Abstract
Traumatic brain injury (TBI) is a pathologically heterogeneous disease, including injury to both neuronal cell bodies and axonal processes. Global atrophy of both gray and white matter is common after TBI. This study was designed to determine the relationship between neuroimaging markers of acute diffuse axonal injury (DAI) and cerebral atrophy months later. We performed high-resolution magnetic resonance imaging (MRI) at 3 Tesla (T) in 20 patients who suffered non-penetrating TBI, during the acute (within 1 month after the injury) and chronic stage (at least 6 months after the injury). Volume of abnormal fluid-attenuated inversion-recovery (FLAIR) signal seen in white matter in both acute and follow-up scans was quantified. White and gray matter volumes were also quantified. Functional outcome was measured using the Functional Status Examination (FSE) at the time of the chronic scan. Change in brain volumes, including whole brain volume (WBV), white matter volume (WMV), and gray matter volume (GMV), correlates significantly with acute DAI volume (r = −0.69, −0.59, −0.58, respectively; p < 0.01 for all). Volume of acute FLAIR hyperintensities correlates with volume of decreased FLAIR signal in the follow-up scans (r = −0.86, p < 0.001). FSE performance correlates with acute hyperintensity volume and chronic cerebral atrophy (r = 0.53, p = 0.02; r = −0.45, p = 0.03, respectively). Acute axonal lesions measured by FLAIR imaging are strongly predictive of post-traumatic cerebral atrophy. Our findings suggest that axonal pathology measured as white matter lesions following TBI can be identified using MRI, and may be a useful measure for DAI-directed therapies.
Key words: MR imaging, post-traumatic atrophy, TBI
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity in young people. Each year, approximately 1.5 million Americans suffer from TBI with 1.2 million emergency room (ER) visits and more than 50,000 deaths (Langlois et al., 2004). Severe diffuse axonal injury (DAI) is clinically associated with loss of consciousness and extended deep coma after brain injury, and contributes to disability in approximately 40% of closed head injuries (Buki et al., 2006; Meythaler et al., 2001). The pathology of DAI is characterized histologically by Wallerian-type axonal degeneration in the parasagital white matter, corpus callosum, and dorsal upper brainstem (Meythaler et al., 2001; Povlishock et al., 2005; Adams et al., 1989; Strich, 1956). Current neuroimaging techniques are insensitive for detecting DAI (Levine et al., 2006). Computed tomography (CT), while universally done for the management of TBI, is not sensitive to DAI lesions, and only rarely shows punctate hemorrhages of small penetrating vessels. Recently, several magnetic resonance imaging (MRI) techniques have been used to characterize DAI, and explore the correlation between DAI lesion burden and long-term neurological outcomes (van der et al., 1999; Weiss et al., 2007). Pierellini et al. (2000) reported that the total lesion volume measured 60–90 days after injury using fluid-attenuated inversion-recovery (FLAIR) scans correlated significantly with 1-year Glasgow Outcome Score (GOS), while volume of shear hemorrhage detected on fast field-echo images did not (Pierallini et al., 2000). Our group extended these findings showing that DAI lesion volume on acute (within 14 days after injury) FLAIR scans significantly correlated with 6-month Glasgow Outcome Scale–Extended (GOS-E) (Marquez de la Plata et al., 2007). These data indicate that the quantification of lesion volume using FLAIR scans is a useful tool to measure acute DAI lesion burden.
Cerebral atrophy is common after TBI (Bigler et al., 2006; MacKenzie et al., 2002; Tomaiuolo et al., 2004, 2005; Trivedi et al., 2007). Blatter et al. (1997) studied cerebral atrophy cross-sectionally in 123 TBI patients grouped according to time between injury and MRI scanning. They concluded that there was a progressive decrease in total brain volume starting 3 weeks after moderate to severe TBI, and reaching significance at 8–12 months later. Subsequent brain volume loss continued at a rate greater than that seen with normal aging for up to 3 years after injury. A similar finding was reported in patients with mild TBI (Hofman et al., 2001). Numerous studies have focused on the relationship between post-TBI atrophy and severity of injury (Trivedi et al., 2007; Wilde et al., 2006; Blatter et al., 1997) or functional outcome (Gale et al., 2005; Himanen et al., 2005; Hofman et al., 2001; Tomaiuolo et al., 2004, 2005). Summarizing this extensive literature, global brain volume loss correlated with several indicators of injury severity, including admission GCS, duration of coma, and post-traumatic amnesia. The association between post-traumatic global atrophy and functional outcome is more complex, but in general, cerebral atrophy in the chronic phase is better related to the injury severity than functional outcome (Bigler, 2001). Levine et al. (2008) demonstrated a stepwise, dose-response relationship between TBI severity and parenchymal volume loss using Partial Least Squares (PLS) technique (Levine et al., 2008). Although cerebral atrophy has been widely used as a gross neuropathological hallmark of diffuse injury, especially in the subacute (>30 days after injury) and chronic (>6 months later) phases, its relationship with DAI lesion load defined by abnormal FLAIR signals has not been addressed.
The purpose of the present prospective longitudinal pilot study is to determine whether global cerebral atrophy is associated with DAI lesion load in adult patients with TBI, by performing quantitative analysis of abnormal FLAIR signals and global brain volumes over time. Additionally, this study aims to determine whether TBI related cerebral atrophy correlates with long-term functional outcomes.
