Abstract
Infection by HIV is associated with the expansion of monocytes expressing CD16 antigens, but the significance of this in HIV pathogenesis is largely unknown. In rhesus macaques, at least three subpopulations of blood monocytes were identified based on their expression of CD14 and CD16: CD14highCD16−, CD14highCD16low, and CD14lowCD16high. The phenotypes and functions of these subpopulations, including CD16+ monocytes, were investigated in normal, uninfected rhesus macaques and macaques that were infected with SIV or chimeric SHIV. To assess whether these different monocyte subpopulations expand or contract in AIDS pathogenesis, we conducted a cross-sectional study of 54 SIV- or SHIV-infected macaques and 48 uninfected controls. The absolute numbers of monocyte populations were examined in acutely infected animals, chronically infected animals with no detectable plasma virus RNA, chronically infected animals with detectable plasma virus RNA, and animals that died with AIDS. The absolute numbers of CD14highCD16low and CD14lowCD16high monocytes were elevated significantly in acutely infected animals and chronically infected animals with detectable plasma virus RNA compared with uninfected controls. Moreover, a significant, positive correlation was evident between the number of CD14highCD16low or CD14lowCD16high monocytes and plasma viral load in the infected cohort. These data show the dynamic changes of blood monocytes, most notably, CD14highCD16low monocytes during lentiviral infection, which are specific to disease stage.
Keywords: subsets, macrophage, CD16, HIV, AIDS
Introduction
HIV infection in humans results in hematologic abnormalities and immune suppression, which is well represented by changes in the CD4/CD8 T cell ratio. As obviously seen in T cell populations, HIV-induced immune perturbation is also easily observed within the mononuclear phagocyte system including macrophages and monocytes. Circulating monocytes in HIV disease display marked phenotypic and functional alterations associated with progression to AIDS [1,2,3,4,5]. Notably, cell surface antigens that exhibit a bimodal expression on all of the resting monocyte populations (thereby indicating the presence of subpopulations) have been recognized as the most affected by HIV infection. For example, an increase in FcγRIII (CD16) expression and decrease in FcγRI (CD64) and L-selectin (CD62L) expression on blood monocytes were significant in HIV-infected patients compared with uninfected controls [6,7,8,9,10,11,12]. It is likely that these changes in HIV-infected patients are largely a result of expansion and contraction of monocyte subpopulations expressing these markers. Indeed, it has been shown repeatedly that CD14+ monocytes, expressing CD16, expand during HIV infection [13,14,15,16,17,18] and SIV infection [19,20,21]. We have observed recently that monocytes leave the bone marrow and enter the blood in response to monocyte/macrophage apoptosis in monkeys that are SIV-infected [22].
The presence of phenotypically definable subpopulations of human monocytes has been recognized for over 20 years. Using two-color flow cytometry of human monocytes for the correlated expression of CD14 (LPS receptor) and CD16, Ziegler-Heitbrock et al. [23] first showed two monocyte subpopulations: CD14highCD16− and CD14lowCD16high. Following this initial description, refined analyses of chemokine receptors and adhesion molecules have better defined subpopulations of monocytes and shown additional phenotypic heterogeneity of human monocytes [24,25,26,27]. These data show a CD14highCD16− subpopulation of monocytes that express CD62L, CD64, and CCR2 with low levels of CX3CR1 expression. In contrast, the CD14lowCD16high subpopulation expresses no detectable CD62L, CD64, or CCR2 but high levels of CX3CR1.
Studies in mice have demonstrated in addition to their phenotypic differences differential homing potential of i.v.-injected CX3CR1lowCCR2+Gr1+ and CX3CR1highCCR2−Gr1− monocytes (two rodent counterparts of human CD14highCD16− and CD14lowCD16high monocytes, respectively) [28, 29]. A more recent study by Auffray et al. [30] showed that mouse CD11a+CX3CR1highCCR2−Gr1− monocytes (equivalent to human CD14lowCD16high monocytes) are the first to exit into inflamed tissues. In this study, Auffray et al. [30] demonstrated that this subset crawls on normal endothelium of blood vessels, invades tissues rapidly upon tissue damage, and becomes tissue macrophages. This study is also in line with the work showing that the human counterpart CD14lowCD16high monocytes preferentially reside in the marginal pool and are mobilized quickly [31].
Functional in vitro studies analyzing endocytotic and phagocytotic functions and the ability to produce inflammatory cytokines in response to LPS also confirmed the existence of two distinct monocyte subsets in humans [14, 32]. It is important to note the presence of a third, less well-described subpopulation that expresses high levels of CD14 and low levels of CD16 (CD14highCD16low monocytes). Adding one additional layer of complexity to monocyte heterogeneity, these monocytes with an “intermediate” or “transient” phenotype may also represent a subset with distinctive functions. Their role in HIV and SIV pathogenesis is understudied.
SIV- or chimeric SHIV-infected rhesus macaques are the prime model to study HIV pathogenesis. In this study, we investigated the phenotypic and functional differences of monocyte subpopulations in normal, uninfected and SIV- or SHIV-infected monkeys. We initially identified by flow cytometry four distinct subpopulations of blood mononuclear phagocytes in rhesus monkeys based on differential expression of CD14 and CD16: CD14highCD16−, CD14highCD16low, CD14lowCD16high, and CD14−/lowCD16−. To better characterize these subpopulations, we have further examined the expression of a broad spectrum of other monocyte- and DC-associated markers. The differential expression of these markers was largely confirmed by gene array studies using FACS-sorted monocyte subpopulations. To investigate the relative contribution of subsets that replicate virus in vivo, we examined levels of SIV RNA associated with freshly sorted monocyte subpopulations in infected animals and found their differential ability to sustain SIV infection. Changes in monocyte subpopulations during lentivirus infection were assessed by a cross-sectional study of the uninfected and infected cohorts of rhesus macaques. In this report, by performing a comprehensive analysis of the association between lentiviral infection and changes in monocytes, we identify disease-specific changes in monocyte subpopulations.
MATERIALS AND METHODS
Animals
One hundred and two rhesus macaques (Macaca mulatta) were recruited for this study, including 48 uninfected control macaques and 54 animals infected with SIV or SHIV. The infected cohort of monkeys consisted of animals infected with SIVmac239 (n=3), SIVmac251 (n=30), SHIV-89.6P (n=14), and SHIV-SF162P3 (n=7), all of which have similar magnitudes of peak viremic, chronic infection and AIDS-related plasma virus. Most blood samples were collected once at necropsy, from killed animals that were not allowed to develop AIDS and death or from animals that died with AIDS. Four animals were used for serial blood samples, two to three times over the course of disease.
Flow cytometric immunophenotyping
Peripheral blood samples were used to determine the immune phenotype of rhesus monocytes. We and others [33, 34] found that heparinized blood is not suitable to assess expression of some monocyte-associated antigens as a result of high, nonspecific background staining (e.g., CCR2) or inconsistency of staining (e.g., CD163). Similarly, as circulating immune complexes bind CD16 that mask recognition by anti-CD16 antibodies [35], it is essential to remove plasma to detect CD16 consistently. Therefore, all subsequent immunofluorescence staining for flow cytometry was performed using EDTA-anticoagulated, plasma-depleted blood. For this, aliquots of 100 μL EDTA-anticoagulated whole blood were washed with PBS, and the pellets were incubated at room temperature for 15 min with a mixture of antibodies conjugated with FITC, PE, PerCP/PerCP-Cy5.5, or APC. Three four-color immunophenotyping panels were used: One panel included anti-CD14-FITC (clone M5E2, BD PharMingen, San Diego, CA, USA), anti-CD16-PE (3G8, BD PharMingen), and anti-HLA-DR-PerCP (L243, Becton Dickinson, San Diego, CA, USA); the second panel included anti-CD16-PE, anti-HLA-DR-PerCP, and anti-CD14-APC; and the third panel included anti-CD16-FITC, anti-CD14-PerCP-Cy5.5, and anti-HLA-DR-APC. Using these three basic panels, monocyte subpopulations were examined further for expression of other monocyte- and DC-associated antigens. The mAb used for this included: CD1c-APC (AD5-8E7, Miltenyi Biotec, Auburn, CA, USA), CD11b-APC (M1/70.15.11.5, Miltenyi Biotec), CD11c-APC (S-HCL-3, Becton Dickinson), CD31-APC (WM59, eBioscience, San Diego, CA, USA), CD45RA-FITC (2H4, Beckman Coulter, Fullerton, CA, USA), CD45RB-FITC (PD7/26, Dako, Denmark), CD62L-PE (SK11, BD PharMingen), CD64-FITC (10.1, BD PharMingen), CD163-FITC (Mac2-158, Trillium Diagnostics, Brewer, ME, USA), CD123-PE (7G3, BD PharMingen), CD141-PE (1A4, BD PharMingen), CCR2-APC (48607, R&D Systems, Minneapolis, MN, USA), CCR8 (191704, R&D Systems), and CX3CR1-APC (rabbit polyclonal, Torrey Pines Biolabs, Houston, TX, USA). Isotype control mAb were used in all panels. These included: mouse IgG2b-APC (27-35, BD PharMingen), rat IgG2b-APC (141945, R&D Systems), mouse IgG1-APC (MOPC-21, BD PharMingen), mouse IgG1-FITC (MOPC-21), mouse IgG2a-FITC (G155-178, BD PharMingen), and mouse IgG2a-PE (G155-178). After staining, cells were fixed in 2% paraformaldehyde and analyzed on a FACSCalibur (BD Biosciences, San Jose, CA, USA). To establish the flow cytometric identification of monkey monocytes in blood, a gate was set based on light-scatter properties of blood monocytes to contain predominantly monocytes. The monocyte gate constituted 80–90% CD14+ cells in all samples tested, and contaminating CD3+ T cells were consistently found to be <5%. Isotype controls were used to set gates for positive cells, allowing <1% positive cells in the negative control samples. Absolute counts of monocytes and monocyte subsets were calculated by multiplying the percentage of each subpopulation within the blood monocyte gate by the number of monocytes/μL blood, as determined by complete blood cell counts (ADVIA 120 hematology analyzer, Bayer Diagnostics, Germany).
