Abstract
Purpose
Treatment with cyclophosphamide (CYC) confers up to a 40% risk of ovarian failure in women of reproductive age. The use of GnRH agonists (GnRHa) to preserve ovarian function has been investigated in several small studies. We performed a systematic review of studies examining whether a GnRHa administered during chemotherapy is protective of ovarian function and fertility.
Methods
We searched the English-language literature (1966–April 2007) using MEDLINE and meeting abstracts and included studies that reported an association between GnRHa and ovarian preservation in women receiving chemotherapy. Studies without a control group were excluded. Ovarian preservation was defined as the resumption of menstrual cycles and a premenopausal follicle-stimulating hormone (FSH) after chemotherapy. Fertility was determined by a woman's ability to become pregnant. We estimated the summary relative risk (RR) and associated 95% confidence intervals (95% CI) using a random-effects model.
Results
Nine studies included 366 women. Three studies included women with autoimmune disease receiving CYC; six included women with hematologic malignancy receiving combination chemotherapy. In total, 178 women were treated with GnRHa during chemotherapy, 93% of whom maintained ovarian function. Of the 188 women not treated with GnRHa, 48% maintained ovarian function. The use of a GnRHa during chemotherapy was associated with a 68% increase in the rate of preserved ovarian function compared with women not receiving a GnRHa (summary RR = 1.68, 95% CI 1.34-2.1). Among the GnRHa-treated women, 22% achieved pregnancy following treatment compared with 14% of women without GnRHa therapy (summary RR = 1.65, CI 1.03–2.6).
Conclusions
Based on the available studies, GnRHa appear to improve ovarian function and the ability to achieve pregnancy following chemotherapy. Several randomized trials are underway to define the role and mechanism of GnRHa in ovarian function preservation. In the meantime, premenopausal women facing chemotherapy should be counseled about ovarian preservation options, including the use of GnRHa therapy.
Introduction
Ovarian failure following chemotherapy has long been viewed as an unfortunate but unavoidable consequence of potentially lifesaving therapy. In recent years, however, this life-altering side effect has become less acceptable for several reasons. First, the survival rates following chemotherapy have begun to increase. This is raising the importance of postchemotherapy quality of life in many arenas. Second, exciting advances in reproductive medicine allow more and more previously infertile women to bear children. Third, several small studies have demonstrated that techniques undertaken prior to chemotherapy may allow a woman to have children following chemotherapy.
The effectiveness of a GnRH agonist (GnRHa) during chemotherapy to preserve ovarian function was first demonstrated in rodents and monkeys in the 1980s.1–3 Over the last 10 years, more than a dozen reports of small cohorts of women undergoing chemotherapy with concomitant GnRHa therapy have been published, most with resoundingly positive results. In this paper, we performed a meta-analysis of the published literature that compares GnRHa cotherapy during chemotherapy with chemotherapy alone to determine if GnRHa can improve ovarian preservation and maintain fertility.
Materials and Methods
Data sources and searches
This meta-analysis was conducted and reported according to recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group.4 MEDLINE (1966–April 2007) and American College of Rheumatology (ACR), American Society of Clinical Oncology (ASCO), and American Society of Reproductive Medicine (ASRM) abstracts (2000–2006) were searched using crossing keywords. Terms included GnRH agonist, fertility, ovarian failure, ovarian preservation, and chemotherapy. Reference lists were also searched for additional papers.
Study selection
Two investigators reviewed each study. Included studies met the following criteria: (1) females < age 50 undergoing potentially ovarian-toxic therapy for either malignancy or rheumatology disease, (2) included a control group of women with similar illness and chemotherapy who did not receive GnRHa therapy, and (3) an acceptable definition of ovarian function was included in patient assessment using menstrual history, follicle-stimulating hormone (FSH) levels, or antral follicle counts. Studies that reported pregnancy rates were included in the fertility portion of the meta-analysis. To determine the likelihood of preserving fertility following chemotherapy, we counted the number of women who became pregnant during follow-up. Some women had more than one pregnancy; in these cases, only the initial pregnancy was included in this analysis. Study and author data were examined to ensure that every included dataset was unique.
Data extraction and quality assessment
Data were extracted into contingency tables to facilitate the calculation of the odds of ovarian preservation and the odds of pregnancy following chemotherapy. The data in each study were assessed for consistency among abstract, tables, and text. One paper had a discrepancy, and we elected to use the numbers in the text, not the abstract, which were least favorable toward GnRHa.5 All studies except the larger Blumenfeld report10 come from small cohorts. In addition, only two studies report being randomized.5,7 However, in Loverro et al.,7 the method of randomization is not described, and the difference in the length of patient follow-up for the two groups suggests that it was not well randomized.
Data synthesis and analysis
For the meta-analysis, we combined the calculated relative risks (RR) for ovarian preservation and pregnancy. Our first step was to test for homogeneity of RRs using a chi-square test to determine if we needed to combine estimates using a fixed-effect or random-effect model. Summary estimates for both types of models were generated using the software program FAST-PRO (Academic Press, San Diego, CA).8,9 The summary estimate from a fixed-effect model was calculated on the natural log transformations of RRs from the individual studies using the variance-weighted method. Estimates from random-effect models were calculated using an empirical Bayes or restricted likelihood estimation method.