Methods
Research participants
All TBI patients were referred from the Department of Neurological Surgery at the University of Texas (UT) Southwestern Medical Center at Dallas. The inclusion criteria for TBI patients included (1) involvement in a non-penetrating TBI that required hospitalization; (2) age between 16 to 65 years old; (3) victims of high-speed motor vehicle/motorcycle collision. Exclusion criteria consisted of (1) patients with focal lesions (including contusion, extraaxial hematoma, and intraparenchymal hemorrhages) greater than 10 ml; (2) a prior history of TBI; (3) other conditions which may result in abnormal MRI findings and compromise cognitive functions (i.e., prior brain tumor, multiple sclerosis, encephalitis/men-meningitis, Alzheimer's disease/mild cognitive impairment, brain abscess, HIV encephalitis, vascular malformation, stroke, and psychiatric disease); (4) prisoners, homeless patients, and pregnant women; and (5) patients who were not medically stable enough to have MRI within a month after the injury. Twenty age- and gender-matched healthy volunteers were recruited as controls.
This study was approved by the Institutional Review Board at UT Southwestern Medical Center at Dallas. Written consent was obtained from patients (or their legally authorized representative) prior to participation in the study.
Magnetic resonance imaging
Structural MRI was performed using either a General Electric Signa Excite 3.0 Tesla (T) MR scanner (GE, Milwaukee, WI) or Siemens Trio 3T MR scanner (Siemens AG, Erlangen, Germany). Each patient was scanned using the same scanner for both acute and chronic time points to eliminate the influence of the different slice thickness/interslice gaps on measurements. The scanning parameters for the GE 3T scanner are as follows: three-dimensional (3D) T1-weighted structural FSPGR images were obtained with slice thickness 1.3 mm, the field of view (FOV) 240 mm, TR/TE 8.0/2.4 ms, flip angle 25, and NEX 2; T2 FLAIR images were acquired in the axial plane with 3-mm slice thickness, interslice gap of 3.5 mm, FOV 200–210 mm, and TR/TE/TI 9500/136.6/2500 ms. For the Siemens 3T scanner: 3D T1-weighted MP-RAGE structural images were obtained with slice thickness 1.0 mm, FOV 240 mm, and TE/TI/TR 4/900/2250 ms; T2 FLAIR images were acquired in the axial plane with 2-mm slice thickness, no gap, FOV 210 mm, and TR/TE/TI 9800/78/2500 ms.
Image processing
Diffuse axonal lesion measurements
FLAIR images were converted from DICOM to ANALYZE format for further analysis. They were then analyzed via a semi-automatic quantification tool developed in our lab on MATLAB (Math Works, Inc., Natick, MA). The detailed processing steps have been described previously (Marquez de la Plata et al., 2007). Briefly, the volume of hyperintense FLAIR signal seen in white matter was quantified for the acute scans. The hyper-intensity lesion index was calculated as a ratio of hyperintense lesion volume to whole brain volume (WBV). Both high and low FLAIR signal seen in white matter in the follow-up scans were quantified to create a hyperintensity lesion index and a hypointensity lesion index, respectively. The volume of total abnormal FLAIR lesions, including both hyperintense and hypointense signals, on the follow-up scans were normalized to the WBV in order to generate total lesion index.
Whole brain volume measurements
Structural Image Evaluation, using Normalization of Atrophy, Single-Time-Point Estimation (SIENAX) within the FMRIB Software Library (FSL) was used for the automated assessment of total brain volume, and the volume of gray and white matter. The high-resolution T1 images were converted from ANALYZE to NIFTI format. SIENAX starts the quantification process by extracting brain from skull (Smith, 2002; Smith et al., 2002, 2004). The brain image is then affine-registered to MNI 152 space (Jenkinson et al., 2001, 2002). Next, tissue-type segmentation with partial volume estimation is taken in FMRIB's Automated Segmentation Tool (FAST) to calculate total volume of brain tissue (including separate estimates of volumes of gray matter and white matter). The WBV change (WBV %) was obtained as the ratio of the difference of WBV between two-time points and the initial WBV. The white matter volume change (WMV %) and the gray matter volume change (GMV %) are volume changes as percent of initial WMV and GMV, respectively.
Functional outcome measure
Functional outcome was determined at the time of the second scans using GOS-E and Functional Status Examination (FSE). FSE is a semi-structured interview conducted in person or via telephone, and typically takes 20 min to complete. The 10-item interview covers a broad range of everyday activities within physical, social, and psychological domains to determine the nature and degree of limitations that have occurred as a result of TBI. For each category, outcome is rated along a four-point ordinal scale: 1 signifies no change from pre-injury; 2 signifies difficulty performing the activity, but maintains total independence; 3 signifies dependence upon others to perform the activity sometimes or a significant decrease in the frequency of performing the activity; 4 signifies complete dependence on others or not performing the activity at all. Total FSE scores range from 10 to 40, with lower scores associated with better outcome. Score 41 is assigned to subjects who die before follow-up. Dikmen et al. (2001, 2003) demonstrated that FSE is more sensitive to recovery at 1–6 months compared to GOS. Our previous study also showed that FSE and GOS-E scores correlated well with each other (r = −0.83), while FSE is a more sensitive outcome measurement (Hudak et al., 2005).
