Abstract
Primary suture ACL repair was abandoned in favor of reconstruction due to a high rate of clinical failures. However, the insertion of a collagen scaffold loaded with platelets into the wound at the time of suture repair (“enhanced primary repair”) has been shown to improve functional healing in animal models. Our objectives were to determine if using a collagen scaffold alone (without platelets) would be sufficient to increase the structural properties of the repaired ACL and decrease postoperative knee laxity compared to suture repair without the scaffold. Eight Yucatan minipigs underwent bilateral ACL transection and suture repair. In one knee, the repair was augmented with a collagen scaffold (SCAFFOLD group) while the other had suture alone (SUTURE group). After thirteen weeks of healing, knee joint laxity and the structural properties of the ACL were measured. The addition of the collagen scaffold to suture repair of a transected ACL did not significantly improve the mean anteroposterior knee laxity [SCAFFOLD vs SUTURE: 6.1±1.4 vs 4.4±2.0 mm (p=0.07), 8.1±2.0 vs 7.6±2.0 mm (p=0.66), and 6.2±1.2 vs 6.1±1.8 mm (p=0.85) at 30°, 60°, and 90° flexion, respectively]. Likewise, there were no significant differences in the structural properties [SCAFFOLD vs SUTURE: 367±185.9 vs 322±122.0 N (p=0.66) and 90.7±29.5 vs 85.0±30.3 N/mm (p=0.74) for the yield load and linear stiffness, respectively]. The use of a collagen scaffold alone to enhance suture repair of the ACL was ineffective in this animal model. Future work will be directed at stimulating biological activity in the scaffold.
Keywords: ACL, Ligament, Collagen, Scaffold, Fibroblast, Regeneration
INTRODUCTION
Anterior cruciate ligament (ACL) injuries are common among people of all ages, especially those active in athletics 1. Unlike extra-articular ligaments like the MCL,2 the ACL does not readily heal3–6 Thus, primary suture repair, in which the torn ends are sutured back together, was abandoned, and ACL reconstruction, in which the torn ACL is replaced with a tendon graft, became the popular treatment option. However, biological enhancement of ACL healing using a combination of growth factors and a scaffold placed between the ruptured ends of the ACL in conjunction with primary suture repair (“enhanced primary repair”) could provide a better alternative to reconstruction if such a tissue engineered construct could be developed. Enhanced ACL repair has theoretical advantages over ACL reconstruction because it would preserve the native ligament insertions and proprioceptive nerve fibers of the native ACL, would eliminate the need for graft harvesting, and would permit a less invasive procedure.
Enhanced suture repair using a collagen-based scaffold saturated with platelet rich plasma (a “collagen-platelet composite” or “CPC”) has been shown to improve ACL healing1,7–9. Collagen-based scaffolds are resistant to degradation by catabolic enzymes of synovial fluid,7 and the use of atelocollagen, collagen in which the telopeptides are removed, to minimize any immune response.8 Atelocollagen scaffolds when combined with platelets and fibrin have been shown to produce an ACL wound healing response mimicking that seen in the MCL.9–11 In an effort to better understand the mechanisms behind enhanced repair, a study was performed to determine if the independent use of platelet-rich plasma (PRP) without a collagen scaffold could improve the healing response of the ACL.12 No significant improvement in healing was found with PRP alone when compared to ACL repair with sutures alone. One possible reason may be the relatively easy degradability of the unprotected PRP clot in the synovial environment. Another prior study had demonstrated that the use of an atelocollagen hydrogel with platelet poor plasma did not result in any significant increase in biomechanical properties over suture repair alone13; however, the numbers of animals in that group was small (n=2), and a hydrogel was used, as opposed to sponge type scaffold which would be capable of absorbing blood at the time of surgery.
The objective of this current study was to determine whether the use of a collagen sponge-type scaffold alone to an ACL suture repair would improve the properties of the healing ligament. Use of a sponge-type scaffold to absorb any local bleeding could stabilize and activate those platelets at the surgical site. In theory, the collagen scaffold could provide a stabilized region in the wound site for ACL cells to invade, proliferate and produce collagen1,7–9. Prior studies in tendon repair looking at the use of both acellular dermis14,15, polyglycolic acid sheets16 and a woven poly-L-lactide device17 without any biologic adjunct have demonstrated increased mechanical properties in animal models, while others have found use of a scaffold made of polylactic acid had no beneficial effect18. Our primary hypothesis was that the collagen scaffold alone would increase the structural properties of the healing ACL and decrease postoperative anterior-posterior (AP) laxity of the knee joint when compared to traditional ACL suture repair without the supplemental scaffold.
