY infusion into the CeA on alcohol intake (last 3 days of 9% ethanol intake) in P rats (lower panel). No significant differences are observed in the total fluid intake (ml/day) of P rats from different groups (upper panel). Values are mean ± S.E.M. of 7 rats in each group. *Significantly different from P rats infused with aCSF (p < 0.001; repeated measures using a two-way ANOVA followed by the Tukey test). B) The effect of NPY infusion (0.5 μl of 100 pM NPY or aCSF once daily for the last 3 days of 9% ethanol voluntary intake) and alcohol consumption on the total ambulations (upper panel) and percent time spent by the P rats in each of the light and dark compartments (lower panel), during the light/dark box exploration test. Values are the mean ± S.E.M. of 7 rats per group (WW+aCSF, Water/Water + aCSF; WW+NPY, Water/Water + NPY; WE+aCSF, Water/Ethanol + aCSF; WE+NPY, Water/Ethanol + NPY). *Significantly different from the P (WW+ aCSF) rats (p < 0.001; one-way ANOVA followed by the Tukey test).
We found that NPY infusion and ethanol intake significantly increased (p<0.001) the time spent in the light compartment and decreased (p<0.001) the time spent in the dark compartment by P rats during the LDB exploration test (Fig. 2B). The general activity of rats analyzed in terms of total ambulations in the compartments did not reveal significant changes (Fig. 2B). Furthermore, NPY-infused P rats consumed less alcohol (Fig. 2A) and spent more time in the light compartment during the LDB test (Fig. 2B). Immediately after anxiety measurements, the blood ethanol levels (mean ± S.E.M.) in ethanol drinking P rats, infused with aCSF (n = 6) or NPY (n = 7), were 182 ± 15 and 59 ± 9.8 mg%, respectively. Blood ethanol data also indicated that NPY-infused P rats consumed less alcohol compared to aCSF-infused P rats. Taken together, these results suggest that NPY infusion into the CeA of P rats may decrease both alcohol intake and anxiety-like behaviors.
Effect of Central Amygdaloid Infusion of NPY on CREB Phosphorylation in P Rats
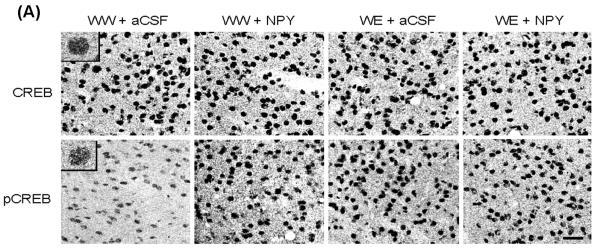
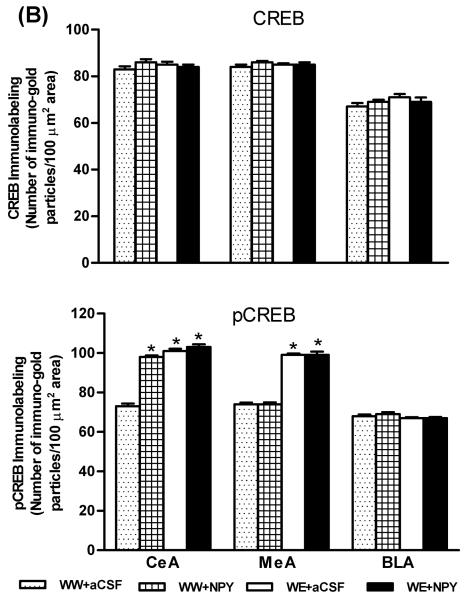
Brains were collected to examine the protein levels of CREB and pCREB in amygdaloid structures of P rats, with or without alcohol drinking, and infused with NPY or aCSF. The nuclei containing either CREB (upper panel) or pCREB (lower panel) in the CeA of the P rat can be seen in Figure 3A. We found that NPY infusion into the CeA increased the protein levels of pCREB (p<0.001), but did not have any effects on CREB levels in the CeA of P rats (Fig. 3A,B). Central amygdaloid infusion of NPY had no effects on the protein levels of CREB or p-CREB in either the MeA or BLA of P rats. In addition, we found that alcohol consumption significantly increased the protein levels of p-CREB in both the CeA (p<0.001) and MeA (p<0.001) but not in the BLA of P rats. Alcohol exposure did not modify total CREB protein levels in the amygdaloid structures of P rats (Fig. 3A,B). We also found that NPY infusion did not cause any toxicity in the CeA of P rats, as demonstrated by Nissl staining (Fig. 1). These results suggest that NPY infusion into the CeA may increase CREB phosphorylation, specifically in the CeA, and may also attenuate anxiety-like and excessive alcohol drinking behaviors of P rats.