FSE and GOS-E were administered by clinical research coordinators who were blinded to circumstances of each patient's injury and imaging results. Inter-rater reliability for scoring FSE was assessed by auditing 20% of the scoring sheet every 3 months. Reproducibility was >99%. Whenever possible, the GOS-E and FSE were completed by the survivor, although completion of the questionnaires by the caregiver was also acceptable. Published data from our group and others (Hudak et al., 2005; Dikmen et al., 2001) support the validity of survivor or caregiver completion of the outcome instruments.
Statistical analysis
D'Agostino-Pearson omnibus normality test was used to test the distribution of sample in terms of injury severity, brain volume, and outcome measurement. Independent group t-tests were used to compare brain volumes of normal volunteers, patients in the acute phase, and patients in the chronic phase. Paired t-tests were used to detect the change in brain volume among patients over the time. Spearman's nonparametric correlations were performed to determine the relationship among DAI lesion volume, the change of brain volume, and outcome measure. Hierarchical regression analysis was performed to determine factors of importance in predicting outcome. Statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL) and GraphPad Prism 5 (GraphPad software, Inc., La Jolla, CA).
Results
Demographic characteristics
The 20 patients included in this study had a median age of 20 years (range, 16–62 years) and were predominantly male (65%). Four patients (20%) had complicated-mild TBI, as their first post-resuscitation GCS was above 13, but they required hospitalization; four patients (20%) had moderate TBI with GCS of 9–12; and 12 patients (60%) suffered severe TBI with GCS lower than 8. Initial MRI was performed in the acute period (median, 7 days; range, 1–35 days). The follow-up MRI was performed with a median of 8 months after the injury (range, 6–11 months). Age- and gender- matched normal healthy volunteers were scanned one time using the same protocol based on the assumption that no significant brain volume change during a period of 1 year in healthy young adults. Patients' age, initial GCS, and time to follow-up scan were normally distributed, while as expected, time to initial scan was not. Age was normally distributed among the sample of controls. Demographic information for both the patient and control groups is summarized in Table 1.
Table 1.
Demographic Information
| n | Median | Range | Mean | SD | Normality distribution | |
|---|---|---|---|---|---|---|
| Controls | ||||||
| Age, years | 20 | 26 | 16–60 | 28 | 11 | Yes |
| Male (n) | 13 | — | — | — | — | — |
| Patients | ||||||
| Age, years | 20 | 20 | 16–62 | 26 | 12 | Yes |
| Male (n) | 13 | — | — | — | — | — |
| Time to initial scan (days) | 20 | 6.5 | 1–35 | 11 | 10 | No |
| Time to follow-up scan (months) | 20 | 8 | 6–11 | 8 | 1 | Yes |
| GCS at ER | 20 | 6 | 3–15 | 7 | 5 | Yes |
| FSE | 20 | 14 | 12–38 | 20 | 10 | Yes |
Normality test was performed using D'Agostino-Pearson normality test with alpha = 0.05.
Volumetric study
The median hyperintensity lesion volume for all 20 patients was 1.3 mm3 (mean ± SD, 4.5 ± 5.5 mm3). Fewer hyperintense signal lesions were found on follow-up FLAIR scans with a median volume of 1.2 mm3 (mean ± SD, 1.4 ± 1.3 mm3). Several areas of hyperintense signal seen on acute FLAIR scans were identified as areas of decreased attenuation on follow-up FLAIR scans, and were measured as hypointensity lesions with a median volume of 0.19 mm3 (mean ± SD, 0.66 ± 1.1 mm3; Fig. 1). There are no hypointense lesions large enough to measure in the acute scans.
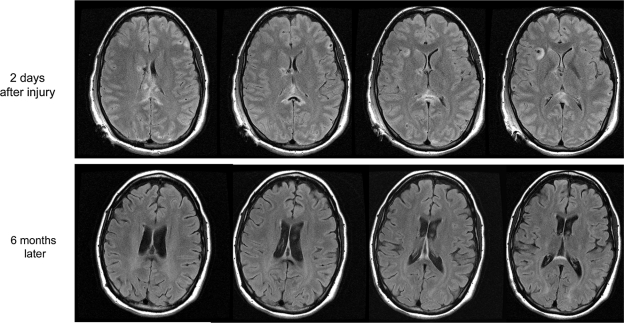
FIG. 1.
Magnetic resonance imaging (MRI) in a case of severe traumatic brain injury (TBI). The patient was a 20-year-old man involved in a motorcycle collision accident. The first post-resuscitation Glasgow Coma Scale (GCS) score was 8. The first MRI scan was performed 2 days after his injury: hyperintense fluid-attenuated inversion-recovery (FLAIR) signal lesions were seen at corpus callosum, fornix, and subcortical white matter. The follow-up scan was performed 6 months later: decreased attenuation FLAIR lesions were found in corpus callosum and fornix, where hyperintense lesions were noted in the acute scans. The volume of bright FLAIR lesions in corpus callosum decreased compared to the acute scans. He lost 13% of whole brain volume in 6 months. Functional Status Examination (FSE) score at the time of his follow-up scan was 32, and Glasgow Outcome Scale—Extended (GOS-E) score was 5.
The results of the brain volume measurements can be seen in Table 2. No significant difference was found between acute scans and normal healthy controls; however, paired t-tests revealed significant differences between acute and follow-up scans in terms of WBV, GMV, and WMV. Unpaired comparison of brain volume of normal controls with the follow-up volume of brain injured patients showed a non-significant trend toward smaller volumes for TBI patients. This is likely due to limited sample size. Seven of 20 patients (35%) had at least 5% WBV loss.