Results
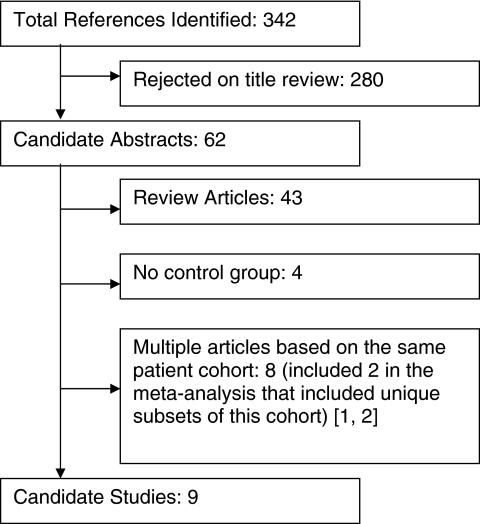
The systematic search yielded 342 references, which were narrowed to nine eligible papers and abstracts based on the meta-analysis inclusion criteria (Fig. 1). Of the nine studies, only two were randomized5,7 (Table 1). The others included either historic controls or women who elected not to receive GnRHa therapy for undefined reasons.6,10–15 Six studies included women undergoing a range of chemotherapy for lymphoma or leukemia. Three studies included women undergoing monthly intravenous infusions of cyclophosphamide (CYC) for severe lupus.
FIG. 1.
Results of systematic literature search.
Table 1.
Review of Studies Included in the Meta-Analysis
| Author, location | Patient population | Study type | GnRHa, dose, timing | Chemotherapy | Definition of premature ovarian failure | Duration of patient follow-up |
|---|---|---|---|---|---|---|
| Blumenfeld et al. 20056 Israel | Lymphoma (Hodgkin and non-Hodgkin) Age 14–40 | Prospective Controls: referred after therapy started or did not want GnRHa |
Triptorelin 3.75 mg i.m. q4wks | Various XRT in 65% |
Amenorrhea, estradiol <100 pmol/L, and FSH > 25 IU/L | Not reported |
| Blumenfeld et al. 200010 Israel | SLE (7) Nephrotic syndrome (1) |
Prospective. Nonrandomized |
Triptorelin 3.75 mg i.m. q4wks | CYCa or chlorambucil with CYC dose GnRHa: 6–11 g No GnRHa: 4–26.5 g |
Amenorrhea, estradiol <100 pmol/L, and FSH > 25 IU/L | GnRHa: 2–15 years (average 8.3 years) No GnRHa: 4–15 years (average 9 years) |
| Castelo-Branco et al. 200711 Spain | Hodgkin's Age 14–45 |
Prospective, Nonrandomized. | Triptorelin 3.75 mg i.m. q4wks (1st dose 1–2 wks before chemotherapy) And Tibolone qd to decrease low estrogen effects |
Various: ABVD: 10 of each group ABVD + XRT: 10 GnRH, 7 control |
No regular Menses | Not reported, but all patients enrolled “during the same period” of time |
| Dann et al. 200512 Israel | Non-Hodgkin's lymphoma Age 18–40 (median 27) |
Prospective. Offered GnRHa to women under 40. Nonrandomized |
Triptorelin 3.75 mg i.m. q4wks | Cumulative CYC dose: 8,000–12,000 mg/m2 10: CYC 3,000 mg/m2 over 2 days + doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, prednisone 1: CYC 2500 mg/m2 2: CYC 2000 mg/m2 |
Cyclic ovarian function = regular menses + normal gonadotropin and sex steroid levels or follicles on U/S or pregnancy | Follow-up: 70 months (23–99) GnRHa: 23–95 months No GnRHa: 23–99 months |
| Loverro, et al. 20077 Italy | Hodgkin's lymphoma Mean age 24.3 ± 6.6 | Prospective randomized | Triptorelin 3.25 mg i.m. qmonth Or Triptorelin 11.25 mg i.m. q3months |
13 patients: ABVD × 6 13 patients: ABVD × 5 alternating with C(M)OPP 3 patients: C(M)OPP alternative ABV, then DHAP 24 patients: Supradiaphragmatic radiation |
No menses within 12 months | GnRHa: 2.4 ± 1.7 years No GnRHa: 5.9 ± 4.5 years |
| Pereyra Pacheco et al. 200113 Argentina | Adolescents with leukemia Group B: Postmenarche, treated with GnRHa Group C: Postmenarche, no GnRHa. |
Nonrandomized, nonblinded. Prospective for GnRHa patients, retrospective for controls. |
Leuprolide 3.75 mg IM q4wks Short-acting leuprolide given daily the 1st 2 weeks of treatment so chemotherapy could be started immediately |
Group B1: BMT6 Group B2: CVPP7 or ABVD, no BMT Group C: BMT |
Amenorrhea | Group B: 5 yrs. Group C: 6 yrs |
| Petri et al. 200414 Johns Hopkins | Severe SLE Age < 40 |
Prospective cohort | Leuprolide 3.75 mg i.m. q4wks | Monthly CYC × 6, then q3months for 18 months Or High-dose CYC (50 mg/kg × d) |
FSH > 25 | Not reported |
