Abstract
The authors describe a 9 month old female with recurrent atypical central neurocytoma and leptomeningeal spread treated with high dose chemotherapy, autologous stem cell rescue, and adjuvant therapy. She had a complete response to therapy and was disease free at 4 years of age until a recurrence 6 months later. The use of intensive chemotherapy followed by autologous stem cell rescue for atypical neurocytoma may be considered as an adjunct to surgical therapy in young patients with atypical neurocytoma not amenable to radiation therapy.
Keywords: Atypical central neurocytoma, Autologous stem cell rescue, Myeloablative chemotherapy
Introduction
Atypical central neurocytomas are rare central nervous system tumors in the pediatric population [1, 2]. They are treated unsuccessfully with gross total resection and require consolidative radiation therapy or chemotherapy to prevent recurrence [3–5]. The atypical nature and aggressive behavior of the central neurocytoma we describe in our patient along with her young age led to the use of systemic chemotherapy. The use of chemotherapy in the treatment of typical and atypical central neurocytoma is reported in adults and children [6–16]. This is the second report describing the use of high dose chemotherapy followed by autologous stem cell rescue in a child with atypical central neurocytoma [17].
Case report
We describe a 14 month old female with episodic agitation upon awakening followed by a very intense episode of agitation with associated respiratory compromise. She was transported to a local hospital where CT imaging demonstrated a large hemorrhagic lesion in the right frontal lobe extending into the right lateral ventricle (see Fig. 1a). A gross total resection of this hemorrhagic lesion was completed as demonstrated by CT imaging (see Fig. 1b). Microscopic evaluation showed an atypical neurocytic tumor (see Fig. 2a–d). The histology was that of a mildly pleomorphic round cell tumor with diffuse, strong immunolabeling for synaptophysin. The tumor cells were negative for glial fibrillary acidic protein (GFAP). Atypical features were noted including the presence of mitoses, glomeruloid vascular proliferation, and necrosis. The Ki-67 labeling index was 10% overall though focally was higher. A decision to avoid consolidative radiation therapy was made secondary to her young age.
Fig. 1.
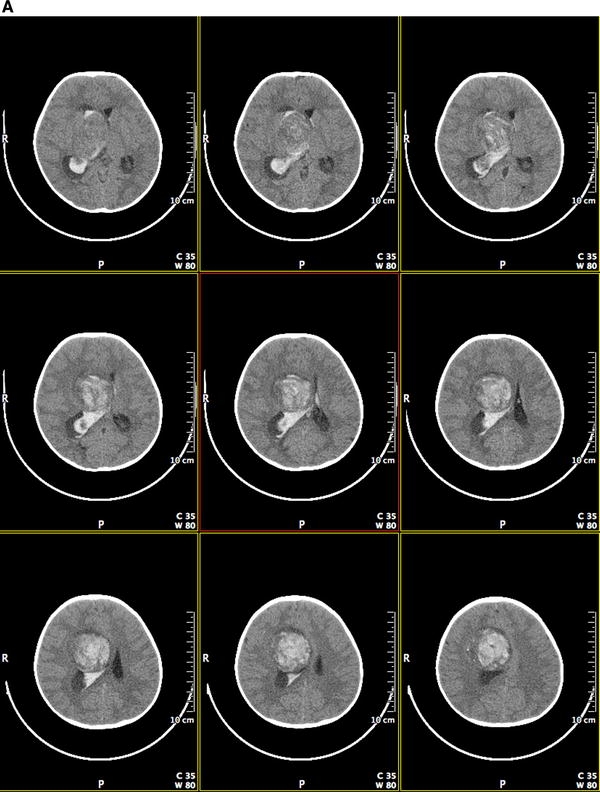
a Preoperative post contrast coronal CT images demonstrating a hemorrhagic lesion in the right frontal lobe extending into the right lateral ventricle. b Postoperative post contrast coronal CT images demonstrating a gross total resection of the right frontal lesion
Fig. 2.
