Abstract
Immune and inflammatory cells play a critical role in the pathogenesis of atherosclerotic plaques. We have demonstrated that A2ARs inhibit foam cell formation and stimulate production of ABCA1, the primary transporter of lipoproteins. We asked whether the effects of A2ARs on foam cell formation in vitro are mediated by transporters involved in reverse cholesterol transport, ABCA1 and ABCG1. Foam cells were generated from THP-1 cells by incubation with 100 nM PMA for 2 days and incubated with acLDL (50 μg/mL) plus IFN-γ (500 U/mL) ± A2AR agonist CGS-21680 (1 μM). Radiolabeled cholesterol (0.2 μCi/ml) was added to cells, and efflux was measured using a liquid scintillation counter. Lentiviral siRNA infection markedly reduces ABCA1 or ABCG1 mRNA in THP-1 cells. Despite diminished ABCG1 expression (KD), CGS-21680 inhibits foam cell formation (81+5% inhibition; P<0.0001 vs. IFN-γ alone; n=3) but has no effect on foam cell formation in ABCA1 KD cells (5+3% inhibition; P<0.85 vs. IFN-γ alone; n=3). The A2A agonist increases apoA-I-mediated cholesterol efflux nearly twofold in THP-1-derived macrophages (from 9.5% to 17.5+2.5% [3H]-cholesterol efflux; P<0.0090 vs. control; n=3) but not in ABCA1 KD cells. Activation of Epac, a signaling molecule downstream of the A2AR, increased ABCA1 (23+5%; P<0.0007 vs. control; n=3) and phospho-ABCA1 (13+5%; P<0.0003 vs. control; n=3) protein. These results demonstrate that A2AR occupancy diminishes foam cell formation by stimulating increased reverse cholesterol transport via ABCA1.
Keywords: atherosclerosis, inflammation, macrophages
Introduction
In extrahepatic tissues, such as the vasculature, cholesterol is esterified and accumulates in lipid droplets; lipid-laden macrophages or foam cells are one of the earliest recognizable hallmarks of atherosclerotic plaque. Eliminating excess cellular cholesterol requires the active transport of free cholesterol out of the cell [1]. Reverse cholesterol transport from the periphery, including vascular deposits, to the liver, where it is excreted in the bile, is mediated by HDLs.
One major gatekeeper of reverse cholesterol transport is ABCA1 [2], which belongs to a superfamily of proteins that use ATP to shuttle substrates between different cellular compartments and out of the cell. ABCA1 mediates the transport of cholesterol, phospholipids, and other lipophilic molecules across cellular membranes, where they are then removed from cells by lipid-poor HDL species [3]. Many factors are thought to contribute to the activity level of ABCA1, including excess cellular cholesterol, the conversion of intracellular cholesterol to the second messenger oxysterol, interaction of ABCA1 with apolipoproteins, and metabolites associated with diseases, such as inflammation and diabetes [2]. Some of these factors might operate through their activation of the nuclear hormone LXR, which transcriptionally activates ABCA1 [3].
Other proteins and mechanisms, in addition to ABCA1, mediate cellular cholesterol efflux. For example, passive diffusion of aqueous cholesterol created by cholesterol gradients acts to remove extracellular cholesterol. Additionally, ABCG1 has been implicated in mediating macrophage cholesterol efflux to mature HDL [4]. Studies have shown that ABCA1 and ABCG1 actually work in concert to promote reverse cholesterol transport [5]. More recent studies indicate that ABCA1 may lipidate lipid-poor apoA-I to generate nascent HDL, which can then act as an acceptor for ABCG1-mediated cholesterol efflux [4]. Taken together, these studies indicate that regulating ABCA1 and ABCG1 expression may be an effective strategy in enhancing macrophage reverse cholesterol transport and thereby reducing atherosclerosis.
The macrophage-activating factor IFN-γ increases the activity and expression of acyl CoA:cholesterol acyltransferase and reduces cholesterol efflux to HDL in macrophage-derived foam cells [6]. IFN-γ also reduces cholesterol efflux significantly when apoA-I serves as the cholesterol acceptor [6]. The role of the proinflammatory cytokine IFN-γ in promoting the development of atherosclerotic lesions has also been supported in human atherosclerotic plaque formation and in the apoE IFN-γ double-knockout mouse [6].
We have demonstrated previously that adenosine, acting at anti-inflammatory A2ARs, inhibits the formation of lipid-laden foam cells and stimulates the expression of ABCA1 [7]. We therefore determined whether A2AR-mediated ABCA1 expression or increased ABCA1 function is required to prevent foam cell formation. We report here that A2AR stimulation promotes cholesterol efflux from THP-1-derived macrophages (a model of human macrophages) and their transformation into foam cells by an ABCA1-dependent mechanism and that ABCA1 expression and function are enhanced by adenosine receptor occupancy.
MATERIALS AND METHODS
Cell culture reagents
Tissue-culture media (RPMI 1640) and reagents were obtained from Invitrogen (Carlsbad, CA, USA) and Sigma Chemical Co. (St. Louis, MO, USA). FBS, BSA, L-glutamine, and penicillin were purchased from Invitrogen.
A2AR agonists and antagonists
The selective A2AR agonist CGS-21680 was purchased from Sigma Chemical Co. The A2AR antagonist ZM-241385 was purchased from Tocris Cookson (Bristol, UK). Epac agonist 8-pcPT-2′-O-Me-cAMP and PKA agonist 6-Benz-cAMP were purchased from Axxora (San Diego, CA, USA).
