Abstract
T cell activation is an important mechanism in HIV-associated immune depletion. We have previously demonstrated an association between the hyperactivation of CD4+ and CD8+ T cells and low CD4 status in HIV-infected Ugandan children. In this study, we explore differences in activation between naive and memory T cells in HIV-infected Ugandan children. A significant correlation between CD4- and CD8-mediated immune activation and CD4 status was observed only in the memory T cells. Antiretroviral (ART) untreated and treated HIV-positive and HIV-negative children displayed similar profiles of activation and distribution within the CD4+ naive T cells. In contrast, significantly higher immune activation of the memory CD4+ T cell subset was seen in ART-untreated children when compared to ART-treated or HIV-negative children. ART-mediated viral suppression led to the correction of CD4+ immune activation to levels seen in uninfected children but did not increase the size of the memory CD4+ T cell population. High levels of CD8+ immune activation were also found in both naive and memory cell subsets. Antiretroviral treatment led to the normalization of CD8+ T cell activation but did not correct the distribution of naive CD8+ T cells. We also assessed PD-1 expression on CD8+ T cells as a measure of immune dysfunction. Upregulation of PD-1 was highest in untreated children but persisted in ART-treated children compared to uninfected children. The mechanisms of immunopathogenesis in pediatric HIV infection likely involve distinct contributions from individual naive and memory T cells subsets.
Introduction
Human immunodeficiency virus (HIV) infection in children leads to progressive immunodeficiency and immune dysfunction, although our understanding of T cell dynamics during viral infection remains limited.1,2 High levels of immune activation have been associated with HIV disease progression in children. The gradual decline of naive T cell pools in HIV-infected children has been attributed to continuous activation of naive T cells and to thymic dysfunction. In addition, depletion of memory T cells likely occurs through direct lysis of infected cells and apoptosis of uninfected bystander cells via activation-induced cell death.3
The mechanisms of immune dysfunction and exhaustion are not well understood. The receptor programmed cell death-1 (PD-1), a member of the CD28 family, is involved in signaling T cell death, and negatively regulates T cell function.4,5 The level of PD-1 expression on HIV-specific T cells is associated with a decrease in functional capacity.6–8 High levels of PD-1 expression on HIV-1-specific T cells reflect a state of cellular exhaustion and may prevent optimal antiviral function. In the presence of high viral load, increased expression of PD-1 can be attributed to T cell activation.6,7
The level to which T cells are depleted in HIV infection depends on the level of immune activation. Homeostatic levels of naive and memory T cell subsets may be disrupted in chronic infection. We previously showed that the CD4+ T cell count, but not the viral load, was significantly associated with both CD4+ and CD8+ T cell activation in Ugandan children.9 Given the variable level of T cell maturity in children, the contributions of different subsets to immune exhaustion are uncertain. To clarify this area, we next evaluated the pattern of immune activation in different T cell subsets in HIV-infected children in Uganda.
Materials and Methods
Study subjects and samples
Samples were obtained from the study cohort “Children with HIV and Malaria Project” (CHAMP, N = 61), a prospective observational study investigating interactions between HIV and malaria coinfection in children. CHAMP has enrolled 300 HIV-infected children (ages 1–10 years) from the Pediatric Infectious Disease Clinic at Mulago Hospital in Kampala.
For comparison, samples from a total of 25 children were selected from a local parallel cohort of 601 HIV-uninfected children.10 Samples from HIV-1-positive adult volunteers (N = 27) were obtained from an existing cross-sectional cohort study in Uganda.11–15
Demographic information, CD4+ T cell status (percentage or absolute count), and plasma HIV RNA levels were also obtained at the time of enrollment and blood draw. Peripheral blood mononuclear cells (PBMCs) were separated and cryopreserved in liquid nitrogen until assay time as previously described.13,14,16–18
Approvals for these studies were obtained from the California Department of Public Health, the University of California, San Francisco, the Makerere University Research and Ethics Committee, the Ugandan National Council of Science and Technology, and the Joint Clinical Research Centre Institutional Board Review. All study participants gave written informed consent. Parents or legal guardians consented on behalf of the children.
