Abstract
Despite HAART, patients infected with HIV develop NHL at a significantly higher level than the noninfected population. The primary difference between lymphoma in non-HIV-infected individuals and those with ARL is that ARL is consistently high-grade and metastatic. The emergence of ARL is associated with the presence of macrophage viral reservoirs, similar to what has been observed for HAD. HIV-infected macrophages, as seen by histology and HIV p24 staining, are present in approximately half of ARLs. Macrophage reservoirs recruit additional immune cells, including monocytes/macrophages, through the release of chemoattractants. Additionally, TAM are known to promote tumor progression for most cancer types, including lymphomas. This review will highlight and discuss the role of macrophage viral reservoirs in the development and progression of ARLs and hopefully, shed light on this new and interesting field.
Keywords: HIV, reservoir, metastasis, cancer
Introduction
The incidence of NHL, a disease of malignant lymphocytes, is increased in the HIV-infected population. While the advent of HAART has decreased the occurrence of NHL in HIV-infected individuals, the risk of developing NHL is still ∼60 times greater than in the noninfected population, making ARL one of the most common AIDS-defining cancers in HIV-infected individuals. Additionally, little change in morbidity as a result of ARL has been observed in HIV-infected patients [1, 2].
Since the introduction of HAART therapy and the resulting immune reconstitution in HIV patients, the epidemiology of AIDS-related cancers has changed. For instance, the incidence of CNS lymphoma has dropped to nearly one-tenth the level observed in the pre-HAART era [3]. The incidence of KS has also decreased dramatically, suggesting that immune reconstitution has had a large effect on preventing these AIDS-defining cancers. In contrast, the occurrence of ARLs has not declined to the same extent. Although HAART therapy has been reported to decrease ARL incidence by almost 50%, there has been little change amongst the various CD4 strata [4]. Therefore, even though more patients are living longer on HAART, the overall number of ARL cases is expected to rise in the future.
The greatest difference between lymphoma in non-HIV and HIV-infected individuals is that ARLs are usually high-grade and aggressively metastatic, with death occurring in as little as 2 weeks after diagnosis [2]. ARLs predominantly present as systemic NHLs with frequent involvement of extranodal sites, including the gastrointestinal tract, liver, and bone marrow [5]. Approximately 80% of ARLs arise systemically, and the remaining cases arise within the CNS [6, 7]. These systemic, high-grade ARLs are primarily of B cell origin and are usually histologically classified as either large cell lymphoma (variants include centroblastic, immunoblastic, and anaplastic), Burkitt lymphoma, and primary effusion lymphoma [1].
During the pre-HAART era, it seemed certain that ARL occurred primarily as a result of HIV replication-associated immunosuppression. However, due to HAART, this disease would be seen much less frequently if immune suppression alone were the cause for the lymphoma or only occurred in a subset of individuals who are intolerant of HAART [2]. Since the molecular studies performed on ARL tissues to date have shown that HIV is not inserted within malignant B cells, it has been suggested that HIV contributes to ARL incidence through indirect mechanisms of immunosuppression and genetic abnormalities due to EBV infection, HHV-8 infection, c-MYC and BCL-6 rearrangements, RAS mutations, p53 inactivation, and 6q deletions [8, 9]. Additionally, ARL onset is often preceded by an overproduction of B cell stimulatory cytokines, resulting in B cell activation and possibly, activation-induced cytidine deaminase-associated DNA modification errors and oncogenic translocations [10,11,12]. However, much is still unknown about the development and progression of ARL. The focus of this review is to discuss the role of HIV viral reservoirs and TAM in lymphoma development in the HIV-positive patient population.
MACROPHAGES: PROMOTERS OF TUMOR PROGRESSION
Macrophages form a large portion of the inflammatory infiltrate in most, if not all, cancer types. TAM constitute up to 80% of the total tumor mass, depending on the tumor type [13]. Although the role of TAM in tumorigenesis has been controversial, there is increasing evidence that macrophages actively promote tumor progression for numerous human cancers (reviewed in ref. [14]). Tumor-derived chemoattractants signal monocytes to extravasate out of the bloodstream and infiltrate the tumor mass, resulting in a continuous influx of macrophages throughout the lifespan of the tumor [15, 16]. Within the tumor microenvironment, macrophages are exposed to IL-4 and IL-10, which likely induce TAM to become alternatively activated/M2 macrophages [17]. These M2 macrophages scavenge debris, promote tissue repair and angiogenesis, and lack tumoricidal capabilities [17]. Interestingly, high numbers of TAM, as measured by CD68 positivity within the tumor mass, are associated with poor prognosis in many types of human cancer to include, brain, breast, prostate, ovarian, bladder, kidney, melanoma, and FL (reviewed in refs. [14, 18]).