Figure 3.
A) Representative photomicrographs of CREB (upper panel) and p-CREB (lower panel) gold-immunolabeling in CeA structures of P rats infused with aCSF or NPY during voluntary alcohol drinking. These photomicrographs show either CREB- or p-CREB-positive nuclei in the CeA of P rats infused with aCSF or NPY. The inset figures show the immuno-gold particles within a single nucleus (CREB, or p-CREB). Scale bar = 40 μm. B) Effect of NPY infusion into the CeA and voluntary alcohol drinking on CREB or p-CREB protein levels in CeA, MeA, and BLA of P rats. Values are mean ± S.E.M. of 7 rats per group (WW+aCSF, Water/Water + aCSF; WW+NPY, Water/Water + NPY; WE+aCSF, Water/Ethanol + aCSF; WE+NPY, Water/Ethanol + NPY). *Significantly different from the P (WW+ aCSF) rats (p < 0.001; one-way ANOVA followed by the Tukey test).
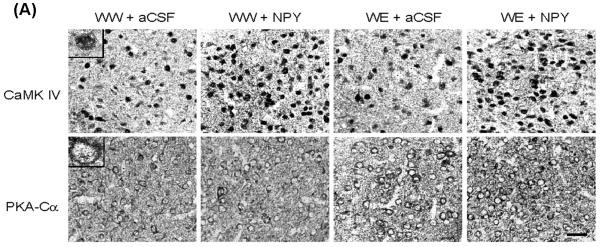
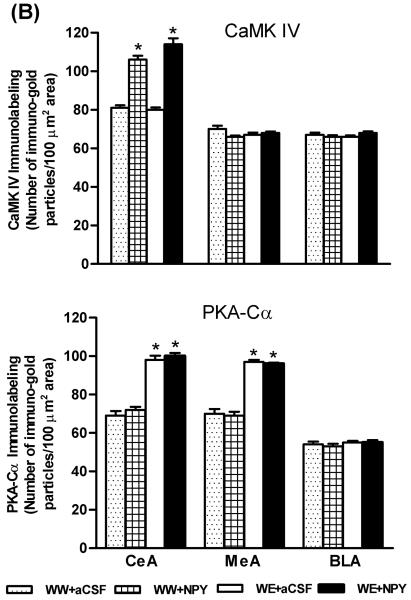
Effects of Central Amygdaloid Infusion of NPY on CaMK IV and PKA-Cα Protein Levels in P Rats
In order to examine whether increased CREB phosphorylation by NPY infusion or by ethanol consumption may be attributable to increased protein levels of CaMK IV or PKA-Cα, we also measured the levels of these protein kinases in amygdaloid structures of P rats. The nuclei containing CaMK IV (upper panel) in the CeA of the P rat can be seen in Figure 4A. The cell bodies and fibrous labeling of PKA-Cα can also be seen in Figure 4A (lower panel). We found that NPY infusion into the CeA increased protein levels of CaMK IV (p<0.001) but had no effect on protein levels of PKA-Cα in the CeA of P rats (Fig. 4A,B). CeA-NPY infusion also had no effect on protein levels of CaMK IV or PKA-Cα in the MeA or BLA of P rats. On the other hand, while alcohol consumption did not change CaMK IV protein levels, it did increase protein levels of PKA-Cα in both the CeA (p<0.001) and MeA (p<0.001), but not in the BLA of P rats (Fig. 4A,B). These results clearly suggest that NPY-induced increases in CREB phosphorylation in the CeA may be related to increases in protein levels of CaMK IV, whereas an alcohol-induced increase in CREB phosphorylation may be related to increases in PKA-Cα levels in the CeA and MeA of P rats.