Lymphocyte immunophenotyping was performed using combinations of following mAb: CD3-PerCP-Cy5.5 or -APC (SP34, BD PharMingen); CD4-FITC (19thy5d7, Beckman Coulter) or CD8-FITC or -PE (SK1, Becton Dickinson); and CD20-PerCP-Cy5.5 (L27, Becton Dickinson), CD28-PE (28.2, Beckman Coulter), and CD95-APC (DX2, BD PharMingen). Naïve T lymphocytes were determined by their CD28+CD95− phenotype in CD3+CD4+ or CD3+CD8+ cells, whereas central and effector memory T cell subsets were defined as being CD28+CD95+ and CD28−CD95+, respectively.
Isolation of monocyte subpopulations
FACS sorting of monocyte subpopulations was performed as described previously [20]. Briefly, Ficoll-separated PBMC isolated from monkey blood were stained with anti-CD14-FITC, anti-CD16-PE, anti-CD4-PerCP-Cy5.5, and anti-CD3-APC. Cell sorting was done using a BD FACSVantage flow cytometer in a Biosafety Level 2+ facility. CD3-negative cells in the monocyte gate were fractionated based on CD14 and CD16 expression into CD14high CD16−, CD14highCD16low, and CD14lowCD16high cells. Sorted cells were washed and snap-frozen rapidly for gene array or SIV-DNA and -RNA analysis described below. In addition, CD3+CD4+ lymphocytes were sorted for comparison. Purity of all FACS-sorted populations was confirmed by flow cytometry and was routinely >95%.
Intracellular cytokine staining
The phenotype and frequency of nonstimulated monocytes spontaneously producing TNF-α and granzyme B in normal, uninfected and SIV-infected animals were determined by intracellular cytokine staining followed by seven-color flow cytometry. PBMC were isolated from heparinized blood by Ficoll gradient centrifugation and then incubated at 37°C in a 5% CO2 incubator for 3 h in the presence of monensin (GolgiStop, BD Biosciences). Cultured cells were stained with mAb specific for cell surface molecules, including anti-CD3-Pacific Blue (SP34-2, Becton Dickinson), anti-CD4-AmCyan (L200, Becton Dickinson), anti-CD8-PerCP-Cy5.5 (RPA-T8, Becton Dickinson), anti-CD16-PE (3G8, BD PharMingen), and anti-CD14-PE-Texas Red (RMO52, Beckman Coulter). Cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences) and stained with anti-TNF-α-FITC (mAb11, BD PharMingen) and anti-granzyme B-Alexa Fluor 700 (GB11, BD PharMingen). Labeled cells were resuspended and fixed with 1% formaldehyde in PBS. Samples were collected and analyzed on a BD LSR II instrument using FlowJo software (Tree Star, Ashland, OR, USA). Events (500,000–1 million) were collected/sample.
Determination of plasma viral RNA and cell-associated viral RNA and DNA
Copies of plasma SIV and SHIV RNA and cell-associated SIV RNA and DNA were determined using a quantitative real-time RT-PCR assay as described previously [20].
Statistical analysis
The Kruskal-Wallis test was used for nonparametric-independent group comparisons of the medians. Only if the test were significant (P<0.05) were comparisons of each of the infected groups with an uninfected control group performed using the two-tailed Wilcoxon test. All combinations of the independent variables were analyzed with 95% confidence level for each variable. Spearman correlation coefficients were computed between two of all variables.
RESULTS
Phenotypic heterogeneity of monkey mononuclear phagocytes in blood
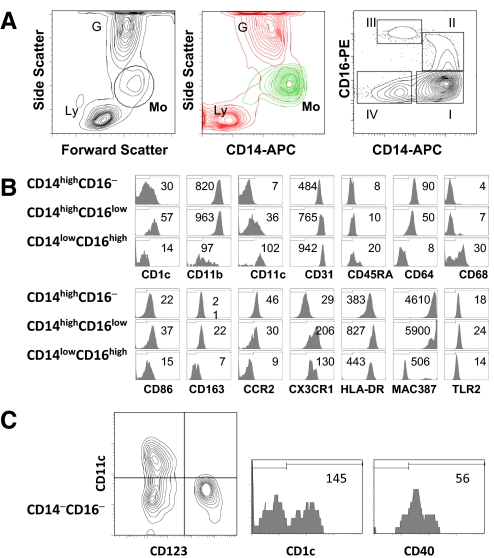
Within the blood, the mononuclear phagocyte system is represented by monocytes, which can be identified by flow cytometry as cells within a “gate” of high forward-scatter and intermediate side-scatter. This light-scatter gate contained cells displaying different levels of CD14 expression (Fig. 1A). Flow cytometric analysis of cells within the gate for the correlated expression of CD14 and CD16 delineated four distinct subpopulations. A major subpopulation is CD14highCD16−. Two additional subpopulations express variable levels of CD14 and CD16: CD14highCD16low and CD14lowCD16high. A fourth population has CD14low/−CD16−. This CD14low/−CD16−population is heterogeneous, containing immature monocytes, CD1c+CD11c+ mDCs, and CD1c−CD123+ pDCs, as well as lymphocytes (Fig. 1C). Monocyte subpopulations, CD14highCD16−, CD14highCD16low, and CD14lowCD16high, in normal, uninfected macaques (n=48) comprised 65% ± 8% (mean±sd), 8% ± 4%, and 9% ± 4%, respectively, of the total monocytes, gated on forward- versus side-scatter.
Figure 1.
Phenotypic heterogeneity of blood mononuclear phagocytes in monkeys. (A) Definition of mononuclear phagocytes in peripheral blood of rhesus macaques. (Left) Typical light-scatter contour map of whole blood cells; Ly, lymphocytes; Mo, monocytes; G, granulocytes (middle) overlay of gated mononuclear phagocytes (Mo; green) on side-scatter versus CD14 contour profile (red); (right) correlated expression of CD14 and CD16 defines four distinct subpopulations of mononuclear phagocytes in normal monkey blood. The regions indicated in the CD14 versus CD16 contour plot of gated mononuclear phagocytes were used to differentiate the following monocyte and DC populations in the blood. (B) Phenotypic analysis of blood monocyte subpopulations. The expression of indicated molecules was determined by four-color flow cytometry. The histograms exhibit the phenotypic characteristics of the three monocyte subpopulations under study: CD14highCD16−, CD14highCD16low, and CD14lowCD16high. The mean fluorescence intensity is shown. The discriminators shown were set at the foot of the isotype control. The results for each marker are representative of at least 10 uninfected animals. (C) Phenotypic characterization of CD14low/−CD16− cells (CD14–CD16–). These cells are heterogeneous, containing CD11c+ mDCs and CD123+ pDCs (left panel). CD11c+ mDCs express another DC marker CD1c (middle). Some CD14low/−CD16− cells express CD40 antigen, unlike monocyte cells (right).