Table 2.
Brain Volumetric Measurement
| Control M (SD) (×106 mm3) | Acute M (SD) (×106 mm3) | Follow-up M (SD) (×106 mm3) | Unpaired p-value (acute vs. control) | Paired p-value (acute vs. follow-up) | |
|---|---|---|---|---|---|
| WBV | 1.67 (0.085)a | 1.68 (0.093)a | 1.63 (0.097)a | NS | 0.01 |
| GMV | 0.89 (0.063)a | 0.89 (0.087)a | 0.86 (0.080)a | NS | 0.02 |
| WMV | 0.78 (0.043)a | 0.79 (0.045)a | 0.77 (0.048)a | NS | 0.02 |
Pass D'Agostino-Pearson normality test (alpha = 0.05).
WBV, white brain volume; GMV, gray matter volume; WMV, white matter volume; M, mean; SD, standard deviation; NS, non-significant (p ≥ 0.05).
Correlations among volumetric measurements and functional outcomes
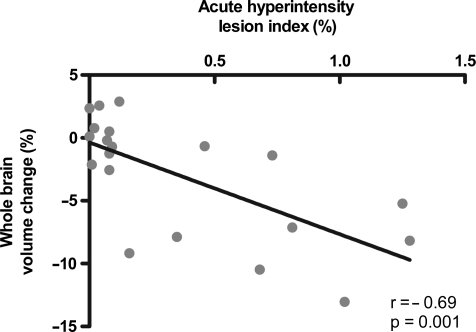
Spearman correlations demonstrated that WBV change, WMV change, and GMV change were all significantly correlated with the acute hyperintensity lesion index (r = −0.69, p = 0.001; r = −0.59, p = 0.006; r = −0.58, p = 0.008, respectively), such that greater amounts of acute hyperintensity FLAIR lesions correlate with greater brain volume loss after injury (Fig. 2).
FIG. 2.
Scatter plot of the association between acute fluid-attenuated inversion-recovery (FLAIR) hyperintense lesion volume and 6-month post-traumatic whole brain volume (WBV) change. The WBV change (%) is a ratio of the difference of WBV between two time points and the initial WBV. Hyperintensity lesion index is a ratio of the volume of hyperintensity lesion to the WBV. Correlation was performed using Spearman's nonparametric correlation coefficient (r).
Acute hyperintensity lesion index was strongly correlated with chronic hypointensity lesion index and total lesion index in follow-up FLAIR scans. Additionally, the acute hyper-intensity index was only moderately correlated with the hyperintensity index on follow-up FLAIR scans. Similar results were noted when FLAIR lesion volume was not normalized to WBV. These data show that greater amounts of acute DAI lesions are associated with greater amounts of chronically abnormal FLAIR signal. Furthermore, the acute hyper-intensity index was moderately correlated with first post-resuscitation GCS score in the ER, such that greater amounts of acute DAI are associated with lower GCS scores (Table 3).
Table 3.
Univariate Correlations with Acute Hyperintensity Lesions
| Acute hyperintensity lesion index | p | |
|---|---|---|
| ER GCS | −0.59 | 0.01 |
| Hypointensity lesion index on F/U | 0.86 | <0.001 |
| Hypointensity lesion volume on F/U | 0.88 | <0.001 |
| Hyperintensity lesion index on F/U | 0.46 | 0.04 |
| Hyperintensity lesion volume on F/U | 0.38 | 0.09 |
| Total lesion index on F/U | 0.72 | <0.001 |
| Total lesion volume on F/U | 0.66 | <0.01 |
Correlations among acute lesion volumes and chronic lesion volumes.
Significant correlation in bold.
Patients with greater degree of brain volume loss had poorer FSE performance. Likewise, acute hyperintensity lesion volume and chronic total lesion volume were significantly correlated with FSE score, while the correlation between chronic hypointensity lesion and FSE score approached statistical significance (p = 0.08). Similar correlations were found when GOS-E was used as the outcome measure. GCS in the ER and age were not significantly associated with functional outcome (Table 4).
Table 4.
Correlations Among Injury Severity Indicators and FSE
| Follow-up FSE | ρ | |
|---|---|---|
| Age | 0.35 | 0.13 |
| ER GCS | −0.34 | 0.14 |
| Acute hyperintensity lesion | 0.53 | 0.02 |
| Hypointensity lesion on F/U | 0.41 | 0.08 |
| Hyperintensity lesion on F/U | 0.30 | 0.21 |
| Total lesion on F/U | 0.45 | 0.05 |
| WBV% | −0.49 | 0.03 |
| WMV% | −0.45 | 0.05 |
| GMV% | −0.44 | 0.05 |
Correlations between potential injury severity indicators and functional outcome.
Significant correlations in bold.
F/U, follow-up; WBV%, whole brain volume change (%); WMV%, white matter volume change (%); GMV%, gray matter volume change (%); total lesion on F/U = (Hypointensity lesion on F/U + Hyperintensity lesion on F/U).