Cell culture
The human monocyte-macrophage cell line (THP-1) was purchased from American Type Culture Collection (Manassas, VA, USA). THP-1 cells were grown in RPMI 1640 (37°C, 5% CO2), supplemented with 10% FBS, 1% penicillin, and 1% L-glutamine.
Infection of THP-1-derived macrophages
THP-1-derived macrophages suspended in medium (1×106/ml) are treated with hexadimethrine bromide from Sigma Chemical Co. (8 ug/ml, final) and 108 transducing units of lentiviral particles corresponding to human ABCA1 shRNA (SHVRS 10030716MN), ABCG1 shRNA (SHVRS 09060701MN), or scrambled shRNA (SHC002V 05210717MN) with a puromycin selection marker (Sigma Chemical Co.). Media are changed 24 h following infection and replaced with medium containing puromycin (1 ug/ml). After 7 days, cells are isolated and resuspended in medium; some cells were lysed and assayed for mRNA for ABCA1 and ABCG1 before and after treatment with IFN-γ in the presence or absence of the A2A-selective agonist CGS-21680, the selective antagonist ZM-241385, or their combination (all at 1 μM final concentration).
Quantitative real-time PCR
For quantification of ABCA1 and ABCG1 message by real-time PCR, the following primer pairs are used: 5′-gttgctgctgtggaagaacctc-3′, 5′-agataatcccctgaacccaagg-3′ (ABCA1) and 5′-aggtctcagcccttctaaagttcctc-3′, 5′-tctctcgaagtgaatgaaatttatcg-3′ (ABCG1). Human GAPDH expression is used as an internal standard (5′-aacatcatccctgcctctac-3′, 5′-ccctgttgctgtagccaaa-3′) under parallel conditions. PCR amplifications are detected using the SYBR Green kit from Applied Biosystems (Foster City, CA, USA).
Foam cell formation and staining
THP-1 cells (106 cells/ml), in four-well glass-chamber slides, are treated with 100 nM PMA (Sigma Chemical Co.) for 2 days at 37°C to stimulate differentiation into macrophages, which are washed three times with PBS and incubated further in RPMI 1640 (37°C, 5% CO2) for 24 h under the following conditions: acLDL (50 μg/ml, Intracel, Frederick, MD, USA) plus IFN-γ (500 U/ml) in the presence or absence of A2AR agonist CGS-21680 (1 μM) or the selective A2AR antagonist ZM-241385 (1 μM). Cholesterol acceptors apoA-I or HDL (50 μg/ml, Invitrogen) are added to some cultures to facilitate cholesterol efflux. Following incubation, media are aspirated, and slides are washed with PBS and fixed in 10% formalin (Sigma Chemical Co.) for 10 min. Slides are washed in distilled water, rinsed in 60% isopropanol, and stained with 0.2% Oil Red O (Sigma Chemical Co.) for 15 min. Slides are rinsed again with 60% isopropanol and dipped in hematoxylin to stain for cell nuclei. After washing with distilled water, coverslips are mounted on slides using glycerine jelly. Foam cells, recognized as macrophages stained with Oil Red O, are visualized via light microscope (Olympus 820) with 100× magnification and photographed using a Kodak DC 290 zoom digital camera. Foam cells, formed in each condition as well as the total cell number/well on each slide, are quantitated manually, and the percentage of foam cells present is calculated as the number of foam cells present divided by the total number of cells in each well.
Western immunoblot
Equal amounts of protein (40 μg/lane) are separated by 7.5% SDS-PAGE and electrotransferred to nitrocellulose membranes. Nonspecific antibody binding is blocked with 3% skim milk in TBS. Membrane is incubated with a primary antibody for ABCA1 (mouse monoclonal) or ABCG1 (rabbit polyclonal; Abcam, Cambridge, MA, USA) overnight and then with HRP-conjugated secondary antibody for another hour in TBS. After each antibody incubation, the blot is washed (three times; 5 min each) with TBS containing 0.1% Tween 20. The blot is exposed to SuperSignal West Pico chemiluminescent substrate (Perbio, Thermo Scientific, Rockford, IL, USA) and scanned using a Kodak Gel Logic 2200 imaging system (Eastman Kodak Co., Rochester, NY, USA). The band intensity is quantitated using Kodak software. To screen multiple proteins after probing with an antibody, the blot is stripped with stripping buffer at 50°C for 30 min and reprobed for β-actin or other proteins of interest.
ABCA1 phosphorylation and immunoprecipitation
THP-1-derived macrophages are treated with apoA-I (50 ug/ml) or apoA-I (50 ug/ml) and selective Epac agonist 8-pcPT-2′-O-Me-cAMP (100 μM) or the PKA agonist 6-Benz-cAMP (100 μM; Axxora) overnight. Cells are washed with 2 ml ice-cold PBS and incubated with 300 ml lysis buffer for 1 h at 4°C. Cellular debris is pelleted by centrifugation at 10,000 g for 10 min at 4°C. Protein L-agarose beads (20 μl; Santa Cruz Biotechnology, Santa Cruz, CA, USA) are added to the supernatant (100 ug total cellular protein) along with the ABCA1 antibody (Abcam) and incubated on a rocker at 4°C overnight. Immunoprecipitate is collected by centrifugation at 2500 rpm for 5 min at 4°C. Pellets are washed four times with PBS. Supernatant is discarded and resuspended in 40 μl 1× electrophoresis buffer. Samples are analyzed by SDS-PAGE. Nitrocellulose membranes are probed with 1 μl phosphoserine antibody (mouse monoclonal; Santa Cruz Biotechnology). Band intensity is quantitated using Kodak software.
