Abstract
Conclusions
Reduction in bone formation may have been the main reason for the lower calcium content of the otoconia after simulated weightlessness in rats. The head-ward distribution of blood volume may explain the morphological changes observed in the middle and inner ears.
Objective
To observe morphological changes in the vestibular organs and measure the calcium content of otoconia in rats after simulated weightlessness.
Material and methods
We used a tail suspension model of simulated weightlessness and then investigated changes in the vestibular organs using scanning electron microscopy and X-ray microanalysis.
Results
In comparison to untreated rats, the vestibular otoconia of the rats subjected to simulated weightlessness were small, irregularly shaped or fissured, and were arranged loosely and out of order. In addition, the calcium content of the otoconia was markedly decreased.
Keywords: Otolith, saccular, simulated weightlessness, space motion sickness, tail suspension, utricle, X-ray microanalysis
Introduction
Since Yuri Gagarin’s historic first flight into space in April 1961, it has become evident that the environment of outer space influences the human body in many different ways and causes it to adapt in ways that can lead to problems when returning to Earth’s gravity. However, many questions, particularly those regarding how to counteract the changes that we now know take place, still need to be addressed through studies on the International Space Station and through simulations on the ground. Space motion sickness is a common problem during space flight and severely influences the ability of astronauts to work effectively. However, at present, the mechanism underlying this condition is not very clear. The function of astronauts’ vestibular organs plays an important role in their space activities. The role of the vestibular organs during space exploration has been studied extensively during the past two decades. Many investigators [1] have shown that some people experience ill effects during the transition from normal gravity to microgravity or weightlessness. In humans, altered gravity may lead to vestibular dysfunction and space motion sickness. It was hypothesized that asymmetric inner ear statoliths might be the morphological basis of space sickness. An animal model, using fish [2], revealed further information: inner ear “stone” (otolith) growth is dependent on the amplitude and direction of gravity, as regulated by a feedback mechanism.
Loss of skeletal mass is a potentially serious consequence of long-term space flight. Skylab astronauts exhibited a significant decline in the bone mineral density of the calcaneus after 84 days of orbital flight [3]. The vestibular systems of animals reared in altered gravity have been studied in several species, with varied results having been reported. Early Russian reports [4] of Xenopus larvae reared in space indicated no qualitative differences in the vestibular organs compared to ground-reared controls. Lim et al. [5] found no differences in the volume of the saccular otolith between centrifuged and control adult rats. To further study the changes in vestibular organs and the mechanism of space motion sickness, we used scanning electron microscopy and X-ray microanalysis to observe the morphological changes of the vestibular organs in rats after simulated weightlessness and then tested the calcium content of the otoconia.
Material and methods
Simulating weightlessness
We suspended Wistar rats (mean weight 200 g) from their tails (head-down tilt at 30° for 160 days) in order to initiate a shift of body fluids similar to that experienced during space flight. Total mechanical demobilization of the hind limbs occurred, but the forelimbs remained in contact with the floor to allow movement and continuous access to food and water. The weight and food consumption of 18 rats were monitored. Another 18 control rats were housed individually. Their food consumption was matched to that of the suspended rats. All animals were housed in a room maintained at 24°C and illuminated with fluorescent light for 12 h daily.
Preparation of specimens and observations
The rats were anesthetized by i.m. injection of pentobarbital (50 mg/kg) and necropsied under an anatomy microscope. The temporal bones were dissected in 2.5% glutaraldehyde and then fixed for 2 h at room temperature. Microdissection was performed to expose the surface of the vestibular sensory epithelium. Samples were then treated with 1% osmium tetroxide for 15 min, dehydrated in ethanol, critical point-dried and sputter-coated with gold. The morphology and the calcium content of otoconia were examined using a scanning electron microscope (S500; Hitachi, Japan) and an X-ray microanalysis instrument (UX-7000; Kevex, Japan), respectively. An acceleration voltage of 20.48 keV was used when measuring the calcium content of the otoconia of control and suspended rats. Values for the y-axis varied between 2048 and 163 848 counts/s. Amplifications were ×1000. Differences between the groups were compared by means of Student’s t-test.