To better define the cells of these subpopulations, we examined their expression of monocyte- and DC-associated markers including CD1c, CD11b, CD11c, CD31, CD40, CD45RA, CD64, CD68, CD86, CD123, CD163, CCR2, CX3CR1, HLA-DR, MAC387, and TLR2 (Fig. 1B). CD1c (blood DC antigen-1) expression in the CD14highCD16− population was bimodal and demonstrated CD1c+ or CD1c− phenotypes. Virtually, all of the CD14highCD16low cells were CD1c+, whereas all CD14lowCD16high cells were CD1c−. CD11b expression was uniformly high on CD14highCD16− and CD14highCD16low cells but low on CD14lowCD16high cells. CD11c expression was bimodal in the CD14highCD16low population with CD11c+ and CD11c− subsets. Essentially, all of the CD14low CD16high cells were CD11c+, and all of the CD14highCD16− cells were CD11c−. PECAM-1 (CD31) was expressed on nearly all cells in the CD14highCD16−, CD14highCD16low, and CD14lowCD16high populations, with the highest levels on CD14lowCD16high cells. CD45RA expression was limited to a subset of the CD14low CD16high population. All CD14highCD16− cells and a majority of CD14highCD16low cells were CD64+, but no cells expressing this marker were detected in the CD14lowCD16high population. A subset of CD86-positive cells was detected in all of the populations, where the highest levels of CD86 were found on CD14high CD16low cells. CD163, a macrophage marker, was expressed on all CD14highCD16− and CD14highCD16low cells but not on CD14lowCD16high cells. The expression of CCR2 followed the pattern of CD64. High CX3CR1 was found on all cells in CD14high CD16low and CD14lowCD16high, whereas CX3CR1low cells were detected in about one-half of the CD14highCD16− population; the level of CX3CR1 expression on CD14highCD16low cells was greater than that on CD14lowCD16high cells. Virtually, all of the CD14highCD16−, CD14highCD16low, and CD14lowCD16high cells were HLA-DR+, and CD14highCD16low cells had the highest levels. Overall, these data demonstrate that the CD14highCD16−, CD14highCD16low, and CD14lowCD16high populations comprise the majority of blood monocytes/macrophages, and the CD14low/−CD16− cells are heterogeneous and consist of mDC and pDC, CD3+ T lymphocytes, and likely, monocyte precursors. Table 1 summarizes the data described above with regard to the CD14highCD16−, CD14highCD16low, and CD14lowCD16high phenotypes.
TABLE 1.
Monkey Monocyte Subpopulations: Phenotypic Characteristics of Three Identified Subpopulations Determined by Flow Cytometry Are Summarized
| Marker | Subpopulation
|
||
|---|---|---|---|
| CD14highCD16– | CD14highCD16low | CD14lowCD16high | |
| CD14 | ++ | ++ | + |
| CD16 | – | + | ++ |
| CD1c | +/– | + | – |
| CD11b | ++ | ++ | + |
| CD11c | – | +/– | ++ |
| CD31 | + | ++ | +++ |
| CD45RA | – | – | +/– |
| CD64 | ++ | + | – |
| CD68 | – | – | + |
| CD163 | + | + | – |
| CCR2 | + | +/– | – |
| CX3CR1 | +/– | ++ | + |
| HLA-DR | + | ++ | + |
| MAC387 | + | + | – |
| TNF-α | + | + | – |
CD14 and CD16 were listed first, as these markers were used to differentiate the monocyte populations.
It has been suggested that within blood, especially during disease states, populations of monocytes are present with phenotypes similar to maturing or mature macrophages. We examined the different monocyte populations for two such markers: intracellular expression of CD68 (a pan-macrophage marker) and MAC387 (a heterodimer of S100A8 and S100A9, expressed by monocytes, which is down-regulated and lost during macrophage differentiation) antigens. We found two mutually exclusive subsets: a CD68+MAC387− subset and CD68−MAC387+ subset. All CD14highCD16− and CD14high CD16low cells were CD68−MAC387+, and a majority of CD14lowCD16high cells were CD68+MAC387−, suggesting that compared with CD14highCD16− and CD14highCD16low cells, the CD14lowCD16high populations represent more mature and macrophage-like cells.
To investigate whether CD16+ monocytes are comprised of distinct subpopulations of monocytes rather than a continuum of CD14+ monocytes with differing levels of cell activation, we used gene array analysis that compared overall gene expression profiles of FACS-sorted CD14highCD16−, CD14highCD16low, and CD14lowCD16high cells (Supplemental Data Section). Microarray analysis confirmed the phenotypic differences between these subpopulations, differentiated on the basis of markers described above (Supplemental Fig. 1) and discerned further between these subpopulations by revealing additional differences (Supplemental Tables). In comparison with CD14highCD16− cells, a large number of genes (9098/29361, 30.9%) were expressed differentially in CD14highCD16low and CD14lowCD16high cells, underscoring the fundamental differences between CD16− and CD16+ monocytes (Supplemental Table 1). Thirty-one genes and 94 genes were associated specifically with CD14highCD16low and CD14lowCD16high subpopulations (Supplemental Tables 2 and 3). A relatively small set of genes that was expressed differentially between the two CD16+ subpopulations highlights similarity between the two cell types, but differentially expressed genes of function observed in CD14highCD16low and CD14lowCD16high subpopulations suggest different roles that these two subpopulations may play in vivo.
Blood monocyte subpopulation dynamics during SIV infection
In an effort to understand how the distribution of monocytes and monocyte subpopulations changes in HIV/SIV infection, we analyzed SIV- or SHIV-infected (n=54) and naïve (n=48) rhesus monkeys (Table 2). The percentage of total monocytes was significantly higher in infected animals than in uninfected controls (mean±sd; infected, 7.5%±5.3% vs. uninfected, 4.5%±1.7%; P<0.01, unpaired t-test). Additionally, the expansion of monocytes in blood, termed monocytosis (defined as >500 cells/μL), was seen more frequently in infected animals (20/54, 37.4%) than in uninfected animals (6/48, 12.5%). However, the mean absolute numbers of total monocytes were not significantly different between the infected and uninfected groups (506±582 and 327±170 counts/μL, respectively). The percentage and absolute number of monocytes in the infected cohort were highly variable and displayed a more positively skewed distribution as compared with uninfected controls (skewness of the absolute numbers, 5.58 and 1.06, for the infected vs. uninfected animals). For these reasons, we analyzed the data from these cohorts using nonparametric tests of medians.
TABLE 2A.
Distribution of Monocyte Subpopulations
| Animal subgroup | CD4 T cells (μL) | CD4/CD8 ratio | Viral load (copies/mL) | Monocytes (%) | Monocytes (μL) | CD14highCD16– (μL) | CD14highCD16low (μL) | CD14lowCD16high (μL) | M2/M1 ra tio | M3/M1 ratio | (M2+M3)/ M1 ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected control (n=48) | |||||||||||
| median (range) | 1026 | 1.32 | NA | 4.1 | 285 | 172 | 21 | 24 | 0.12 | 0.14 | 0.25 |
| (344–2683) | (0.98–2.31) | (2.3–10.1) | (120–740) | (67–527) | (5–121) | (5–129) | |||||
| Peak viremic (n=6) | |||||||||||
| median (range) | 417a | 1.22 | ND | 8.2b | 265 | 76 | 66b | 47c | 1.02a | 0.79b | 1.81a |
| (185–461) | (0.63–1.48) | (5.0–31.9) | (200–4360) | (32–728) | (44–2158) | (39–909) | |||||
| Infected with no detectable plasma virus RNA (n=16) | |||||||||||
| median (range) | 544d | 0.91b | <125 | 6.1b | 360 | 222 | 33c | 30 | 0.19 | 0.13 | 0.31 |
| (139–1156) | (0.29–1.76) | (30–228) | (2.9–13.2) | (120–730) | (75–543) | (9–98) | (5–75) | ||||
| Infected with detectable plasma virus RNA (n=26) | |||||||||||
| median (range) | 814a | 0.67d | 66,180 | 5.7a | 370c | 194 | 53a | 41c | 0.25d | 0.18c | 0.50d |
| (17–1653) | (0.17–1.37) | (270–26,000,000) | (3.9–12.5) | (150–1430) | (62–393) | (10–293) | (4–75) | ||||
| Terminal AIDS (n=6) | |||||||||||
| median (range) | 111a | 0.23a | 7,850,000 | 9.2c | 685b | 231 | 96 | 101 | 0.36c | 0.24 | 0.60 |
| (10–260) | (0.02–0.96) | (49,000–30,000,000) | (0.6–29.8) | (310–1120) | (31–601) | (8–213) | (5–279) |
P values were determined using the Wilcoxon rank-sum test for nonparametric data and refer to comparisons of each animal group of infected animals with uninfected control animals. Significance indicated with
P < 0.001;
P < 0.01;
P < 0.05;
P < 0.0001. M2/M1, CD14highCD16low-to-CD14highCD16–; M3/M1, CD14lowCD16high-to-CD14highCD16–; (M2+M3)/M1, (CD14highCD16low+CD14lowCD16high)-to-CD14highCD16–; NA, not applicable; ND, not determined.