| Somers et al. 2005.15 University of Michigan | SLE Age 17–32 follow-up minimum 3 years |
Prospective for GnRHa patients, retrospective for controls | Leuprolide 3.75 mg i.m. q4wks (10 days prior to next CYC dose) 1st dose usually given after 1st CYC dose Depot provera q3m and estrogen patch |
CYC i.v. monthly × 6 months, then AZA or MMF Or CYC i.v. qm × 4 |
FSH > 40 and amenorrhea × 12 months Median time to POF: 4.3 years |
Minimum 3 years GnRHa: 3–9 years (median 5 years) No GnRHa: 4–17 years (median 10 years) |
| Waxman et al. 19875 U.K. | Advanced Hodgkin's disease | Prospective, randomized | Buserelin 200 μg t.i.d. intranasal, starting 1 week before chemotherapy |
Up to 6 cycles of MVPP | Amenorrhea | Mean: GnRHa: 2.3 years No GnRHa: 2.0 years |
CYC, cyclophosphamide, ABVD; adriamycin, bleomycin, vinblastine, dacarbazine; C(M)OPP, cyclophosphamide, vincristine, procarbazine, prednisone; ABV, adriamycin, bleomycin, vinblastine; DHAP, dexamethasone, cytarabine, cisplatin; BMT, bone marrow transplant; CVPP, cyclophosphamide, vinblastine, procarbazine, prednisone; AZA, azathioprine; MMF, mycophenolate mofetil; MVPP, mustine, vinblastine, procarbazine, prednisolone; XRT, radiation; U/s, ultrasound.
Three different GnRHa formulations were used in the studies: leuprolide (Lupron, Tap Pharmaceuticals, Deerfield, IL) and triptorelin (Decapeptyl) intramuscularly or buserelin intranasally. Except for the study using buserelin, dosing was fairly standard between studies, with most women receiving 3.75 mg of GnRHa every 4 weeks throughout chemotherapy administration (Table 1). Most studies described starting treatment about 2 weeks prior to chemotherapy or treatment with short-acting GnRHa to avoid chemotherapy during the expected ovarian flare that follows GnRHa therapy by 5–10 days. In one study, many women received their first leuprolide dose following their initial CYC dose to avoid treatment during the ovarian flare.15
Ovarian preservation and failure were defined differently in the studies. Most used regular menses after cessation of chemotherapy as an indication of continued ovarian function. Some included FSH, luteinizing hormone (LH), and estradiol levels, and others included ultrasound-derived follicle counts to define ovarian function. Pregnancy following chemotherapy was described in seven of the nine included studies.
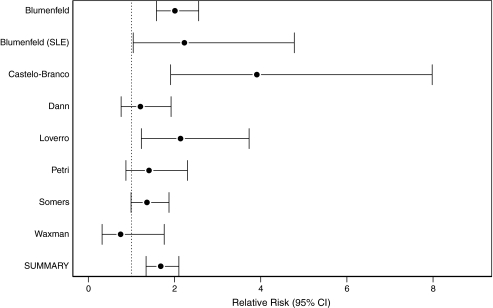
From these nine papers, eight studies were included in the meta-analysis of ovarian preservation (Table 2). One study that met eligibility criteria could not be included in the meta-analysis because we were unable to calculate an RR.13 In this study, no women without GnRHa maintained ovarian function (0 of 4 women). This led to a zero in the denominator of the RR for ovarian preservation and, therefore, an unreliable RR and confidence interval. For the eight included studies, we rejected the test of homogeneity for RR (chi-square 16.3, p = 0.02). Based on the nonhomogeneity of RRs between studies, the summary RR estimate was generated using a random-effects model. The estimated summary RR from the random-effects model for those given GnRHa therapy was associated with ovarian preservation (summary RR 1.68, CI 1.34-2.1) compared with women not exposed. If the excluded study is included with 0.5 in the denominator, the summary RR is 1.70 (CI 1.36-2.13). The estimated summary RR from the fixed-effects model for those given GnRHa therapy was associated with ovarian preservation (summary RR 1.70, CI 1.46-1.98) compared with women not exposed (Fig. 2).
Table 2.