MATERIALS AND METHODS
Experimental Design
The study was approved by the Institutional Animal Care and Use Committee. Five female and three male adolescent (50.8 kg ± 13.7 kg and 16.1 ± 1.2mos; mean ± SD) Yucatan mini-pigs underwent bilateral ACL transection and suture repair. All animals were treated with suture repair alone (SUTURE) in one knee, and suture repair augmented with a collagen scaffold (SCAFFOLD) in the contralateral knee (n=8). The knees were block randomized to treatment. All animals were euthanized after thirteen weeks of healing. The response measures included the graft structural properties, AP knee laxity, clinical assessment, blood composition, and histology.
Scaffold Preparation
The collagen scaffold was made by solubilizing bovine fascia. It was harvested, minced, and solubilized in a pepsin solution to cleave the telopeptides from the collagen molecules to create an atelocollagen solution. Atelocollagen is highly conserved between animal species and thus less immunogenic.19,20 The resulting solution was then frozen, lyophilized, and rehydrated with a specified amount of water to create the atelocollagen solution equal to 8.2 mg/ml. The slurry then was neutralized using HEPES buffer (Mediatech Inc, Herndon, VA), sodium hydroxide (Fisher Scientific, Fair Lawn, NJ), PBS (HyClone, Logan UT), and calcium chloride (Sigma-Aldrich, St. Louis, MO) and kept on ice until use. The neutralized solution was placed into cylindrical molds with an inner diameter of 16 mm. The solution was then lyophilized producing a scaffold 14 mm in diameter by 40 mm in length.
Surgical Procedure
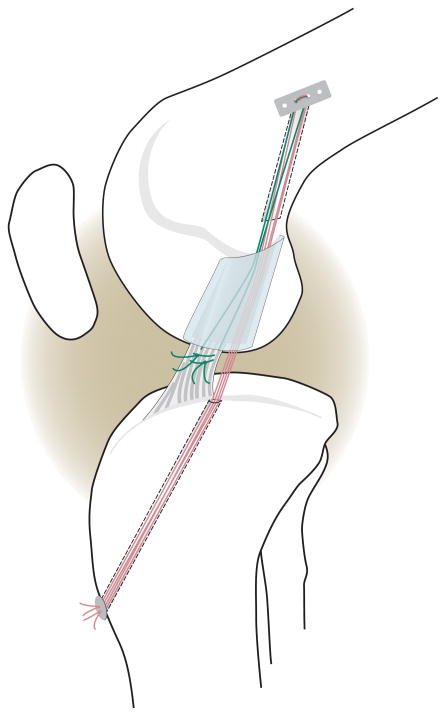
After the induction of general anesthesia, a medial parapetellar arthrotomy was made and the ligamentum mucosum released. A Lachman test was performed to verify knee stability. The ACL was then cut at the junction of the proximal and middle thirds using a knife. The Lachman test was repeated to verify functional loss of the ACL. A variable depth suture was placed in the tibial stump with sutures exiting through the proximal cut end of the tibial stump of the ACL (Fig. 1). A 2.4 mm drill pin was placed in the tibia using a commercial drill guide (Acufex gold guide; Smith and Nephew, Andover, MA) with tunnel entrance posterior to the intermeniscal ligament and medial to the tibial insertion site of the ACL. The knee was irrigated with sterile saline. The femoral tunnel was made using the Endobutton drill. The Endobutton had three #1 Vicryl sutures placed through the central holes and was brought up through the femur and engaged on the proximal femoral cortex. For the “SCAFFOLD” group (Fig. 1), the collagen scaffold was threaded onto four of the six sutures coming distally from the Endobutton and passed up into the notch. These four sutures were passed though the tibial tunnel and tied over a button with the knee held in extension. The variable depth suture was then tied to the remaining Vicryl sutures from the Endobutton. For the “SUTURE” knees the identical procedure was performed on the contralateral knee; however, no collagen scaffold was used in the repair site. Both knees were closed in layers. After 13 weeks of healing, the animals were euthanized with an injection of pentobarbital solution (Fatal Plus; Vortech Pharmaceuticals, Dearborn, Michigan). The knees were harvested and frozen at −20°C until testing.
Fig. 1.
Schematic diagram depicting the primary suture repair with the collagen scaffold in place. Sutures were fixed proximally with an Endobutton. The sponge was threaded onto four of the trailing suture ends (RED) which were then passed through the tibial tunnel and tied over a button to provide initial knee stability. The remaining two suture ends (GREEN) were tied to the sutures in the tibial stump of the ACL. All sutures were resorbable, and there was no sign of suture material remaining at the time of post-mortem testing.