Results
Scanning electron microscopy
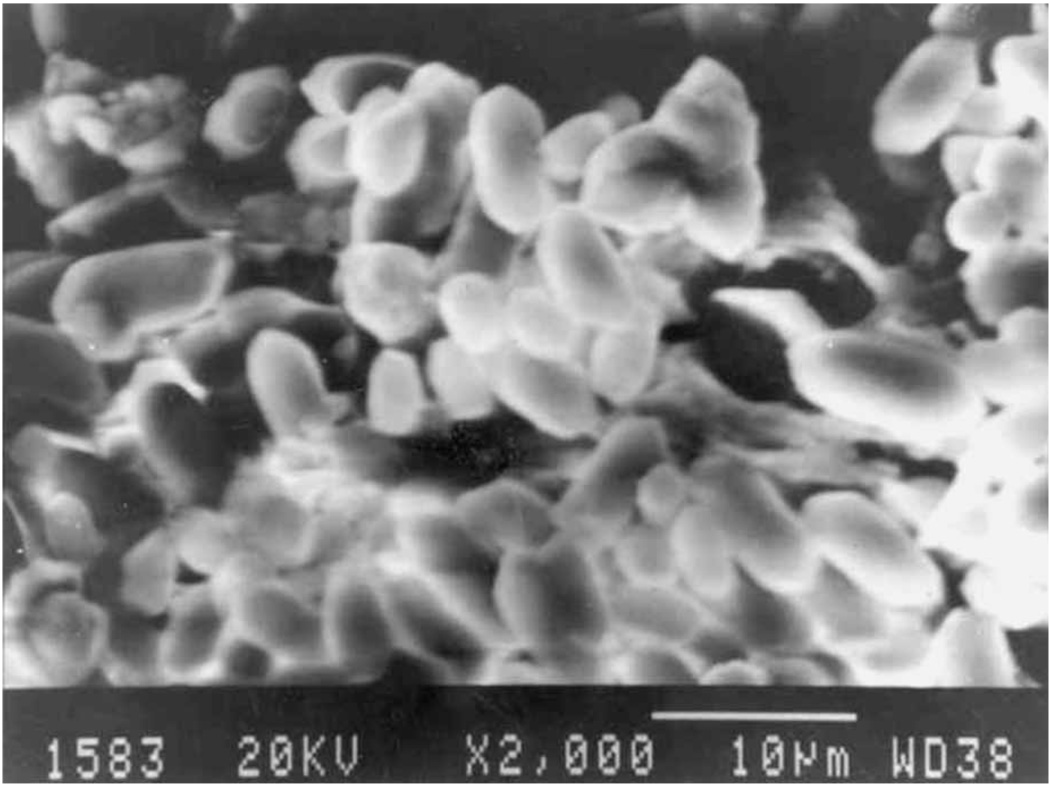
The stone-like otoliths were comprised of white crystals. Using scanning electron microscopy, we observed that in the control group of rats, the otoconia of the utricles and saccules were regular and cylindrical (Figure 1). Their surfaces were smooth and two conical ends were clearly observable. Only a small portion of them were polyhedra. All of them were arranged in a orderly fashion. In the suspended rats, the otoconia were irregularly shaped (Figure 2). Some resembled small rough balls and they were arranged loosely and out of order, unlike those in the control rats (Figure 1), which were tightly packed. Many small otoconia were observed. On their surfaces, we found small globular substances, fine granular substances and fissures.
Figure 1.
Scanning electron micrograph of the otoconia of the utricle in a control rat. Original magnification ×2000.
Figure 2.
Scanning electron micrograph of the otoconia of the utricle in a rat suspended by the tail for 160 days. Original magnification ×2000.
X-ray microanalysis
The otoconia of the utricle, saccule and ossicle (stapes) were tested with X-ray microanalysis in both the suspended and control rats. In the X-ray spectra, peaks for Na, Au, K and Ca were identified in most analyses. The calcium content was calculated using the calcium peak, the number of photons and the photon beam intensity (kiloelectronvolts). The results in Table I show that the calcium content of the otoconia was remarkably lower in the suspended group compared to the control group.
Table I.
Calcium content (mean ± SD; CPS) of the utricle otolith, saccule otolith and stapes in rats after tail suspension for 160 days.
| Source | Control group | Suspended group | p |
|---|---|---|---|
| Utricle | 4350.50±894.40 | 1855.50±432.20 | ≤0.01 |
| Saccule | 1443.17±280.23 | 946.44±289.07 | ≤0.01 |
| Ossicle (stapes) | 10 744.00±2050.11 | 7888.17±887.39 | ≤0.01 |
Discussion
The relative infrequency and prohibitive expense of space experimentation studies emphasize the need to develop ground-based models of weightlessness. One of these complementary alternatives is the head-down tilt bed-rest study, in which volunteers are confined to beds that are tilted 6° below the horizontal at the head end. Every activity, including eating, reading, showering, etc., is performed in this position for the duration of the study. This leads to changes in the human body that are very similar to those seen during space flight, such as loss of bone and muscle mass and cardiovascular and neurosensory reconditioning [6]. Bed rest has been used to mimic space flight in humans. A less traumatic method of simulating weightlessness was developed in which rats were suspended by their tails; this has proven to be a good model of weightlessness in rats [7,8] and to be better than back-suspended methods.