TAM are known to play a role in every hallmark step of tumor progression. TAM promote tumor growth through the expression and release of factors that increase tumor cell proliferation and survival, such as EGF, platelet-derived growth factor, hepatocyte growth factor, basic fibroblast growth factor, and TGF-β1 [19, 20]. As tumor cells begin to divide, and hypoxic areas develop, TAM release numerous proangiogenic cytokines, growth factors, and angiogenesis-modulating enzymes to support tumor neovascularization [18, 21]. Macrophages within hypoxic areas also act as immunosuppressors via the release of PGE2 and IL-10, two factors that impair immune cell development [22, 23]. Additionally, TAM release various proteases that break down the basement membrane, providing invading tumor cells with access to surrounding tissues. Metastasis is further promoted by macrophages that release chemoattractants located near the tumor vasculature, including EGF, which act as a beacon, guiding tumor cells into the circulation [18].
It has been proposed that macrophages may play a direct role in cancer progression, as some reports have suggested that metastatic cells arise from cells of the myeloid/macrophage lineage [24,25,26]. The behavior of normal macrophages in response to tissue injury or disease is similar to the behavior of metastatic tumor cells during the metastatic cascade. Both macrophages and metastatic cells enter and exit the circulation, invade tissues, release inflammatory cytokines, and exhibit phagocytic behaviors [27]. Additionally, many metastatic tumor cells have been reported to express macrophage-specific antigens, lipids, and behaviors [25, 28,29,30]. Due to the abundant behavioral and biological similarities between macrophages and metastatic cells, a myeloid origin of metastatic cancer has been proposed [24, 25, 29, 31]. The myeloid origin of metastasis stands apart from the more widely accepted hypotheses of random somatic mutations and the epithelial-mesenchymal transition [32,33,34]. Although aberrant antigen expression may be seen in a subset of tumors, cancer cells that express behavioral, biochemical, and genetic properties of macrophages are likely of myeloid origin.
STUDIES ON MACROPHAGES IN NON-HODGKIN LYMPHOMA
TAM have been reported to negatively impact NHL tumor grade and patient survival, similar to that seen for many solid tumor types. FL, the second most common type of NHL in the HIV-negative population, is a clinically heterogeneous disease with survival ranging from 2 to 20 years after diagnosis [35]. A study examining a group of FL patients, who were uniformly staged and treated, revealed that those with high TAM levels had a median OS of only 5 years, in contrast to those with low TAM levels, who had an OS of 16.3 years [36]. These findings were further supported by gene-expression studies showing that FLs with a macrophage gene signature had reduced survival when compared with FLs with a T cell gene signature [37,38,39,40,41]. It has also been shown in other forms of B cell NHLs that TAM content increases with malignancy grade and is highly correlated with tumor vascularity [42]. However, a conflicting report suggested that TAM did not correlate with progression-free survival, OS, or tumor grade in patients with DLBCL [43]. These discrepancies are likely a result of differences in sample population, as one group analyzed TAM levels in specimens obtained from patients that were treated with curative intent, and the other examined node biopsies taken before any therapy was administered [42, 43]. Although further studies will be needed to clarify the role of TAM in DLBCL pathogenesis, these findings suggest that TAM in HIV-negative B cell NHLs have a similar negative impact on prognosis, as seen in many other cancer types. The role of TAM in HIV-positive NHL is currently unknown.
STUDIES ON AIDS-RELATED NHL: MACROPHAGES AS VIRAL RESERVOIRS
A major obstacle in HIV eradication is the ability of the virus to establish reservoirs within cells that are resistant to apoptosis and HAART therapy. HIV infection and replication mainly occur within CD4+ T cells and macrophages. Shortly after HIV infects CD4+ T cells, they undergo apoptosis; however, macrophages are much more resistant to HIV-induced apoptosis and are able to sustain a continuous low-level of viral replication [44]. In fact, infectious HIV can be detected in circulating monocytes from patients that have been on HAART therapy for long periods of time [45, 46]. While macrophages represent a major viral reservoir, other cells, including resting CD4+ T cells, dendritic cells, hematopoietic progenitor cells, and astrocytes, can also harbor HIV for long periods of time (reviewed in ref. [44]).