Multiple regression analysis
A hypothesis-driven hierarchical regression analysis was performed to determine factors of importance in predicting outcome. Age and first post-resuscitation GCS, which are commonly used outcome predictors in clinical practice (Flanagan et al., 2005; Katz et al., 1994; Livingston et al., 2005; Marquez de la Plata CD et al., 2008; Stuss et al., 2000), were entered in the first step of this analyses as the base model with which all subsequent models are compared. This base model explained 34% of the variance in FSE performance. Subsequently, three alternative models were created by entering three different variables (i.e., acute DAI lesion volume, chronic hypointensity lesion, and brain volume change) into the base model separately to determine whether these variables account for significant amounts of variance in FSE performance. The three alternative regression models (Table 5) each added a significant amount of explained variance in functional outcome after TBI.
Table 5.
Hierarchical Regression Model for Predicting FSE Performance
| Models | R square | R square change | Significant F change |
|---|---|---|---|
| Base model | 0.34 | 0.34 | 0.03 |
| Alt model 1 | 0.52 | 0.18 | 0.03 |
| Alt model 2 | 0.65 | 0.31 | 0.002 |
| Alt model 3 | 0.50 | 0.16 | 0.04 |
Base Predictors: ER_GCS, AGE.
1 Predictors: ER_GCS, AGE, acute hyperintensity lesion volume.
2 Predictors: ER_GCS, AGE, follow-up hypointensity lesion volume.
3 Predictors: ER_GCS, AGE, whole brain volume change.
Relative contributions of explained variance in functional outcome.
Alt, alternative.
Discussion
The present study is the first longitudinal study to directly demonstrate the association between acute FLAIR hyper-intensity lesion and post-traumatic atrophy. We excluded patients who suffered large or medium focal lesions including contusions, extra- or intra- axial hematomas from the present study in order to focus on the relationship between diffuse injury, particularly DAIs, and cerebral atrophy after TBI. DAIs can be visualized indirectly through shear hemorrhages caused by tearing lesions of blood vessels (Scheid et al., 2003; Tong et al., 2003) or more directly by analyzing white matter hyperintensities on FLAIR MRI (Marquez de la Plata et al., 2007; Pierallini et al., 2000; Takaoka et al., 2002). We found that acute hyperintensity FLAIR lesions are strongly predictive of post-traumatic cerebral atrophy. Although the pathophysiology of the post-TBI cerebral atrophy remains unknown, axonal injury and subsequent Wallerian degeneration may be a possible mechanism. The current working hypothesis, based on animal data (Povlishock et al., 2005; Smith et al., 2003), is that at the acute stage (hours to days after non-penetrating brain injury), the axons swell due to the local ionic homeostatic disruption, increased permeability of the axolemma, with immediate mechanical damage to the axonal cytoskeleton (primary axotomy) seen in severe cases. Days to months after the injury, pathological changes of DAI are believed to consist of progressive disorganization of the axonal cytoskeleton and progressive protein accumulation, leading to disconnection of axons (secondary axotomy). The primary and secondary axotomy also triggers the local or even global metabolic changes which would lead to further cell death and Wallerian degeneration (Smith et al., 2003). This pathologic process may lead to cerebral atrophy in the chronic phase after TBI. Our data is consistent with this working hypothesis, and also indicates that tracking of macroscopic lesions visible in FLAIR scans may be a useful method to monitor progressive tissue pathology associated with DAI.
The current study confirmed our prior finding that acute hyperintensity FLAIR lesion was an important factor for predicting functional outcome, though only moderately correlated with FSE (Marquez de la Plata et al., 2007). Combined with GCS in the ER and age, the acute DAI lesion volume could be used to stratify injury severity when selecting patients for TBI clinical trials. Our study indicates that the follow-up MRI, especially on high magnetic field, could offer useful information on the pathological change of DAI, which would be potentially useful in DAI-directed therapies.
While FLAIR is a commonly used clinical sequence which all physicians are familiar with, it may not be the most sensitive MR sequence to detect DAI. Several other MR techniques have been proposed to increase the sensitivity of detecting DAI in vivo. T2-weighted gradient echo is excellent in detecting acute small punctuate hemorrhagic lesions. The number of traumatic microbleeds detected on T2-weighted gradient echo sequence at chronic stage (≥3 months after TBI) correlated significantly with GCS, but not with long-term outcome measured by GOS-E (Pierallini et al., 2000; Scheid et al., 2003). A new high-resolution 3D gradient-echo MR imaging technique, known as susceptibility-weighted imaging (SWI) is much more sensitive than conventional T2-weighted gradient-echo sequences in detecting hemorrhagic DAI. Number of traumatic microhemmorhagic lesion detected by SWI correlated better with GOS-E than that detected by gradient echo (Tong et al., 2003, 2004, 2008). No longitudinal data is available to assess whether SWI lesions correlate with post-traumatic brain atrophy. Diffusion-weighted imaging (DWI) has proven to be highly sensitive for the detection of early cytotoxic edema in the setting of acute stroke. DWI has not been widely used in clinical TBI, though the sensitivity of DWI to identify DAI lesions is similar to that of FLAIR. It is less sensitive than T2 gradient echo for detecting hemorrhagic lesions (Huisman, 2002; Huisman et al., 2003; Kinoshita et al., 2005). The volume of DWI lesions in white matter is moderately correlated with functional outcome (Ayala et al., 2008). Unlike conventional DWI, diffusion tensor imaging (DTI) characterizes the diffusion of water along white matter tracts. Our group and others are studying DTI in the hope that it would be more sensitive to axonal pathology after traumatic injury (Bazarian et al., 2007; Kim et al., 2008; Sidaros et al., 2008; Wang et al., 2008). DTI is able to visualize changes that were not seen on conventional scans and strongly correlated with functional outcome. However the analysis of DTI data is time-consuming, experience-dependent and may not be ideally suitable for routine clinical practice. Overall, the combination of FLAIR and other MRI sequences may provide additional information about injury severity and correlate with the functional outcome better.