Clinical and Hematology Assessments
At the time of surgery and at euthanasia, the knee was flexed and extended while the flexion and extension limits were measured with a goniometer. A Lachman test was performed. Blood was drawn into a 60 cc syringe containing 10% acid-citrate-dextrose at both time points. The anticoagulated blood samples were evaluated using a VetScan HM5 (Abaxis: Union City, CA) to determine the WBC count, platelet count and monocyte percentage.
Biomechanical Testing
The specimens were removed from the freezer approximately 24 hours prior to testing. All extraneous muscle and soft tissue were carefully removed leaving the knee capsule intact. Specimens were kept moist throughout the test protocol with a wrap of normal saline-soaked gauze. The femur and tibia were then rigidly potted in a urethane compound (Smooth On, Easton, PA) leaving approximately 5 cm of exposed bone to the joint line. The bones were visually oriented such that the long axes of the bone and cannon were coaxial.
Cyclic AP laxity testing was performed with the knee flexed at 30°, 60° and 90°. Knees were supported on custom fixtures and rigidly attached to an MTS 810 servohydraulic load frame (MTS Systems Corporation, Eden Prairie MN) as previously described.12,21,22 Sinusoidal anterior-posterior directed shear loads of ±30 N were applied at 0.0833 Hz for 12 cycles. ±30 N were selected since they occur within in the linear regions of the load-displacement curve.12,21 Load and displacement data were acquired at 20 Hz and plotted.12 The knee flexion angle was checked with a goniometer prior to initiating each test. During the AP laxity tests, axial rotation was locked in the neutral position, while the varus-valgus angulation and the coronal plane translations were left unconstrained as previously described.12,21,22
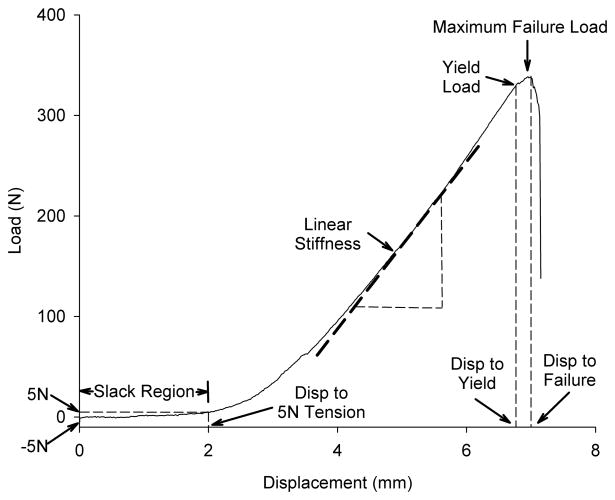
Following AP laxity testing, the knees were positioned for tensile testing.12 The joint capsule, menisci, collateral ligaments and the PCL were dissected from the joint leaving the femur-ACL scar mass-tibia complex intact. The tibia and femur were positioned so that the mechanical axis of the ACL was collinear with the load axis of the MTS with the knee flexion angle initially set at 30°. The tibia was mounted to the base of the MTS via a sliding X-Y platform. The femur was unconstrained to rotation. This enabled the complex reposition such that the load was distributed over the ligament cross section during tensile loading. The crosshead was lowered until a −5 N compressive load was applied across the joint to provide a reproducible starting point for tensile loading. A ramp at 20 mm/min was performed, 12 and the load-displacement data were recorded at 25 Hz. The displacement data were based on grip-to-grip measurements. The yield load, maximum failure load, linear stiffness, displacement to yield, displacement to failure, and displacement to 5 N of tensile load were determined (Fig. 2).
Fig. 2.
Schematic of the load-displacement curve for tensile testing of the femur-ACL-tibia complex. The toe region (the displacement of the joint surfaces at low loads) was defined by the displacement between −5 and +5N of tensile load. After the slack of the ligament was removed, the slope increased as it entered the linear stiffness region until the yield load was reached. Maximum failure load was defined as the maximum load supported by the construct. Yield load is defined as the location on the load-displacement curve where the slope of the curve first deviates by 2% from the linear region. The displacements to yield and failure were defined as the amount of excursion of joint surfaces at the yield load and the maximum failure load, respectively.
Histology
After biomechanical testing, the ACL repair tissue was removed and fixed in formalin. The tissue was embedded in paraffin and central sagittal sections, 7 μm thick, were stained with hematoxylin and eosin. The sagittal sections were maintained by marking the front and proximal sides of the ligament tissue with sutures at the time of removal of the sections. The midpoint of the central cross section was scored using the 24 point Ligament Tissue Maturity Index, an index previously validated for large animal in vivo ligament healing.8 This index has subscores for cellularity, vascularity and collagen organization. One reviewer, who was blinded to the identification of the samples, scored each slide.