Macrophage tropic HIV strains are detectable during all stages of HIV infection [47]. It is estimated that macrophages constitute a small percentage of the total infected cell population during the chronic stage of HIV disease. However, when CD4+ T cells become depleted during the late stages of HIV infection, macrophages likely account for a larger portion of the infected cell population. Due to the long macrophage lifespan and the continual recruitment of these cells to infected tissues, macrophages are highly susceptible to super-infection with multiple HIV viral strains. Interestingly, up to 50% of viral sequences from ARL tissues contain intra-host viral recombinants, suggesting that the macrophage reservoirs within these tissues are acting as a viral haven, thus generating novel viral genotypes through HIV evolution and recombination [48, 49]. The genetic diversity of HIV strains has the potential to generate drug-resistance mutations, change coreceptor usage, and aid in immune evasion [49, 50]. Therefore, future drug therapies targeting HIV-infected macrophages should greatly enhance the ability to eradicate HIV infection.
MACROPHAGES IN ARL
The existence of long-lived, HIV-infected macrophage populations resistant to HAART suggests separate pathogenic potentials for viruses within this reservoir than those associated with pathologies related to T cell depletion. Currently, it is believed that HIV is not localized to malignant B cells in ARL [2]. However, in preliminary studies, HIV protein expression within ARL tissues has been localized to TAM, similar to what has been observed in the SIV-associated lymphomas [51,52,53,54]. Therefore, it appears that HIV infection, when present in ARL, is localized to the macrophages within the tumor stroma.
HIV-infected macrophages have been shown to have lymphomagenic potential in SCID mice, suggesting a viral component to lymphomagenesis [51]. Zenger et al. [51] isolated TAM from two patients, who died from ARL, and implanted them into SCID mice. Before macrophage implantation, PCR analysis confirmed the presence of HIV within the TAM isolated from both patients. Interestingly, within 3 months, 74% of the animals developed aggressive murine lymphomas. Human lysozyme staining of the mouse tumors confirmed that the spontaneous lymphomas contained human macrophages within the tumor stroma. The HIV-positive TAM isolated from the human ARLs were responsible for the murine tumor development, as T cells isolated from the same patients with ARL and control macrophages isolated from healthy donors were not capable of inducing tumors in SCID mice [51]. This study directly implicated HIV-infected macrophages in the induction of ARL.
COULD HIV INFECTION CONTRIBUTE TO CANCER DEVELOPMENT?
Since HIV is not localized within malignant B cells, it is unlikely that HIV contributes to lymphomagenesis through inducing B cell immortalization. However, this is not the case for other viruses that are associated with ARLs. EBV is known to promote lymphoma onset in a subset of ARLs. Although almost all ARLs of the CNS contain EBV-infected cells, it has been reported that up to 66% of systemic ARLs also contain detectable EBV [55], and EBV infection is thought to contribute to tumorigenesis, as in vitro EBV infection leads to B cell immortalization and proliferation [56]. The role EBV plays as a directly transforming agent is unclear in most classes of ARL. A recent report by Chadburn et al. [57] demonstrated that the presence of EBV within ARL tissues did not correlate with the patients’ CD4 counts or clinical outcome, suggesting that more subtle immunologic abnormalities allow EBV-infected ARL cells to proliferate. Additionally, HHV-8 is commonly associated with primary effusion lymphoma, a subtype of ARL, and has been suggested to play a role in some solid ARLs. HHV-8 is also believed to trigger B cell proliferation and inhibit T cell cycle regulation, potentially resulting in lymphomagenesis [58,59,60].
A study by Mack et al. [54] revealed that HIV-induced macrophage “immortalization” may be a potential mechanism for ARL induction. Tissues from individuals with ARL contained populations of HIV-expressing macrophages with HIV genomes integrated within or nearby genes associated with signal transduction, receptor activation, and oncogene activation [54]. Thus, in late-stage HIV disease, HIV may generate immortalized macrophage populations through insertional mutagenesis, contributing to ARL pathogenesis. These findings also lend additional support to the myeloid origin of cancer hypothesis.