Immune activation and functional immunophenotype
Activation staining was performed by incubating PBMCs with the following antibodies: HLADR FITC, CD38 PE, CD3 PerCp-Cy5.5, CD4 APC-CY7, CD27 APC, CD45RA-PECy7, and CD8 Pacific Blue, and analyzed by flow cytometry.18 Dead cells were first gated out using a violet excited viability dye (LIVE/DEAD Fixable Dead Cell Stain; Invitrogen). Immune activation was defined as the percent of CD38+ HLA DR+ T cells within the naive and memory subsets. We defined naive T cells as CD3+CD8+ (or CD3+CD4+) CD27+CD45RA+ and memory T cells as CD3+CD8+ (or CD3+CD4+) CD27−CD45RA−. PD-1 level was defined as the percent of expression of PD-1-APC on CD3+CD8+ T cells. Gating was standardized and set using fluorescence controls for HLADR, CD38, and PD-1.
A minimum of 30,000 CD3+ cells per sample was acquired using an eight-color flow cytometer (LSRII, BD Biosciences). Analysis was performed by FLOWJO software (TreeStar, San Carlos, CA).
Statistical analysis
Groups were compared using the Mann–Whitney U test and analysis was performed with PRISM software version 4.02 (GraphPad, San Diego, CA). Spearman's correlation coefficient was used to determine the correlation between two variables, and a linear least-squares regression model was used in multivariate analysis. Statistical significance was defined as p < 0.05.
Results
Characteristics of study participants
The clinical characteristics of the pediatric and adult study volunteers are shown in Table 1. HIV-infected children on antiretroviral (ART) treatment in this study have undetectable HIV plasma RNA. Overall, there was no statistical difference in age (p ≥ 0.05) between the HIV-positive and HIV-negative children (median 8 years, range = 3–11). The median age, CD4+ T cell count, and plasma HIV-1 RNA levels of the adult study population were 35 years (range = 31–42), 240 cells/mm3 (range = 4–687), and 101,122 copies/ml (range = 678–844,279), respectively.
Table 1.
Clinical Characteristics of Study Participantsa
| Study participants | Age (years, range) | CD4 (percent, range) | HIV plasma RNA (copies per milliliter, range) |
|---|---|---|---|
| Untreated HIV+ children | 8 | 27 | 72,493 copies/ml |
| N = 41 | (2–11) | (12–43) | (962–232,582) |
| ART-treated children | 6 | 25 | <400 copies/ml |
| N = 20 | (2–10) | (11–33) | — |
| HIV-negative children | 8 | — | — |
| N = 25 | (3–11) | ||
| Untreated HIV+ adults | 35 | 240 | 101,122 copies/ml |
| N = 27 | (31–42) | (4–687) | (678–844,279) |
Median values and range.
Profile of immune activation in CD4+ T cell subsets
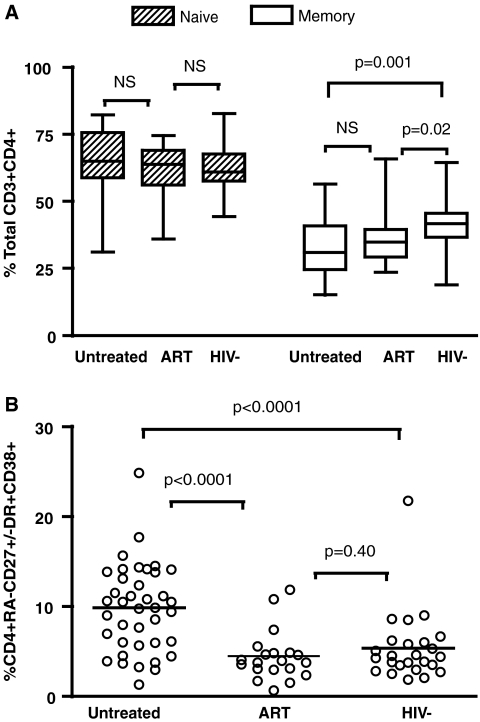
T cell activation is believed to play a critical role in T cell depletion and immune recovery in children chronically infected with HIV.19 We assessed immune activation, as measured by coexpression of surface CD38 and HLA-DR, in different CD4+ T cell subsets in Ugandan children. Naive T cell subsets were defined as CD4+ T cells expressing both CD27 and CD45RA. Memory T cells were defined as CD45RA− with variable expressions of CD27 in order to include both memory effector and central memory subpopulations. Because there was no significant difference in the level of immune activation between different subsets of memory T cells, data from memory T cells were grouped together as CD4+ and CD45RA−CD27+/−.