Figure 4.
A) Representative photomicrographs of CaMK IV (upper panel) or PKA-Cα (lower panel) gold-immunolabeling in CeA structures of P rats infused with aCSF or NPY during voluntary alcohol drinking. The representative photomicrographs show CaMK IV or PKA-Cα-positive cells in the CeA of P rats infused with aCSF or NPY. The inset figures show the immuno-gold particles within a single nucleus for CaMK IV and within a cell body for PKA-Cα. Scale bar = 40 μm. B) Effect of NPY infusion into the CeA and voluntary alcohol drinking on CaMK IV and PKA-Cα protein levels in CeA, MeA, and BLA of P rats. Values are the mean ± S.E.M. of 5-7 rats per group (WW+aCSF, Water/Water + aCSF; WW+NPY, Water/Water + NPY; WE+aCSF, Water/Ethanol + aCSF; WE+NPY, Water/Ethanol + NPY). *Significantly different from the P (WW+ aCSF) rats (p < 0.001; one-way ANOVA followed by the Tukey test).
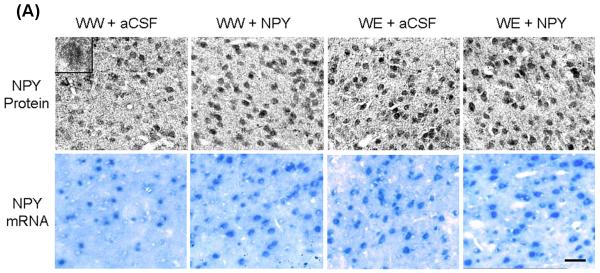
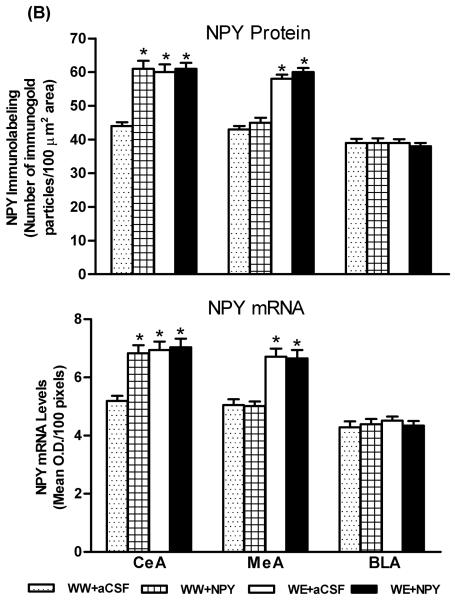
Effects of Central Amygdaloid Infusion of NPY on Protein and mRNA Levels of NPY in P Rats
Since NPY expression is regulated by CREB (Higuchi et al., 1988; Pandey et al., 2004), we also determined the protein and mRNA levels of NPY in amygdaloid structures of P rats, with or without alcohol drinking, and infused with NPY or aCSF. NPY protein or mRNA containing cell bodies in the CeA can be seen in Figure 5A. We found that NPY infusion into the CeA significantly increased (p<0.01-0.001) the mRNA and protein levels of NPY (Fig. 5A,B) in the CeA but not in the MeA or BLA of P rats. On the other hand, alcohol consumption significantly increased NPY expression (mRNA and protein levels) in both the CeA (p<0.001) and MeA (p<0.001), but not in the BLA of P rats (Fig. 5A,B). These results suggest that exogenous NPY infusion may have increased CaMK IV-dependent CREB phosphorylation, thereby increasing NPY expression in the CeA of P rats.
Figure 5.
A) Representative photomicrographs of NPY gold-immunolabeling (upper panel) and NPY mRNA expression (in situ RT-PCR; lower panel) in the CeA of P rats with aCSF or NPY infusion during alcohol drinking. Inset figure for NPY protein shows gold particles in a cell body at high magnification (100x). Scale bar = 40 μm. B) Effect of alcohol consumption with or without central amygdaloid NPY infusion on mRNA and protein levels of NPY in the CeA, MeA and BLA structures of rats. Values are the mean ± S.E.M. derived from 5 P rats per group (WW+aCSF, Water/Water + aCSF; WW+NPY, Water/Water + NPY; WE+aCSF, Water/Ethanol + aCSF; WE+NPY, Water/Ethanol + NPY). *Significantly different from P (WW+ aCSF) rats (p < 0.01-0.001; one-way ANOVA followed by the Tukey test).