STUDIES ON HIV-INFECTED MACROPHAGES WITHIN ARL
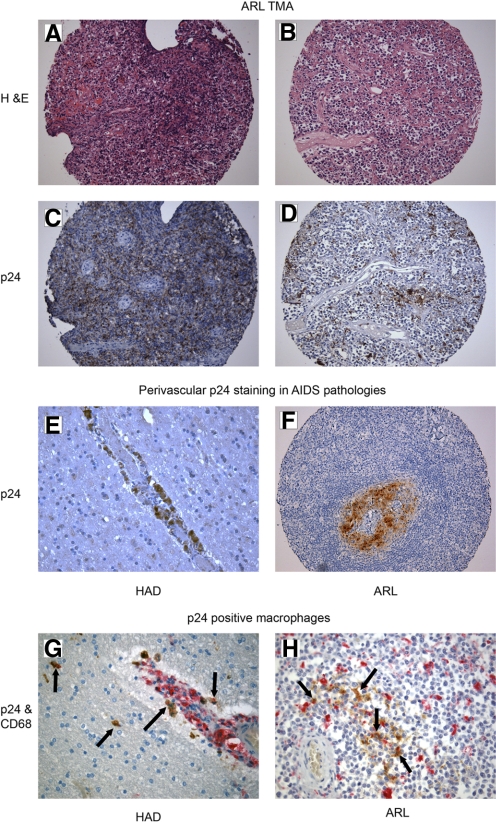
We were interested in determining if HIV-infected macrophages could be commonly identified within ARL tumors. To answer this question, we focused on the HIV p24 antigenic reactivity of macrophages in ARL biopsies from HIV+ patients at time of diagnosis. In this preliminary study, a tissue microarray obtained from the ACSR containing HIV-positive ARL tissues (from 1984 to 2005) was used to screen for p24 and CD68 expression by immunohistochemistry (Fig. 1, A–D and F). Of the 60 ARL samples analyzed, 24 (40%) contained p24+ macrophages identified by CD68 and p24 double-staining. EBV status was available from the ACSR, as determined by EBV-EBER in situ hybridization for all of the cases (additional clinical information was not available). HIV and EBV status for these cases are summarized in Table 1. None of these ARL biopsies was HHV-8-positive (not shown). Here, we have identified a subset of ARLs that are not infected with EBV but contain HIV-positive TAM, suggesting by extension from the animal model data [51], that in these tumors, HIV might be contributing to tumorigenesis (Table 1). Although preliminary, these data suggest the existence of a novel class of ARL that contains HIV-infected macrophages without evidence for EBV or HHV-8 infection.
Figure 1.
Histopathology of p24-expressing macrophages. H&E and p24 staining revealed p24-positive macrophages in ARL tumors (A–D and F). HAD and ARL biopsies contained perivascular p24 staining (E and F). ARLs and HAD biopsies were stained with CD68 (red) and p24 (brown), confirming p24 expression within CD68-positive macrophages (G and H), as we recently described [61]. Examples of double-positive cells are highlighted by the black arrows. Mayer’s hematoxylin was used for counterstaining, and slides were examined by light microscopy. Original magnification (A–D and F), ×200; (E, G, and H), ×400 (representative tissues shown).
TABLE 1.
HIV and EBV Status of ARL Cases
| HIV/EBVa | No. of cases |
|---|---|
| +/+ | 10 |
| +/– | 14 |
| –/+ | 11 |
| –/– | 25 |
HIV was accessed by p24 reactivity. HIV colocalized mostly with CD68-positive macrophages on serial sections. EBV status was available from the ACSR. Tumors were considered EBV-postive if EBV-EBER in situ staining was above background.
HIV-INFECTED MACROPHAGES MAY PROMOTE A TUMORIGENIC ENVIRONMENT
Although NHLs are one of the most common forms of cancer in the HIV-infected population, other non-AIDS-defining cancers, including skin (basal cell carcinoma, melanoma), lung (squamous cell carcinoma), liver (hepatocellular carcinoma), anal (squamous cell carcinoma), and Hodgkin lymphoma, are increasing in incidence [62]. Many cancers that affect HIV-infected patients are associated with viral infection such as HHV-8 (KS), human papilloma virus (cervical and anal), EBV (NHL), and the hepatitis viruses (liver) [63]. Interestingly, chronic inflammation has been linked to the development of cancer and likely contributes to tumor development in patients infected with these tumorigenic viruses [64, 65]. In patients coinfected with HIV and a tumorigenic virus, it has been generally accepted that HIV plays a passive role in cancer development through impairing the host’s immune system’s ability to eliminate tumorigenic viral replication. However, HIV may contribute directly to cancer development and progression through the creation of a tumor-promoting inflammatory environment. Long-lived HIV macrophage reservoirs represent a source of chronic infection and inflammation in HIV patients. Additionally, HIV replication in macrophages results in increased production of MIP-1α, a strong monocyte/macrophage chemoattractant, resulting in an increase in the inflammatory cell population within the tissues [66, 67]. Long-term inflammation produces a microenvironment that fosters genomic abnormalities and tumor progression through the release of reactive oxygen species and cytokines associated with wound repair (reviewed in refs. [64, 65]). HIV proteins have also been shown to induce tumor angiogenesis [68]. Therefore, it is possible that HIV-induced macrophage infiltration and chronic inflammation contribute to the risk in developing various types of cancers in the infected population. If the HIV-induced inflammatory response increases TAM content, this may help to explain why lymphomas in the HIV-infected population tend to be more aggressive, resulting in a poor prognosis. It would be of interest to determine if TAM are elevated in HIV-positive tumors when compared with HIV-negative tumors.