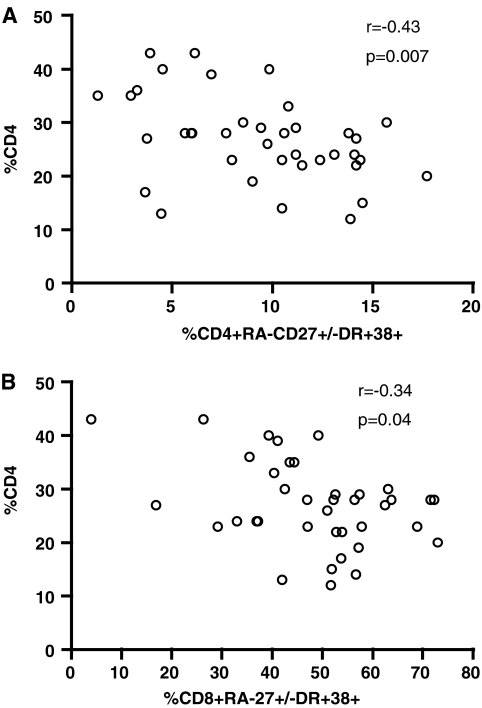
The level of activation and the distribution of the naive CD4+ T cell subset did not differ between HIV-infected and uninfected children (Fig. 1, and data not shown, p > 0.05). In contrast, the level of memory CD4+ T cell population was lower in HIV-positive children, both untreated and ART treated, compared to that of HIV-negative children (p = 0.0014 and 0.02, respectively). CD4+ memory T cell activation was comparable in the ART-treated and HIV-negative children (p = 0.40) and was significantly lower than in the untreated HIV-positive group (p < 0.0001). Furthermore, only memory, and not naive CD4+ T cell immune activation correlated significantly with CD4 cell status (Fig. 2A, r = −0.43, p = 0.007 and p > 0.05, data not shown). CD4 percent, instead of absolute count, was used for correlation analyses of data from children because it is a more clinically relevant measure of CD4 status. Nevertheless, the statistical significance persisted when using absolute CD4 cell count in correlation analysis (p < 0.05, data not shown). Consistent with our previous findings,9 we found no correlation between immune activation and viral load in any of the T cell subsets (p > 0.05, data not shown).
FIG. 1.
(A) Distribution of CD4+ T cell subsets as defined by total naive (striped pattern) or memory (clear pattern) cells in a group of antiretroviral-naive children (N = 40), ART experienced with undetectable viral load (N = 20), or healthy HIV-negative children (N = 25). Horizontal lines represent medians and interquartile ranges (25th and 75th percentiles). (B) Immune activation in CD4+ T cell memory subsets was defined as the percentage of CD4+ T cells with the CD45RA−CD27+/− phenotypes with surface coexpression HLA-DR and CD38. Immune activation was significantly higher in untreated children compared to ART-suppressed or HIV-negative children (p < 0.001).
FIG. 2.
Correlation between the CD4% and memory T cell activation in children. The percentage of (A) CD4+ memory T cell activation or (B) CD8+ memory T cell activation is plotted against CD4%. A significant negative was observed in (A) (r = −0.43, p = 0.007) and in (B) (r = −0.034, p = 0.04).