Our findings of reduced WBV, WMV, and GMV at approximately 8 months after TBI are consistent with previous volumetric studies (MacKenzie et al., 2002; Trivedi et al., 2007). This indicated that our method of brain volume measurement is reliable and consistent. In our study, SIENAX, a fully automated method, has been used to measure atrophy. This program has been shown to be an accurate approach to measure cross-sectional normalized brain volume with 0.5–1% brain volume accuracy for a single-time point. It has successfully coped with both de-skulling and tissue segmentation, and can be used for the subjects with extreme parenchymal loss (Smith et al., 2002). Recently, its reliability has be demonstrated in other neurological conditions, such as multiple sclerosis (MS) and Alzheimer's disease (Anderson et al., 2006, 2007; Smith et al., 2007). A particular advantage of SIENAX is that it is relatively insensitive to different scanning parameters. For MS-related brain atrophy, the inter-center agreement assessed with the concordance correlation coefficient was 0.94 between two centers regardless of the difference of magnetic field strength and scanning parameters (Jasperse et al., 2007). SIENA is a similar fully automated program developed to measure longitudinal atrophy rate. Our finding of cerebral atrophy measured using SIENAX was commensurate to the results of Trivedi et al. (2007) using SIENA. The development of SIENA/SIENAX makes it practical to use atrophy rate/state as an index of disease progression in clinical studies.
The present investigation was a pilot study, and a larger scale investigation is under way in our group to confirm these pilot results. While many initial scans took place within the first week, many occurred several days after injury. The heterogeneity in the interval from injury to first scan may confound results, as it is possible that FLAIR lesions increase in conspicuity over time. In order to establish the utility of MRI as a biomarker in clinical trials, scans obtained within 24 h of injury must be studied. Furthermore, neuropathologic studies are needed to correlate the cellular and tissue abnormalities with lesions detected by MRI.
The present study provides in vivo evidence of pathological change of DAI after TBI. It demonstrates a strong association between post-traumatic cerebral atrophy and DAI. The strategy presented in this study could be a practical method to monitor the efficacy of DAI-directed therapies in future clinical trials.
Acknowledgments
The present study was supported by the U.S. Department of Education (grant NIDRR H133 A020526) and the National Institutes of Health (grants NIH R01 HD48179 and NIH U01 HD42652).
Author Disclosure Statement
No competing financial interests exist.
References
- Adams J.H. Doyle D. Ford I. Gennarelli T.A. Graham D.I. McClellan D. Diffuse axonal injury in head injury: Definition, diagnosis, and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Anderson V.M. Fernando K.T. Davies G.R. Rashid W. Frost C. Fox N.C. Miller D.H. Cerebral atrophy measurement in clinically isolated syndromes and relapsing remitting multiple sclerosis: a comparison of registration-based methods. J. Neuroimaging. 2007;17:61–68. doi: 10.1111/j.1552-6569.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- Anderson V.M. Fox N.C. Miller D.H. Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J. Magn. Reson. Imaging. 2006;23:605–618. doi: 10.1002/jmri.20550. [DOI] [PubMed] [Google Scholar]
- Ayala R.R. Redfern S. Ding K. Moore M. Harper C. Madden C.J. Diaz-Arrastia R. Diffusion-weighted MR imaging in closed head injury: identification of distinct injury subtypes and correlation with functional outcome. Neurology. 2008;70:A362. [Google Scholar]
- Bazarian J.J. Zhong J. Blyth B. Zhu T. Kavcic V. Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Quantitative magnetic resonance imaging in traumatic brain injury. J. Head Trauma Rehabil. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Ryser D.K. Gandhi P. Kimball J. Wilde E.A. Day-of-injury computerized tomography, rehabilitation status, and development of cerebral atrophy in persons with traumatic brain injury. Am. J. Phys. Med. Rehabil. 2006;85:793–806. doi: 10.1097/01.phm.0000237873.26250.e1. [DOI] [PubMed] [Google Scholar]
- Blatter D.D. Bigler E.D. Gale S.D. Johnson S.C. Anderson C.V. Burnett B.M. Ryser D. MacNamara S.E. Bailey B.J. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. Am. J. Neuroradiol. 1997;18:1–10. [PMC free article] [PubMed] [Google Scholar]
- Buki A. Povlishock J.T. All roads lead to disconnection?–traumatic axonal injury revisited. Acta Neurochir. (Wien.) 2006;148:181–193. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- Dikmen S. Machamer J. Miller B. Doctor J. Temkin N. Functional status examination: a new instrument for assessing outcome in traumatic brain injury. J. Neurotrauma. 2001;18:127–140. doi: 10.1089/08977150150502578. [DOI] [PubMed] [Google Scholar]
- Dikmen S.S. Machamer J.E. Powell J.M. Temkin N.R. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2003;84:1449–1457. doi: 10.1016/s0003-9993(03)00287-9. [DOI] [PubMed] [Google Scholar]