Cholesterol efflux from THP-1-derived macrophages
THP-1 cells, ABCA1 KD THP-1 cells, or ABCG1 KD THP-1 cells are maintained in RPMI-1640 medium containing 10% FBS and 1% penicillin/streptomycin at 37°C, 5% CO2. Cells are incubated at 106cells/ml in the presence of PMA for 4 days to induce differentiation into macrophages. Untreated ABCA1 KD or ABCG1 KD macrophages are labeled with 3H-cholesterol (0.2 μCi/ml) for 24 h (37°C, 5% CO2). The cells are washed two times with serum-free medium containing 0.2% BSA and then cultured overnight in medium. To measure the effect of adenosine receptor agonists/antagonists on cholesterol efflux, cells are incubated for 24 h with 50 μg/ml native HDL (for ABCG1-mediated efflux) or 50 μg/ml apoA-I (for ABCA1-mediated efflux) in the absence or presence of CGS-21680 (1 μg/ml), ZM-241385 (1 μg/ml), or their combination and IFN-γ. At the end of the incubation, cells are centrifuged (10,000 g for 5 min) to remove medium and then lysed. Aliquots (200 μl) from the medium and cells are counted separately using liquid scintillation counting.
Statistics
Reported as the mean ± sem, differences between treatment groups were analyzed by using one-way ANOVA, followed by unpaired Student’s t-test to evaluate levels of significance. P < 0.05 was accepted as being statistically significant. All statistical analyses are performed with SigmaStat software v. 3.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Lentiviral constructs reduce ABCA1 and ABCG1 mRNA in human THP-1-derived macrophages
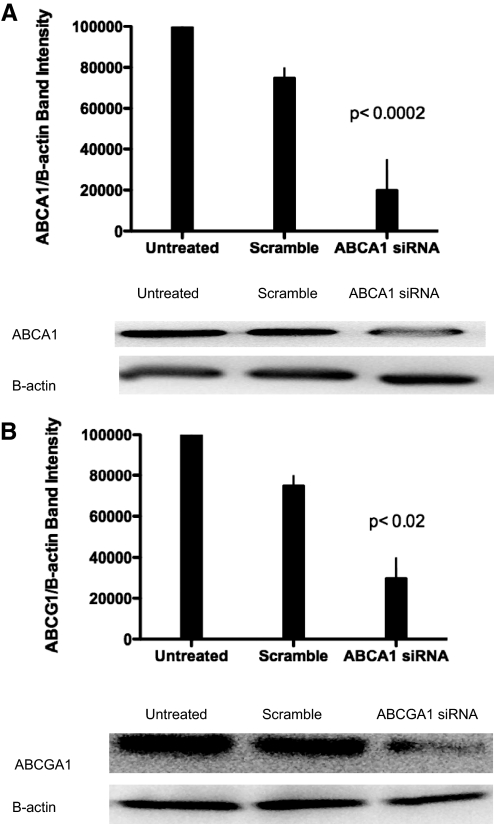
We have shown that adenosine acting at A2A diminishes foam cell formation by human monocytoid cells and murine peritoneal macrophages [5] (Fig. 1). To test the effects of adenosine receptor activation on foam cell suppression, we decided to down-regulate molecules involved in reverse cholesterol transport. Using siRNA technology, we were able to reduce ABCA1 (P<0.0002; ABCA1 KD vs. control; n=3; Fig. 2A) and ABCG1 mRNA (P<0.0200; ABCG1 KD vs. control; n=3; Fig. 2B). In later experiments, we compare the function of the cell lines lacking these molecules with THP-1-derived macrophages treated with A2AR agonists and antagonists. Using these stable KDs, we hope to understand better how A2AR activation regulates cholesterol balance in THP-1-derived macrophages.
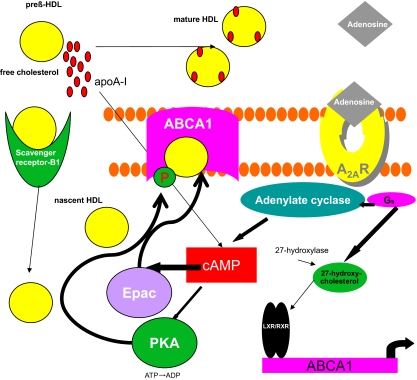
Figure 1.
Molecular mechanism by which A2AR activation decreases foam cell formation. Activation of the A2AR stimulates transcription of ABCA1. Downstream cAMP activation of PKA and Epac leads to increased phoshpo-ABCA1 and subsequently, enhanced cholesterol efflux.
Figure 2.
Lentiviral constructs reduce ABCA1 and ABCG1 protein levels in human THP-1 cells. (A) Monocytes were transduced by lentivirus with shRNA for ABCA1. Protein was isolated after 7 days of puromycin selection (10 μg/ml), and Western blot analysis was performed. Data are presented as means ± sem (n=3). Results are representative of three experiments and compared with those in macrophages transduced with lentiviral scramble (P<0.0002). Immunoblot is shown below. (B) Monocytes were transduced by lentivirus with shRNA for ABCG1. Protein was isolated after 7 days of puromycin selection (10 μg/ml), and Western blot analysis was performed. Data are presented as means ± sem (n=3). Results are representative of three experiments and compared with those in macrophages transduced with lentiviral scramble (P<0.02). Immunoblot is shown below.