Statistical Methods
To address the primary hypotheses, paired t-tests were used to evaluate the differences between means of the two treatment groups (SUTURE vs SCAFFOLD) for the AP laxity structural property values. Paired t-tests were used to compare the Ligament Tissue Maturity Indices between treatment groups, and to compare the pre-operative systemic platelet values, WBC, and monocyte counts to those obtained after 13 weeks of healing. Terminal flexion and extension and the clinical Lachman assessments were compared between treatment groups (SUTURE vs SCAFFOLD) over time (pre-op vs 13 weeks post-op) using repeated measures analyses of variance. The level of significance was set at p<0.05 (2-tailed).
RESULTS
Laboratory and Physical Examination Results
All animals were weight bearing on both hind limbs within 12 hours of surgery. They resumed normal food intake within 24 hours. Normal gait, as determined via visual assessment, was observed in all animals at a mean of 6 (range 2 to 11) days of surgery.
The mean values of systemic WBC counts at the beginning of the study and after thirteen weeks were 11.2±3.29 and 13.1±3.22 (×106/ml) respectively, a change that was not significant (p=0.10). The mean values of systemic platelet count at the beginning of the study and after thirteen weeks were 340.5±77.1and 288.8±79.6 (×106/ml) respectively, a change that was also not significant (p=0.33). The average monocyte percentage found in WBC pre- and postoperatively was 3.1±4.1% and 1.4±1.0%, respectively, which was not statistically significant (p=0.30).
There was no significant treatment effect on terminal flexion (p=0.685) or terminal extension (p= 0.504; Table 1). There was no significant effect of time on terminal flexion (p=0.196); however, there was a significant decrease in the terminal extension angle at the time of euthanasia as compared to the preoperative values at the 13 week time point (p=0.027), though this was not group specific (interaction p=0.785). There was no significant effect of treatment on the Lachman sign (p=0.598).
Table 1.
Flexion and extension measurements for SUTURE and SCAFFOLD treatment groups (mean±SD) before and after surgery are shown. Mean difference and p-values are listed. Both groups experienced a significant loss of terminal extension after surgery that was not treatment-specific. There were no significant differences between the SUTURE and SCAFFOLD groups for any of the measured physical exam parameters.
| SUTURE (n=8) | SCAFFOLD (n=8) | |||
|---|---|---|---|---|
| Pre-op | 13 wk post-op | Pre-op | 13 wk post-op | |
| Extension (deg) | 43.8±5.2 | 37.5±4.6* | 45.0±7.6 | 38.1±5.3* |
| Flexion (deg) | 142.5±4.6 | 136.3±12.7 | 142.5±4.6 | 135±15.8 |
| Lachman (mm) | 3.0±0.8 | 3.5±1.8 | 3.0±0.8 | 4.0±2.1 |
p = 0.02 for the change in Extension angle with time for both the SUTURE and SCAFFOLD groups.
Gross observations
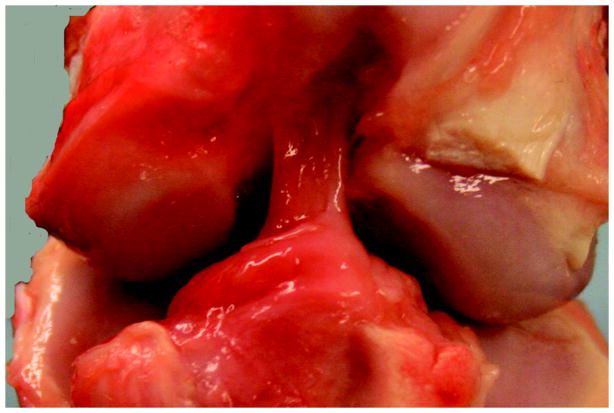
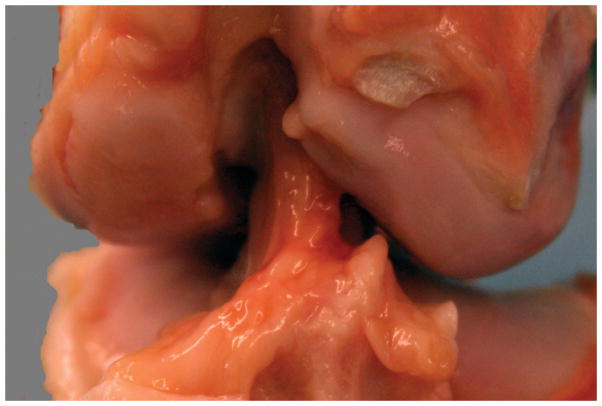
For all knees, repair tissue was visible in the notch at the time of harvest (Fig. 3). The repair tissue bridged between the anterior slope of the tibial spines and the lateral aspect of the femoral notch, and had the appearance of multiple individualized synovialized bands. There was no evidence of cyclops lesions in any knee, and there were no visible sutures remaining in the notch. No synovial hypertrophy, meniscal tears, palpable effusions or cartilage damage were evident after 13 weeks of healing.