- Flangan S.R. Hibbard M.R. Gordon W.A. The impact of age on traumatic brain injury. Phys. Med. Rehabil. Clin. North Am. 2005;16:163–177. doi: 10.1016/j.pmr.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Gale S.D. Baxter L. Roundy N. Johnson S.C. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen L. Portin R. Isoniemi H. Helenius H. Kurki T. Tenovuo O. Cognitive functions in relation to MRI findings 30 years after traumatic brain injury. Brain Inj. 2005;19:93–100. doi: 10.1080/02699050410001720031. [DOI] [PubMed] [Google Scholar]
- Hofman P.A. Stapert S.Z. Van Kroonenburgh M.J. Jolles J. De Kruijk J. Wilmink J.T. MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. Am. J. Neuroradiol. 2001;22:441–449. [PMC free article] [PubMed] [Google Scholar]
- Hudak A.M. Caesar R.R. Frol A.B. Krueger K. Harper C.R. Temkin N.R. Dikmen S.S. Carlile M.C. Madden C. Diaz-Arrastia R. Functional outcome scales in traumatic brain injury: a comparison of the Glasgow Outcome Scale (Extended) and the Functional Status Examination. J. Neurotrauma. 2005;22:1319–1326. doi: 10.1089/neu.2005.22.1319. [DOI] [PubMed] [Google Scholar]
- Housman T.A. Sorensen A.G. Hergan K. Gonzalez R.G. Schaefer P.W. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J. Comput. Assist. Tomogr. 2003;27:5–11. doi: 10.1097/00004728-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Huisman T.A.G.M. Diffusion-weighted imaging: basic concepts and application in cerebral stroke and head trauma. Eur. Radiol. 2002;13:2283–2297. doi: 10.1007/s00330-003-1843-6. [DOI] [PubMed] [Google Scholar]
- Jasperse B. Valsasina P. Neacsu V. Knol D.L. De Stefano N. Enzinger C. Smith S.M. Ropele S. Korteweg T. Giorgio A. Anderson V. Polman C.H. Flippi M. Miller D.H. Rovaris M. Barkhof F. Vrenken H. Intercenter agreement of brain atrophy measurement in multiple sclerosis patients using manually-edited SIENA and SIENAX. J. Magn. Reson. Imaging. 2007;26:881–885. doi: 10.1002/jmri.21101. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Bannister P. Brady M. Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Katz D. Alexander M.P. Predicting course of recovery and outcome for patients admitted to rehabilitation. Arch. Neurol. 1994;51:661–670. doi: 10.1001/archneur.1994.00540190041013. [DOI] [PubMed] [Google Scholar]
- Kim J. Avants B. Patel S. Whyte J. Coslett B.H. Pluta J. Detre J.A. Gee J.C. Structural consequences of diffuse traumatic brain injury: a large deformation tensor-based morphometry study. Neuroimage. 2008;39:1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T. Moritani T. Hiwatashi A. Wang H.Z. Shrier D.A. Numaguchi Y. Westesson P.L. Conspicuity of diffuse axonal injury lesions on diffusion-weighted MR imaging. Eur. J. Radiol. 2005;56:5–11. doi: 10.1016/j.ejrad.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Keller M. Butler J. Gotsch K.E. Johnson R.L. Reichard A.A. Webb K.W. Coronado V.G. Selassie A.W. Thurman D.J. Traumatic brain injury-related hospital discharges. Results from a 14-state surveillance system, 1997. MMWR Surveill. Summ. 2003;52:1–20. [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K. Traumatic Brain Injury in the United States: Emergency Department visits, Hospitalizations, and Deaths. National Center for Injury Prevention and Control; Atlanta: [Google Scholar]
- Levine B. Fujiwara E. O'Connor C. Ricard N. Kovacevic N. Mandic M. Restagno A. Easdon C. Robertson I.H. Graham S.J. Cheung G. Gao F. Schwartz M.L. Black S.E. In vivo characterization of traumatic brain injury neuropathology with structural and functional neuroimaging. J. Neurotrauma. 2006;23:1396–1411. doi: 10.1089/neu.2006.23.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. Kovacevic N. Nica E.I. Cheung G. Gao F. Schwartz M.L. Black S.E. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70:771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Livingston D.H. Lavery R.F. Mosenthal A.C. Knudson M.M. Lee S. Morabito D. Manley G.T. Nathens A. Jurkovich G. Hoyt D.B. Coimbra R. Recovery at one year following isolated traumatic brain injury: a Western Trauma Association prospective multicenter trial. J. Trauma. 2005;59:1298–1304. doi: 10.1097/01.ta.0000196002.03681.18. [DOI] [PubMed] [Google Scholar]
- Mackenzie J.D. Siddiqi F. Babb J.S. Bagley L.J. Mannon L.J. Sinson G.P. Grossman R.I. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am. J. Neuroradiol. 2002;23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- Marquez de la Plata C.D. Hart T. Hammond F.M. Frol A.B. Hudak A. Harper C.R. O'Neil-Pirozzi T.M. Whyte J. Carlile M. Diaz-Arrastia R. Impact of age on long-term recovery from traumatic brain injury. Arch. Phys. Med. Rehabil. 2008;89:896–903. doi: 10.1016/j.apmr.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de la Plata C. Ardelean A. Koovakkattu D. Srinivasan P. Miller A. Phuong V. Harper C. Moore C. Whittemore A. Madden C. Diaz-Arrastia R. Devous M. Magnetic resonance imaging of diffuse axonal injury: quantitative assessment of white matter lesion volume. J. Neurotrauma. 2007;24:591–598. doi: 10.1089/neu.2006.0214. [DOI] [PubMed] [Google Scholar]