TABLE 2B.
Distribution of Monocyte Subpopulations
| Animal subgroup | CD4 T cells (μL) | CD4/CD8 ratio | Viral load (copies/mL) | Monocytes (%) | Monocytes (μL) | CD14highCD16– (μL) | CD14highCD16low (μL) | CD14lowCD16high (μL) | M2/M1 ra tio | M3/M1 ratio | (M2+M3)/ M1 ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| n=14 Monkeys with VL <70,000 (median) | |||||||||||
| median (range) | 634 | 0.66 | 10,950 | 5.1 | 295 | 163 | 21 | 24 | 0.18 | 0.16 | 0.31 |
| (17–1275) | (0.07–1.37) | (270–68,000) | (2.6–9.2) | (150–560) | (62–318) | (10–92) | (4–65) | ||||
| n=12 Monkeys with VL >70,000 (median) | |||||||||||
| median (range) | 851 | 0.71 | 225,000 | 6.3 | 565a | 281 | 111b | 65a | 0.38a | 0.29 | 0.60c |
| (178–1653) | (0.17–1.18) | (70,000–16,000,000) | (4.1–12.5) | (280–1430) | (123–393) | (53–293) | (34–95) |
Animals with detectable plasma virus RNA are subdivided into two subgroups: one with low viral load (<median, 70,000 copies/ml); and one with high viral load (>70,000 copies/ml). Comparison of animals with high viral load vs. animals with low viral load performed with the Wilcoxon test. Significance at **, P < 0.01; ***, P < 0.001, and ****, P < 0.0001.
To characterize in detail distribution patterns of monocyte subpopulations reflecting the progression of the disease, we stratified infected monkeys using the following disease stages at the time of specimen collection: (1) acute phase (2 weeks p.i.; n=6) and chronic phase (>12 weeks p.i.); (2) without (n=16) or (3) with (n=26) detectable plasma virus RNA; and (4) terminal AIDS (at necropsy; n=6; Table 2A). The absolute numbers of total monocytes were significantly higher in chronically infected monkeys with detectable plasma virus RNA (median 370 counts/μL, P=0.0392, Wilcoxon rank-sum test) and monkeys with terminal AIDS (median 685 counts/μL, P= 0.0083, Wilcoxon rank-sum test) than in normal, uninfected controls (median 285 counts/μL). In acutely infected animals, the monocyte number (median 265 counts/μL) was comparable with that in uninfected controls, and the median monocyte percentage was significantly higher than in uninfected controls (P=0.0014, Wilcoxon rank-sum test). In acutely infected animals, monocytes expressed lower levels of CD14 (data not shown), in confirmation of previous reports [19, 36]. The median absolute numbers of both CD16+ monocyte subpopulations (CD14highCD16low: 66 counts/μL; and CD14lowCD16high: 47 counts/μL) were significantly greater in acutely infected animals than in uninfected controls (CD14highCD16low: 21 counts/μL; and CD14lowCD16high: 24 counts/μL; Table 2A). In parallel to these increases, the relative percentage of the CD14highCD16− population decreased significantly (P=0.0003, Wilcoxon rank-sum test). This may indicate that a rapid shift in the circulating monocyte pool occurred 2 weeks p.i. from the CD14highCD16− subpopulation to CD14highCD16low and CD14lowCD16high subpopulations. Whether this occurs in blood or from cells emerging from bone marrow is not clear.
The distribution of monocyte subpopulations in chronically infected animals with no detectable plasma virus is comparable with that in uninfected controls (Table 2A). In contrast, in chronically infected animals with detectable plasma virus, CD14highCD16low and CD14lowCD16high subpopulations increased significantly (medians 53 and 41 counts/μL, respectively), and the absolute number of the CD14highCD16− subpopulation (median 194 counts/μL) was similar to that in uninfected controls (median 172 counts/μL). This indicates that CD14highCD16low and CD14lowCD16high subpopulations were expanded during this stage and that the marginal rise in the total monocyte count could be exclusively a result of the expansion of CD14highCD16low and CD14lowCD16high subpopulations. This chronically infected group had highly variable plasma viral load among animals, and these variations may result in variations in the distribution of monocyte subpopulations. Therefore, chronically infected animals with detectable plasma virus were stratified further into two potential subgroups, according to median viral load (70,000 copies/mL): those with viral load <70,000 copies/ml (the low viral load subgroup, n=14) and those with viral load >70,000 copies/ml (the high viral load subgroup, n=12; Table 2B). Absolute numbers of total monocytes and CD14highCD16low and CD14lowCD16high subpopulations were significantly and markedly higher in the high viral load subgroup than in the low viral load subgroup. In fact, in the low viral load subgroup, the distribution of monocyte subpopulations is similar to that in the uninfected cohort.
The animals that died with AIDS had significantly greater numbers of monocytes than uninfected controls. Absolute numbers of the CD14highCD16−, CD14highCD16low, and CD14lowCD16high populations were found increased markedly in this group as compared with the uninfected cohort. In the animals that died with AIDS, the median number of the CD14highCD16low subpopulation was highest among all of the groups, and the difference with the uninfected controls almost achieved statistical significance (P=0.0592, Wilcoxon rank-sum test). The median number of the CD14lowCD16high population in this group was also highest, but this increase was not statistically significant (P=0.1965). Two animals with terminal AIDS had strikingly low counts of CD14lowCD16high cells (five and six counts/μL), whereas the other four animals had high CD14lowCD16high cell numbers of 279, 150, 106, and 95 counts/μL, respectively.
To minimize interindividual variations in the numbers of monocyte subpopulations, the ratio of CD14highCD16low, CD14lowCD16high, and both to CD14highCD16− in individual monkeys was calculated. The median CD14highCD16low/CD14highCD16− ratio in uninfected controls was 0.12. In the acutely infected animals, this ratio increased and was changed significantly in favor of the CD14highCD16low population (1.02, P=0.0002). The CD14highCD16low/CD14highCD16− ratio in chronically infected animals with no detectable plasma virus (0.19) or with low viral load (0.18) is comparable with that of uninfected controls. Then, the ratio increased to median levels of 0.38 in the high viral load subgroup and 0.36 in the AIDS group. The CD14lowCD16high/CD14highCD16− ratio followed the similar pattern, but an increase of this ratio in the AIDS group was not significant. Collectively, these data indicate that the distribution of monocyte subpopulations was altered in a manner specific to disease stages, where an expansion of cells with more mature macrophage-like properties emerges.
Immunologic correlations with monocyte heterogeneity
For each animal, important immunological parameters of HIV infection, including CD4+ and CD8+ T-lymphocyte counts, the number of CD4+ and CD8+ central memory T cells, and plasma viral load, were analyzed in parallel to monocyte subsets. We sought to determine in this cross-sectional study whether any immunologic parameter correlates with the changes in distribution of monocyte subpopulations. Initially, we found significant correlations between some of these parameters and monocyte subpopulations, especially the CD14highCD16low, when we used a pool of all of the animals studied. Upon further investigation, we realized that these correlations were mostly attributed to disease-specific clustering of data. Thus, correlations were sought separately in the uninfected and infected cohorts (Table 3). In the uninfected controls, the CD4 and CD8 T cell counts were positively correlated with numbers of total monocytes and CD16+ monocytes (CD14highCD16low and CD14lowCD16high). However, we found significant negative correlations between the CD4/CD8 ratio and monocyte subpopulations. Interestingly, CD8+ central memory T cells were negatively correlated with monocytes and monocyte subpopulations with strong Spearman correlation coefficients, suggesting a role of CD8+ central memory T cells in the control of the numbers of monocytes. We have found recently similar observations in HIV-infected humans [37]. In infected animals, viral load is the only parameter amongst those tested in our study that correlated with monocytes and monocyte subpopulations. Statistically significant, positive correlations were found between the CD14highCD16low count and the viral load (r=0.3757, P=0.0085) or between the CD14lowCD16high count and the viral load (r=0.3781, P=0.0081), suggesting that virus or viral proteins could play a role in controlling monocyte dynamics.