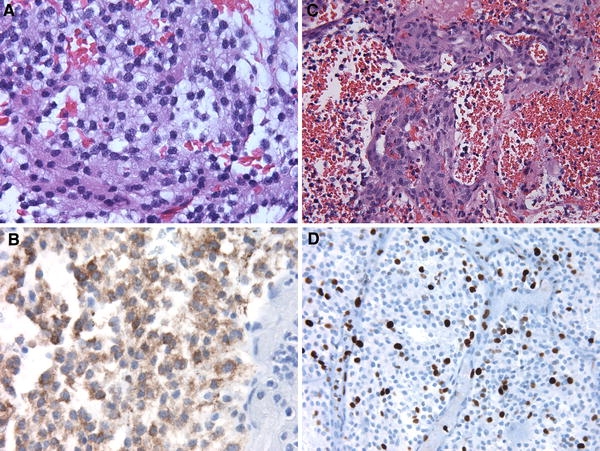
a Microscopic evaluation showed an atypical neurocytic tumor composed of cells with round to oval nuclei. (H&E 400×). b Diffuse, strong immunolabeling for synaptophysin was demonstrated. (400×). c Atypical features were noted including prominent glomeruloid vascular proliferation (H&E 400×). d The Ki-67 labeling index was approximately 10% overall (400×)
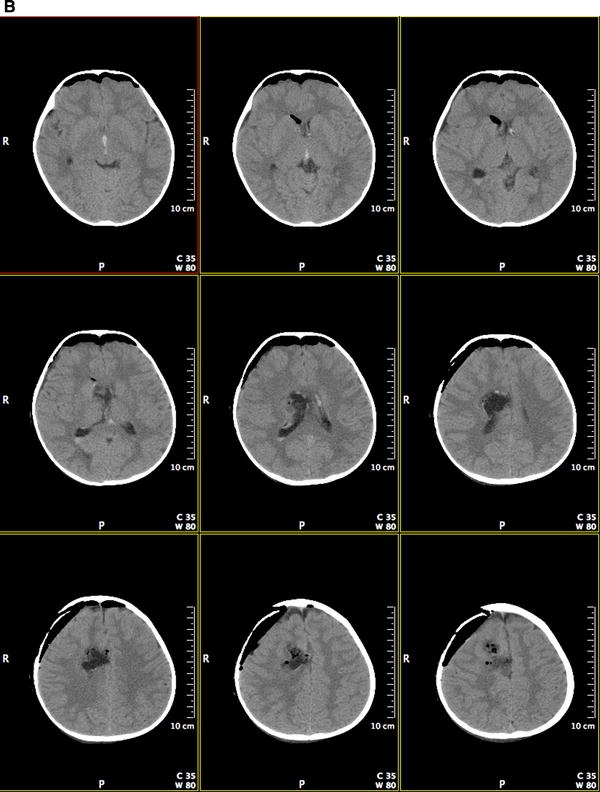
Two months after her resection she developed a recurrence with obstructive hydrocephalus and midline shift requiring placement of a ventriculo-peritoneal shunt. Imaging demonstrated diffuse meningeal enhancement as well as interval development of an area of local enhancement concerning for recurrence (see Fig. 3). Her CSF was negative for evidence of malignant cells. She subsequently received 3 cylces of chemotherapy including vincristine (0.05 mg/kg/dose, Days 1 & 8), cisplatin (3.5 mg/kg/dose, Day 2), and etoposide (2.5 mg/kg/dose, Days 1–3). This was followed by GCSF beginning on day 3. Imaging demonstrated a partial response with decreased leptomeningeal and local enhancement in the resection cavity. She was then given 2 cycles of temodar for (Days 1–5).
Fig. 3.
Post contrast coronal and axial T1-weighted images show nodular enhancement (black arrow) along the margin of the right posterior frontal resection cavity, suggesting recurrent tumor
At 24 months she was noted to have development of a cystic lesion in the resection cavity and increased leptomeningeal enhancement. As a result, she then received 2 cycles of cytoxan (55 mg/kg/dose, Days 1–2) and MESNA; however, the second cycle was complicated by hyponatremia and seizures. She was then placed back on the chemotherapy regimen consisting of vincristine, cisplatin, and etoposide. Repeat imaging demonstrated stable disease.
At 28 months of age prior to the completion of additional chemotherapy she underwent GCSF mobilized peripheral blood stem cell collection. A total of 12 × 106 CD34+ cells/kg were collected in anticipation of high dose chemotherapy and autologous stem cell rescue. Additional chemotherapy was provided with 3 cycles of ifosfamide (1800 mg/m2/dose Days 1–5), carboplatin (400 mg/m2/dose Days 1–2), and etoposide (100 mg/m2/dose Days 1–5). Repeat imaging was completed demonstrating stable disease.