As a direct consequence of exposure to microgravity, astronauts experience a number of physiological changes, which can have serious medical implications when they return to Earth. Most immediate and significant are the head-ward shift of body fluids and the removal of gravitational loading from bone and muscles, which leads to progressive changes in the cardiovascular and musculoskeletal systems [9]. Loss of skeletal mass is a potentially serious consequence of long-term space flight. Skylab astronauts exhibited a significant decline in the bone mineral density of the calcaneus after 84 days of orbital flight [3]. Tail suspension methods are a more appropriate model for evaluating the effects of simulated weightlessness [7]. Therefore, we used this method to observe the morphological changes of the vestibular organs after simulated weightlessness and then tested the calcium content of the otoconia in rats in order to study the mechanism of space motion sickness. Rats placed in orbit aboard Soviet Cosmos biosatellites were characterized by a reduced mass of trabecular bone and an accumulation of marrow fat [10], although bone resorption was not altered in these animals [11]. Our findings indicate that the morphology of the otoconia of the utricle and saccule changes, and that the calcium content of the otoconia decreases due to tail suspension [10]. Some investigators reported that weightlessness inhibited periosteal bone formation and induced a decline in the metaphyseal osteoblast population. Bone loss during space flight is primarily due to a reduction in bone formation. Fu et al. [12] showed that, under conditions of simulated weightlessness, mineralization was inhibited and demineralization of the femur and the mineralized matrix in cartilage was prominent. Demineralization was more prominent in 28-day-old rats. In conclusion: osteocalcin levels in the bone and marrow of rats were lower after tail suspension; calcium deposition was inhibited in the bone and cartilage; and demineralization was prominent after long-term hind-limb unloading.
As the vestibular system is the only sensory organ whose primary function is self-motion detection, some investigators [13] examined the conditions under which the otoliths, which detect the linear acceleration of the head, could be used to estimate distance traveled. Calcium is very important in the functioning of the inner ear and is the main component of the otoliths. Brookes [14] reported that rickets may lead to cochlear deafness. Sun et al. [15] established the vitamin D-deficient rickets model in white rats. The capillary structure of the stria vascularis was observed in most cochleae in the rickets group, and segmental deletions of outer hair cells on the first coil of the cochlea were found in a small number of rickety rats. The results indicated that the effect of vitamin D deficiency on rat cochleae mainly involves the cochlear microcirculation. Wang et al. [16] believed that a long-term (120-day) simulation of the head-ward distribution of blood volume and the hind-limb underloading effect induced by weightlessness may cause morphological changes and a lower calcium content of the otoconia. The findings of Anniko [17] indicate that during the incorporation of calcium into the otoconia, calcium is likely to emanate largely from the surface cells of the utricle, because the endolymphatic space does not contain much calcium. In addition, otoconial formation is stratified, with that in the upper layer forming first and that in the lower layer forming last [18].
Based on the results of the current study, it is proposed that a reduction in bone formation may be the main reason for the lower calcium content of the otoconia. The results of Wang et al. [19] showed that the otoconium was a dynamic structure, which took up 45Ca over a time course generally comparable to that of bone. Although chemical and physical analyses have revealed many details of the structure and organization of mineral in bone, much remains unclear about the process by which calcium and phosphate ions are sequestered from the soluble phase to form crystals in association with the bone matrix. It remains unclear whether mineralization of bone principally reflects passive chemical processes, requiring only the presence of appropriate local concentrations of the precipitating ions, or instead involves active biological processes requiring higher-order functions of cells and their macromolecular components. Therefore, our hypothesis that the formation of bone and otoconia share similar mechanisms may help to answer questions that have been raised about bone mineralization. The head-ward distribution of blood volume caused by tail suspension may also lead to the above-noted morphological changes in the middle and inner ears. We are planning further studies in the areas of vestibular function and compensation after simulated weightlessness in order to understand alterations in the vestibule and the mechanism of space motion sickness. These studies will be very beneficial in enhancing the health and performance of astronauts during and after space flight.