Profile of immune activation in CD8+ T cell subsets
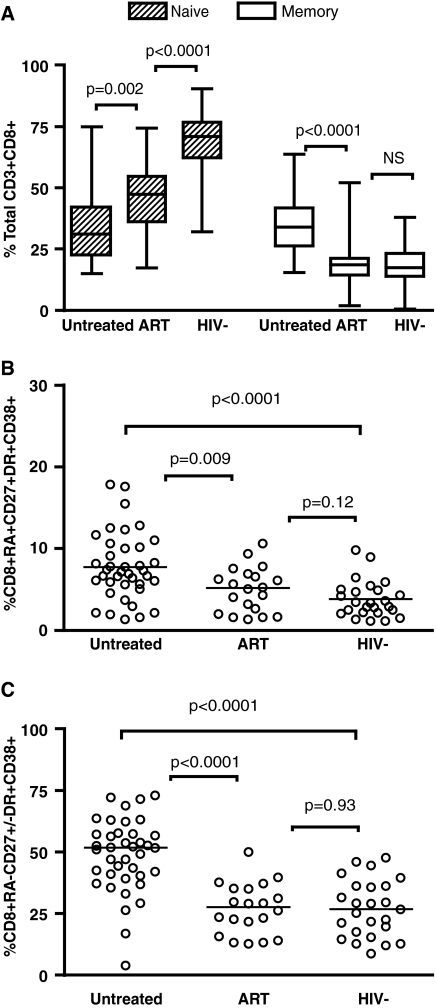
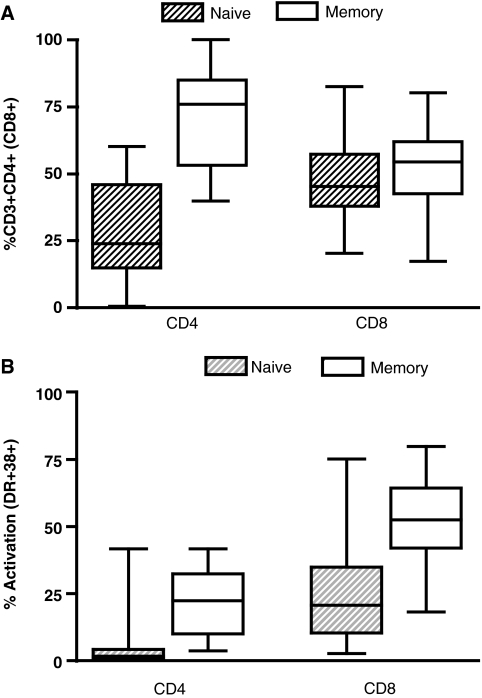
High CD8+ T cell immune activation carries poor prognosis in HIV-infected children.20,21 We first assessed the distribution of naive and memory T cells as defined above for CD4+ T cells. HIV-infected children not treated with ART drugs had a distinct distribution of naive and memory CD8+ T cells compared to ART-treated and HIV-negative children (Fig. 3A). Similarly, high levels of immune activation in the naive and memory T cell subsets were found in untreated children when compared to the ART-treated or uninfected groups (Fig. 3B and C). The level of activation of memory CD8+ T cells negatively correlated with CD4 percent in the untreated population (r = −0.34, p = 0.04; Fig. 2C). This correlation was not observed for the naive CD8+ T cell subset (p > 0.05, data not shown). In contrast to the CD4+ immune profile, we found lower frequencies of the naive but not memory CD8+ T cells in ART-treated children compared to the uninfected group, despite no detectable differences in immune activation in these respective T cell subsets (Fig. 3).
FIG. 3.