Ovarian Function following Chemotherapy with and without GnRHa Cotherapy
| |
GnRHa |
No GnRHa |
|
||||
|---|---|---|---|---|---|---|---|
| Author Location of study | n | n with ovarian function | % with ovarian function | n | n with ovarian function | % with ovarian function | RR of ovarian function RR 95% CI |
| Blumenfeld et al. 20056 Israel | 75 | 70 | 93 | 82 | 38 | 46 | 2.01 (1.58–2.56) |
| Blumenfeld et al. 200010 Israel | 8 | 8 | 100 | 9 | 4 | 44 | 2.23 (1.04–4.78) |
| Castelo-Branco et al. 200711 Spain | 30 | 27 | 90 | 26 | 6 | 23 | 3.91 (1.91–7.98) |
| Dann et al. 200512 Israel | 7 | 7 | 100 | 6 | 5 | 83 | 1.21 (0.76–1.92) |
| Loverro et al. 20077 Italy | 14 | 14 | 100 | 15 | 7 | 47 | 2.14 (1.23–3.73) |
| Pereyra Pacheco et al. 200113 Argentina | 12 | 12 | 100 | 4 | 0 | 0 | No RRa |
| Petri et al. 200414 Johns Hopkins | 4 | 4 | 100 | 17 | 11 | 65 | 1.41 (0.87–2.3) |
| Somers et al. 200515 University of Michigan | 20 | 19 | 95 | 20 | 14 | 70 | 1.36 (0.99–1.87) |
| Waxman et al 19875 U.K. | 8 | 4 | 50 | 9 | 6 | 67 | 0.75 (0.32–1.76) |
| Summary RR | 1.68 (1.34–2.1)b | ||||||
As we are unable to calculate this RR, this study is not included in the meta-analysis.
Empirical Bayes.
FIG. 2.
Ovarian preservation with and without GnRHa cotherapy.
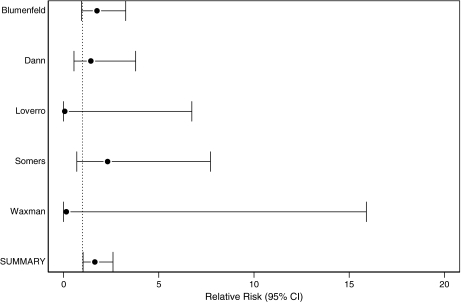
Only seven of the included papers provided pregnancy data, and five of these studies were included in the meta-analysis (Table 3). Two studies were excluded because no women without GnRHa therapy became pregnant, leaving it impossible to determine a reliable RR for these studies. Pregnancy was far less frequent than ovarian function preservation in all studies. Some studies had large numbers of pregnancies and others very few. The rate of pregnancy postchemotherapy differed significantly between studies (homogeneity p = 0.37). Despite the small number of pregnancies, the estimated summary RR from the fixed-effects model was RR 1.65, CI 1.03-2.6, demonstrating that women who received GnRHa had more pregnancies than women without this treatment. If the excluded studies are included with 0.5 in the denominator, the summary RR is 1.64 (CI 1.01-2.65). The estimated summary RR from the random-effects model for those given GnRHa therapy was associated with pregnancy (summary RR 1.63, CI 1.004–2.6) compared with women not exposed (Fig. 3).
Table 3.
Pregnancies following Chemotherapy with and without GnRHa Cotherapy
| |
GnRHa |
No GnRHa |
|
||||
|---|---|---|---|---|---|---|---|
| Author Location of study | n | n of pregnant womena | % pregnant | n | n of pregnant women | % pregnant | RR of pregnancy RR (95% CI) |
| Blumenfeld et al. 2005.6 Israel | 75 | 21 | 28 | 82 | 13 | 16 | 1.76 (0.95–3.27) |
| Castelo-Branco et al. 200711 Spain | 30 | 1 | 3 | 26 | 0 | 0 | No RR2 |
| Dann et al. 200512 Israel | 7 | 5 | 71 | 6 | 3 | 50 | 1.44 (0.55–3.79) |
| Loverro et al. 20077 Italy | 14 | 0 | 0 | 15 | 2 | 13 | 0.07 (0–6.74) |
| Pereyra Pacheco et al. 200113 Argentina | 12 | 2 | 17 | 4 | 0 | 0 | No RRb |
| Somers et al. 200515 University of Michigan | 20 | 7 | 35 | 20 | 3 | 15 | 2.32 (0.7–7.72) |
| Waxman et al. 19875 U.K. | 8 | 0 | 0 | 9 | 1 | 11 | 0.15 (0–15.9) |
| Summary RR | 1.65 (1.03–2.6)c | ||||||
If a woman had more than one pregnancy, only the first pregnancy was counted in this analysis.
As we are unable to calculate this RR, this study is not included in the meta-analysis.
Empirical Bayes.
FIG. 3.
Pregnancy following chemotherapy with and without GnRHa cotherapy.
Discussion
This meta-analysis demonstrates that cotreatment with a GnRHa during chemotherapy is associated with increased odds of a woman maintaining ovarian function and having a pregnancy following chemotherapy by 68%. Larger randomized trials are underway to further define the role of GnRHa in ovarian preservation. In the meantime, women requiring ovarian-toxic chemotherapy should be offered GnRHa cotherapy if they desire to preserve ovarian function and fertility.