Due to a lack of significant clinical response and nephrotoxicity (hypomagnesaemia, hypokalemia, and academia) she was then treated with cytoxan and topotecan per Pediatric Oncology Group Protocol 9464. She received one cycle of therapy followed by imaging demonstrating interval progression of her leptomeningeal disease.
At 34 months of age, high dose chemotherapy per the consolidation phase of Children’s Cancer Group Protocol 99703 was started. This chemotherapy regimen consisted of carboplatin (17 mg/kg, Days 0–1) and thiotepa (10 mg/kg, Days 0–1). This was followed by a day of rest, stem cell infusion, and then GCSF. This phase was repeated every 4 weeks for 3 cycles with the infusion of 2.4 × 106 CD34+ cells/kg, 3.68 × 106 CD34+ cells/kg, and 3.86 × 106 CD34+ cells/kg, respectively. Toxicity included fever and neutropenia.
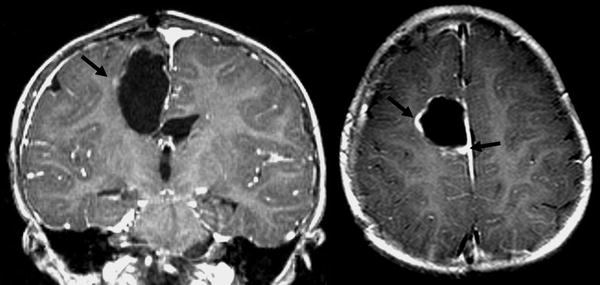
At 40 months of age she was disease free by imaging criteria and was started on isotretinoin (8 mg/kg/day, 14 days/month) and oral etoposide (3 mg/kg/day, 21 days/month) for 8 months until the development of neutropenia. Unfortunately, 6 months later abnormal enhancement in the cervical, thoracic, or lumbar spine was noted on MRI, suggesting recurrent disease (see Fig. 4). Surgical biopsy confirmed the diagnosis. Following her most recent relapse, a decision was made to begin therapy with temodar (5 days per week every 2 weeks) and irinotecan (every 2 weeks). She is tolerating this therapy without complications and is currently waiting for re-imaging.
Fig. 4.
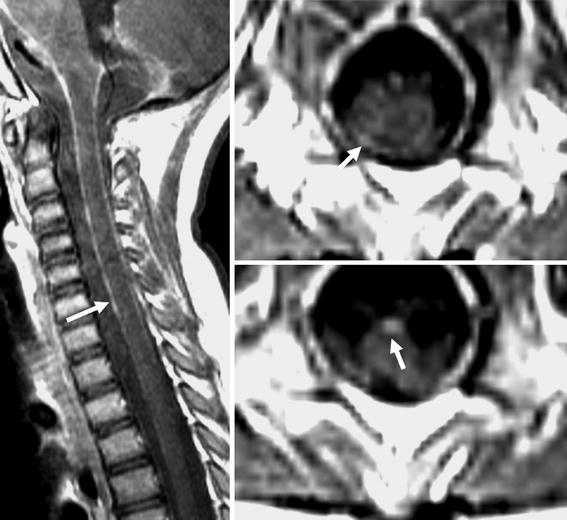
Post contrast sagittal T1 and axial T1-weighted images show enhancement of the surface of the cord (white arrow), suggesting recurrent disease
Discussion
Central neurocytoma, first described in 1982, is a rare central nervous system (CNS) tumor that accounts for 0.1–0.5% of CNS tumors [1, 2]. This tumor typically affects young adults in their third decade of life [1, 2]. Neurocytomas are believed to arise from subependymal plate of lateral ventricles and are believed to be neuronal or neuroglial in origin [1, 2]. Histologically they are divided into typical and atypical central neurocytomas [1, 2]. Microscopically they appear as small round cells with round nuclei and scant cytoplasm and will often stain positive for synaptophisin. Typical central neurocytomas are well differentiated and benign appearing; however, atypical central neurocytomas are less well differentiated and malignant appearing. The presence of necrosis, increased mitotic activity, and vascular proliferation is not uncommon. Clinically they also behave aggressively.