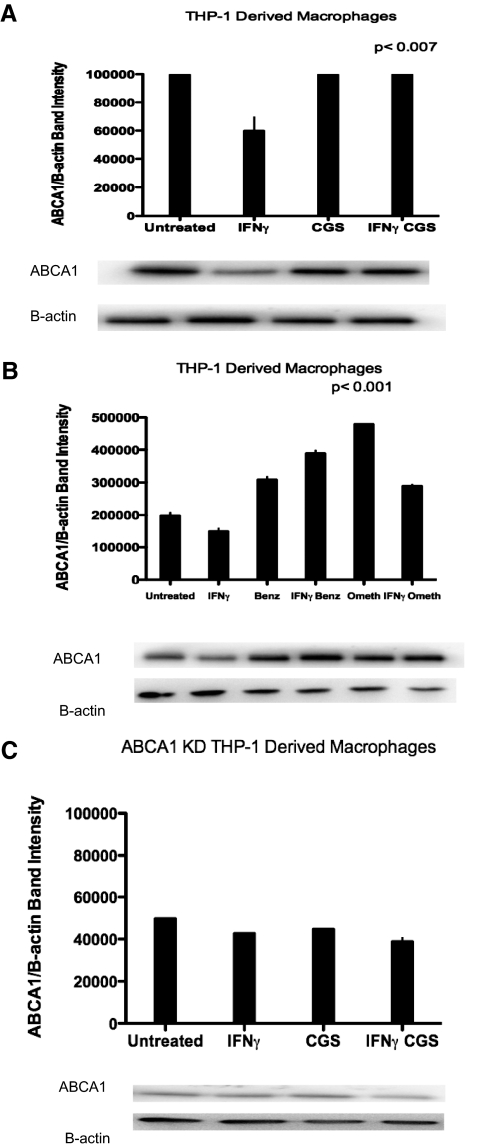
A2AR agonist CGS-21680 and Epac agonist 8-pcPT-2′-O-Me-cAMP increase ABCA1 and phospho-ABCA1 protein expression in THP-1-derived macrophages
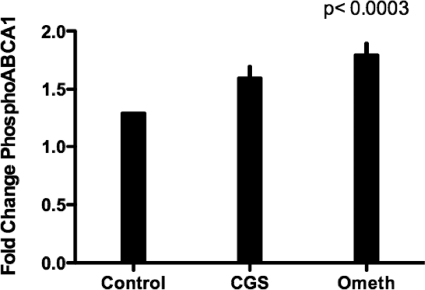
The A2AR signals via activation of adenylate cyclase and cAMP-dependent effector mechanisms, including PKA and via Epac, a member of a family of cAMP-regulated guanine nucleotide exchange factors that mediate protein PKA-independent signal transduction [8]. Others have shown that activation of PKA induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts [9]. We therefore determined the effects of selective stimuli for these signaling pathways on foam cell formation and cholesterol efflux. We determined whether Epac regulates ABCA1 protein levels. We found that CGS-21680 treatment increased ABCA1 protein levels significantly in THP-1-derived macrophages (P<0.0007 vs. IFN-γ; n=3; Fig. 3A). Similarly, the selective Epac agonist 8-pcPT-2′-O-Me-cAMP also increased ABCA1 protein expression (by 23±5%; P<0.0001 vs. IFN-γ; n=3; Fig. 3B). Adenosine receptor activation had no effect on ABCA1 protein levels in ABCA1 KD THP-1-derived macrophages (Fig. 3C). In addition to ABCA1, Epac agonist 8-pcPT-2′-O-Me-cAMP increased phospho-ABCA1 significantly (P<0.0003 vs. control; n=3; Fig. 4).
Figure 3.
ABCA1 protein expression is induced by adenosine receptor activation in THP-1-derived macrophages but not in ABCA1 KD THP-1-derived macrophages. (A) THP-1-derived macrophages were incubated with apoA-I (50 μg/ml) ± IFN-γ (500 U/ml), CGS-21680 (1 uM), or IFN-γ (500 U/ml) and CGS-21680 (1 uM) for 24 h. Protein levels were quantified by calculating the average band intensity of ABCA1/band intensity B-actin. Protein levels were measured in triplicate. (B) THP-1-derived macrophages were incubated with apoA-I (50 μg/ml) ± IFN-γ (500 U/ml), PKA agonist 6-Benz-cAMP (Benz; 100 uM), or Epac agonist pcPT-2′-O-Me-cAMP (Ometh; 100 uM) for 24 h. Protein levels were quantified by calculating the average band intensity of ABCA1/band intensity B-actin. Protein levels were measured in triplicate. (C) ABCA1 KD THP-1-derived macrophages were incubated with apoA-I (50 μg/ml) ± IFN-γ (500 U/ml), CGS-21680 (1 uM), or IFN γ (500 U/ml) and CGS-21680 (1 uM) for 24 h. Protein levels were quantified by calculating the average band intensity of ABCA1/band intensity B-actin. Protein levels were measured in triplicate.
Figure 4.
Phospho-ABCA1 protein expression is induced Epac agonist pcPT-2′-O-Me-cAMP in THP-1-derived macrophages. ABCA1 protein was immunoprecipitated with phospho-ABCA1 antibody in THP-1-derived macrophages after treatment with CGS-21680 (1 μM) or pcPT-2′-O-Me-cAMP (100 uM). Protein levels were quantified by calculating the average band intensity of ABCA1/band intensity preimumune complex (13±5%; P<0.0003; n=3).