(A) Distribution of CD8+ T cell subsets as defined by total naive (striped pattern) or memory (clear pattern) cells in a group of antiretroviral-naive children (N = 40), ART experienced with undetectable viral load (N = 20), or healthy HIV-negative children (N = 25). Horizontal lines represent medians and interquartile ranges (25th and 75th percentiles). Immune activation in CD8 T cell subsets was defined as the percentage of total naive (B) or memory (C) CD8+ T cells expressing HLA-DR and CD38. Significant differences were observed in immune activation of the memory CD8+ T cell subpopulation between HIV-positive and HIV-negative children (p < 0.001).
Profile of T cell function in children
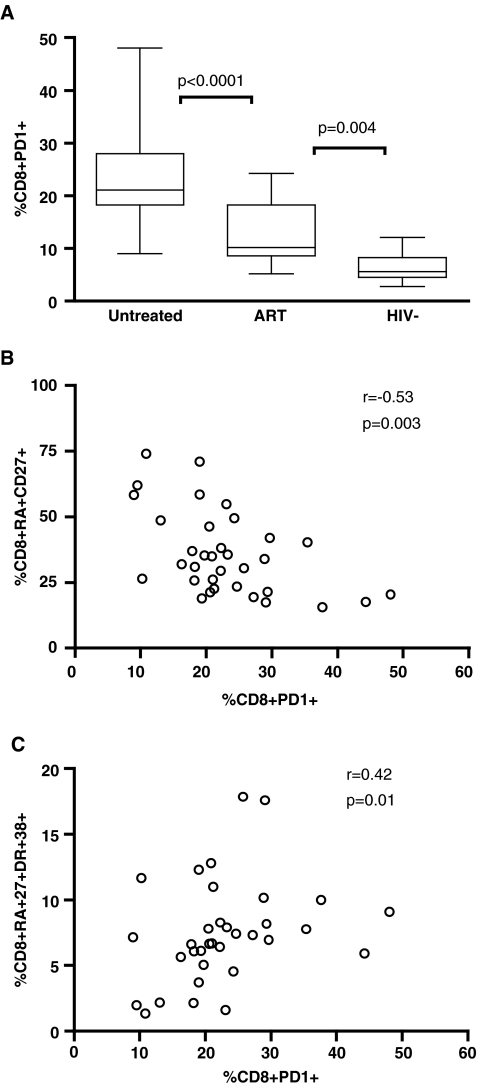
PD-1 is a member of the CD28 family and is expressed on T cells. It is primarily expressed on activated cells and its expression level is determined by T cell differentiation stage.5,22,23 For CD8+ T cells, PD-1 was primarily expressed on CD45RA−CD27+ T cells (data not shown). The highest PD-1 expression on CD8+ T cells was found in untreated children, although viral suppression by ART treatment did not lower PD-1 expression to levels seen in HIV-negative children (Fig. 4A). High PD-1 expression was negatively correlated with the frequency of total naive CD8+ T cells (Fig. 4B, r = −0.53, p = 0.003), and positively associated with naive CD8+ T cell activation (r = 0.42, p = 0.01, Fig. 4C). Surprisingly, no association was observed between PD-1 levels and the distribution or the level of activation in the memory CD8+ T cell subsets (p > 0.05, data not shown), suggesting variable expression of PD-1 in the memory T cell population.
FIG. 4.
PD-1 expression on CD8+ T cells is higher in HIV-positive children. Dead cells were first gated out using a violet excited viability dye (LIVE/DEAD Fixable Dead Cell Stain; Invitrogen). Samples were subsequently gated on the CD3+/CD8+ lymphocyte population then the percent of PD-1-positive cells was determined. Gating was standardized and set using the fluorescence minus one (FMO) control for PD-1. (A) PD-1 expression on CD8+ T cells was highest in children not receiving ART (p < 0.0001). Viral suppression does not lead to normalization of PD-1 expression compared to samples from HIV-negative children (p = 0.004). (B) The presence of PD-1 expression negatively correlated with the frequency of naive CD8+ T cells (r = −0.53, p = 0.003) and (C) positively associated with naive CD8+ T cell activation (r = 0.42, p = 0.01).