TABLE 3.
Correlations Between Immunologic Parameters and Monocyte Subpopulations
| Animal group | Monocyte count | CD14highCD16− count | CD14highCD16low count | CD14lowCD16high count |
|---|---|---|---|---|
| Uninfected control (n=48) | ||||
| CD4 T cells | 0.3667a (0.0132)b | 0.3715 (0.0120) | 0.4211 (0.0040) | |
| CD8 T cells | 0.5256 (0.0002) | 0.4026 (0.0061) | 0.5495 (<0.0001) | 0.5936 (<0.0001) |
| CD4/CD8 ratio | –0.3795 (0.0101) | –0.3085 (0.0330) | –0.3156 (0.0347) | –0.3924 (0.0077) |
| CD4 CM T cells | ||||
| CD8 CM T cells | –0.7199 (0.0001) | –0.5994 (0.0025) | –0.5617 (0.0053) | –0.5874 (0.0032) |
| Infected (n=54) | ||||
| CD4 T cells | ||||
| CD8 T cells | –0.2781 (0.0417) | |||
| CD4/CD8 ratio | ||||
| CD4 CM T cells | ||||
| CD8 CM T cells | ||||
| Plasma viral loadc | 0.4089 (0.0039) | 0.3757 (0.0085) | 0.3781 (0.0081) |
The correlation coefficients were calculated using the Spearman’s rank test.
P values are shown in parentheses. The nonsignificant correlation coefficients are not presented.
n = 48. CM, Central memory.
Functional heterogeneity of monocyte subpopulations in SIV infection
The phenotypic and transcriptional heterogeneity of monocyte subpopulations and their dynamics in SIV infection suggest that there exist functionally distinct subsets. We examined the expression of TNF and the replication of SIV in these subsets.
Differential ex vivo expression of TNF in monocyte subsets.
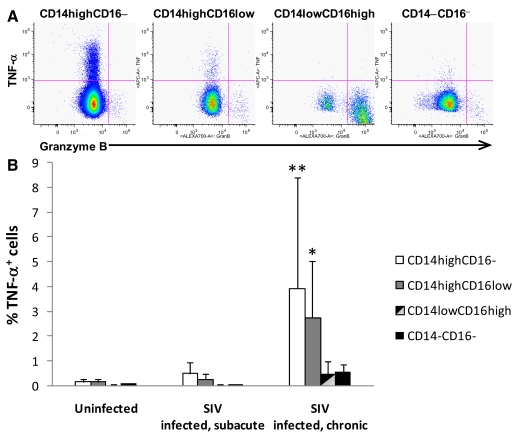
CD14+ monocytes/macrophages are the dominant source of TNF-α in vivo, and it has been shown that the CD14lowCD16high monocyte is a major source of TNF in response to LPS in vitro. To determine which subsets express this cytokine, intracellular cytokine staining was used to compare the phenotype and frequency of monocytes spontaneously producing (without in vitro stimulation) TNF-α as well as granzyme B in monkeys with subacute (n=5; 8 weeks p.i.) and chronic (n=8; 2.6 years p.i.) SIV infection and uninfected animals as controls (n=5). Monocytes from normal, uninfected controls expressed little-to-no TNF-α but produced granzyme B at low frequency. Monocytes from infected monkeys produced TNF-α spontaneously, where expression of TNF was preferentially associated with CD14highCD16− and CD14highCD16low cells but not with the CD14lowCD16high (Fig. 2). The percentage of TNF-expressing cells in the CD14highCD16− population was 7.5–25 times greater than that in the CD14lowCD16high. The frequency of monocyte subsets spontaneously producing TNF-α was increased significantly in infected monkeys compared with uninfected controls. The magnitude of the increase in frequency was greater in chronically infected animals than in subacutely infected animals. These results support SIV infection-dependent, spontaneous production of TNF-α, which may reflect the immune activation in infected monkeys. Granzyme B was expressed exclusively in CD14lowCD16high cells, and the frequency of granzyme B-expressing cells in the CD14lowCD16high population in infected animals was not significantly higher than that in uninfected controls (infected, mean±sd, 17.25%±13.38% vs. uninfected, mean±sd, 9.43%±7.43%). It appears that granzyme B expression is not associated specifically with SIV infection.
Figure 2.
Differential expression of TNF-α and granzyme B in monocyte subpopulations. (A) The expression of TNF-α and granzyme B in monocyte subpopulations. Intracellular expression of TNF-α and granzyme B was assessed with intracellular cytokine staining, followed by flow cytometry. CD3+, CD4+, or CD8+ cells were excluded from the CD14+ monocyte population to ensure no contaminating cells. The figure shown was the analysis of one chronically infected animal with a detectable viral load; representative of n = 8. (B) The expression of TNF-α in peripheral blood monocyte subsets defined by CD14 and CD16 was evaluated in healthy, uninfected monkeys (n=5), monkeys with subacute SIV infection (n=5), and monkeys with chronic SIV infection (n=8). Results are expressed as means ± sd; significant at *, P < 0.05, and **, P < 0.01 (one-way ANOVA followed by Tukey test).
Differential susceptibility to SIV infection in monocyte subsets.
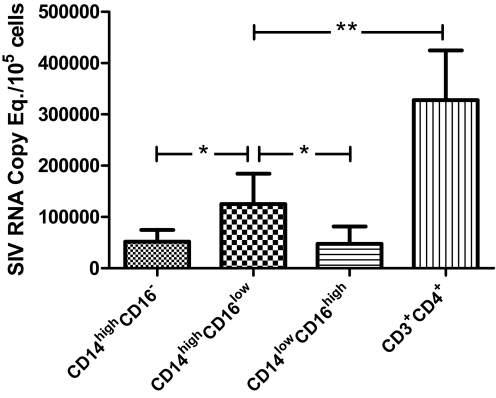
Peripheral blood monocytes are a major reservoir for HIV-1 in infected individuals on virally suppressive HAART [38, 39]. We examined levels of SIV-DNA and -RNA associated with monocyte subpopulations in acutely infected animals (12 days p.i., n=4; Fig. 3). Although levels of SIV DNA were comparable in all monocyte subpopulations (data not shown; P>0.05), significantly higher levels of SIV RNA were found in CD14highCD16low cells (P<0.05, paired t-test). However, the level of viral replication in CD14highCD16low cells was not comparable with that of CD4+ T lymphocytes. SIV replication, which occurred preferentially in CD14highCD16low cells, likely involves high levels of cell-specific factors in CD14highCD16low cells such as SerpinB2 (Supplemental Table 2).
Figure 3.
Differential levels of SIV replication in monocyte subpopulations, which with CD4+ T cells, were FACS-sorted from the blood of acutely infected animals (12 days p.i., n=4). Levels of SIV RNA associated with each cell type were measured by quantitative RT-PCR; significant at *, P < 0.05, and **, P < 0.01 (paired t-tests).
DISCUSSION
The present study was designed to identify monkey monocyte subpopulations by gene array and flow cytometry and to characterize the distribution of these subpopulations during SIV infection. Our data demonstrate for the first time large-scale gene expression differences between CD16− monocytes (CD14highCD16−) and the two CD16+ subpopulations (CD14highCD16low and CD14lowCD16high). Between the two CD16+ subpopulations, differential expression of relatively few but distinct genes was found (Supplemental Data Section). Expansion of these CD16+ monocytes during SIV infection was associated with SIV disease stages and correlated to plasma viral load, suggesting effects of virus infection on monocyte heterogeneity.
Immature monocytes are derived from CD34+ myeloid progenitor cells and become mature in the bone marrow. Monocytic maturation takes place in sequential stages, which is controlled by sequential expression of a specific set of transcription factors including PU.1, acute myelogenous leukemia 1, C/EBPβ, IFN regulatory factor-8, and MafB [40,41,42]. The sequential expression and combination of these transcription factors lead to gradual loss of CD34 phenotype and sequential acquisition of CD11a, CD33, CD11b, CD14, and CD16 phenotypes [43, 44]. Mature CD14+ monocytes continuously leave bone marrow, enter the circulation, and marginate to the vessel wall. The monocytes that pass through the vessel wall become tissue macrophages.