A2AR occupancy blocks IFN-γ-mediated foam cell formation in THP-1-derived macrophages and ABCG1 KD THP-1-derived macrophages but not in ABCA1 KD THP-1-derived macrophages
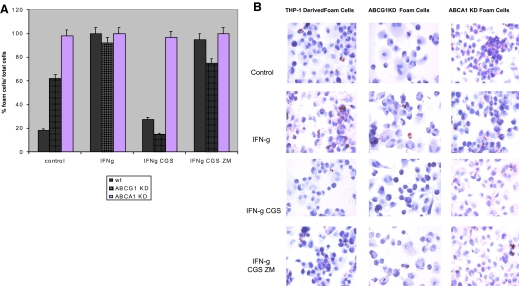
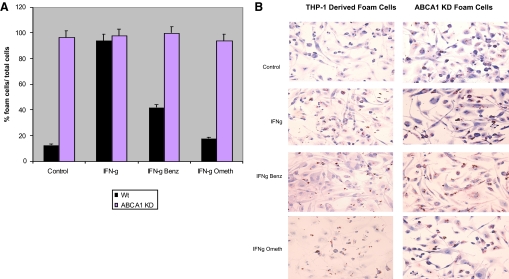
We have reported previously that IFN-γ promotes foam cell formation in THP-1-derived macrophages [7]. We therefore determined whether CGS-21680 decreased foam cell formation by an ABCA1- or ABCG1-dependent mechanism. Consistent with what we have reported previously, the A2AR agonist CGS-21680 (1 μM) inhibits foam cell formation (59±6% inhibition; P<0.004 vs. IFN-γ alone; n=3) in THP-1-derived macrophages (Fig. 5, A and B). Similarly, CGS-21680 inhibited foam cell formation (81±5% inhibition; P<0.0001 vs. IFN-γ alone; n=3) in ABCG1 KD THP-1-derived macrophages (Fig. 6, A and B). The A2AR antagonist ZM-241385 reversed the effects of CGS-21680 on foam cell formation in THP-1-derived macrophages (62±2% inhibition; P<0.0001 vs. IFN-γ CGS-21680; n=3) and ABCG1 KD THP-1-derived macrophages (65±6% inhibition; P<0.0002 vs. IFN-γ CGS-21680; n=3). CGS-21680 did not decrease foam cell formation in ABCA1 KD-derived macrophages (Fig. 5, A and B). In addition to adenosine receptor activation, we wanted to test whether molecules downstream of the adenosine receptor contributed to decreased foam cell formation. PKA and Epac are activated by the adenosine receptor and are thought to enhance ABCA1 function. The selective Epac agonist pcPT-2′-O-Me-cAMP decreased foam cell formation in THP-1-derived macrophages (76±5% inhibition; P<0.0001 IFN-γ pcPT-2′-O-Me-cAMP vs. IFN-γ alone; n=3) but not in ABCA1 KD THP-1-derived macrophages (Fig. 6, A and B). PKA agonist 6-Benz-cAMP behaved similarly.
Figure 5.
A2AR agonist CGS-21680 decreases IFN-γ-stimulated foam cell formation in untreated and ABCG1 KD cells but not in ABCA1 KD cells. (A) THP-1 cells, ABCG1 KD cells, or ABCA1 KD cells were incubated with PMA (106 cells/ml) for 48 h. Cells were then incubated with acLDL (50 μg/ml) ± proinflammatory cytokine IFN-γ (500 U/ml), selective A2AR agonist CGS-21680 (1 μM/ml), and ± selective A2A antagonist ZM-241385 (1 μM/ml) for an additional 24 h. Foam cells were quantified and are expressed as a percentage of the total number of cells present in each chamber. wt, Wild-type. (B) Oil Red O stain was used to visualize lipid droplets in THP-1/macrophages.
Figure 6.
PKA agonist 6-Benz-cAMP and Epac agonist 8-pcPT-2′-O-Me-cAMP decrease foam cell formation in THP-1-derived macrophages but not in ABCA KD THP-1-derived macrophages. (A) THP-1 cells or ABCA1 KD THP-1 cells were incubated with PMA (106 cells/ml) for 48 h. Cells were then incubated with acLDL (50 μg/ml) ± proinflammatory cytokine IFN-γ (500 U/ml) and selective PKA agonist 6-Benz-cAMP (50 μM/ml) or Epac agonist 8-pcPT-2′-O-Me-cAMP (50 μM/ml) for an additional 24 h. Foam cells were quantified and are expressed as percentage foam cells divided by the total cells/chamber. (B) Oil Red O stain was used to visualize lipid droplets in foam cells.