The results presented in this meta-analysis are in keeping with previously published studies of ovarian function following chemotherapy. On average, 40% of women who undergo chemotherapy will develop ovarian failure. The rate of ovarian failure depends largely on the type of chemotherapy, the cumulative dose, and the age of the woman receiving the treatment.16,17 Among women < age 40 with early stage breast cancer, amenorrhea occurred in 54% of women after 6–12 cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF ). Menses resumed in 23% of these young women. In comparison, among women aged > 40 years treated in the same manner, 96% developed premature ovarian failure during therapy, and 92% never resumed menses.18
In addition to older age, the type and cumulative dose of chemotherapy determine the risk of ovarian failure. For example, in treatment of early stage breast cancer, rates of amenorrhea have been reported as high as 69% 1 year postchemotherapy among premenopausal women receiving 6 months of oral CMF chemotherapy. This compares to a 34% risk among those receiving 12 weeks of adriamycin plus cyclophosphamide (AC) chemotherapy.19 Historically, noncell cycle-specific alkylating agents (i.e., CYC, iphosphamide, nitrosoureas, melphalan, bulsulfan, chorambucil, and procarbazine) have proven to be directly toxic to the ovarian granulosa cell, leading to depletion of finite ovarian follicles such that permanent menopause ensues.20,21
The rate of ovarian failure for women with autoimmune diseases who undergo therapy with alkylating agents is similar to that of women with malignancy. Intravenous CYC is a widely used therapy for aggressive lupus, particularly renal and central nervous system (CNS) disease, as well as scleroderma lung disease and severe vasculitis. Several studies of women with lupus found premature ovarian failure rates between 16% and 54%, with an average of 30%.22–26 Both age at the time of CYC therapy and the cumulate dose of the drug were the main determinants of risk for ovarian failure, with the risk sharply increasing over age 30 and with doses over 10 g.22,23 This meta-analysis includes studies with varying intensities and types of chemotherapy, making comparisons between studies difficult. It does, however, allow the results to be more generalizable, demonstrating that GnRHa may be effective during a variety of chemotherapeutic regimens.
All the studies in this meta-analysis included a control group. There are several studies, however, that detailed success with GnRHa cotherapy during chemotherapy, but did not include a control group. At the University of North Carolina, only 2 of 25 women cotreated with leuprolide during CYC treatment for lupus nephritis developed premature ovarian failure. The 2 patients who lost ovarian function were both older (> age 35) and received two 6-month cycles of CYC/leuprolide for recurrent lupus activity.27 In another study of 18 women with lupus who underwent treatment with both monthly CYC and leuprolide, only 3 (17%) developed ovarian failure.28
Several oncology studies showed similar benefits from GnRHa cotherapy. In a study of 24 young women with early stage breast cancer, only 1 woman developed amenorrhea following chemotherapy and cotherapy with GnRHa.29 A large retrospective study of 100 women with breast cancer cotreated with goserelin 3.5 mg every 4 weeks or 11.25 mg every 12 weeks found that 33% of the women developed ovarian failure following chemotherapy; none of the 25 women < age 40 had ovarian failure.30
On the other hand, one study of 30 women undergoing stem cell transplant for hematological malignancy showed no benefit from GnRHa therapy; all 30 women had premature ovarian failure.31 In addition, the first study of GnRHa cotherapy failed to show benefit. In this study, 8 women were randomized to no cotreatment or intranasal buserelin 200 μg t.i.d. starting 1 week prior to chemotherapy for advanced Hodgkin's disease. Following chemotherapy, 4 of 8 women continued to have menses, compared with 6 of 9 women without buserelin therapy.5 Intranasal buserelin is not as effective as i.m. dosing in suppressing ovarian function, leaving women with this treatment at risk for ovarian toxicity.32 By allowing only a 1-week interval between initial buserelin administration and chemotherapy, women may have still been having the expected ovarian flare at the time of chemotherapy. This period of ovarian overactivity places the ovary at particular risk from the toxic effects of chemotherapy.
It should be noted that there may be a publication bias in that studies that do not show a benefit from GnRHa may not be not published. By searching in the abstracts of the major reproductive endocrinology, oncology, and rheumatology groups, however, we hope to have identified smaller negative studies in addition to positive published work.
In this meta-analysis, the pregnancy rate was higher among women who received GnRHa cotherapy. As most of the studies in this analysis included nonrandomized samples, it is possible that more women interested in future childbearing elected to receive GnRHa cotherapy. It is also unclear if pregnancies in the GnRHa groups were spontaneous or assisted, which may also lead to bias and overstatement of the overall fertility benefits. None of the studies report the number of women who tried unsuccessfully to become pregnant. Several of the studies, however, have longer follow-up periods for women without GnRHa cotherapy, giving this cohort of women the unfair advantage of more time to become pregnant. Given these biases, only a truly randomized study will be able to fully answer whether GnRHa cotherapy improves the chances for childbearing following chemotherapy.