This transit population, however, appears not to be homogeneous. It has been suggested that bone marrow monocytes are kinetically heterogeneous in their transit time through bone marrow [45, 46]. Blood monocytes are heterogeneous in terms of physical and functional properties. Two or more subsets of monocytes that differ in size, density, or granularity [47,48,49] have been isolated, and they show differential peroxidase and lysozyme production, cytokine production, phagocytotic and cytotoxic activities, and responses to GM-CSF [50,51,52,53]. Phenotypically, the heterogeneity of human blood monocytes can be demonstrated by the presence of CD16+ monocytes. As CD16 is expressed only on a minor subset of mature CD14high monocytes in bone marrow [44, 54], CD14+ monocytes that express CD16 in bone marrow and peripheral blood represent a subpopulation at the more mature differentiation stages. Our genome-wide transcriptional profiling demonstrates the transcriptional heterogeneity of monkey monocytes and identifies CD16+ monocytes as even more mature cell types than mature CD16− monocytes (CD14highCD16−). Our study differentiated CD16+ monocytes further into two subpopulations: CD14highCD16low and CD14lowCD16high. Although CD14highCD16low cells appeared phenotypically intermediate between CD14highCD16− and CD14lowCD16high in terms of expression levels of CD11c, CD31, CD64, and CCR2, CD14highCD16low cells do not seem to represent a transient subpopulation from the CD14highCD16− to the CD14lowCD16high. The highest levels of CD1c, CD86, CX3CR1, HLA-DR, and TLR2 were expressed on CD14highCD16low cells. Our gene array data showed that the gene expression profile of the CD14highCD16low population is substantially different from that of the CD14highCD16− and closely shared with that of the CD14lowCD16high (Supplemental Data Section). In addition, we showed that CD14highCD16low cells are a functionally distinct subpopulation that preferentially takes up dextran polymers, produces TNF, and supports SIV replication. Recently, it was shown that influenza virus infection and severe asthma are associated with this particular subpopulation [55,56,57]. Our study also reports for the first time a “cytotoxic” phenotype (granzyme+lymphotoxinß+perforin+TRAIL+), which specifically defines CX3CR1highCD14lowCD16high cells. Whether CD14highCD16low cells are the precursor of CD14lowCD16high cells or whether CD14highCD16low and CD14lowCD16high cells are macrophage-like cells with two different phenotypes remains to be determined.
Monocytes are susceptible targets for HIV infection, and macrophages, their tissue counterparts, constitute an important reservoir of virus, especially during the chronic phase of infection. A study of the relationship between monocyte differentiation and HIV replication demonstrated that restricted HIV replication and no evident cytopathic effects in monocytes are important mechanisms of virus persistence [58]. Indeed, monocytes function as a major reservoir for persisting virus during antiretroviral therapy [38]. In a cohort of 39 HIV-positive patients, increases in the number of HIV-positive monocytes in the blood preceded the increase in the plasma viral load, suggesting their contribution to viral load [59]. On the other hand, HIV infection appears to induce phenotypic and functional changes in monocytes. Myelomonoblastic cells, upon in vitro HIV infection, become a more mature, monocytic phenotype [60]. In HIV-infected patients, phenotypic and functional changes driven by HIV infection occurred in the monocyte population, which includes expansion of CD16+ monocytes [8, 13, 16, 61, 62]. It is important to note that only a small fraction of the monocyte populations becomes infected and that infection by HIV is not needed to develop such dysfunctions. In vitro HIV gp120 stimulation is sufficient to induce dysfunctions and phenotypic changes in monocytes/macrophages [63,64,65]. HIV gp120 is known to induce IL-10 production in monocytes [66, 67] and induce the ability of monocytes to kill CD4+ T cells [68], which may contribute to HIV-induced immune suppression in vivo. Notably, gp120 mimics the expansion of CD16+ monocytes, which is observed in the blood of HIV-infected patients, increasing CD16 expression on cultured monocytes [64]. We suggest that HIV infection induces differential monocyte maturation and kinetics, thereby affecting the heterogeneity of monocytes in the peripheral blood.
Relevance of this heterogeneity in the pathogenesis of HIV is beginning to be understood. It has been shown recently that in HIV-infected patients receiving HAART, CD16+ monocytes are more susceptible to HIV infection and harbor preferentially HIV DNA [39]. A direct comparison between this human study and our monkey study cannot be made, as Ellery et al. [39] measured HIV DNA detected in sorted CD16+ monocytes including CD16low and CD16high subsets, whereas we examined separately SIV RNA detected in CD16+ monocytes, which were fractionated further into CD14highCD16low and CD14low CD16high monocytes. We found that SIV RNA is detected in all three monocyte subsets with a highest level in CD14high CD16low monocytes. Our data in SIV-infected monkeys are somewhat different than these human data. One reason for this might be species differences, where SIV is detected readily in monocytes, and HIV is less abundant in human monocytes [20, 21, 38, 39, 69, 70]. Another difference is that Ellery et al. [39] analyzed HIV DNA from patients on HAART, and we assessed SIV RNA from animals that were not on antiretroviral therapy. The relationship between phenotypes and functions of different monocyte subpopulations remains to be established. Interestingly, a recent mouse study by Auffray et al. [30] demonstrated CD11a+CX3CR1highGr1− monocytes (equivalent to human CD14lowCD16high monocytes) as a subset that crawls on normal endothelium of blood vessels, invades tissues rapidly upon tissue damage, and becomes tissue macrophages. Whether such cells do so in the course of viral infection has yet to be studied thoroughly.
We were interested in establishing the contribution of different monocyte subpopulations in HIV pathogenesis. In this study, we have conducted a cross-sectional study of SIV- or SHIV-infected animals to investigate changes in the distribution of monocyte subpopulations during infection. We report the positive correlations between plasma viral load and absolute numbers of total monocytes and the two CD16+ subpopulations. Expansion of CD16+ monocytes was evident in the acute and chronic phase of infection with high plasma viral load, suggesting a biphasic increase (first phase early after primary infection and second phase prior to development of AIDS) in the absolute number of CD16+ monocytes. Expansion or a depletion of CD16+ monocytes was seen in cases of terminal AIDS. This may signify impaired maturation at the late chronic state of the disease. Collectively, our observations suggest that monocyte maturation and/or activation are related to plasma viral load.
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grants NS040237 (K. W.), NS037654 (K. W.), NS0500041 (K. W.), and MH81835 (K. W. and M. S. M.).