Treatment of atypical central neurocytoma in children relies upon surgical therapy and radiation therapy [3–5]. Rades and colleagues provided a retrospective review 438 cases of central neurocytoma [5]. Of these 438 cases 73 patients were 18 years of younger at the time of initial surgery. The median age of the sample was 16 years. Typical central neurocytomas comprised 62 individuals in the sample and atypical central neurocytomas only 11 individuals. A median follow up of 36 months was provided. Those individuals that had a complete resection with or without radiation therapy had an excellent 5 year overall survival rate. Those individuals that had an incomplete resection followed by radiation therapy achieved improved local control rates, but did not benefit from improved overall survival. This data suggests that durable cures are likely in individuals that achieve a complete resection; however, this data did not parse out responses of atypical central neurocytomas from typical neurocytomas.
Based upon the extant literature present in Table 1, there is little experience with the use of conventional chemotherapy for atypical central neurocytoma and high dose chemotherapy followed by autologous stem cell transplant for atypical central neurocytoma [6–17]. Our patient was disease free by imaging criteria following the completion of high dose chemotherapy and autologous stem cell rescue which is a notable difference when looking at the cases described previously; however, the duration of this remission was brief. Myeloablative chemotherapy followed by autologous stem cell rescue has been reported as an effective consolidative therapy for patients with malignant brain tumors and those individuals that are not candidates for radiation therapy [18–21]. Avoidance or delay of radiation therapy use may also help limit some of the potential long-term complications of craniospinal irradiation including increased risk of future second cancers, neuro-cognitive dysfunction, and endocrinologic deficits [22–24].
Table 1.
Summary of reported cases of neurocytoma treated with chemotherapy
| Study | Age/gender | Clinical history/therapy | Chemotherapy | Outcome |
|---|---|---|---|---|
| Amini et al. [17] | 5 years old/male | (1) Resected and treated with adjuvant chemotherapy | (1) Adjuvant therapy: VCR and cisplatin monthly alternating with cyclophosphamide. He completed 5 cycles | Stable disease with no evidence of recurrence or progression |
| (2) Local recurrence one year later which was reselected | (2) No chemotherapy | |||
| (3) Disseminated recurrence 5 months later | (3) Gleevec (200 mg/m2 twice daily) with a lack of response followed by local radation and temozolamide (75 mg/m2/day) followed by maintenance (175 mg/m2/day) for 5 days every 4 w aeks, complete response | |||
| (4) Local recurrence 9 months later | (4) IT liposomal Ara-C (2 courses) and radiosurgery followed by Temodar maintenance, complet | |||
| (5) Spinal metastasis treated with induction chemotherapy | ||||
| Brandes et al. [6] | 43 years old/female | Stereotactic radiotherapy led to a complete response Recurrence was noted six years later and was treated with chemotherapy | Etoposide (40 mg/m2/day, Days 1–4), cisplatin (25 mg/m2/day, Days 1–4), and cytoxan (1000 mg/m2, Day 4) Cycles were repeated every 4 weeks. She completed 5 cycles | Partial repsonse for a follow-up of 15 months |
| Brandes et al. [6] | 61 years old/male | Limited field radiation was completed with a partial response noted and stable disease for 5 years Recurrent disease developed and was treated with chemotherapy. | Etoposide (40 mg/m2/day, Days 1-4), cisplatin (25 mg/m2/day, Days 1–4), and cytoxan (1000 mg/m2, Day 4) Cycles were repeated every 4 weeks. He completed 5 cycles | Stable disease for a follow-up of 18 months |
| Brandes et al. [6] | 22 years old/female | Total resection was completed Recurrent disease 3 years later with ventrcular disease and a spinal | Etoposide (40 mg /m2/day, Days cisplatin (25 mg/m2/day, Days 1–4), and cytoxan (1000 mg/m2, Day 4) Cycles were repeated every 4 weeks She completed 3 cycles | Complete response of the spinal lesion and stable ventricular disease Craniospinal radiation led to a complete response for a follow-up of 36 months |
| Coelho et al. [7] | 6 years old/male | Recurrent ventrile and thalamus and peritoneal dissemination 3 5 yrs after subtotal resection VP shunt for hydrocephalus | Etoposide, carboplatin, doxorubicin, cyclophosphamide | Died 3 days after diagnosis of disemmination |