The mechanism by which GnRHa may protect the ovary during chemotherapy is debated. Some have proposed that GnRHa protects the ovary by shutting down the hypothalamic-pituitary-ovarian (HPO) axis and inducing a prepubertal state. Natural human GnRH is released in a pulsatile fashion to stimulate the gonadotropin release that drives the ovulatory cycle and subsequent ovarian steroidogenesis. Sustained-release synthetic GnRHa binds the GnRH receptors on the pituitary, initially inducing gonadotropin release, commonly known as the flare response. With this increase in gonadotropin, the ovary is briefly hyperstimulated. Following the flare, pituitary GnRH receptors are downregulated, and gonadotropin release is prevented. This inhibits the pituitary-ovarian cycle, resulting in postmenopausal levels of estrogen.33 In central precocious puberty (CPP), however, higher doses of GnRHa are required for full HPO suppression. The effect of GnRHa in these patients is dose dependent, with leuprolide 7.5 mg monthly suppressing ovarian function more effectively than either 3.75 mg monthly or 11.25 mg every 3 months.34
Another proposed mechanism by which GnRHa may provide ovarian protection is via a decrease in ovarian blood flow, causing a decrease in the amount of chemotherapy reaching the ovary. Studies on the effect of GnRHa on blood flow are few and contradictory. Reinsch et al.35 documented a 21% decrease in uterine blood flow after 3 months of depot-Lupron 3.75 mg; ovarian blood flow was not examined. Ng et al.36 and Jarvela et al.37 did not detect a difference in ovarian stromal blood flow before and after GnRHa pituitary downregulation for in vitro fertilization.
A direct effect of GnRHa on the ovary has also been proposed. GnRH receptors have been identified in ovarian cancer cell lines, ovarian surface epithelium, preovulatory follicles, and the corpus luteum but not in primordial or early antral follicles.38 Granulosa cells from preovulatory follicles aspirated for in vitro fertilization exposed to GnRHa prior to doxorubicin and FSH produce estradiol at levels similar to those of controls. Granulosa cells exposed to doxorubicin and FSH without GnRHa premedication produce lower levels of estradiol.39 This study provides in vitro evidence that GnRHa may decrease doxorubicin-induced damage to granulosa cell function when applied to granulosa from preovulatory follicles, but it does not provide a mechanism by which GnRHa would protect the primordial and preantral follicles that make up the majority of the follicle pool and lack GnRH receptors.
Challengers of GnRHa suppression during chemotherapy express concern that offering GnRHa suppression in the absence of proof of efficacy from a large randomized controlled trial may divert patients from proven fertility preservation options, such as embryo cryopreservation. They also propose that GnRHa may decrease the effectiveness of the chemotherapy via antiproliferative and antiapoptotic activity in tumor cells, specifically among hormone-sensitive malignancies, such as estrogen receptor-positive breast cancer,40 or increase the gonadotoxicity of chemotherapy via reduction of detoxifying enzymes in the granulosa cells.41,42 Given the observational evidence available to date, both proponents and opponents of GnRHa suppression agree that a large randomized controlled trial is needed.
Alternative measures to protect fertility are available to some women preparing to undergo chemotherapy. Options include embryo or oocyte cryopreservation, for which a woman must undergo ovarian hyperstimulation for oocyte harvest. This process takes 2–3 weeks, and the best success is derived from fertilized embryos, requiring either a male partner or the use of anonymous sperm.43 The elevated estrogen levels associated with this treatment are contraindicated in women with lupus or estrogen receptor-positive breast cancer. The addition of aromatase inhibitor to gonadotropins has been shown to decrease peak estrogen levels compared with traditional in vitro fertilization stimulation protocols. Few women have had the opportunity to return for their frozen embryos, so pregnancy outcome data and long-term data on breast cancer outcome have yet to be reported for this low estrogen technique.44, 45 Several sites are preserving ovarian tissue, obtained through laparoscopy. Although animal models of in vitro ovarian tissue maturation are promising, this technique has yet to result in a successful human pregnancy from the oocytes in the cryopreserved tissue.46
Based on the results of this meta-analysis, we recommend offering GnRHa therapy to premenopausal women who desire future fertility as they prepare to undergo chemotherapy. Our analysis shows that GnRHa cotherapy increases the chances of maintaining ovarian function and childbearing by 65%–68% over chemotherapy alone.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Ataya K. Rao LV. Lawrence E. Kimmel R. Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52:365–372. doi: 10.1095/biolreprod52.2.365. [DOI] [PubMed] [Google Scholar]