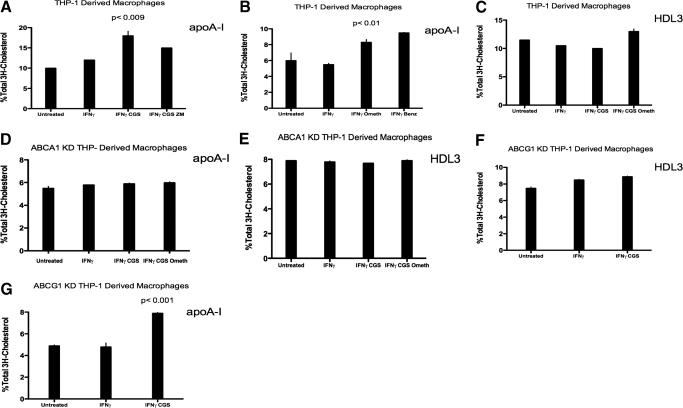
Selective A2AR agonist CGS-21680 and Epac agonist pcPT-2′-O-Me-cAMP increase apoA-I-mediated cholesterol efflux in THP-1-derived macrophages but not in ABCA1 KD THP-1-derived macrophages
Cholesterol efflux is mediated via ABCA1 and ABCG1 in the presence of the acceptor molecules apoA-I and HDL, respectively. We therefore wanted to determine whether A2AR stimulation contributes to enhanced cholesterol efflux. The A2AR agonist CGS-21680 nearly doubled apoA-I-mediated [3H]-cholesterol efflux (from 9.5±2% to 17.5 ±2.5% [3H]-cholesterol efflux; P<0.0090 vs. control; n=3) in IFN-γ-treated THP-1-derived macrophages (Fig. 7A). Selective Epac agonist 8-pcPT-2′-O-Me-cAMP increased apoA-I-mediated cholesterol efflux by 1.5-fold (from 5±1% to 7.5% ±1 [3H]-cholesterol efflux; P<0.0100 vs. control; n=3) in IFN-γ-treated THP-1-derived macrophages (Fig. 7B). CGS-21680 and Epac agonist 8-pcPT-2′-O-Me-cAMP failed to enhance cholesterol efflux in THP-1-derived macrophages when HDL3 was used as the cholesterol acceptor (Fig. 7C). Adenosine receptor activation was unable to increase apoA-I- or HDL3-mediated cholesterol efflux in ABCA1 KD THP-1-derived macrophages (Fig. 7, D and E). In ABCG1 KD THP-1-derived macrophages, the A2AR agonist CGS-21680 was unable to enhance HDL3-mediated cholesterol efflux but increased apoA-I-mediated efflux significantly (from 4.7±1% to 7.8% ±1 [3H]-cholesterol efflux; P<0.001 vs. control; n=3; Fig. 7, F and G). These findings support the hypothesis that A2AR occupancy inhibits foam cell formation by increasing cholesterol efflux via ABCA1, as adenosine receptor-stimulated cholesterol efflux increases only when ABCA1 and cholesterol acceptor apoA-I are present.
Figure 7.
Adenosine receptor activation increases cholesterol efflux in THP-1-derived macrophages and ABCG1 KD THP-1-derived macrophages but not in ABCA1 KD THP-1-derived macrophages. THP-1-derived macrophages or ABCA1 KD THP-1-derived macrophages were incubated with PMA (106 cells/ml) for 48 h. Cells were then incubated with acLDL (50 μg/ml). (A) Induction of apolipoprotein-mediated cholesterol efflux from THP-1-derived macrophages, which were incubated for 24 h, with or without IFN-γ (500 U/ml) and A2AR agonist CGS-21690 (1 μM) and A2AR antagonist ZM-241385 and (B) PKA agonist 6-Benz-cAMP (100 μM) or Epac agonist 8pcPT-2′-O-Me-cAMP (100 uM). [3H]-Cholesterol efflux was measured after 4 h incubation with the appropriate cholesterol acceptor apoA-I (50 ug/ml). (C) Induction of HDL3-mediated cholesterol efflux from THP-1-derived macrophages, which were incubated for 24 h, with or without IFN-γ (500 U/ml), A2AR agonist CGS-21690 (1 μM), or Epac agonist 8pcPT-2′-O-Me-cAMP (200 uM). [3H]-Cholesterol efflux was measured after 4 h incubation with the appropriate cholesterol acceptor apoA-I (50 ug/ml). (D) Induction of apolipoprotein-mediated cholesterol efflux from ABCA1 KD THP-1-derived macrophages, which were incubated for 24 h, with or without IFN-γ (500 U/ml), A2AR agonist CGS-21680 (1 μM), or PKA agonist 6-Benz-cAMP (100 uM). [3H]-Cholesterol efflux was measured after 4 h incubation with the cholesterol acceptor apoA-I (50 ug/ml). (E) Induction of HDL3-mediated cholesterol efflux from ABCA1 KD THP-1-derived macrophages, which were incubated for 24 h, with or without IFN-γ (500 U/ml), A2AR agonist CGS-21680 (1 μM), or Epac agonist 8pcPT-2′-O-Me-cAMP (100 uM). [3H]-Cholesterol efflux was measured after 4 h incubation with the cholesterol acceptor HDL3 (50 ug/ml). (F) Induction of HDL3-mediated cholesterol efflux from ABCG1 KD THP-1-derived macrophages, which were incubated for 24 h, with or without IFN-γ (500 U/ml) and A2AR agonist CGS-21680 (1 μM). [3H]-Cholesterol efflux was measured after 4 h incubation with the cholesterol acceptor HDL3 (50 ug/ml). (G) Induction of apoA-I-mediated cholesterol efflux from ABCG1 KD THP-1-derived macrophages, which were incubated for 24 h, with or without IFN-γ (500 U/ml) and A2AR agonist CGS-21690 (1 μM). [3H]-Cholesterol efflux was measured after 4 h incubation with the cholesterol acceptor apoA-I (50 ug/ml). Values are means of three experiments ± sem.