HIV-INFECTED MACROPHAGES DRIVE DEMENTIA PATHOGENESIS
The pathogenic potential of HIV-infected macrophages in HAD is widely accepted. HAD affects ∼40% of HIV-infected individuals, generally during late stages of the disease [69]. Since HIV does not infect neurons directly, the loss of cognitive and motor function in infected individuals is believed to be a pathological side-effect of macrophage HIV reservoirs in the CNS [70, 71]. Presumably, HIV enters the brain early after the initial infection, via infected macrophages that cross the blood brain barrier and likely persist as a result of the inability of antiretroviral drugs to cross the barrier [72,73,74]. Once in the CNS, HIV-infected macrophages release cytokines, proinflammatory molecules, and viral proteins, which stimulate additional macrophage recruitment and activation, resulting in neuronal damage [70, 71, 75]. Therefore, HIV-infected macrophages play a role in modulating their surrounding microenvironment, thus confirming that infected macrophages can drive a pathogenic process without directly infecting cells affected by the disease (i.e., neurons in HAD). We suggest that HIV-infected macrophages play a similar role in ARL. In fact, the histology of HIV-positive macrophages in some ARLs looks markedly similar to those cells in HAD with a perivascular localization contributing to the attraction of other macrophages in the altered environment (Fig. 1, E and F).
CONCLUSION
Macrophages are among the most versatile cells of the body with respect to their roles in innate immunity, viral persistence, and tumor progression. When taking into consideration that macrophages facilitate tumor progression and that nearly half of ARLs contain HIV-positive macrophages, it seems highly probable that this cell population is contributing to AIDS-related lymphomagenesis. Therefore, a macrophage-targeted therapy has the potential to decrease the incidence, morbidity, and mortality of ARL and other AIDS-related pathologies. Future studies are needed to elucidate the role of the macrophage HIV reservoir in lymphomagenesis.
AUTHORSHIP
Leanne C. Huysentruyt contributed to the conception, design, analysis/interpretation of data, and writing of this manuscript. Michael S. McGrath contributed to the conception, design, analysis/interpretation of data, and editing of this manuscript.
ACKNOWLEDGMENTS
This work was supported from National Institutes of Health grants 5 U01 CA096230-06 and 5 U01 CA 066529-14. We thank Laura M. Shelton and Alanna Morris for helpful comments and technical assistance.
Footnotes
Abbreviations: ACSR=AIDS and Cancer Specimen Resource, ARL=AIDS-related lymphoma, DLBCL=diffuse large B cell lymphoma, EBER=EBV-encoded RNA, EGF=epidermal growth factor, FL=follicular lymphoma, HAART=highly active antiretroviral therapy, HAD=HIV-associated dementia, HHV-8=human herpesvirus type 8, KS=Kaposi sarcoma, NHL=non-Hodgkin lymphoma, OS=overall survival, TAM=tumor-associated macrophage(s)
References
- Beral V, Peterman T, Berkelman R, Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;337:805–809. doi: 10.1016/0140-6736(91)92513-2. [DOI] [PubMed] [Google Scholar]
- Rabkin C S. AIDS and cancer in the era of highly active antiretroviral therapy (HAART) Eur J Cancer. 2001;37:1316–1319. doi: 10.1016/s0959-8049(01)00104-6. [DOI] [PubMed] [Google Scholar]
- Kirk O, Pedersen C, Cozzi-Lepri A, Antunes F, Miller V, Gatell J M, Katlama C, Lazzarin A, Skinhoj P, Barton S E. Non-Hodgkin lymphoma in HIV-infected patients in the era of highly active antiretroviral therapy. Blood. 2001;98:3406–3412. doi: 10.1182/blood.v98.12.3406. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Chene G. Evolving epidemiology of malignancies in HIV. Curr Opin Oncol. 2008;20:534–540. doi: 10.1097/CCO.0b013e32830a5080. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Dalla-Favera R. Biologic aspects of human immunodeficiency virus-related lymphoma. Curr Opin Oncol. 1992;4:900–906. doi: 10.1097/00001622-199210000-00013. [DOI] [PubMed] [Google Scholar]