- 2.Ataya K. Ramahi-Ataya A. Reproductive performance of female rats treated with cyclophosphamide and/or LHRH agonist. Reprod Toxicol. 1993;7:229–235. doi: 10.1016/0890-6238(93)90229-z. [DOI] [PubMed] [Google Scholar]
- 3.Bokser L. Szende B. Schally AV. Protective effects of D-Trp6-luteinising hormone-releasing hormone microcapsules against cyclophosphamide-induced gonadotoxicity in female rats. Br J Cancer. 1990;61:861–865. doi: 10.1038/bjc.1990.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stroup DF. Berlin JA. Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 5.Waxman JH. Ahmed R. Smith D, et al. Failure to preserve fertility in patients with Hodgkin's disease. Cancer Chemother Pharmacol. 1987;19:159–162. doi: 10.1007/BF00254570. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld Z. Eckman A. Preservation of fertility and ovarian function and minimization of chemotherapy induced gonadotoxicity in young women by GnRH-a. J Natl Cancer Inst Monogr. 2005;34:40–43. doi: 10.1093/jncimonographs/lgi015. [DOI] [PubMed] [Google Scholar]
- 7.Loverro G. Attilio G. Edoardo DN. Loredana G. Cristina L. Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD) Hematology. 2007;12:141–147. doi: 10.1080/10245330600954072. [DOI] [PubMed] [Google Scholar]
- 8.Hedges LV. Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 9.Eddy DM. Hasselblad V. Shachter R. An introduction to a Bayesian method for meta-analysis: The confidence profile method. Med Decis Making. 1990;10:15–23. doi: 10.1177/0272989X9001000104. [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld Z. Shapiro D. Shteinberg M. Avivi I. Nahir M. Preservation of fertility and ovarian function and minimizing gonadotoxicity in young women with systemic lupus erythematosus treated by chemotherapy. Lupus. 2000;9:401–405. doi: 10.1191/096120300678828596. [DOI] [PubMed] [Google Scholar]
- 11.Castelo-Branco C. Nomdedeu B. Camus A. Mercadal S. Martinez de Osaba M. Balash J. Use of gonadotropin releasing hormone agonists in patients with Hodgkin's disease for preservation of ovarian function and reduction of gonadotoxicity related to chemotherapy. Fertil Steril. 2007;87:703–706. doi: 10.1016/j.fertnstert.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Dann EJ. Epelbaum R. Avivi I, et al. Fertility and ovarian function are preserved in women treated with an intensified regimen of cyclophosphamide, adriamycin, vincristine and prednisone (Mega-CHOP) for non-Hodgkin lymphoma. Hum Reprod. 2005;20:2247–2249. doi: 10.1093/humrep/dei018. [DOI] [PubMed] [Google Scholar]
- 13.Pereyra Pacheco B. Mendez Ribas JM. Milone G, et al. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: A preliminary report. Gynecol Oncol. 2001;81:391–397. doi: 10.1006/gyno.2001.6181. [DOI] [PubMed] [Google Scholar]
- 14.Petri M. Brodsky R. Jones R. Johnson L. Magder L. High dose cyclophosphamide can cause ovarian failure. Arthritis Rheum. 2004;50(Suppl 9):408. [Google Scholar]
- 15.Somers EC. Marder W. Christman GM. Ognenovski V. McCune WJ. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52:2761–2767. doi: 10.1002/art.21263. [DOI] [PubMed] [Google Scholar]
- 16.Laml T. Schulz-Lobmeyr I. Obruca A. Huber J. Hartmann B. Premature ovarian failure: Etiology and prospects. Gynecol Endocrinol. 2000;14:292–302. doi: 10.3109/09513590009167696. [DOI] [PubMed] [Google Scholar]
- 17.Hensley M. Reichman B. Fertility and pregnancy after adjuvant chemotherapy for breast cancer. Crit Rev Oncol Hematol. 1998;28:121–128. doi: 10.1016/s1040-8428(98)00013-4. [DOI] [PubMed] [Google Scholar]
- 18.Bonadonna G. Valagussa P. Adjuvant systemic therapy for resectable breast cancer. J Clin Oncol. 1985;3:259–275. doi: 10.1200/JCO.1985.3.2.259. [DOI] [PubMed] [Google Scholar]
- 19.Cobleigh M. Amenorrhea following adjuvant chemotherapy for breast cancer. Proc Am Soc Clin Oncol. 1995;14:158. [Google Scholar]
- 20.Lee S. Schover L. Partridge A, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 21.Walshe J. Denduluri N. Swain S. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 22.Huong du L. Amoura Z. Duhaut P, et al. Risk of ovarian failure and fertility after intravenous cyclophosphamide. A study in 84 patients. J Rheumatol. 2002;29:2571–2576. [PubMed] [Google Scholar]
- 23.Park MC. Park YB. Jung SY. Chung IH. Choi KH. Lee SK. Risk of ovarian failure and pregnancy outcome in patients with lupus nephritis treated with intravenous cyclophosphamide pulse therapy. Lupus. 2004;13:569–574. doi: 10.1191/0961203304lu1063oa. [DOI] [PubMed] [Google Scholar]