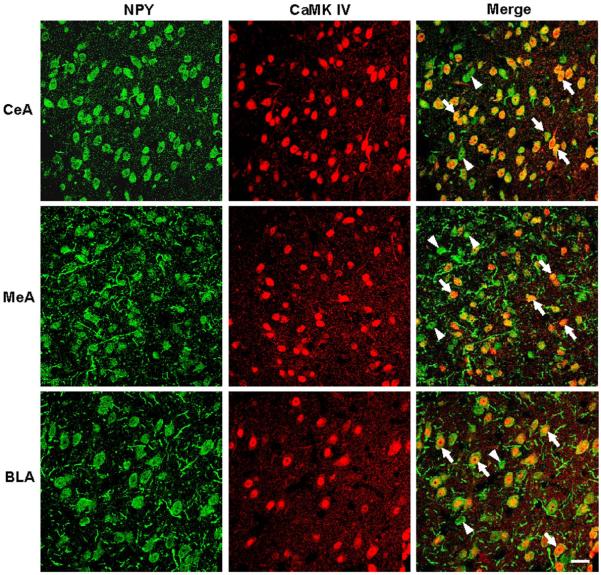
Colocalization of NPY and CaMK IV in Amygdala of P rats
Since NPY infusion increased CaMK IV protein levels, we determined whether these two proteins are colocalized. We employed the double immunofluorescence protocol to examine colocalization of NPY and CaMK IV proteins in the amygdaloid structures of P rats (Fig. 6). The majority of NPY-immunoreactive cells in the amygdala (CeA, MeA, and BLA) co-expressed CaMK IV. However, some NPY-positive cells were devoid of CaMK IV immunoreactivity (Fig. 6). These results suggest that NPY is co-expressed in the majority of CaMK IV-positive cells in the amygdaloid structures of P rats.
Figure 6.
Representative photomicrographs showing the immunofluorescence staining for NPY (green) and CaMK IV (red) in the cells of CeA, MeA and BLA. The colocalization of the NPY and CaMK IV in cells is indicated by the yellow color development (merge). These photomicrographs show that most of the NPY-positive cells co-express CaMK IV (arrows) with a few exceptions (arrowheads). Scale bar = 20 μm.
DISCUSSION
An interesting observation of the present study was that NPY infusion-induced increases in CaMK IV-dependent CREB phosphorylation and increases in the expression of endogenous NPY in the CeA may contribute to the attenuation of anxiety-like and alcohol-drinking behaviors of P rats. We reported earlier that both NPY infusion into the CeA and alcohol drinking produced anxiolytic effects in P rats as measured by the EPM test. We have also previously reported that NPY infusion into the CeA can also attenuate alcohol intake in P rats (Pandey et al., 2005). Here, we have confirmed both of these findings and anxiolytic effects of NPY and ethanol in P rats were measured using the LDB test, another test for measuring anxiety-like behavior. In addition, we extended the behavioral observations to investigate the molecular mechanisms of NPY action and found that bilateral NPY infusion into the CeA increased protein levels of CaMK IV, but not PKA-Cα, thereby increasing both CREB phosphorylation and NPY expression in the CeA of P rats. Alcohol exposure also increased both CREB phosphorylation and NPY expression in the CeA and MeA, however the biochemical effects of alcohol were not caused by increased CaMK IV, but instead may have occurred due to increased PKA-Cα levels in the CeA and MeA of P rats, as reported earlier by us (Pandey et al., 2005) and confirmed in the present study.