- Meythaler J.M. Peduzzi J.D. Eleftheriou E. Novack T.A. Current concepts: Diffuse axonal injury-associated traumatic brain injury. Arch. Phys. Med. Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- Pierallini A. Pantano P. Fantozzi L.M. Bonamini M. Vichi R. Zylberman R. Pisarri F. Colonnese C. Bozzao L. Correlation between MRI findings and long-term outcome in patients with severe brain trauma. Neuroradiology. 2000;42:860–867. doi: 10.1007/s002340000447. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Scheid R. Preul C. Gurber O. Wiggins C. Von Cramon D.Y. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3T. AJNR Am. J. Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- Sidaros A. Engberg A.W. Sidaros K. Liptrot M.G. Herning M. Petersen P. Paulson O.B. Jernigan T.L. Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Meaney D.F. Shull W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003;18:307–306. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Jenkinson M. Woolrich M.W. Bechmann C.F. Behrens T.E. Johansen-Berg H. Bannister P.R. De Luca M. Drobnjak I. Flitney D.E. Niazy R.K. Saunders J. Vickers J. Zhang Y. De Stefano N. Brady J.M. Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Rao A. De Stefano N. Jenkinson M. Schott J.M. Matthews P.M. Fox N.C. Longitudinal and cross-sectional analysis of atrophy in Alzheimer's disease: cross-validation of BSI, SIENA and SIENAX. Neuroimage. 2007;36:1200–1206. doi: 10.1016/j.neuroimage.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Zhang Y. Jenkinson M. Chen J. Matthews P.M. Federico A. De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Strich S.J. Diffuse degeneration of cerebral white matter in severe dementia following head injury. J. Neurol. Neurosurg. Psychiatry. 1956;19:163–185. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T. Binns M.A. Carruth F.G. Levine B. Brandys C.F. Moulton R.J. Snow W.G. Schwartz M.L. Prediction of recovery of continuous memory after traumatic brain injury. Neurology. 2000;54:1337–1344. doi: 10.1212/wnl.54.6.1337. [DOI] [PubMed] [Google Scholar]
- Takaoka M. Tabuse H. Kumura E. Nakajima S. Tsuzuki T. Nakamura K. Okada A. Sugimoto H. Semiquantitative analysis of corpus callosum injury using magnetic resonance imaging indicates clinical severity in patients with diffuse axonal injury. J. Neurol. Neurosurg. Psychiatry. 2002;73:289–293. doi: 10.1136/jnnp.73.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F. Carlesimo G.A. Di Paola M. Petrides M. Fera F. Bonanni R. Formisano R. Rasqualetti P. Caltagirone C. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1-weighted MRI study. J. Neurol. Neurosurg. Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F. Worsley K.J. Lerch J. Di Paola M. Carlesimo G.A. Bonanni R. Caltagirone C. Paus T. Changes in white matter in long-term survivors of severe non-missile traumatic brain injury: a computational analysis of magnetic resonance images. J. Neurotrauma. 2005;22:76–82. doi: 10.1089/neu.2005.22.76. [DOI] [PubMed] [Google Scholar]
- Tong K.A. Ashwal S. Holshouser B.A. Nickerson J.P. Wall C.J. Shutter L.A. Osterdock R.J. Haacke E.M. Kido D. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann. Neurol. 2004;56:36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- Tong K.A. Ashwal S. Holshouser B.A. Shutter L.A. Herigault G. Haacke E.M. Kido D. Hemorrhagic shearing lesions in children and adolescents with post-traumatic diffuse axonal injury. Radiology. 2003;227:332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- Tong K.A. Ashwal S. Obenaus A. Nickerson J.P. Kido D. Haacke E.M. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am. J. Neuroradiol. 2008;29:9–17. doi: 10.3174/ajnr.A0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi M.A. Ward M.A. Hess T.M. Gale S.D. Dempsey R.J. Rowley H.A. Johnson S.C. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: relationship with duration of coma. J. Neurotrauma. 2007;24:766–771. doi: 10.1089/neu.2006.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der N.J. Hew J.M. Van Zomeren A.H. Sluiter W.J. Minderhoud J.M. Computed tomography and magnetic resonance imaging in mild to moderate head injury: early and late imaging related to outcome. Ann. Neurol. 1999;46:70–78. doi: 10.1002/1531-8249(199907)46:1<70::aid-ana11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wang J.Y. Bakhadirov K. Devous M. Abdi H. McColl R. Moore C. Marquez de la Plata C. Ding K. Whittemore A. Babcock E. Rickbeil T. Dobervich J. Kroll D. Dao B. Mohindra N. Diaz-Arrastia R. Diffusion tensor tractography in traumatic diffuse axonal injury. Arch. Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- Weiss N. Galanaud D. Carpenter A. Naccache L. Puy-basset L. Clinical review: prognostic value of magnetic resonance imaging in acute brain injury and coma. Crit. Care. 2007;11:230. doi: 10.1186/cc6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A. Bigler E.D. Pedroza C. Ryser D.K. Post-traumatic amnesia predicts long-term cerebral atrophy in traumatic brain injury. Brain Inj. 2006;20:695–699. doi: 10.1080/02699050600744079. [DOI] [PubMed] [Google Scholar]