- 24.Boumpas DT. Austin HA., 3rd Vaughan EM. Yarboro CH. Klippel JH. Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993;119:366–369. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 25.McDermott EM. Powell RJ. Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann Rheum Dis. 1996;55:224–229. doi: 10.1136/ard.55.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok CC. Wong RW. Lau CS. Ovarian failure and flares of systemic lupus erythematosus. Arthritis Rheum. 1999;42:1274–1280. doi: 10.1002/1529-0131(199906)42:6<1274::AID-ANR26>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Dooley MA. Patterson CC. Hogan SL, et al. Preservation of ovarian function using depot leuprolide acetate during cyclophopsphamide therapy for severe lupus nephritis. Arthritis Rheum. 2000;43(Suppl 9):2858. [Google Scholar]
- 28.Perez Pampin E. Mera Varela A. Maceiras Pan F. Gomez-Reino JJ. Impact on bone mineral density on the treatment with gonadotropin-releasing hormone antagonists (GnRH-a) in SLE patients treated with IV cyclophosphamide. Arthritis Rheum. 2006;54(Suppl 9):S431. [Google Scholar]
- 29.Fox KR. Scialla J. Moore H. Preventing chemotherapy related amenorrhea using leuprolide during adjuvant chemotherapy for early-stage breast cancer. Proc Am Soc Clin Oncol. 2003:22. Abstract 50. [Google Scholar]
- 30.Recchia F. Saggio G. Amiconi G, et al. Gonadotropin releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514–523. doi: 10.1002/cncr.21646. [DOI] [PubMed] [Google Scholar]
- 31.Chiusolo P. Salutari P. Sica S, et al. Luteinizing hormone-releasing hormone analogue: Leuprorelin acetate for the prevention of menstrual bleeding in premenopausal women undergoing stem cell transplantation. Bone Marrow Transplant. 1998;21:821–823. doi: 10.1038/sj.bmt.1701187. [DOI] [PubMed] [Google Scholar]
- 32.Falkson CI. Falkson HC. Falkson G. Effect of chemotherapy with or without buserelin on serum hormone levels in premenopausal women with breast cancer. Eur J Cancer. 1991;27:1208–1211. doi: 10.1016/0277-5379(91)90082-o. [DOI] [PubMed] [Google Scholar]
- 33.Periti P. Mazzei T. Mini E. Clinical pharmacokinetics of depot leuprorelin. Clin Pharmacokinet. 2002;41:485–504. doi: 10.2165/00003088-200241070-00003. [DOI] [PubMed] [Google Scholar]
- 34.Badaru A. Wilson DM. Bachrach LK, et al. Sequential comparisons of one-month and three-month depot leuprolide regimens in central precocious puberty. J Clin Endocrinol Metab. 2006;91:1862–1867. doi: 10.1210/jc.2005-1500. [DOI] [PubMed] [Google Scholar]
- 35.Reinsch RC. Murphy AA. Morales AJ. Yen SS. The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: A prospective, randomized study. Am J Obstet Gynecol. 1994;170:1623–1627. [PubMed] [Google Scholar]
- 36.Ng EHY. Tang OS. Chan CCW. Ho PC. Ovarian stromal blood flow in the prediction of ovarian response during in vitro fertilization treatment. Hum Reprod. 2005;20:3147–3151. doi: 10.1093/humrep/dei166. [DOI] [PubMed] [Google Scholar]
- 37.Jarvela IY. Sladkevicius P. Kelly S. Ojha K. Campbell S. Nargund G. Effect of pituitary down-regulation on the ovary before in vitro fertilization as measured using three-dimensional power Doppler ultrasound. Fertil Steril. 2003;79:1129–1135. doi: 10.1016/s0015-0282(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 38.Choi JH. Gilks CB. Auersperg N. Leung PC. Immunolocalization of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and type I GnRH receptor during follicular development in the human ovary. J Clin Endocrinol Metab. 2006;91:4562–4570. doi: 10.1210/jc.2006-1147. [DOI] [PubMed] [Google Scholar]
- 39.Imai A. Sugiyama M. Furui T. Tamaya T. Ohno T. Direct protection by a gonadotropin-releasing hormone analog from doxorubicin-induced granulosa cell damage. Gynecol Obstet Invest. 2007;63:102–106. doi: 10.1159/000096062. [DOI] [PubMed] [Google Scholar]
- 40.Emons G. Grundker C. Gunthert AR. Westphalen S. Kavanagh J. Verschraegen C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Rel Cancer. 2003;10:291–299. doi: 10.1677/erc.0.0100291. [DOI] [PubMed] [Google Scholar]
- 41.Toft E. Becedas L. Soderstrom M. Lundqvist A. Depierre JW. Glutathione transferase isoenzyme patterns in the rat ovary. Chem Biol Interact. 1997;108:79–93. doi: 10.1016/s0009-2797(97)00095-1. [DOI] [PubMed] [Google Scholar]
- 42.Oktay K. Sonmezer M. Oktem O. Ovarian cryopreservation versus ovarian suppression by GnRH analogues: Primum non nocere: Reply. Hum Reprod. 2004;19:1681–1683. doi: 10.1093/humrep/deh300. [DOI] [PubMed] [Google Scholar]
- 43.Oktay K. Cil AP. Bang H. Efficiency of oocyte cryopreservation: A meta-analysis. Fertil Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Oktay K. Buyuk E. Libertella N. Akar M. Rosenwaks Z. Fertility preservation in breast cancer patients: A prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Oktay K. Hourvitz A. Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 42.Xu M. Kreeger PK. Shea LD. Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]