Abstract
It is broadly accepted that HIV DNA in lymphoid and myeloid cells persists despite combination antiretroviral therapy. Recognized as the Achilles heel to HIV eradication, the role of these peripheral reservoirs in HIV morbidity is less well developed. The burden of HIV DNA in peripheral mononuclear cells is linked to HIV disease outcomes such as time to AIDS diagnosis, survival, and CD4 T-lymphocyte counts. Monocytes are a minor HIV DNA reservoir, and the burden of HIV DNA in these cells appears to be linked to dementia, suggesting that residual infection in this subset is linked to tissue-related HIV complications. Since monocytes are likely involved in trafficking virus to the brain, there is a strong mechanistic link underlying this discovery. Herein, we summarize our current understanding of monocyte HIV DNA and central nervous system dysfunction in humans. We present a model to understand these relationships and suggest possible treatment approaches to be tested.
Keywords: AIDS dementia complex, antiretroviral therapy, lipodystrophy
HIV-associated dementia (HAD), the most severe form of HIV-related cognitive dysfunction, is characterized by abnormalities in motor skills (slowed movements, abnormal gait, hypertonia), behavior (apathy, irritability, emotional lability), and cognitive function (attention, concentration, memory, information processing, language) [1]. Prior to widespread use of highly active antiretroviral therapy (HAART), the prevalence of HAD was 20–30% among patients with advanced HIV and low CD4 lymphocyte counts. The widespread availability of HAART led to a marked decline in the reported incidence of HAD to 10/1000 person-years in 1996–1998 [2].
The characteristics of HIV-related cognitive dysfunction have also changed. Cognitive complications are noted with higher CD4 lymphocyte counts and milder degrees of cognitive impairment are more typical [3]. Paradoxically, while HAD incidence has decreased, prevalence may be rising as individuals on HAART live longer. The HAART-era prevalence of HAD or a milder variant referred to as mild neurocognitive disorder (MND) may be as high as 37% [2, 4, 5]. Fluctuation in disease course with a waxing and waning pattern has now been described [3, 6]. Taken together, these findings highlight the failure of HAART to universally eradicate cognitive impairment. The identification of mechanistic underpinnings has been disappointing, leading some to believe that much of the impairment is due to comorbid illnesses or pre-HAART (permanent) brain injury.
THE NEUROIMMUNOLOGY OF HIV
Human immunodeficiency virus (HIV) encephalitis is the classically described substrate of HIV brain injury, characterized by gliosis, microglial nodules, perivascular macrophage accumulation, and the presence of multinucleated giant cells [7]. These findings are associated with immune activation and inflammation seemingly out of proportion to the amount of HIV virus present in the brain [8,9,10]. Although viral particles, such as nef, gp120, and tat, are neurotoxic in vitro, the mechanisms of brain injury likely involve a significant contribution from indirect immunological processes, including a prominent role for inflammatory pathways mediated by cells of the monocyte/macrophage lineage [10,11,12,13,14].
On the basis of evidence from animal models and autopsy data, the clinical onset of neurological disease and its acceleration as immune function fails, directly relate to the dysregulation and accumulation in the central nervous system (CNS) of activated perivascular macrophages, some of which are infected [8]. The number of inflammatory macrophages rather than the concentration of virus is the best indicator of neural damage and cognitive deterioration in simian immunodeficiency virus (SIV)-encephalitis, and the majority of the SIV in the CNS is within these perivascular macrophages and not in parenchymal microglia [8, 9, 15, 16]. Recent studies suggest that the accumulation of perivascular macrophages in late-stage disease is due to increased trafficking of peripheral monocytes into the CNS [8, 16,17,18]. A unifying hypothesis is proposed that HIV encephalitis following virally induced failure of the immune system is primarily a disease resulting from blood-borne activated macrophages capable of stimulating inflammatory responses in the CNS.
The phenotype of the perivascular macrophage is similar to that of a minor monocyte population found in the peripheral blood defined by the coexpression of CD14 and CD16 and/or CD69 [19]. Populations of CD14+/CD16+ and CD14+/CD69+ monocytes are expanded in HIV-infected patients [20] and correlate to HAD [21, 22]. On the basis of these and other such data, it is hypothesized that critical events initiating the development of dementia occur outside of the brain [15]. This concept is further supported by the finding that sequences of the HIV viral gp160 gene, which encode the highly variable HIV envelope protein, taken from the deep white matter of the brain in an individual with HAD were more closely related to sequences from the bone marrow and to sequences from blood monocytes taken 5 mo earlier than to those from other tissues [15].
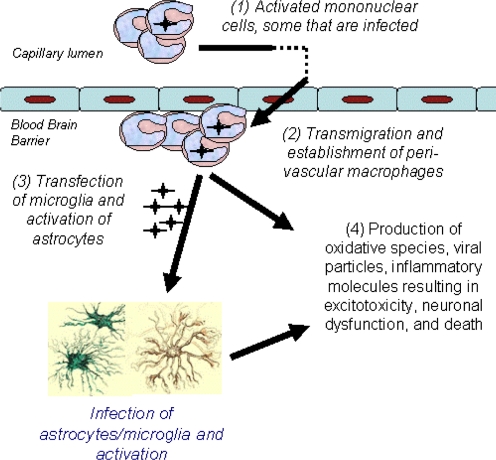
These findings are consistent with leading theories of encephalitis development in HIV [10, 15]. Here, peripherally infected monocytes, upon meeting the brain microvascular endothelium, are able to self-initiate transmigration to the brain because of their activated state. These cells then become perivascular macrophages and may also transfect other resident cells (astrocytes, microglia). The result is a proinflammatory environment characterized by cellular activation, heightened secretion of cytokines and chemokines, and increased oxidative stress ultimately contributing to neuronal dysfunction and HAD (Fig. 1).