The actions of NPY in the central nervous system are mediated by at least five receptor subtypes, namely the Y1, Y2, Y4, Y5, and Y6 receptors that are negatively linked to the cAMP second messenger pathway (Michel, 1991; Blomqvist and Herzog, 1997; Larhammar and Salaneck, 2004). The role of NPY-Y1 (Thiele et al., 2002), Y2 (Thorsell et al., 2002; Thiele et al., 2004), and Y5 (Schroeder et al., 2003, 2005) receptors have been implicated in alcohol drinking behaviors. Evidence accumulated suggests that NPY can positively regulate CREB phosphorylation. For example, infusion of NPY into the rat hypothalamus has been shown to increase CRE-DNA binding and CREB phosphorylation (Sheriff et al., 1997). Furthermore, it has been shown that NPY can activate NPY-Y1 receptors and increase CREB phosphorylation via Ca2+ mobilization and activation of CaM kinases (Sheriff et al., 1998; 2002). Here, we have found that NPY infusion into the CeA significantly increased the levels of both CaMK IV and pCREB in the CeA of P rats. Since NPY is a cAMP inducible gene, increased CREB phosphorylation may be responsible for the NPY-induced increase in endogenous NPY expression. Voluntary ethanol consumption had no effect on CaMK IV protein levels, but did increase protein levels of PKA-Cα and pCREB and NPY expression. Later findings support our previous studies showing that acute ethanol injection or voluntary ethanol consumption can increase protein levels of PKA-Cα, pCREB and NPY in both the CeA and MeA, but not in BLA of P rats (Pandey et al., 2005). Taken together, these studies suggest that ethanol-induced increases in CREB phosphorylation and NPY expression in the CeA and MeA of P rats may be related to increased PKA activation. In addition, NPY may be involved in the upregulation of CREB phosphorylation in the CeA via activation of CaMK IV. This is further supported by the observation that CaMK IV and NPY are co-expressed in the CeA of P rats. Therefore, increased expression of NPY, due to increased CREB phosphorylation, may be involved in the anxiolytic effects of both NPY and ethanol in P rats. Future studies are needed to determine how different doses of NPY infused into the CeA or MeA may affect anxiety and alcohol intake in P rats.
Previous preclinical studies suggest that a deficiency in the NPY gene may be involved in promoting alcohol intake and anxiety-like behaviors (Thiele et al., 1998; Primeaux et al., 2006; Badia-Elder et al., 2007; Thorsell et al., 2007). Heightened anxiety levels may also predispose individuals to excessive alcohol drinking behaviors (Koob, 2003; Pandey, 2003; Novak et al., 2003). The CeA, which is a part of the extended amygdala, appears to be important in the regulation of anxiety and alcohol drinking behaviors (Koob, 2003; McBride, 2002). Direct evidence supporting this notion was derived from rat studies involving lesions to the CeA, but not the BLA, that led to decreased anxiety levels and alcohol intake (Moller et al., 1997). NPY in the CeA has also been shown to regulate anxiety and alcohol drinking behaviors (Pandey, 2003; Pandey et al., 2005; Thorsell et al., 2007). Over-expression of NPY in the CeA of anxious rats using viral vectors was also shown to significantly decrease alcohol preference (Primeaux et al., 2006). Furthermore, over-expression of NPY via viral vectors or direct NPY infusion into the CeA significantly diminished alcohol intake in alcohol dependent rats (Thorsell et al., 2005; 2007; Gilpin et al., 2008). Interestingly, NPY over-expression or NPY infusion into the CeA of non-anxious or normal rats did not have any effects on alcohol intake (Primeaux et al., 2006; Thorsell et al., 2005). In addition, mRNA and protein levels of NPY were decreased in the CeA and MeA but not in the BLA during withdrawal after chronic ethanol exposure in Sprague Dawley (SD) rats (Roy and Pandey, 2002; Zhang and Pandey, 2003; Pandey et al., 2008)). NPY in the CeA has also been shown to regulate anxiety-like behaviors via activation of NPY-Y1 receptors in rats (Heilig et al., 1993). Previously, we found that increased CREB phosphorylation and NPY expression, due to PKA activation (Sp-cAMP infusion) in the CeA, significantly decreased the anxiety-like and alcohol drinking behaviors of P rats. On the other hand, decreased CREB phosphorylation and NPY expression due to PKA inhibition (Rp-cAMP infusion) into the CeA significantly increased anxiety levels and alcohol intake in both NP and SD rats (Pandey et al., 2003b; 2005). Combining these results with our current results clearly suggests that deficits in NPY signaling in the CeA may be involved in anxiety-like and alcohol drinking behaviors of P rats.