Figure 3.
Figure 3A: Gross appearance of a knee treated with SUTURE repair alone. Note the repair tissue arising from just posterior to the fat pad, from the anterior slope of the tibial spines, and coursing from the tibia to the lateral wall of the intercondylar notch. There is no significant change noted in the femoral condylar cartilage either medially or laterally. The repair tissue itself is composed of multiple individual synovialized bands.
Figure 3B. Gross appearance of a knee treated with SCAFFOLD supplementation of the suture repair. Note the similar appearance with the SUTURE knee in 3A. Again, there is repair tissue coursing from tibia to femur in one continuous mass. There is no change noted in the femoral condylar cartilage. The repair tissue itself is composed of multiple individual synovialized bands.
Biomechanical Testing
AP laxity at 30° was 34% higher in the SCAFFOLD group than in the SUTURE group, a difference that did not reach statistical significance (p=0.07) (Table 2). AP laxity was only 6% higher at 60° and only 2% higher at 90° in the SCAFFOLD group than in the SUTURE group, neither differences were statistically significant (p=0.66 and p=0.85, respectively; Table 2).
Table 2.
Biomechanical test measurements for SUTURE and SCAFFOLD treatment groups (mean±SD). Results from cyclic AP laxity testing performed with knees flexed at 30°, 60° and 90° are shown in the top three rows. Values for linear stiffness, maximum/yield load and displacement and displacement to 5N that were calculated or extrapolated from the tensile failure test load/displacement plots are also listed below. Mean differences and p values are also listed. No values reached the threshold for statistical significance (p < 0.05).
| Variable | SUTURE (n=8) | SCAFFOLD (n=8) | Mean Difference | p |
|---|---|---|---|---|
| AP Laxity 30° (mm) | 4.4±2.0 | 6.1±1.4 | 1.7±2.2 | 0.07 |
| AP Laxity 60° (mm) | 7.6±2.0 | 8.1±2.0 | 0.4±2.6 | 0.66 |
| AP Laxity 90° (mm) | 6.1±1.8 | 6.2±1.2 | 0.1±1.2 | 0.85 |
| Linear stiffness (N/mm) | 85.0±30.3 | 90.7±29.5 | 5.8±46.4 | 0.74 |
| Maximum tensile load (N) | 362.7±117.9 | 380.3±181.8 | 17.6±264.2 | 0.86 |
| Yield tensile load (N) | 322.3±122.0 | 367.0±185.9 | 44.7±273.9 | 0.66 |
| Max displacement (mm) | 10.1±3.0 | 10.0±2.0 | −0.08±1.6 | 0.89 |
| Yield displacement (mm) | 8.9±2.5 | 9.6±1.7 | 0.72±1.44 | 0.20 |
| Displacement to 5N (mm) | 3.1±2.7 | 3.7±1.8 | 0.61±3.1 | 0.59 |
All specimens failed within the ligament midsubstance. There was no significant difference in the yield load, maximum failure load, linear stiffness, displacement to yield, displacement to failure, or displacement to 5N of tensile load between the two groups (Table 2).
Histology
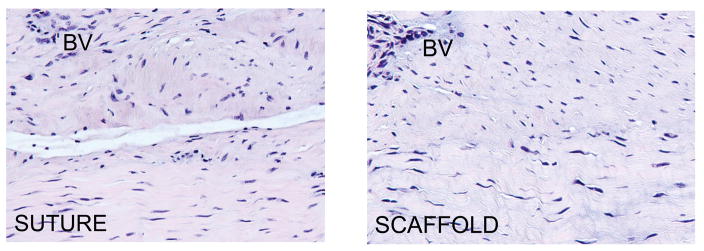
The tissue in both the SUTURE and SCAFFOLD repairs was highly cellular and vascular at 13 weeks. The cells had the characteristic shape of mature fibroblasts, with nuclear aspect ratios of over 2 in both groups. No inflammatory cells or acellular or avascular areas were noted in any sections, and there was no residual collagen scaffold visualized. Arterioles, venules and capillaries were found throughout the sections for all ligaments. The degree of collagen organization varied among the ligaments, but no specific difference between groups was noted. The Ligament Tissue Maturity index 11 was similar in the two groups (17±4 and 15±5 for the SCAFFOLD and SUTURE groups respectively, mean±SD, p=0.37). The cellularity subscores (7.3±1.8 vs 6.5±1.9; p=0.42), the collagen subscores (3.4±2.7 vs 4.5±2.0; p=0.37) and the vascularity subscores (5.6±0.5 vs 5.4±0.7; p=0.45) between the SCAFFOLD and SUTURE groups, respectively, were not significantly different.