- Knowles D M, Pirog E C. Pathology of AIDS-related lymphomas and other AIDS-defining neoplasms. Eur J Cancer. 2001;37:1236–1250. doi: 10.1016/s0959-8049(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Baumgartner J E, Rachlin J R, Beckstead J H, Meeker T C, Levy R M, Wara W M, Rosenblum M L. Primary central nervous system lymphomas: natural history and response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg. 1990;73:206–211. doi: 10.3171/jns.1990.73.2.0206. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Carbone A, Dalla-Favera R. Pathogenesis of AIDS-related lymphomas: molecular and histogenetic heterogeneity. Am J Pathol. 1998;152:623–630. [PMC free article] [PubMed] [Google Scholar]
- Carbone A. AIDS-related non-Hodgkin’s lymphomas: from pathology and molecular pathogenesis to treatment. Hum Pathol. 2002;33:392–404. doi: 10.1053/hupa.2002.124723. [DOI] [PubMed] [Google Scholar]
- Epeldegui M, Breen E C, Hung Y P, Boscardin W J, Detels R, Martinez-Maza O. Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis. AIDS. 2007;21:2265–2270. doi: 10.1097/QAD.0b013e3282ef9f59. [DOI] [PubMed] [Google Scholar]
- Epeldegui M, Hung Y P, McQuay A, Ambinder R F, Martinez-Maza O. Infection of human B cells with Epstein-Barr virus results in the expression of somatic hypermutation-inducing molecules and in the accrual of oncogene mutations. Mol Immunol. 2007;44:934–942. doi: 10.1016/j.molimm.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Martinez-Maza O, Breen E C. B-cell activation and lymphoma in patients with HIV. Curr Opin Oncol. 2002;14:528–532. doi: 10.1097/00001622-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Morantz R A, Wood G W, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- Bingle L, Brown N J, Lewis C E. The role of tumor-associated macrophages in tumor progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Giannoudis A, Lewis C E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Lewis C E, Pollard J W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Goswami S, Sahai E, Wyckoff J B, Cammer M, Cox D, Pixley F J, Stanley E R, Segall J E, Condeelis J S. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- O'Sullivan C, Lewis C E, Harris A L, McGee J O. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet. 1993;342:148–149. doi: 10.1016/0140-6736(93)91348-p. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen B E, Forster I, Pawlinski R, Mackman N, Haase V H, Jaenisch R, Corr M, Nizet V, Firestein G S, Gerber H P, Ferrara N, Johnson R S. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgert K D, Alleva D G, Mullins D W. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother. 1997;20:165–177. [PubMed] [Google Scholar]
- Pawelek J M. Tumor cell hybridization and metastasis revisited. Melanoma Res. 2000;10:507–514. doi: 10.1097/00008390-200012000-00001. [DOI] [PubMed] [Google Scholar]
- Huysentruyt L C, Mukherjee P, Banerjee D, Shelton L M, Seyfried T N. Metastatic cancer cells with macrophage properties: evidence from a new murine tumor model. Int J Cancer. 2008;123:73–84. doi: 10.1002/ijc.23492. [DOI] [PubMed] [Google Scholar]
- Munzarova M, Lauerova L, Capkova J. Are advanced malignant melanoma cells hybrids between melanocytes and macrophages? Melanoma Res. 1992;2:127–129. doi: 10.1097/00008390-199207000-00008. [DOI] [PubMed] [Google Scholar]
- Burke B, Lewis C E. Oxford, UK; New York, NY, USA: Oxford University Press; The Macrophage. (2nd Edition) 2002:434–474. [Google Scholar]
- Munzarova M, Kovarik J. Is cancer a macrophage-mediated autoaggressive disease? Lancet. 1987;1:952–954. doi: 10.1016/s0140-6736(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Ruff M R, Pert C B. Small cell carcinoma of the lung: macrophage-specific antigens suggest hemopoietic stem cell origin. Science. 1984;225:1034–1036. doi: 10.1126/science.6089338. [DOI] [PubMed] [Google Scholar]
- Calvo F, Martin P M, Jabrane N, De Cremoux P, Magdelenat H. Human breast cancer cells share antigens with the myeloid monocyte lineage. Br J Cancer. 1987;56:15–19. doi: 10.1038/bjc.1987.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysentruyt L C, Shelton L M, Seyfried T N. Influence of methotrexate and cisplatin on tumor progression and survival in the VM mouse model of systemic metastatic cancer. Int J Cancer. 2010;126:65–72. doi: 10.1002/ijc.24649. [DOI] [PubMed] [Google Scholar]