DISCUSSION
We have demonstrated previously that adenosine, acting at A2ARs, diminishes foam cell formation by human monocytoid cells and murine peritoneal macrophages and that the functional modulation of the adenosine receptor is a key factor in the regulation of the inflammatory state [5]. In the same experiments, we have also demonstrated that adenosine, acting at the same receptor, stimulates the expression of ABCA1 [7]. We found that ligation of A2ARs prevents foam cell formation by promoting reverse cholesterol transport via increased expression and/or function of ABCA1. In support of this hypothesis, we observed that in confirmation of prior observations, the A2AR agonist CGS-21680 increased ABCA1 mRNA in THP-1 cells. Moreover, using ABCA1 and ABCG1 lentiviral siRNA technology, we found that the effects of A2AR ligation on cholesterol transport and foam cell formation were observed only in wild-type and ABCG1 KD but not ABCA1 KD cells. In addition to stimulating increased ABCA1 expression, the A2AR agonist CGS-21680 promotes ABCA1 phosphorylation and ABCA1-mediated cholesterol transport and foam cell formation by an Epac-dependent pathway. In contrast to ABCA1, ABCG1 expression/function was not influenced by A2AR activation.
Cholesterol homeostasis is regulated by a number of pathways, including cholesterol efflux. We looked at effects of adenosine receptor activation on cholesterol efflux and found that A2AR agonist CGS-21680 mediates cholesterol efflux via reverse cholesterol transport protein ABCA1 but not ABCG1 (Fig. 7). CGS-21680 was only able to increase cholesterol efflux when the acceptor apoA-I for ABCA1 and ABCA1 was present. The cholesterol acceptor HDL3 for ABCG1 and ABCG1 did not contribute to enhanced adenosine receptor mediated-cholesterol efflux (Fig. 7).
Foam cell formation during the development of atheromatous plaque depends on the balance between uptake and efflux of cholesterol [10, 11]. ABCA1 is a key contributor to this exchange. In addition to ABCA1, ABCG1 plays important roles in maintaining cholesterol homeostasis. ABCA1 mediates cholesterol efflux from extrahepatic cells via apoA-I, and ABCG1 mediates cholesterol efflux via HDL [6, 12]. Dysregulation of ABCA1 or ABCG1 can lead to development of atherosclerosis. Our results indicate that A2AR activation may help protect against the development of atherosclerosis by promoting ABCA1-mediated cholesterol efflux from macrophages. In this light, it is interesting to note that dipyridamole, an agent that blocks adenosine uptake and thereby increases extracellular adenosine concentrations, has been used to prevent vascular events and may also help prevent plaque development.
Occupancy of the anti-inflammatory A2AR minimizes early atherosclerotic changes in the arterial wall [13], and one potential mechanism for this effect is enhanced macrophage expression of ABCA1 mRNA [7]. The A2AR is a member of the family of G protein (Gs)-coupled receptors and is known for its role in vasodilation as well as for its anti-inflammatory effects. Ligation of A2AR prevents reperfusion injury and atherosclerosis in animal models, inhibits foam cell formation, and prevents IFN-γ-mediated down-regulation of ABCA1 by a PKA cAMP- mediated mechanism [7, 14]. Interestingly, in contrast to prior studies in injury-induced models of atherosclerosis [13], recent studies about atherosclerosis-prone apoE-deficient mice lacking A2ARs suggest that loss of A2ARs prevents the development of atherosclerosis [15]. The protective effect of loss of A2ARs in this model was attributed to apoptosis of macrophages that had taken up lipid. Although protection may be afforded by macrophage apoptosis at an early stage in the pathogenesis of atherosclerosis, at later stages, this leads to accumulation of extracellular lipid and cholesterol and a complicated plaque. Thus, the apoptosis-mediated protection seen in this model may be peculiar to the model and does not reflect the slow accumulation of plaque that occurs in humans.
Recent interest has been directed at the proatherogenic effects of inflammatory cytokines [14]. We have shown that T cell clones from human atherosclerotic plaques respond to oxysterols by secreting cytokines such as IFN-γ [5]. It has also been shown that IFN-γ promotes foam cell formation in human monocytic THP-1 cells [14]. Oxidized lipids in the atherosclerotic plaque induce many genes that give rise to a chronic inflammatory response. Oxidized lipids also induce cAMP production, which has been shown to mediate adenosine receptor signaling [9, 16].
cAMP induces apoA-I-binding activity and promotes cellular cholesterol efflux in a PKA-dependent manner. In addition to PKA, Epac is a cAMP-regulated guanine nucleotide exchange factor activated by A2AR signaling [8]. We investigated whether the signaling molecule Epac was able to phosphorylate ABCA1 and promote cholesterol efflux in human macrophages and were surprised to find that Epac- and PKA-dependent pathways are involved in phosphorylation and activation of ABCA1.
We report here evidence that A2AR suppresses foam cell formation by regulating the expression and function of ABCA1, a transporter that plays a key role in cholesterol efflux [5]. A2AR stimulation inhibits foam cell formation but not when ABCA1 expression is diminished. Moreover, A2AR stimulation has no effect on cholesterol uptake but increases cholesterol efflux from macrophages but not when ABCA1 expression is suppressed by siRNA, and siRNA-mediated down-regulation of ABCG1 does not affect the capacity of A2AR stimulation to suppress foam cell formation and to stimulate ABCA1-mediated cholesterol efflux. Furthermore, we provide evidence that A2AR-mediated stimulation of adenylate cyclase with downstream stimulation of PKA-dependent and -independent signaling regulates ABCA1 function and foam cell formation. Here, we provide a potential mechanism by which activation of A2ARs increases ABCA1 expression and function: The proatherogenic effects exerted by proinflammatory cytokines such as IFN-γ can be overcome by A2AR activation.
AUTHORSHIP
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
ACKNOWLEDGMENTS
We acknowledge Sackler NYU School of Medicine and the Department of Pharmacology (NIH T32GM66704) for their support. This work was funded by grants from NIH (AA13336, AR41911, GM56268), the Vilcek Foundation, and the General Clinical Research Center (M01RR00096).