DISCUSSION
The results of this study show that the use of an atelocollagen scaffold alone to enhance primary suture repair of the ACL was not effective. There were no significant improvements in any of the evaluated biomechanical parameters (AP laxity or structural properties). It should be noted that the AP laxity of the intact adolescent Yucatan minipig knee is approximately 2 mm using the testing methods reported here (unpublished data). In the present study, the average values for the SUTURE and SCAFFOLD repairs were 350% of that value. The average value of AP laxity at 30° was 34% higher in the SCAFFOLD group than in the SUTURE group, a difference that did not reach statistical significance, likely due in part to the small sample size of our study. The AP laxity at 30° is likely dominated by the posterior capsular constraints and the effect of scaffold placement on those constraints deserves further study. However, the measured difference in means of AP laxity at 60° and 90° of flexion (where the ACL repair tissue would be expected to play a larger role) were far smaller at these more physiologic testing conditions. In addition, the maximum tensile load of an intact ACL of an adolescent Yucatan minipig is approximately 1600N (unpublished data). Thus, the maximum failure loads of the SUTURE and SCAFFOLD knees on average only reached 25% of the intact ACL load. As material properties were not evaluated in this study, it is possible that the tissue quality was different between the two groups; however, as the structural properties dictate joint function and laxity, these were chosen as our primary outcome measures. Future studies including material property measurements would be useful, particularly for evaluating repair techniques that result in significant improvements in AP laxity or structural properties.
In this study, there were no significant differences in either the mechanical or histologic outcomes when a collagen scaffold was added to the suture repair. In prior studies of the healing ACL at similar time points9 when a functional improvement in mechanical properties is seen with treatment with a collagen-platelet composite, this has been associated with an increase in the cellular density of the repair tissue. Similarly, when improvements in ACL graft strength are noted with the addition of a collagen-platelet adjunct, histologic differences are noted between groups, with elimination of the avascular areas of the graft10. However, the specific cellular mechanisms which are responsible for the improvements in mechanical properties with biologic enhancement of the healing ACL remain unknown. Future studies to investigate these mechanisms are warranted for treatments which result in improved functional outcomes.
Atelocollagen is more resistant to synovial fluid degradation than a fibrin clot; however, there were no signs of residual scaffold at 13 weeks. Therefore, the lack of improvement in the functional properties could be due to the premature loss of the scaffold. This could also be the explanation for the lack of improvement seen with use of an atelocollagen hydrogel in our earlier study1. However, other prior studies using acellular implants have also shown failure to improve failure loads in tendon repair23–30, where other studies utilizing collagen scaffolds combined with mesenchymal stem cells (MSCs) as a biologic stimulus have been effective in stimulating tendon repair24–30. For example a collagen matrix seeded with MSCs reached 75% of the maximum force of the intact tendon, whereas the control group of the collagen matrix without the MSCs only reached 40% strength of the maximum force.26 Based on this collection of results, one might hypothesize that platelets or MSCs add a biologic stimulus, while the collagen scaffold provides the structural support, and both are required to stimulate ligament or tendon healing.
Prior studies of the tensile properties of ligaments have typically applied a small pre-load to the bone-ligament-bone construct to ensure consistency in testing.31 However, in prior studies of repaired ACLs,10 we noted differences in the amount of displacement of the joint surfaces with the application of the small pre-load between specimens. As one of the principal functions of ligaments is to maintain a specific bone-to-bone distance relationship, we felt that this displacement difference from tibiofemoral contact (−5 N compression) to a low tensile “pre-load” (+5 N tension) was worth recording as an additional outcome measure. A repaired ligament with a long slack region that allows the joint to shift by 10 mm before restraining joint motion is likely to be non-functional, even if it can eventually support high yield and failure loads.