- Fidler I J. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg R A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Thiery J P. Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- Farinha P, Masoudi H, Skinnider B F, Shumansky K, Spinelli J J, Gill K, Klasa R, Voss N, Connors J M, Gascoyne R D. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- Alvaro T, Lejeune M, Camacho F I, Salvado M T, Sanchez L, Garcia J F, Lopez C, Jaen J, Bosch R, Pons L E, Bellas C, Piris M A. The presence of STAT1-positive tumor-associated macrophages and their relation to outcome in patients with follicular lymphoma. Haematologica. 2006;91:1605–1612. [PubMed] [Google Scholar]
- Dave S S, Wright G, Tan B, Rosenwald A, Gascoyne R D, Chan W C, Fisher R I, Braziel R M, Rimsza L M, Grogan T M. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- Kelley T, Beck R, Absi A, Jin T, Pohlman B, Hsi E. Biologic predictors in follicular lymphoma: importance of markers of immune response. Leuk Lymphoma. 2007;48:2403–2411. doi: 10.1080/10428190701665954. [DOI] [PubMed] [Google Scholar]
- Lee A M, Clear A J, Calaminici M, Davies A J, Jordan S, MacDougall F, Matthews J, Norton A J, Gribben J G, Lister T A, Goff L K. Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol. 2006;24:5052–5059. doi: 10.1200/JCO.2006.06.4642. [DOI] [PubMed] [Google Scholar]
- Leich E, Hartmann E M, Burek C, Ott G, Rosenwald A. Diagnostic and prognostic significance of gene expression profiling in lymphomas. APMIS. 2007;115:1135–1146. doi: 10.1111/j.1600-0463.2007.apm_867.xml.x. [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Ruco L, Giacchetta F, Nico B, Quondamatteo F, Ria R, Iurlaro M, Dammacco F. Angiogenesis extent and macrophage density increase simultaneously with pathological progression in B-cell non-Hodgkin’s lymphomas. Br J Cancer. 1999;79:965–970. doi: 10.1038/sj.bjc.6690154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselblom S, Hansson U, Sigurdardottir M, Nilsson-Ehle H, Ridell B, Andersson P O. Expression of CD68+ tumor-associated macrophages in patients with diffuse large B-cell lymphoma and its relation to prognosis. Pathol Int. 2008;58:529–532. doi: 10.1111/j.1440-1827.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins J I, Corey L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambotte O, Taoufik Y, de Goer M G, Wallon C, Goujard C, Delfraissy J F. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- Schuitemaker H, Kootstra N A, de Goede R E, de Wolf F, Miedema F, Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhu H, Wilcox C K, van't Wout A, Andrus T, Llewellyn N, Stamatatos L, Mullins J I, Corey L, Zhu T. Blood monocytes harbor HIV type 1 strains with diversified phenotypes including macrophage-specific CCR5 virus. J Infect Dis. 2008;197:309–318. doi: 10.1086/524847. [DOI] [PubMed] [Google Scholar]
- Lamers S L, Salemi M, Galligan D C, de Oliveira T, Fogel G B, Granier S C, Zhao L, Brown J N, Morris A, Masliah E, McGrath M S. Extensive HIV-1 intra-host recombination is common in tissues with abnormal histopathology. PLoS One. 2009;4:e5065. doi: 10.1371/journal.pone.0005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier C, Nora T, Tenaillon O, Clavel F, Hance A J. Extensive recombination among human immunodeficiency virus type 1 quasispecies makes an important contribution to viral diversity in individual patients. J Virol. 2006;80:2472–2482. doi: 10.1128/JVI.80.5.2472-2482.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenger E, Abbey N W, Weinstein M D, Kapp L, Reis J, Gofman I, Millward C, Gascon R, Elbaggari A, Herndier B G, McGrath M S. Injection of human primary effusion lymphoma cells or associated macrophages into severe combined immunodeficient mice causes murine lymphomas. Cancer Res. 2002;62:5536–5542. [PubMed] [Google Scholar]
- Habis A, Baskin G B, Murphey-Corb M, Levy L S. Simian AIDS-associated lymphoma in rhesus and cynomolgus monkeys recapitulates the primary pathobiological features of AIDS-associated non-Hodgkin’s lymphoma. AIDS Res Hum Retroviruses. 1999;15:1389–1398. doi: 10.1089/088922299310098. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Herndier B G, McGrath M S. Identification of a common clonal human immunodeficiency virus integration site in human immunodeficiency virus-associated lymphomas. Cancer Res. 1994;54:2069–2072. [PubMed] [Google Scholar]