Profile of immune activation in adults
We previously reported higher immune activation in HIV-positive adults compared to children in Uganda.9 We further studied the distribution of naive and memory CD4+ and CD8+ T cells and their respective level of activation in adults (Fig. 5). The median (and range) of memory CD4+ and CD8+ T cells in this adult population was 76% (41–99) and 55% (18–79), respectively. The median naive and memory CD4+ T cell activation was 2% (0.4–42) and 22% (4–42), respectively. The median naive and memory CD8+ T cell activation was 21% (18–78) and 53% (3–75), respectively. The frequencies of naive and memory CD4+ and CD8+ T cells in HIV-positive adults were significantly different compared to frequencies found in the untreated pediatric population (p < 0.0001, data not shown). Immune activation levels in the naive and memory CD4+ T cell subsets were higher in adults compared to children. Interestingly, despite similar viral loads (Table 1, p = 0.56), only the activation of CD8+ T cells within the naive subset, not in the memory subset, was higher in untreated adults when compared to children (p > 0.0001 and 0.34, respectively).
FIG. 5.
(A) Distribution of CD4+ and CD8+ T cell subsets as defined by total naive or memory cells with horizontal lines representing medians and interquartile ranges (25th and 75th percentiles) in a group of untreated HIV-positive Ugandan adults (N = 27). The median distributions of memory phenotypes on CD4+ and CD8+ T cells were 76% and 55%, respectively. (B) Immune activation in CD4+ and CD8+ T cell subsets. Naive and memory T cell activation was defined as the percentage of CD45RA+CD27+ or CD45RA−CD27+/− cells expressing HLA-DR and CD38, respectively. Median naive and memory CD4+ T cell activation was 22% and 2%, respectively. Median naive and memory CD8+ T cell activation was 53% and 21%, respectively.
Discussion
CD4 T cell loss is a surrogate marker of HIV pathogenesis and the extent of immune activation is associated with T cell apoptosis. We report here studies of activation of naive and memory T cells in a cohort of HIV-positive Ugandan children. Untreated, ART-experienced, and HIV-uninfected children displayed similar levels and a similar profile of activation of naive CD4+ cells. In contrast, the level of memory CD4+ T cells was lower in HIV-positive children, regardless of treatment status, and elevated activation was specifically observed in untreated, HIV-infected children. One explanation for these findings is the presence of an active thymic output that permits the maintenance and relative preservation of the CD4+ naive T cell population. Alternatively, repletion after ART may favor the naive cell subset, which appears to be less susceptible to HIV-induced immune activation. Activation of the CD4 memory population is expected to increase the division rate and relative cell count. However, activated CD4+ T cells are also at higher risk of bystander killing and direct HIV infection.24–26 The fact that ART treatment corrects the abnormal immune activation without achieving full restoration of the memory CD4+ T cell pool indicates ongoing immune destruction. Our data indicate that viral suppression does not fully correct the disrupted CD4+ homeostasis in HIV infection, but may reset the balance between activation and destruction of T cells. One possible consequence is to delay the collapse of the immune system by strengthening its renewal capacity.27,28 Nevertheless, immune homeostasis and restoration in HIV infection is a complex dynamic process and our study number and its cross-sectional design limit any definitive conclusions.
Elevated CD8+ T cell activation is an independent risk factor in HIV-mediated disease progression. The level of immune activation in both naive and memory CD8+ T cell subsets in untreated children was significantly higher than in ART-treated or uninfected children. HIV infection also perturbed the balance between naive and memory CD8+ T cell distribution in children, and this imbalance failed to normalize fully with viral suppression. These findings suggest the presence of ongoing maturation and turnover of the CD8+ T cell population in the absence of detectable viremia and immune activation. Activation markers alone may thus undervalue the dynamics of CD8+ T cell turnover in untreated as well as treated children. We have previously described lower immune activation in children compared to HIV-infected Ugandan adults.9 In this study, the level of CD8+ T cell activation in the memory T cell subset of untreated children was similar to levels seen in untreated HIV-positive adults. These findings underscore the need for a clear designation of cell subsets when measuring immune activation in children.