Complete ACL transection and repair in the larger porcine model offers multiple advantages. The large animal model allows for easy identification of the structures of interest, both at the time of ligament transection and retrieval, and relative ease of structural repair for surgeons. The biomechanical assays are well established for knees of this size. The porcine model was selected since it is anatomically similar to the human knee,32 is dependent on its ACL for joint stability,33 and it has hematologic characteristics that are similar to the human particularly important for wound healing studies involving platelets.34 Adolescent animals were selected for study because they represent the human population at greatest risk for ACL injury. The adolescent minipigs had ascertained 95% of their skeletal growth and were sexually mature. Over the period of the study, the animals increased in weight by an average of only 1.5 Kg.
The porcine model has a few limitations common to large animal studies in quadrupeds. Their gait pattern is different from humans due to the use of four limbs for weight bearing rather than two. While the pig model was selected due to its anatomical and biomechanical similarities to the human knee, there may be differences in gait and rehabilitation which cannot be reliably controlled. Furthermore, there may be subtle differences in the wound healing cascade that are not yet appreciated.
There are also limitations to the histomorphometric approach used in this study. While large differences in cellularity or vascularity can be appreciated using this method, there may be smaller differences between groups that would not be apparent with this qualitative method.
This study was designed to evaluate short-term healing in the porcine model. In a recent evaluation of the porcine model, we determined that the nadir in ligament healing strength occurs between 6 and 9 weeks after surgery.35 For the present study, we selected a time point after this window (i.e. 13 weeks) where an improvement in the structural properties could be detected. Whether differences would occur with additional healing time remains unknown.
In our previous large animal studies, a relatively large variability between animals was observed.10,12,22,35 In order to increase the power of the study, we utilized paired comparison between the two treatments within each animal. This paired design enabled us to control the variability inherent to animal, knee, and ligament size, and growth rate. However, the effects of bilateral surgery (i.e. immediate weight bearing on both repaired joints and the potential for a higher spike in inflammatory response due to bilateral trauma) must be acknowledged.
Due to the expenses of large animal studies, the number of knees in each study group was relatively low (n=8). This can raise concerns about low power in a study in which no significant difference is detected between groups. However, the differences between the SCAFFOLD and SUTURE groups were not only statistically insignificant, they were likely to be clinically insignificant given the small changes between means of the two groups. For example, the 5% improvement in linear stiffness or maximum failure load with use of the collagen scaffold would unlikely justify the expense and complexity of an augmented repair procedure. Nonetheless, the study was more than 80% powered to detect if the enhanced ACL repair would have a return of mechanical strength similar to the successfully healing MCL at a similar time point (60% of normal).36 To determine if the small change noted in maximum load (~5%) of the present study were statistically significant, the study would require over 800 animals to complete.
The goal of this study was to determine if the collagen component of a collagen-platelet composite (CPC) was responsible for enhancing ACL wound healing. The CPC combination has been previously shown to improve wound healing.9–11 In the present study, we determined that the collagen scaffold alone was not sufficient for stimulating the healing process. When taken together, these results suggest that there are at least two core components required to enhance healing of an ACL tear; a collagen scaffold and a stable source of platelets. It is likely that fibroblasts need a scaffold that remains situated between the torn ligament ends and growth factor stimulation by platelets are needed to initiate and drive wound healing. These two factors may work synergistically to simulate the wound healing process that occurs naturally in extra-articular joint healing. Future work will be aimed at methods to stimulate biological activity in collagen scaffolds to enhance intra-synovial soft tissue repairs.
Figure 4.
Representative histomicrographs of healing ligament from the SUTURE and SPONGE groups. Note that the healing ligaments had areas of parallel collagen fascicles (bottom of both micrographs) as well as more irregular areas (top of both micrographs). BV= Blood vessel. Hematoxylin and Eosin, 200× magnification.
Acknowledgments
The authors would like to thank Eduardo Abreu, Matthew Palmer, Ashley Mastrangelo, David Zurakowski and Alison Biercevicz for their assistance. Patrick Bibbins created Figure 1. In addition, funding was received from NIH Grants AR054099 and AR052772 (MMM) and AR049199 (BCF). The first author is a consultant for Connective Orthopaedics (BCF) and the corresponding authors is a founder and shareholder of Connective Orthopaedics (MMM).