Several clinical studies also support the fact that NPY gene polymorphisms may be involved in alcohol dependence (Zhu et al., 2003; Mottagui-Tabar et al., 2005). A functional NPY Leu 7 Pro polymorphism may be associated with alcohol dependence in US populations, however a similar polymorphism in the NPY gene may be protective against alcoholism in Finnish populations (Ilveskoski et al., 2001; Lappalainen et al., 2002). Another study indicates that NPY Leu 7 Pro polymorphism has been associated with severe alcohol withdrawal symptoms in Japanese alcoholics (Okubo and Harada, 2001). A novel polymorphism in the 5′-flanking region of the NPY gene was identified which has been associated with alcoholism in type I (late onset), but not in type II (early-onset) alcoholics (Mottagui-Tabar et al., 2005). Also, several polymorphisms have been identified in the 5′-flanking regions of NPY in P rats that may function to regulate NPY expression in several brain regions including the amygdala (Spence et al., 2005). Lower NPY plasma levels may also be associated with anxiety experienced by combat veterans with post traumatic stress disorder after returning home from war (Rasmusson et al., 2000, Yehuda et al., 2006). Recently, it has been shown that lower haplotype-driven NPY expression was associated with higher emotion-induced activation of the amygdala in humans (Zhou et al., 2008). Both clinical and preclinical studies suggest that the NPY gene may play a crucial role in regulating anxiety and alcoholism.
In conclusion, our findings demonstrate that a deficiency in NPY signaling in the CeA may be involved in regulating both anxiety and alcohol drinking behaviors. Here, we have observed that NPY infusion normalized the decreased expression of NPY in the CeA via increased CaMK IV-dependent CREB phosphorylation and also attenuated the anxiety-like and alcohol drinking behaviors of P rats. The findings of this study, along with our previous studies in P and NP rats (Pandey et al., 2005), clearly implies that a deficiency in CREB and its target gene NPY may be operative in the anxiety-like and excessive alcohol drinking behaviors observed in P rats.
ACKNOWLEDGEMENTS
This study was supported by the grants from the National Institute on Alcohol Abuse and Alcoholism (AA-010005; AA-013341; AA-016690; AA-015626) and the Department of Veterans Affairs (Merit Review Grant; Research Career Scientist award) to SCP. The Alcohol Research Resource Award (R24AA015512) from NIAAA to Indiana University provided support for the selective breeding of P rats. The authors would also like to thank Bela Starkman for her help in editing the manuscript.
REFERENCES
- Akabayashi A, Zaia CT, Gabriel SM, Silva I, Cheung WK, Leibowitz SF. Intracerebroventricular injection of dibutyryl cyclic adenosine 3′, 5′-monophosphate increases hypothalamic levels of neuropeptide Y. Brain Res. 1994;660:323–328. doi: 10.1016/0006-8993(94)91306-4. [DOI] [PubMed] [Google Scholar]
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li T-K. Effect of neuropeptide Y(NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -non preferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–390. [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li T-K. Effects of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–899. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Gilpin NW, Stewart RB. Neuropeptide Y modulation of ethanol intake: effects of ethanol drinking history and genetic background. Peptides. 2007;28:339–344. doi: 10.1016/j.peptides.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes-how many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Pharmacogenomics of alcohol response and addiction. Am J Pharmacogenomics. 2003;3:217–232. doi: 10.2165/00129785-200303040-00001. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JA, Pavia JM, Morris MJ. Neuropeptide Y and [Leu31, Pro34] neuropeptide Y potentiate potassium-induced noradrenaline release in the paraventricular nucleus of the aged rat. Brain Res. 1997;750:301–304. doi: 10.1016/s0006-8993(96)01475-8. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, Widerlöv E. Neuropeptide Y: an overview of central distribution, functional aspects, and possible involvement in neuropsychiatric illnesses. Acta Psychiatr Scand. 1990;82:95–114. doi: 10.1111/j.1600-0447.1990.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Widerlöv E. Neurobiology and clinical aspects of neuropeptide Y. Crit Rev Neurobiol. 1995;9:115–136. [PubMed] [Google Scholar]