Acknowledgements
We thank the staff of the Department of Aerospace Physiology, Fourth Military Medical University, Xi’an for their continuous support. The study was supported by a grant from Xi’an Jiaotong University and by grant Nos. NSFC30440080 and DC005846.
References
- 1.Gerathewohl SJ. Otolith functions in weightlessness. Life Sci Space Res. 1975;13:33–40. [PubMed] [Google Scholar]
- 2.Edelmann E. Function-morphological investigations of fish inner ear otoliths as basis for interpretation of human space sickness. Acta Astronaut. 2002;50:261–266. doi: 10.1016/s0094-5765(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 3.Rambaut PC, Johnston RS. Prolonged weightlessness and calcium loss in man. Acta Astronautica. 1979;6:1113–1122. doi: 10.1016/0094-5765(79)90059-6. [DOI] [PubMed] [Google Scholar]
- 4.Vinnikov IaA, Gazenko OG, Lychakov DV, Pal’mbakh LR. The development of the vestibular apparatus under conditions of weightlessness. Zh Obshch Biol. 1983;44:147–163. (in Russian) [PubMed] [Google Scholar]
- 5.Lim DJ, Stith JA, Stockwell CW, Oyama J. Observations on saccules of rats exposed to long-term hypergravity. Aerosp Med. 1974;45:27–33. [PubMed] [Google Scholar]
- 6.Elmann-Larsen B, Schmitt D. Staying in bed to benefit ESA’s astronauts and Europe’s citizens. ESA Bull. 2003;113:34–39. [PubMed] [Google Scholar]
- 7.Wronski TJ, Morey-Holton ER. Skeletal response to simulated weightlessness: a comparison of suspension techniques. Aviat Space Environ Med. 1987;58:63–68. [PubMed] [Google Scholar]
- 8.Cao XS, Yang LJ, Wu XY, Wu YH, Zhang LN, Zhang LF. Changes of bone morphogenesis proteins and transforming growth factor-beta in hind-limb bones of 21 d tail-suspended rats. Space Med Med Eng (Beijing) 2003;16:269–271. [PubMed] [Google Scholar]
- 9.Hawkey A. The physical price of a ticket into space. J Br Interplanet Soc. 2003;56:152–159. [PubMed] [Google Scholar]
- 10.Jee WS, Wronski TJ, Morey ER, Kimmel DB. Effects of space flight on trabecular bone in rats. Am J Physiol. 1983;244:R310–R314. doi: 10.1152/ajpregu.1983.244.3.R310. [DOI] [PubMed] [Google Scholar]
- 11.Cann CE, Adachi RR. Bone resorption and mineral excretion in rats during spaceflight. Am J Physiol. 1983;244:R327–R331. doi: 10.1152/ajpregu.1983.244.3.R327. [DOI] [PubMed] [Google Scholar]
- 12.Fu CJ, Yu BB, Yang LJ, Zhang LF. Changes of osteocalcin in bone and bone marrow in tail suspended rats. Space Med Med Eng. 2003;16:260–263. [PubMed] [Google Scholar]
- 13.Israel I, Capelli A, Sable D, Laurent C, Lecoq C, Bredin J. Multifactorial interactions involved in linear self-transport distance estimate: a place for time. Int J Psychophysiol. 2004;53:21–28. doi: 10.1016/j.ijpsycho.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Brookes GB. Vitamin D deficiency—a new cause of cochlear deafness. J Laryngol Otol. 1983;97:405–420. doi: 10.1017/s0022215100094330. [DOI] [PubMed] [Google Scholar]
- 15.Sun H, Tao ZD, Li XZH. The cochlear structural impairment of white rats caused by vitamin D deficiency. Bull Hunan Med Univ. 1993;18:371–374. [Google Scholar]
- 16.Wang J, Liu S, Zhang L, Chen J. Changes in morphology and calcium content of otoconia in rats after 120d tail-suspension. Space Med Med Eng. 1997;10:283–287. [PubMed] [Google Scholar]
- 17.Anniko M. Development of otoconia. Am J Otolaryngol. 1980;1:400–410. doi: 10.1016/s0196-0709(80)80021-4. [DOI] [PubMed] [Google Scholar]
- 18.Kido T. Otoconial formation in the chick: changing patterns of tetracycline incorporation during embryonic development and after hatching. Hear Res. 1997;105:191–201. doi: 10.1016/s0378-5955(96)00210-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang E, Wang J, Liu S. Experimental study of streptomycin on 45Ca intake of otolithic membranes. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1996;31:280–282. [PubMed] [Google Scholar]