References
- 1.Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. American Academy of Orthopaedic Surgeons 1999 [Google Scholar]
- 2.Adriani E, Mariani PP, Maresca G, Santori N. Healing of the patellar tendon after harvesting of its mid-third for anterior cruciate ligament reconstruction and evolution of the unclosed donor site defect. Knee Surg Sports Traumatol Arthrosc. 1995;3:138–143. doi: 10.1007/BF01565472. [DOI] [PubMed] [Google Scholar]
- 3.Cabaud HE, Rodkey WG, Feagin JA. Experimental studies of acute anterior cruciate ligament injury and repair. Am J Sports Med. 1979;7:18–22. doi: 10.1177/036354657900700105. [DOI] [PubMed] [Google Scholar]
- 4.O’Donoghue DH, Rockwood C, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg [Am] 1966;48:503–519. [PubMed] [Google Scholar]
- 5.O’Donoghue DH, Frank GR, Jeter GL, Johnson W, Zeiders JW, Kenyon R. Repair and reconstruction of the anterior cruciate ligament in dogs. Factors influencing long-term results. J Bone Joint Surg [Am] 1971;53:710–718. [PubMed] [Google Scholar]
- 6.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg [Am] 2000;82:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kroon ME, van Schie ML, van der Vecht B, van Hinsbergh VW, Koolwijk P. Collagen type 1 retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis. 2002;5:257–265. doi: 10.1023/a:1024540701634. [DOI] [PubMed] [Google Scholar]
- 8.Magarian E, Mastrangelo A, Connolly SA, Vavken P, Murray MM. Safety of intra-articular use of atelocollagen for enhanced tissue repair. Trans Orthop Res Soc. 2009;34:1259. doi: 10.2174/1874325001206010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, Ballard P, Nanney LB, Zurakowski D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 10.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 11.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 12.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: An in vivo study. J Orthop Res. 2009;27:639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 14.Adams JE, Zobitz ME, Reach JS, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Mizuta H. Reconstruction of large rotator-cuff tears with acellular dermal matrix grafts in rats. J Shoulder Elbow Surg. 2009;18:288–295. doi: 10.1016/j.jse.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Yokoya S, Mochizuki Y, Nagata Y, Deie M, Ochi M. Tendon-bone insertion repair and regeneration using polyglycolic acid sheet in the rabbit rotator cuff injury model. Am J Sports Med. 2008;36:1298–1309. doi: 10.1177/0363546508314416. [DOI] [PubMed] [Google Scholar]
- 17.Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg [Am] 2009;91:1159–1171. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacGillivray JD, Fealy S, Terry MA, Koh JL, Nixon AJ, Warren RF. Biomechanical evaluation of a rotator cuff defect model augmented with a bioresorbable scaffold in goats. J Shoulder Elbow Surg. 2006;15:639–644. doi: 10.1016/j.jse.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Davison PF, Levine L, Drake MP, Rubin A, Bump S. The serologic specificity of tropocollagen telopeptides. J Exp Med. 1967;126:331–346. doi: 10.1084/jem.126.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 21.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anterioposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming BC, Spindler KP, Palmer M, Magarian E, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing ACL grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson GP, Breur GJ, Van Sickle D, Yao JQ, Kim J, Blanchard CR. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J Shoulder and Elbow Surg. 2007;16:S184–190. doi: 10.1016/j.jse.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 25.Dressler MR, Butler DL, Boivin GP. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J Orthop Res. 2005;23:287–293. doi: 10.1016/j.orthres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, Butler DL. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12:369–379. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- 27.Shearn JT, Juncosa-Melvin N, Boivin GP, Galloway MT, Goodwin W, Gooch C, Dunn MG, Butler DL. Mechanical stimulation of tendon tissue engineered constructs: effects on construct stiffness, repair biomechanics, and their correlation. J Biomech Eng. 2007;129:848–854. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- 28.Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 29.Butler DL, Awad HA. Perspectives on cell and collagen composites for tendon repair. Clin Orthop. 1999;367:S324–332. doi: 10.1097/00003086-199910001-00031. [DOI] [PubMed] [Google Scholar]
- 30.Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 31.Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effect of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 32.Xerogeanes JW, Fox RJ, Takeda Y, Hyoung-Soo K, Ishibashi Y, Carlin GJ, Woo SL-Y. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Engin. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]
- 33.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Effect of anterior translation on total knee force in a porcine model. International Symposium of Ligament and Tendon VIII; San Francisco. 2008. [Google Scholar]
- 34.Mueller XM, Tevaearai HT, Jegger D, Tucker O, von Segesser LK. Are standard human coagulation tests suitable in pigs and calves during extracorporeal circulation? Artif Organs. 2001;25:579–584. doi: 10.1046/j.1525-1594.2001.025007579.x. [DOI] [PubMed] [Google Scholar]
- 35.Joshi S, Mastrangelo A, Magarian E, Fleming BC, Murray MM. Collagen-Platelet Composite Enhances Biomechanical and Histologic Healing of the Porcine ACL. Am J Sports Med. 2009 doi: 10.1177/0363546509339915. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank C, Amiel D, Woo SL, Akeson W. Normal ligament properties and ligament healing. Clin Orthop. 1985;196:15–25. [PubMed] [Google Scholar]