- Mack K D, Jin X, Yu S, Wei R, Kapp L, Green C, Herndier B, Abbey N W, Elbaggari A, Liu Y, McGrath M S. HIV insertions within and proximal to host cell genes are a common finding in tissues containing high levels of HIV DNA and macrophage-associated p24 antigen expression. J Acquir Immune Defic Syndr. 2003;33:308–320. doi: 10.1097/00126334-200307010-00004. [DOI] [PubMed] [Google Scholar]
- Shibata D, Weiss L M, Hernandez A M, Nathwani B N, Bernstein L, Levine A M. Epstein-Barr virus-associated non-Hodgkin’s lymphoma in patients infected with the human immunodeficiency virus. Blood. 1993;81:2102–2109. [PubMed] [Google Scholar]
- Kilger E, Kieser A, Baumann M, Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadburn A, Chiu A, Lee J Y, Chen X, Hyjek E, Banham A H, Noy A, Kaplan L D, Sparano J A, Bhatia K, Cesarman E. Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma and clinical implications in patients from AIDS malignancies consortium clinical trials 010 and 034. J Clin Oncol. 2009;27:5039–5048. doi: 10.1200/JCO.2008.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Cesarman E, Boshoff C. KSHV G protein-coupled receptor inhibits lytic gene transcription in primary-effusion lymphoma cells via p21-mediated inhibition of Cdk2. Blood. 2006;107:277–284. doi: 10.1182/blood-2005-06-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Vaccher E, Cerri M, Gaidano G, Dalla-Favera R, Tirelli U. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: a tissue-based variant of primary effusion lymphoma. J Mol Diagn. 2005;7:17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epeldegui M, Widney D P, Martinez-Maza O. Pathogenesis of AIDS lymphoma: role of oncogenic viruses and B cell activation-associated molecular lesions. Curr Opin Oncol. 2006;18:444–448. doi: 10.1097/01.cco.0000239882.23839.e5. [DOI] [PubMed] [Google Scholar]
- Salemi M, Lamers S L, Huysentruyt L C, Galligan D C, Gray R R, Morris A, McGrath M S. Distinct patterns of HIV-1 evolution within metastatic tissues in patients with non-Hodgkin lymphoma. PLoS One. 2009 doi: 10.1371/journal.pone.0008153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone N, Hullsiek K H, Marconi V, Weintrob A, Ganesan A, Barthel R V, Fraser S, Agan B K, Wegner S. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Tosato G. Neoplastic conditions in the context of HIV-1 infection. Curr HIV Res. 2004;2:343–349. doi: 10.2174/1570162043351002. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- Swingler S, Mann A, Jacque J, Brichacek B, Sasseville V G, Williams K, Lackner A A, Janoff E N, Wang R, Fisher D, Stevenson M. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:997–1103. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro L A, Burdick M, Low Q E, Kunkel S L, Strieter R M. MIP-1α as a critical macrophage chemoattractant in murine wound repair. J Clin Invest. 1998;101:1693–1698. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- Kolson D L, Gonzalez-Scarano F. HIV and HIV dementia. J Clin Invest. 2000;106:11–13. doi: 10.1172/JCI10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K C, Hickey W F. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden G A, Lipton S A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Maslin C L, Kedzierska K, Webster N L, Muller W A, Crowe S M. Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr HIV Res. 2005;3:303–317. doi: 10.2174/157016205774370401. [DOI] [PubMed] [Google Scholar]
- Pomerantz R J. Reservoirs, sanctuaries, and residual disease: the hiding spots of HIV-1. HIV Clin Trials. 2003;4:137–143. doi: 10.1310/80jh-148k-nadq-u927. [DOI] [PubMed] [Google Scholar]
- Gisolf E H, Enting R H, Jurriaans S, de Wolf F, van der Ende M E, Hoetelmans R M, Portegies P, Danner S A. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS. 2000;14:1583–1589. doi: 10.1097/00002030-200007280-00014. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton S A. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]