| Dodds et al. [8] | 15 years old/male | Subtotal resection completed along with placement of a VP shunt Treated with chemotherapy | Carboplatin (500 mg/m2, Day 1–2), etoposide (100 mg/m2, Days 1–3), and ifosfamide (3 g/m2, Days 1–3) The etoposide and ifosfamide are repeated at week. 3 He completed 4 cycles | Response with tumor shrinkage for 22 months until symptoms and tumor growth treated with subtotal resection and radiation therapy Stable disease with a follow-up period of 6 years. Complication of nephrotoxicity |
| Eng et al. [9] | 22 years old/female | Subtotal resection completed along with placement of a VP shunt A local recurrence occurred 20 months later and a second subtotal resection was done. A ventricular recurrence and leptomenningeal enhancement were noted 14 months later and treated with chemotherapy. | Cyclophosphamide, etoposide, and cisplatin | Not reported |
| Kulkarni et al. [10] | 21 years old/male | Radiation therapy and placement of bilateral VP shunts and chemotherapy | Lomustine (7 doses) | Partial response with follow-up for 60 months followed by death |
| Kulkarni et al. [10] | 21 years old/female | Radiation therapy and chemotherapy | Lomustine (9 doses) | Subependymal spread with increased size of primary Lost to follow-up at 15 months |
| Kulkarni et al. [10] | 14 years old/female | Radiation therapy and chemotherapy | Lomustine (7 doses) | No change in tumor with follow-up of 108 months Underwent shunt surgery |
| Kulkarni et al. [10] | 45 years old/male | Radiation therapy and placement of VP shunt and chemotherapy | Lomustine (8 doses) | No change in tumor with follow-up of 90 months |
| Kulkarni et al. [10] | 38 years old/male | Radiation therapy and chemotherapy | Lomustine (9 doses) | No change in tumor with follow-up of 114 months |
| Kulkarni et al. [10] | 27 years old/female | Radiation therapy and placement of bilateral VP shunts and chemotherapy | Lomustine (7 doses) | No change in tumor with follow-up of 96 months |
| Leenstra et al. [11] | 17 years old/female | Radiation therapy followed by 1st recurrence treated with total resection at 45 months Second, third, and fourth recurrences with local and drop metastases treated with chemotherapy at 150, 172, and 185 months, respectively | Etoposide (60 mg/m2 Days 1–3, cisplatin (20 mg/m2 Days 1–5), cytoxan (500 mg/m2 Day 1 every 4 weeks She completed 7 cylces with second recurrence Four more cycles were given for third recurence Then given Carboplatin (250 mg /m2 Day 1 and ifosfamide 1 35 g/m2 Days 1–3) with Mesna for fourth recurrence | Partial responses with 4 relapses as described Had seizures after 1 st cycle of chemotherapy and stroke after 4th cycle Died 28 months after completion of initial salvage chemotherapy |
| Leenstra et al. [11] | 20 years old/male | Total resection with second total resection at 3 months following local recurrence. Second relapse at 6 months treated with chemotherapy | Cisplatin (75 mg/m2/day, Day 1), vincristine (15 mg/m2 Days 7 and 14), cytoxan (1000mg/m2, Days 21 and 22) with MESNA. He completed 4 cycles | Alive with disease at 12 months of follow-up |
| Leenstra et al. [11] | 25 years old/male | Subtotal resection, radiation therapy and chemotherapy | Cisplatin (50 mg/m2/day, Days 1–3), and ranimustine (50 mg/m2 D1) | Stable disease, no recurrence, no progression Alive 3.7 years |
| Leenstra et al. [11] | 8 years old/male | Total resection, radiation therapy and chemotherapy | Cisplatin, vincristine, and prednisone every 8 weeks | No disesase or recurrence Alive at 10.8 years Cognitive deficits |
| Leenstra et al. [11] | 23 years old/female | Total resection, radiation therapy and chemotherapy | lomustine (200 mg/m2/day, Days 1), and carmustine (200 mg/m2 D1), every 6 weeks. She completed 5 cycles |
Alive at 11.2 years. No disease. No recurrence |
| Louis et al. [12] | 17 years old/male | Subtotal resection, radiation therapy and chemotherapy | Cyclophosphamide and cisplatin | Alive and well at 14 months. No recurrence or progression |
| Louis et al. [12] | 26 years old/female | Subtotal resection, radiation therapy and chemotherapy | Cyclophosphamide and cisplatin | Alive and well at 11 months. No recurrence or progression |
| Ogawa et al. [13] | 34 years old/male | Subtotal resection followed by radiosurgery. Continued progression followed by raidtiation therapy and chemotherapy | Etoposide, cisplatin , and cytoxan. She completed 3 cycles | Parital response with eventual dissemination and death one year after radiation therapy |