Footnotes
Abbreviations: APC=allophycocyanin, CCR2=C–C motif chemokine receptor 2, CD14highCD16− monocytes=monocytes with a high density of CD14 and no CD16 antigen, CD14highCD16low=monocytes that coexpress high levels of CD14 and low levels of CD16, CD14lowCD16high monocytes= monocytes that coexpress low levels of CD14 and high levels of CD16, CD62L=CD62 ligand, CX3CR1=C–X3–C motif chemokine receptor 1, Cy5.5=cyanin 5.5, DC=dendritic cell, HAART=highly active antiretroviral therapy, mDC=myeloid dendritic cell, pDC=plasmacytoid dendritic cell, p.i.=postinfection, SHIV=SIV/HIV
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Bender B S, Davidson B L, Kline R, Brown C, Quinn T C. Role of the mononuclear phagocyte system in the immunopathogenesis of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Rev Infect Dis. 1988;10:1142–1154. doi: 10.1093/clinids/10.6.1142. [DOI] [PubMed] [Google Scholar]
- Braun D P, Kessler H, Falk L, Paul D, Harris J E, Blaauw B, Landay A. Monocyte functional studies in asymptomatic, human immunodeficiency disease virus (HIV)-infected individuals. J Clin Immunol. 1988;8:486–494. doi: 10.1007/BF00916955. [DOI] [PubMed] [Google Scholar]
- Rich E A, Toossi Z, Fujiwara H, Hanigosky R, Lederman M M, Ellner J J. Defective accessory function of monocytes in human immunodeficiency virus-related disease syndromes. J Lab Clin Med. 1988;112:174–181. [PubMed] [Google Scholar]
- Dudhane A, Conti B, Orlikowsky T, Wang Z Q, Mangla N, Gupta A, Wormser G P, Hoffmann M K. Monocytes in HIV type 1-infected individuals lose expression of costimulatory B7 molecules and acquire cytotoxic activity. AIDS Res Hum Retroviruses. 1996;12:885–892. doi: 10.1089/aid.1996.12.885. [DOI] [PubMed] [Google Scholar]
- Noursadeghi M, Katz D R, Miller R F. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect Dis. 2006;6:794–804. doi: 10.1016/S1473-3099(06)70656-9. [DOI] [PubMed] [Google Scholar]
- Allen J B, Wong H L, Guyre P M, Simon G L, Wahl S M. Association of circulating receptor Fc γ RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-β. J Clin Invest. 1991;87:1773–1779. doi: 10.1172/JCI115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Vanham G, Kestens L, Kruger M, Ceuppens J L, Vingerhoets J, Gigase P. Expression patterns of Fc γ receptors, HLA-DR and selected adhesion molecules on monocytes from normal and HIV-infected individuals. Clin Exp Immunol. 1994;98:115–122. doi: 10.1111/j.1365-2249.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trial J, Birdsall H H, Hallum J A, Crane M L, Rodriguez-Barradas M C, de Jong A L, Krishnan B, Lacke C E, Figdor C G, Rossen R D. Phenotypic and functional changes in peripheral blood monocytes during progression of human immunodeficiency virus infection. Effects of soluble immune complexes, cytokines, subcellular particulates from apoptotic cells, and HIV-1-encoded proteins on monocytes phagocytic function, oxidative burst, transendothelial migration, and cell surface phenotype. J Clin Invest. 1995;95:1690–1701. doi: 10.1172/JCI117845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes P J, Miao Y M, Gotch F M, Gazzard B G. Alterations in blood leucocyte adhesion molecule profiles in HIV-1 infection. Clin Exp Immunol. 1999;117:331–334. doi: 10.1046/j.1365-2249.1999.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillet S, Prevost M H, Preira A, Girard P M, Rogine N, Hakim J, Gougerot-Pocidalo M A, Elbim C. Monocyte expression of adhesion molecules in HIV-infected patients: variations according to disease stage and possible pathogenic role. Lab Invest. 1999;79:815–822. [PubMed] [Google Scholar]
- Miller L S, Atabai K, Nowakowski M, Chan A, Bluth M H, Minkoff H, Durkin H G. Increased expression of CD23 (Fc(ε) receptor II) by peripheral blood monocytes of AIDS patients. AIDS Res Hum Retroviruses. 2001;17:443–452. doi: 10.1089/088922201750102544. [DOI] [PubMed] [Google Scholar]
- Trial J, Rubio J A, Birdsall H H, Rodriguez-Barradas M, Rossen R D. Monocyte activation by circulating fibronectin fragments in HIV-1-infected patients. J Immunol. 2004;173:2190–2198. doi: 10.4049/jimmunol.173.3.2190. [DOI] [PubMed] [Google Scholar]
- Nockher W A, Bergmann L, Scherberich J E. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol. 1994;98:369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi H M, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- Dunne J, Feighery C, Whelan A. β-2-Microglobulin, neopterin and monocyte Fc γ receptors in opportunistic infections of HIV-positive patients. Br J Biomed Sci. 1996;53:263–269. [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath M S. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Amirayan-Chevillard N, Tissot-Dupont H, Capo C, Brunet C, Dignat-George F, Obadia Y, Gallais H, Mege J L. Impact of highly active anti-retroviral therapy (HAART) on cytokine production and monocyte subsets in HIV-infected patients. Clin Exp Immunol. 2000;120:107–112. doi: 10.1046/j.1365-2249.2000.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A, Ellery P, Maslin C L, Naim E, Heinlein A C, Ryan C E, Paukovics G, Hocking J, Sonza S, Crowe S M. Normal CD16 expression and phagocytosis of Mycobacterium avium complex by monocytes from a current cohort of HIV-1-infected patients. J Infect Dis. 2006;193:693–697. doi: 10.1086/500367. [DOI] [PubMed] [Google Scholar]
- Otani I, Akari H, Nam K H, Mori K, Suzuki E, Shibata H, Doi K, Terao K, Yosikawa Y. Phenotypic changes in peripheral blood monocytes of cynomolgus monkeys acutely infected with simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1998;14:1181–1186. doi: 10.1089/aid.1998.14.1181. [DOI] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim W K, Fuller R A, Kim J P, Autissier P, Sehgal P K, Schinazi R F, Bischofberger N, Piatak M, Lifson J D, Masliah E, Gonzalez R G. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel S J, Wang G, Trichel A M, Murphey-Corb M, Wiley C A. Longitudinal analysis of monocyte/macrophage infection in simian immunodeficiency virus-infected, CD8+ T-cell-depleted macaques that develop lentiviral encephalitis. Am J Pathol. 2006;168:1553–1569. doi: 10.2353/ajpath.2006.050240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A, Liu H, Ling B, Borda J T, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim W K, Williams K C, Ribeiro R M, Lackner A A, Veazey R S, Kuroda M J. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H W, Passlick B, Flieger D. The monoclonal antimonocyte antibody My4 stains B lymphocytes and two distinct monocyte subsets in human peripheral blood. Hybridoma. 1988;7:521–527. doi: 10.1089/hyb.1988.7.521. [DOI] [PubMed] [Google Scholar]
- Grage-Griebenow E, Lorenzen D, Fetting R, Flad H D, Ernst M. Phenotypical and functional characterization of Fc γ receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur J Immunol. 1993;23:3126–3135. doi: 10.1002/eji.1830231213. [DOI] [PubMed] [Google Scholar]
- Szeberenyi J B, Rothe G, Pallinger E, Orso E, Falus A, Schmitz G. Multi-color analysis of monocyte and dendritic cell precursor heterogeneity in whole blood. Immunobiology. 2000;202:51–58. doi: 10.1016/S0171-2985(00)80052-2. [DOI] [PubMed] [Google Scholar]
- Weber C, Belge K U, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber K S, Ziegler-Heitbrock H W. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman D R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon M J, Van Rooijen N, Stehling M, Drevets D A, Leenen P J. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock H W. Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am J Physiol Cell Physiol. 2000;279:C578–C586. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- Kapinsky M, Torzewski M, Buchler C, Duong C Q, Rothe G, Schmitz G. Enzymatically degraded LDL preferentially binds to CD14(high) CD16(+) monocytes and induces foam cell formation mediated only in part by the class B scavenger-receptor CD36. Arterioscler Thromb Vasc Biol. 2001;21:1004–1010. doi: 10.1161/01.atv.21.6.1004. [DOI] [PubMed] [Google Scholar]
- Kim W K, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, Williams K. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniuszko M, Kowal K, Rusak M, Pietruczuk M, Dabrowska M, Bodzenta-Lukaszyk A. Monocyte CD163 and CD36 expression in human whole blood and isolated mononuclear cell samples: influence of different anticoagulants. Clin Vaccine Immunol. 2006;13:704–707. doi: 10.1128/CDLI.00417-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Stallworth J W, Vance P J, Hoxie J A, Fultz P N. Simian immunodeficiency virus (SIV)/immunoglobulin G immune complexes in SIV-infected macaques block detection of CD16 but not cytolytic activity of natural killer cells. Clin Vaccine Immunol. 2006;13:768–778. doi: 10.1128/CVI.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata T, Kodama M, Sato A, Suzuki H, Miyazaki Y, Miura T, Hayami M. Contribution of monocytes to viral replication in macaques during acute infection with simian immunodeficiency virus. AIDS Res Hum Retroviruses. 2007;23:372–380. doi: 10.1089/aid.2006.0208. [DOI] [PubMed] [Google Scholar]