- Higuchi H, Yang HY, Sabol SL. Rat neuropeptide Y precursor gene expression: mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J Biol Chem. 1988;263:6288–6295. [PubMed] [Google Scholar]
- Hwang BH, Zhang J-K, Ehlers CL, Lumeng L, Li T-K. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–1030. [PubMed] [Google Scholar]
- Ilveskoski E, Kajander OA, Lehtimäki T, Kunnas T, Karhunen PJ, Heinälä P, Virkkunen M, Alho H. Association of neuropeptide Y polymorphism with occurrence of type 1 and type 2 alcoholism. Alcohol Clin Exp Res. 2001;25:1420–1422. doi: 10.1097/00000374-200110000-00003. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A. Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol. 2005;516:40–45. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck C, Rosenheck RA, Cramer J, Southwick S, Charney D, Krystal J, Gelernter J. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–831. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38:141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Li T-K, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism:neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Meurs A, Clinckers R, Ebinger G, Michotte Y, Smolders I. Sigma 1 receptor-mediated increase in hippocampal extracellular dopamine contributes to the mechanism of the anticonvulsant action of neuropeptide Y. Eur J Neurosci. 2007;26:3079–3092. doi: 10.1111/j.1460-9568.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- Michel MC. Receptors for neuropeptide Y: multiple subtypes and multiple second messengers. Trends Pharmacol Sci. 1991;12:389–394. doi: 10.1016/0165-6147(91)90610-5. [DOI] [PubMed] [Google Scholar]
- Misra K, Pandey SC. The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: a role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology. 2006;31:1406–1419. doi: 10.1038/sj.npp.1300900. [DOI] [PubMed] [Google Scholar]
- Möller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Mottagui-Tabar S, Prince JA, Wahlestedt C, Zhu G, Goldman D, Heilig M. A novel single nucleotide polymorphism of the neuropeptide Y (NPY) gene associated with alcohol dependence. Alcohol Clin Exp Res. 2005;29:702–707. doi: 10.1097/01.alc.0000164365.04961.b1. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li T-K. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Novak A, Burgess ES, Clark M, Zvolensky MJ, Brown RA. Anxiety sensitivity, self-reported motives for alcohol and nicotine use, and level of consumption. J Anxiety Disord. 2003;17:165–180. doi: 10.1016/s0887-6185(02)00175-5. [DOI] [PubMed] [Google Scholar]
- Okubo T, Harada S. Polymorphism of the neuropeptide Y gene: an association study with alcohol withdrawal. Alcohol Clin Exp Res. 2001;25:59S–62S. doi: 10.1097/00000374-200106001-00014. [DOI] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Pandey SC. The gene transcription factor cyclic AMP-responsive element binding protein: role in positive and negative affective states of alcohol addiction. Pharmacol Ther. 2004;104:47–58. doi: 10.1016/j.pharmthera.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat cortex. J Pharmacol Exp Ther. 2001;296:857–868. [PubMed] [Google Scholar]
- Pandey SC, Carr LG, Heilig M, Ilveskoski E, Thiele TE. Neuropeptide Y and alcoholism: genetic, molecular, and pharmacological evidence. Alcohol Clin Exp Res. 2003a;27:149–154. doi: 10.1097/01.ALC.0000052706.21367.0E. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003b;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24:5022–5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Over expression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Radel M, Goldman D. Pharmacogenetics of alcohol response and alcoholism: the interplay of genes and environmental factors in thresholds for alcoholism. Drug Metab Dispos. 2001;29:489–494. [PubMed] [Google Scholar]
- Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Salimov RM, McBride WJ, Sinclair JD, Lumeng L, Li T. Performance in the cross-maze and slip funnel tests of four pairs of rat lines selectively bred for divergent alcohol drinking behavior. Addict Biol. 1996;1:273–280. doi: 10.1080/1355621961000124886. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Iller KA, Hodge CW. Neuropeptide-Y Y5 receptors modulate the onset and maintenance of operant ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1912–1920. doi: 10.1097/01.ALC.0000098873.80433.BA. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The neuropeptide-Y Y5 receptor antagonist L-152,804 decreases alcohol self-administration in inbred alcohol-preferring (iP) rats. Alcohol. 2005;36:179–186. doi: 10.1016/j.alcohol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Sheriff S, Chance WT, Fischer JE, Balasubramaniam A. Neuropeptide Y treatment and food deprivation increase cyclic AMP response element-binding in rat hypothalamus. Mol Pharmacol. 1997;51:597–604. doi: 10.1124/mol.51.4.597. [DOI] [PubMed] [Google Scholar]
- Sheriff S, Dayal R, Kasckow J, Regmi A, Chance W, Fischer J, Balasubramaniam A. NPY upregulates genes containing cyclic AMP response element in human neuroblastoma cell lines bearing Y1 and Y2 receptors: involvement of CREB. Regul Pept. 1998;75-76:309–318. doi: 10.1016/s0167-0115(98)00083-4. [DOI] [PubMed] [Google Scholar]
- Sheriff S, Qureshy AF, Chance WT, Kasckow JW, Balasubramaniam A. Predominant role by CaM kinase in NPY Y(1) receptor signaling: involvement of CREB. Peptides. 2002;23:87–96. doi: 10.1016/s0196-9781(01)00583-6. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Comparison of anxiety-like behavior in adolescent and adult Sprague-Dawley rats. Behav Neurosci. 2005;119:1477–1483. doi: 10.1037/0735-7044.119.6.1477. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca2+-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Fee JR, Knapp DJ, Breese GR, Thiele TE. Elevated anxiety-like behavior following ethanol exposure in mutant mice lacking neuropeptide Y (NPY) Drug Alcohol Depend. 2007;90:297–300. doi: 10.1016/j.drugalcdep.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JP, Liang T, Habegger K, Carr LG. Effect of polymorphism on expression of the neuropeptide Y gene in inbred alcohol-preferring and - nonpreferring rats. Neuroscience. 2005;131:871–876. doi: 10.1016/j.neuroscience.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li T-K, Murphy JM. Comparison of alcohol-preferring (P) and non-preferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Lumeng L, McBride WJ, Li T-K, Hwang BH. Reduced neuropeptide Y mRNA expression in the central nucleus of amygdala of alcohol preferring (P) rats: its potential involvement in alcohol preference and anxiety. Brain Res. 2004;1014:251–254. doi: 10.1016/j.brainres.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Naveilhan P, Ernfors P. Assessment of ethanol consumption and water drinking by NPY Y(2) receptor knockout mice. Peptides. 2004;25:975–983. doi: 10.1016/j.peptides.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Heilig M. Diverse functions of neuropeptide Y revealed using genetically modified animals. Neuropeptides. 2002;36:182–193. doi: 10.1054/npep.2002.0897. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Rimondini R, Heilig M. Blockade of central neuropeptide Y (NPY) Y2 receptors reduces ethanol self-administration in rats. Neurosci Lett. 2002;332:1–4. doi: 10.1016/s0304-3940(02)00904-7. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in wistar rats with a history of ethanol exposure. Alcohol Clin Exp Res. 2005;29:584–590. doi: 10.1097/01.alc.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O'Dell LE, Chen SA, King AR, Lekic D, Koob GF, Sanna PP. Viral vector-induced amygdala NPY over expression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–1337. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65:397–414. doi: 10.1016/0163-7258(95)98598-k. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pandey SC. Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides. 2003;24:1397–1402. doi: 10.1016/j.peptides.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Pollak L, Mottagui-Tabar S, Wahlestedt C, Taubman J, Virkkunen M, Goldman D, Heilig M. NPY Leu7Pro and alcohol dependence in Finnish and Swedish populations. Alcohol Clin Exp Res. 2003;27:19–24. doi: 10.1097/01.ALC.0000050642.62233.44. [DOI] [PubMed] [Google Scholar]