| Ogawa et al. [13] | 71 years old/female | Radiation therapy followed by chemotherapy | ACNU | Developed disseminated disease and died 1.5 years after diagnosis |
| Sgourous et al. [14] | 19 years old/female | Resection followed by recurrence at 12 months Second resection followed by radiation Response noted, but developed progressive disease treated with chemotherapy | Carboplatin | Partial repsonse for a “few” months |
| Swinson et al. [15] | 58 years old/male | Radiation therapy and chemotherapy followed by progression 5 months later treated with subtotal resection | Temozolamide (175 mg/ 2/day Days 1–5 of 28) He completed 3 cycles | Stable disease on chemotherapy. Developed symptoms of progression 2.5 months later followed by subtotal resection No residual or recurrent tumor at 28 months of follow-up |
| Von Koch et al. [16] | 15 years old/female | Several subtotal resections over a 3 year period followed by tumor progression treated with chemotherapy | Procarbazine (60 mg/m2, Days 8–21), CCNU (110 mg/m2, Day 1), and VCR (14 mg/m2 , Days 8 and 29). She completed 6 cycles | Durable response for at least 16 months of follow-up |
Moreover, the use of novel therapeutic agents should be considered in these patients as we described above. As an example, the use of retinoids in the care of children with brain tumors is under investigation [25, 26]. Their potent anti-tumor effect which occurs by induction of neuronal differentiation, growth arrest, and apoptosis has been demonstrated in childhood cancers with neuronal differentiation such as neuroblastoma [27]. Isotretinoin and other novel retinoids are currently under investigation as therapeutic adjuncts in a host of childhood cancers including brain tumors. Another novel modality used for this patient was low-dose continuous chemotherapy or “metronomic” therapy with oral etoposide. Several recent articles have successfully utilized metronomic combinations of agents such as oral etoposide, isotretinoin, thalidomide, cyclophosphamide, and celecoxib in children and adults with brain tumors [28, 29]. The mechanism of metronomic therapy is believed to be due in part to anti-angiogenic effects although further research is needed to fully understand the complete biology of this approach. A recent publication described the use of metronomic therapy for ten pediatric patients under 5 years old with malignant brain tumors following high-dose chemotherapy and autologous stem cell rescue [30]. Similar to our case, it was tolerated well and had encouraging activity suggesting that this approach may warrant further evaluation in larger clinical trials.
Conclusion
Atypical central neurocytoma is a rare central nervous system tumor in children. Surgical resection and radiation therapy serves as the mainstay of therapy for atypical central neurocytoma. The toxicity of radiation therapy in young children poses a risk to the use of this therapy in the treatment of atypical central neurocytomas including examples such as neuro-cognitive deficits and second cancers. Those young patients with atypical neurocytomas that behave aggressively may also be candidates for the use of systemic chemotherapy. Limited experience with the use of chemotherapy and myeloablative chemotherapy followed by autologous stem cell rescue in central neurocytoma is described. We add to the literature a case describing the use of intensive chemotherapy followed by autologous stem cell rescue. Further exploration of this potentially effective modality of therapy in combination with other novel adjunctive therapeutic agents and approaches is needed in patients with atypical central neurocytoma that is refractory to traditional curative therapy.
Acknowledgements
The authors would also like to acknowledge the support of the Brain Tumor Translational Resource, Jonsson Cancer Center, and Brain Research Institute, David Geffen School of Medicine at UCLA.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
David Buchbinder, Phone: +714-532-8459, FAX: 714-532-8771, Email: dbuchbinder@choc.org.
Moise Danielpour, Phone: +310-423-7900, FAX: +310-423-7955, Email: Moise.Danielpour@cshs.org.
William H. Yong, Phone: +310-825-8269, Email: WYong@mednet.ucla.edu
Noriko Salamon, Phone: +310-206-7308, Email: nsalamon@mednet.ucla.edu.
Joseph Lasky, Phone: +310-222-2345, Email: jlasky@labiomed.org.