- Lentz M R, Kim W K, Lee V, Bazner S, Halpern E F, Venna N, Williams K, Rosenberg E S, Gonzalez R G. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins J I, Corey L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellery P J, Tippett E, Chiu Y L, Paukovics G, Cameron P U, Solomon A, Lewin S R, Gorry P R, Jaworowski A, Greene W C, Sonza S, Crowe S M. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Valledor A F, Borras F E, Cullell-Young M, Celada A. Transcription factors that regulate monocyte/macrophage differentiation. J Leukoc Biol. 1998;63:405–417. doi: 10.1002/jlb.63.4.405. [DOI] [PubMed] [Google Scholar]
- Nagamura-Inoue T, Tamura T, Ozato K. Transcription factors that regulate growth and differentiation of myeloid cells. Int Rev Immunol. 2001;20:83–105. doi: 10.3109/08830180109056724. [DOI] [PubMed] [Google Scholar]
- Gemelli C, Montanari M, Tenedini E, Zanocco M T, Vignudelli T, Siena M, Zini R, Salati S, Tagliafico E, Manfredini R, Grande A, Ferrari S. Virally mediated MafB transduction induces the monocyte commitment of human CD34+ hematopoietic stem/progenitor cells. Cell Death Differ. 2006;13:1686–1696. doi: 10.1038/sj.cdd.4401860. [DOI] [PubMed] [Google Scholar]
- Kansas G S, Muirhead M J, Dailey M O. Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood. 1990;76:2483–2492. [PubMed] [Google Scholar]
- Wood B. Multicolor immunophenotyping: human immune system hematopoiesis. Methods Cell Biol. 2004;75:559–576. doi: 10.1016/s0091-679x(04)75023-2. [DOI] [PubMed] [Google Scholar]
- Whitelaw D M. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 1972;5:311–317. doi: 10.1111/j.1365-2184.1972.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Al Izzi S A, Maxie M G, Valli V E, Wilkie B N, Johnson J A. The kinetics of mononuclear phagocytes in normal calves given Corynebacterium parvum. Can J Comp Med. 1982;46:138–145. [PMC free article] [PubMed] [Google Scholar]
- Arenson E B, Jr, Epstein M B, Seeger R C. Volumetric and functional heterogeneity of human monocytes. J Clin Invest. 1980;65:613–618. doi: 10.1172/JCI109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T, Mantich N M, Boxer L A, Baehner R L. Functions of human monocyte and lymphocyte subsets obtained by countercurrent centrifugal elutriation: differing functional capacities of human monocyte subsets. J Immunol. 1981;127:1515–1518. [PubMed] [Google Scholar]
- Figdor C G, Bont W S, Touw I, de Roos J, Roosnek E E, De Vries J E. Isolation of functionally different human monocytes by counterflow centrifugation elutriation. Blood. 1982;60:46–53. [PubMed] [Google Scholar]
- Akiyama Y, Miller P J, Thurman G B, Neubauer R H, Oliver C, Favilla T, Beman J A, Oldham R K, Stevenson H C. Characterization of a human blood monocyte subset with low peroxidase activity. J Clin Invest. 1983;72:1093–1105. doi: 10.1172/JCI111034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C E, McCarthy S P, Lorenzen J, McGee J O. Heterogeneity among human mononuclear phagocytes in their secretion of lysozyme, interleukin 1 and type-β transforming growth factor: a quantitative analysis at the single-cell level. Eur J Immunol. 1989;19:2037–2043. doi: 10.1002/eji.1830191111. [DOI] [PubMed] [Google Scholar]
- Inamura N, Sone S, Okubo A, Singh S M, Ogura T. Heterogeneity in responses of human blood monocytes to granulocyte-macrophage colony-stimulating factor. J Leukoc Biol. 1990;47:528–534. doi: 10.1002/jlb.47.6.528. [DOI] [PubMed] [Google Scholar]
- Wang S Y, Mak K L, Chen L Y, Chou M P, Ho C K. Heterogeneity of human blood monocyte: two subpopulations with different sizes, phenotypes and functions. Immunology. 1992;77:298–303. [PMC free article] [PubMed] [Google Scholar]
- Passlick B, Flieger D, Ziegler-Heitbrock H W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- Bruno L, Seidl T, Lanzavecchia A. Mouse pre-immunocytes as non-proliferating multipotent precursors of macrophages, interferon-producing cells, CD8α(+) and CD8α(–) dendritic cells. Eur J Immunol. 2001;31:3403–3412. doi: 10.1002/1521-4141(200111)31:11<3403::aid-immu3403>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Toma T, Tsukiji H, Okamoto H, Yamazaki H, Ohta K, Ohta K, Kasahara Y, Koizumi S, Yachie A. Selective expansion of CD16highCCR2– subpopulation of circulating monocytes with preferential production of haem oxygenase (HO)-1 in response to acute inflammation. Clin Exp Immunol. 2005;142:461–470. doi: 10.1111/j.1365-2249.2005.02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol. 2009;130:338–346. doi: 10.1016/j.clim.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Pauza C D, Galindo J, Richman D D. Human immunodeficiency virus infection of monoblastoid cells: cellular differentiation determines the pattern of virus replication. J Virol. 1988;62:3558–3564. doi: 10.1128/jvi.62.10.3558-3564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson B K, Mosiman V L, Cantarero L, Furtado M, Bhattacharya M, Goolsby C. Detection of HIV-RNA-positive monocytes in peripheral blood of HIV-positive patients by simultaneous flow cytometric analysis of intracellular HIV RNA and cellular immunophenotype. Cytometry. 1998;31:265–274. doi: 10.1002/(sici)1097-0320(19980401)31:4<265::aid-cyto6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Roulston A, D'Addario M, Boulerice F, Caplan S, Wainberg M A, Hiscott J. Induction of monocytic differentiation and NF-κ B-like activities by human immunodeficiency virus 1 infection of myelomonoblastic cells. J Exp Med. 1992;175:751–763. doi: 10.1084/jem.175.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel P M, McSharry C, Galloway E, Ross C, Severn A, Toner G, Gruer L, Wilkinson P C. Heterogeneity of peripheral blood monocyte populations in human immunodeficiency virus-1 seropositive patients. FEMS Microbiol Immunol. 1992;5:317–323. doi: 10.1111/j.1574-6968.1992.tb05916.x. [DOI] [PubMed] [Google Scholar]
- van der Kuyl A C, van den Burg R, Zorgdrager F, Groot F, Berkhout B, Cornelissen M. Sialoadhesin (CD169) expression in CD14+ cells is upregulated early after HIV-1 infection and increases during disease progression. PLoS ONE. 2007;2:e257. doi: 10.1371/journal.pone.0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrbaum-Landmann I, Kaltenhauser E, Flad H D, Ernst M. HIV-1 envelope protein gp120 affects phenotype and function of monocytes in vitro. J Leukoc Biol. 1994;55:545–551. doi: 10.1002/jlb.55.4.545. [DOI] [PubMed] [Google Scholar]
- Zembala M, Bach S, Szczepanek A, Mancino G, Colizzi V. Phenotypic changes of monocytes induced by HIV-1 gp120 molecule and its fragments. Immunobiology. 1997;197:110–121. doi: 10.1016/S0171-2985(97)80061-7. [DOI] [PubMed] [Google Scholar]
- Freedman B D, Liu Q H, Del Corno M, Collman R G. HIV-1 gp120 chemokine receptor-mediated signaling in human macrophages. Immunol Res. 2003;27:261–276. doi: 10.1385/IR:27:2-3:261. [DOI] [PubMed] [Google Scholar]
- Gessani S, Borghi P, Fantuzzi L, Varano B, Conti L, Puddu P, Belardelli F. Induction of cytokines by HIV-1 and its gp120 protein in human peripheral blood monocyte/macrophages and modulation of cytokine response during differentiation. J Leukoc Biol. 1997;62:49–53. doi: 10.1002/jlb.62.1.49. [DOI] [PubMed] [Google Scholar]
- Koutsonikolis A, Haraguchi S, Brigino E N, Owens U E, Good R A, Day N K. HIV-1 recombinant gp41 induces IL-10 expression and production in peripheral blood monocytes but not in T-lymphocytes. Immunol Lett. 1997;55:109–113. doi: 10.1016/s0165-2478(97)02695-3. [DOI] [PubMed] [Google Scholar]
- Dudhane A, Wang Z Q, Orlikowsky T, Gupta A, Wormser G P, Horowitz H, Kufer P, Hoffmann M K. AIDS patient monocytes target CD4 T cells for cellular conjugate formation and deletion through the membrane expression of HIV-1 envelope molecules. AIDS Res Hum Retroviruses. 1996;12:893–899. doi: 10.1089/aid.1996.12.893. [DOI] [PubMed] [Google Scholar]
- McElrath M J, Steinman R M, Cohn Z A. Latent HIV-1 infection in enriched populations of blood monocytes and T cells from seropositive patients. J Clin Invest. 1991;87:27–30. doi: 10.1172/JCI114981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini D, Borderi M, De Crignis E, Cicola R, Cimatti L, Vitone F, Chiodo F, Re M C. HIV-1 DNA load analysis in peripheral blood lymphocytes and monocytes from naive and HAART-treated individuals. J Infect. 2008;56:219–225. doi: 10.1016/j.jinf.2008.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.