DISCLOSURES
Conflicts of interest follow. Taiese C. Bingham—none. Bruce N. Cronstein—intellectual property: patents on use of A2AR agonists to promote wound healing and use of A2AR antagonists to inhibit fibrosis; patent on use of adenosine A1R antagonists to treat osteoporosis and other diseases of bone; patent on the use of adenosine A1R and A2BR antagonists to treat fatty liver; patent on the use of A2AR agonists to prevent prosthesis loosening. Consultant (within the past 2 years), all <$10,000—Cypress Bioscience, Inc., King Pharmaceutical (licensee of patents above), CanFite Biopharmaceuticals, Bristol-Myers Squibb, Cellzome, Tap Pharmaceuticals, Prometheus Laboratories, Regeneron (Westat, DSMB), Sepracor, Amgen, Endocyte, Protalex, Allos, Inc., Combinatorx, Kyowa Hakka, Hoffman-LaRoche, Savient, Avidimer Therapeutics. Stock—CanFite Biopharmaceuticals received for membership in Scientific Advisory Board. Grants—King Pharmaceuticals, NIH, Vilcek Foundation, Board Member. Bagels, ice cream, snacks, etc.—Eli Lilly & Co., UCB, Pfizer.
Footnotes
Abbreviations: A2AR=adenosine A2A receptor, ABCA1=ATP-binding cassette transporter A1, ABCG1=ATP-binding cassette transporter G1, acLDL=acetylated human low-density lipoprotein, apoA-I=apolipoprotein A-I, CGS-21680=2-p-(2-carboxyethyl)phenethyllamino-5′-N-ethyl-carboxamido-adenosine, Gs=G stimulatory, HDL=high-density lipoprotein, KD=knockdown, LXR=liver X receptor, NIH=National Institutes of Health, PKA=protein kinase A, shRNA=small hairpin RNA, siRNA=small interfering RNA, ZM-241385=4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
References
- Young S G, Fielding C J. The ABCs of cholesterol efflux. Nat Genet. 1999;22:316–318. doi: 10.1038/11878. [DOI] [PubMed] [Google Scholar]
- Oram J F, Lawn R. ABCA1: the gatekeeper for eliminating excess tissue cholesterol. J Lipid Res. 2001;42:1173–1179. [PubMed] [Google Scholar]
- Oram J F, Vaughan A. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- Wang N, Ranalletta M, Matsuura F, Peng F, Tall A R. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler Thromb Vasc Biol. 2006;26:1310–1316. doi: 10.1161/01.ATV.0000218998.75963.02. [DOI] [PubMed] [Google Scholar]
- Reiss A B, Patel C A, Rahman M, Chan E S, Hasneen K, Montesinos C, Trachman J D, Cronstein B N. Interferon-γ impedes reverse cholesterol transport and promotes foam cell transformation in THP-1 human monocytes/macrophages. Med Sci Monit. 2004;10:BR420–BR425. [PubMed] [Google Scholar]
- Panousis C G, Zuckerman S H. Interferon-γ induces downregulation Tangier disease gene (ATP-binding-cassette transporter 1) in macrophage-derived foam cells. Arterioscler Thromb Vasc Biol. 2000;20:1565–1571. doi: 10.1161/01.atv.20.6.1565. [DOI] [PubMed] [Google Scholar]
- Reiss A B, Awadallah N W, Malhotra S, Montesinos C, Chan E S, Javitt N B, Cronstein B N. Immune complexes and IFN-γ decrease cholesterol 27-hydroxylase in human arterial endothelium and macrophages. J Lipid Res. 2001;42:1913–1922. [PubMed] [Google Scholar]
- Holz G G, Kang G, Harbeck M, Roe M W, Chepurny O G. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar B, Denis M, Krimbou L, Marcil M, Genest J., Jr cAMP induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts. J Lipid Res. 2002;43:2087–2094. doi: 10.1194/jlr.m200235-jlr200. [DOI] [PubMed] [Google Scholar]
- Forcheron F, Legedez L, Chinetti G, Feugier P, Letexier D, Bricca G, Beylot M. Genes of cholesterol metabolism in human atheroma: overexpression of perilipin and genes promoting cholesterol storage and repression of ABCA1 expression. Arterioscler Thromb Vasc Biol. 2005;25:1711–1717. doi: 10.1161/01.ATV.0000174123.19103.52. [DOI] [PubMed] [Google Scholar]
- Williams K J, Feig J E, Fisher E. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- Duong M, Collins H L, Jin W, Zanotti I, Favari E, Rothblat G H. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler Thromb Vasc Biol. 2006;26:541–547. doi: 10.1161/01.ATV.0000203515.25574.19. [DOI] [PubMed] [Google Scholar]
- Chan E S, Zhang H, Fernandez P, Edelman S D, Pillinger M H, Ragolia L, Palaia T, Carsons S, Reiss A B. Effect of cyclooxygenase inhibition on cholesterol efflux proteins and atheromatous foam cell transformation in THP-1 human macrophages: a possible mechanism for increase cardiovascular risk. Arthritis Res Ther. 2007;9:R4. doi: 10.1186/ar2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K, Montesinos M C, Reiss A B, Delano D, Awadallah N, Cronstein B N. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Lestavel S, Bocher V, Remaley A T, Neve B, Torra I P, Teissier E, Minnich A, Jaye M, Duverger N, Brewer H B, Fruchart J C, Clavey V, Staels B. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- Hansson G K. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Feig J E, Shamir R, Fisher E. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]