Upregulation of inhibitory molecules such as PD-1 receptor impairs T cell responses to HIV infection. PD-1 signaling inhibits the CD3/CD28− activation pathway29–31 and blocking by antagonistic PD-1 antibodies restores T cells functions in vitro.7,32 We saw elevated levels of PD-1 on CD8+ T cells in HIV-infected children who were untreated or treated with ART. Interestingly, there was no significant association between PD-1 and the distribution or activation of the memory CD8+ T cell population, suggesting variable expression of PD-1 on this T cell subset. In contrast, PD-1 levels negatively correlated with the frequency and activation of the naive CD8+ T cells. Thus, PD-1 engagement may play a significant role in regulating T cell homeostasis in children in addition to its association with antigen-specific T cell dysfunction. The perturbation of the naive CD8+ T cell population may also be a sensitive measure of the altered homeostasis and immune dysfunction observed in pediatric HIV infection.
The mechanisms involved in HIV pathogenesis in children are not fully understood. Uniquely in children, quantitative and functional factors leading to full immune restoration are confounded by T cell dynamics involved in maturation of the immune system. We describe differential correlations of naive and memory T cells in HIV-infected children in Uganda. Many questions remain unanswered in the treatment of HIV-infected children. In particular, the optimal timing of ART initiation and the consequence of immune repopulation of different T cell subpopulations have not been fully established.19,33–36 Our findings help to further characterize the complex immune derangements elicited by HIV infection in children.
Acknowledgments
This work was supported by NIH Grant AI43885, AI52142, and AI62677.
Disclosure Statement
The authors have no commercial or other association that might pose a conflict of interest. I.S. and C.B. contributed equally to this work.
References
- 1.Douek DC. McFarland RD. Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 2.Plaeger-Marshall S. Isacescu V. O'Rourke S. Bertolli J. Bryson YJ. Stiehm ER. T cell activation in pediatric AIDS pathogenesis: Three-color immunophenotyping. Clin Immunol Immunopathol. 1994;71:19–26. doi: 10.1006/clin.1994.1046. [DOI] [PubMed] [Google Scholar]
- 3.Grossman Z. Meier-Schellersheim M. Sousa AE. Victorino RM. Paul WE. CD4+ T-cell depletion in HIV infection: Are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe AH. Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 5.Ishida Y. Agata Y. Shibahara K. Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day CL. Kaufmann DE. Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 7.Trautmann L. Janbazian L. Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza M. Fontenot AP. Mack DG, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 9.Ssewanyana I. Elrefaei M. Dorsey G, et al. Profile of T cell immune responses in HIV-infected children from Uganda. J Infect Dis. 2007;196:1667–1670. doi: 10.1086/522013. [DOI] [PubMed] [Google Scholar]
- 10.Dorsey G. Staedke S. Clark TD, et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: A randomized trial. JAMA. 2007;297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 11.Eggena MP. Barugahare B. Okello M, et al. T cell activation in HIV-seropositive Ugandans: Differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 12.Eggena MP. Barugahare B. Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 13.Barugahare B. Baker C. K'Aluoch O, et al. Human immunodeficiency virus-specific responses in adult Ugandans: Patterns of cross-clade recognition. J Virol. 2005;79:4132–4139. doi: 10.1128/JVI.79.7.4132-4139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElroy MD. Elrefaei M. Jones N, et al. Coinfection with Schistosoma mansoni is associated with decreased HIV-specific cytolysis and increased IL-10 production. J Immunol. 2005;174:5119–5123. doi: 10.4049/jimmunol.174.8.5119. [DOI] [PubMed] [Google Scholar]
- 15.Elrefaei M. Barugahare B. Ssali F. Mugyenyi P. Cao H. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J Immunol. 2006;176:1274–1280. doi: 10.4049/jimmunol.176.2.1274. [DOI] [PubMed] [Google Scholar]