References
- 1.Schmidt MH, Gottfried ON, von Koch CS, Chang SM, McDermott MW. Central neurocytoma: a review. J Neurooncol. 2004;66:377–384. doi: 10.1023/B:NEON.0000014541.87329.3b. [DOI] [PubMed] [Google Scholar]
- 2.Sharma MC, Deb P, Sharma S, Sarkar C. Neurocytoma: a comprehensive review. Neurosurg Rev. 2006;29:270–285. doi: 10.1007/s10143-006-0030-z. [DOI] [PubMed] [Google Scholar]
- 3.Rades D, Fehlauer F, Schild SE. Treatment of atypical neurocytomas. Cancer. 2004;100:814–817. doi: 10.1002/cncr.20032. [DOI] [PubMed] [Google Scholar]
- 4.Rades D, Schild SE, Fehlauer F. Defining the best available treatment for neurocytomas in children. Cancer. 2004;101:2629–2632. doi: 10.1002/cncr.20695. [DOI] [PubMed] [Google Scholar]
- 5.Rades D, Schild SE. Treatment Recommendations for the various subgroups of neurocytomas. J Neurooncol. 2006;77:305–309. doi: 10.1007/s11060-005-9047-3. [DOI] [PubMed] [Google Scholar]
- 6.Brandes AA, Amistà P, Gardiman M, Volpin L, Danieli D, Guglielmi B, Carollo C, Pinna G, Turazzi S. Monfardini S: Chemotherapy in Patients with Recurrent and Progressive Central Neurocytoma. Cancer. 2000;88:169–174. doi: 10.1002/(SICI)1097-0142(20000101)88:1<169::AID-CNCR23>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Coelho Neto M, Ramina R, de Meneses MS, Arruda WO, Milano JB. Peritoneal dissemination from central neurocytoma: case report. Arq Neuropsiquiatr. 2003;61:1030–1034. doi: 10.1590/s0004-282x2003000600028. [DOI] [PubMed] [Google Scholar]
- 8.Dodds D, Nonis J, Mehta M, Rampling R. Central Neurocytoma: a clinical study of response to chemotherapy. J Neurooncol. 1997;34:279–283. doi: 10.1023/A:1005713909836. [DOI] [PubMed] [Google Scholar]
- 9.Eng DY, DeMonte F, Ginsberg L, Fuller GN, Jaeckle K. Craniospinal dissemination of central neurocytoma: a report of 2 cases. J Neurosurg. 1997;86:547–552. doi: 10.3171/jns.1997.86.3.0547. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni V, Rajshekhar V, Haran RP, Chandi SM. Long-term outcome in patients with central neurocytoma following stereotactic biopsy and radiation therapy. Br J Neurosurg. 2002;16:126–132. doi: 10.1080/02688690220131714. [DOI] [PubMed] [Google Scholar]
- 11.Leenstra JL, Rodriguez FJ, Frechette CM, Giannini C, Stafford SL, Pollock BE, Schild SE, Scheithauer BW, Jenkins RB, Buckner JC, Brown PD. Central neurocytoma: management recommendations based on a 35-year experience. Int J Radiat Oncol Biol Phys. 2007;67:1145–1154. doi: 10.1016/j.ijrobp.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Louis DN, Swearingen B, Linggood RM, Dickersin GR, Kretschmar C, Bhan AK, Hedley-Whyte ET. Central nervous system neurocytoma and neuroblastoma in adults—report of eight cases. J Neurooncol. 1990;9:231–238. doi: 10.1007/BF02341154. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa Y, Sugawara T, Seki H, Sakuma T. Central neurocytomas with MIB-1 labeling index over 10% showing rapid tumor growth and dissemination. J Neurooncol. 2007;79:211–216. doi: 10.1007/s11060-006-9129-x. [DOI] [PubMed] [Google Scholar]
- 14.Sgouros S, Carey M, Aluwihare N, Barber P, Jackowski A. Central neurocytoma: a correlative clinicopathologic and radiologic analysis. Surg Neurol. 1998;49:197–204. doi: 10.1016/S0090-3019(97)00017-7. [DOI] [PubMed] [Google Scholar]
- 15.Swinson BM, Friedman WA, Yachnis AT. Pontine atypical neurocytoma: case report. Neurosurgery. 2006;58:E990. doi: 10.1227/01.NEU.0000210213.12847.1E. [DOI] [PubMed] [Google Scholar]
- 16.von Koch CS, Schmidt MH, Uyehara-Lock JH, Berger MS, Chang SM. The role of PCV chemotherapy in the treatment of central neurocytoma: illustration of a case and review of the literature. Surg Neurol. 2003;60:560–565. doi: 10.1016/S0090-3019(03)00252-0. [DOI] [PubMed] [Google Scholar]