- 16.Baker CA. Bousheri S. Ssewanyana I, et al. HIV subtypes distribution and implication for antiretroviral treatment in a Ugandan population. J Int Assoc Phys AIDS Care. 2007;6:260–263. doi: 10.1177/1545109707303938. [DOI] [PubMed] [Google Scholar]
- 17.Baker CA. Clark R. Ventura F, et al. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin Exp Immunol. 2007;147:533–539. doi: 10.1111/j.1365-2249.2006.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker CA. Emenyonu N. Ssewanyana I, et al. Profile of immunologic recovery in HIV-infected Ugandan adults after antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:900–905. doi: 10.1089/aid.2006.0309. [DOI] [PubMed] [Google Scholar]
- 19.Resino S. Seoane E. Gutierrez MD. Leon JA. Munoz-Fernandez MA. CD4(+) T-cell immunodeficiency is more dependent on immune activation than viral load in HIV-infected children on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42:269–276. doi: 10.1097/01.qai.0000222287.90201.d7. [DOI] [PubMed] [Google Scholar]
- 20.Paul ME. Mao C. Charurat M, et al. Predictors of immunologic long-term nonprogression in HIV-infected children: Implications for initiating therapy. J Allergy Clin Immunol. 2005;115:848–855. doi: 10.1016/j.jaci.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 21.Sherman GG. Scott LE. Galpin JS, et al. CD38 expression on CD8(+) T cells as a prognostic marker in vertically HIV-infected pediatric patients. Pediatr Res. 2002;51:740–745. doi: 10.1203/00006450-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki T. Iwai Y. Honjo T. New regulatory co-receptors: Inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 23.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RW. Ascher MS. Sheppard HW. Direct HIV cytopathicity cannot account for CD4 decline in AIDS in the presence of homeostasis: A worst-case dynamic analysis. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:245–252. doi: 10.1097/00042560-199803010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Finkel TH. Tudor-Williams G. Banda NK, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 26.Holzammer S. Holznagel E. Kaul A. Kurth R. Norley S. High virus loads in naturally and experimentally SIVagm-infected African green monkeys. Virology. 2001;283:324–331. doi: 10.1006/viro.2001.0870. [DOI] [PubMed] [Google Scholar]
- 27.Grossman Z. Meier-Schellersheim M. Paul WE. Picker LJ. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 28.Hunt PW. Deeks SG. Immune-based therapy for HIV infection: Are acute and chronic HIV infection different diseases? J Infect Dis. 2006;194:1632–1634. doi: 10.1086/509627. [DOI] [PubMed] [Google Scholar]
- 29.Riley JL. June CH. The CD28 family: A T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald RJ. Freeman GJ. Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 31.Parry RV. Chemnitz JM. Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber DL. Wherry EJ. Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 33.De Rossi A. Walker AS. Klein N. De Forni D. King D. Gibb DM. Increased thymic output after initiation of antiretroviral therapy in human immunodeficiency virus type 1-infected children in the Paediatric European Network for Treatment of AIDS (PENTA) 5 Trial. J Infect Dis. 2002;186:312–320. doi: 10.1086/341657. [DOI] [PubMed] [Google Scholar]
- 34.Resino S. Galan I. Bellon JM. Navarro ML. Leon JA. Munoz-Fernandez MA. Characterizing the immune system after long-term undetectable viral load in HIV-1-infected children. J Clin Immunol. 2003;23:279–289. doi: 10.1023/a:1024536816684. [DOI] [PubMed] [Google Scholar]
- 35.Anselmi A. Vendrame D. Rampon O. Giaquinto C. Zanchetta M. De Rossi A. Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to anti-retroviral therapy. Clin Exp Immunol. 2007;150:442–450. doi: 10.1111/j.1365-2249.2007.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanchetta M. Anselmi A. Vendrame D, et al. Early therapy in HIV-1-infected children: Effect on HIV-1 dynamics and HIV-1-specific immune response. Antiviral Ther. 2008;13:47–55. [PubMed] [Google Scholar]