- 17.Amini E, Roffidal T, Lee A, Fuller GN, Mahajan A, Ketonen L, Kobrinsky N, Cairo MS, Wells RJ, Wolff J. Central neurocytoma responsive to Topotecan, Ifosfamide, Carboplatin. Pediatr Blood Cancer. 2008;51:137–140. doi: 10.1002/pbc.21551. [DOI] [PubMed] [Google Scholar]
- 18.Mason WP, Grovas A, Halpern S, Dunkel IJ, Garvin J, Heller G, Rosenblum M, Gardner S, Lyden D, Sands S, Puccetti D, Lindsley K, Merchant TE, O’Malley B, Bayer L, Petriccione MM, Allen J, Finlay JL. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 1998;16:210–221. doi: 10.1200/JCO.1998.16.1.210. [DOI] [PubMed] [Google Scholar]
- 19.Finlay JL, Dhall G, Boyett JM, Dunkel IJ, Gardner SL, Goldman S, Yates AJ, Rosenblum MK, Stanley P, Zimmerman RA, Wallace D, Pollack IF, Packer RJ. Children’s Cancer Group. Myeloablative chemotherapy with autologous bone marrow rescue in children and adolescents with recurrent malignant astrocytoma: outcome compared with conventional chemotherapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:806–811. doi: 10.1002/pbc.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broniscer A, Nicolaides TP, Dunkel IJ, Gardner SL, Johnson J, Allen JC, Sposto R, Finlay JL. High-dose chemotherapy with autologous stem-cell rescue in the treatment of patients with recurrent non-cerebellar primitive neuroectodermal tumors. Pediatr Blood Cancer. 2004;42:261–267. doi: 10.1002/pbc.10369. [DOI] [PubMed] [Google Scholar]
- 21.Modak S, Gardner S, Dunkel IJ, Balmaceda C, Rosenblum MK, Miller DC, Halpern S, Finlay JL. Thiotepa-based high dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol. 2004;22:1934–1943. doi: 10.1200/JCO.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 22.Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S, Yasui Y, Kasper CE, Mertens AC, Donaldson SS, Meadows AT, Inskip PD. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 23.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, Stovall M, Yasui Y, Nicholson HS, Wolden S, McNeil DE, Mertens AC, Robison LL. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97:663–673. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 24.Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, Yasui Y, Mertens AC, Sklar CA, Nicholson HS, Zeltzer LK, Neglia JP, Robison LL. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 25.Gumireddy K, Sutton LN, Phillips PC, Reddy CD. All-trans-retinoic acid-induced apoptosis in human medulloblastoma: activation of caspase-3/poly(ADP-ribose) polymerase 1 pathway. Clin Cancer Res. 2003;9:4052–4059. [PubMed] [Google Scholar]
- 26.Damodar Reddy C, Guttapalli A, Adamson PC, Vemuri MC, O’Rourke D, Sutton LN, Phillips PC. Anticancer effects of fenretinide in human medulloblastoma. Cancer Lett. 2006;231:262–269. doi: 10.1016/j.canlet.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB, Villablanca JG. Long-term results for children with high- risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncology. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, Klement G, Laforme A, Gordon A, Thomas A, Neuberg D, Browder T, Folkman J. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 29.Kesari S, Schiff D, Doherty L, Gigas DC, Batchelor TT, Muzikansky A, O’Neill A, Drappatz J, Chen-Plotkin AS, Ramakrishna N, Weiss SE, Levy B, Bradshaw J, Kracher J, Laforme A, Black PM, Folkman J, Kieran M, Wen PY. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9:354–363. doi: 10.1215/15228517-2007-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi LM, Rood B, Kamani N, Fond DL, Packer RJ, Santi MR, MacDonald TJ. Feasibility of metronomic maintenance chemotherapy following high-dose chemotherapy for malignant central nervous system tumors. Pediatr Blood Cancer. 2008;50:970–975. doi: 10.1002/pbc.21381. [DOI] [PubMed] [Google Scholar]