Figure 1.
A model of brain injury in HIV. Activated monocytes transmigrate the blood-brain barrier resulting in inflammation, oxidative stress, and neuronal injury.
PERIPHERAL RESERVOIRS OF HIV DNA BEFORE AND AFTER HAART
The retrovirus, HIV, enters human immune cells through CD4 and the coreceptors CCR5 or CXCR4, and through reverse transcription, incorporates into host DNA [23]. Subsequent transcription results in rapid and extensive HIV RNA production, cell lysis, and the prototypical exponential rise of HIV RNA in the bloodstream. Plasma viremia becomes life-long in the absence of treatment and immunodeficiency ensues in the vast majority of individuals. Because of the archival nature of the immune system, a minor subset of activated and infected CD4+ T-lymphocytes may become memory T-cells that are quiescent with little to no production of virus unless stimulated [24, 25]. It is postulated that monocytes are also a source of residual HIV DNA, likely arising from quiescent and infected cells of the myeloid precursor lineage in bone marrow [26,27,28,29,30,31]. HIV DNA within monocytes may exist integrated into the host genomic DNA or as nonintegrated circular forms and both resistant and wild-type viruses have been demonstrated [32, 33]. Among individuals on HAART, the CD16+ subset of monocytes appears more susceptible than other monocytes to HIV infection and preferentially harbor HIV DNA [34, 35]. CD16+ monocytes produce high levels of chemokines, increasing the susceptibility of resting T cells to HIV infection [36]. Macrophages are generally resistant to the cytopathic effects of the virus and may persist in the tissues for a long period of time despite suppression of plasma HIV RNA [37]. In one study, levels of HIV DNA in purified monocyte-depleted peripheral mononuclear cells were identified in all cases both prior to and 24 mo following effective HAART [38]. In contrast, HIV DNA was detected in monocytes from all naïve cases and 12/34 (35%) of treated cases.
HIV DNA AND DISEASE
The level of HIV DNA in PBMCs predicts HIV events, including rate of decline in CD4 lymphocyte count, time to AIDS diagnosis, and dementia. Early reports identified a relationship to poor antiretroviral response [39] and, more recently, to virological failure [40]. HIV DNA predicts progression to AIDS in the SEROCO cohort, independently of plasma HIV RNA and CD4 counts [41] and disease progression in the PRIMO Cohort [42]. In one study, among HAART-naïve patients, PBMC HIV DNA but not monocyte HIV DNA inversely correlated to CD4 count and PBMC HIV DNA correlated to high (>30,000 copies) compared with low HIV RNA [38]. This finding suggests that HIV DNA within lymphocytes, rather than within monocytes, may have more relevance to primary nontissue-related HIV disease variables such as CD4 lymphocyte reduction.
In the setting of chronic HIV infection, PBMC HIV DNA has been identified as a correlate to HAD. In a cross-sectional study done in our laboratory, the median HIV DNA level among individuals with HAD were roughly 20 times higher than the median level among individuals with normal cognition [43]. This association remained significant among those individuals with undetectable plasma HIV RNA. PBMC HIV DNA is also higher in individuals with milder cognitive impairment although not to the extent seen in individuals with HAD [44]. Further analyses by cellular subsets revealed that the difference in HIV DNA by cognitive category is related specifically to the amount of HIV DNA within the activated monocytes (CD14+/CD16+) subset rather than in nonactivated monocytes (CD14+/CD16neg) or in CD14neg cells, which includes the lymphocyte pool (Fig. 2) [45].
Figure 2.
HIV DNA content in various monocyte blood cell subsets in individuals with HAD vs. normal cognition (NC). (A) HIV DNA is higher in individuals with HIV-associated dementia (HAD) (left) compared with individuals with NC in the CD14+CD16+ subset. (B and C) In CD14+/CD16– (B) and CD14– (C) subsets, the amount of HIV DNA does not differ between individuals with HAD (left) and individuals with NC.
Levels of monocyte HIV DNA in individuals with HAD remain persistently high over time when compared with individuals with normal cognition. In a cohort of HIV-infected individuals in Thailand naïve to HAART, baseline and 48-wk after HAART treatment, monocyte HIV DNA levels strongly correlated to concurrent cognitive performance irrespective of plasma HIV RNA and CD4 lymphocyte counts [46]. At 48 wk, monocyte HIV DNA was below the level of detection of our assay (10 copies/106 cells) in 15/15 non-HAD compared with only 4/12 HAD cases, despite undetectable plasma HIV RNA in 26/27 cases (Fig. 3).
Figure 3.
HIV DNA in circulating monocytes in a naïve cohort and 6 and 12 mo following highly active antiretroviral therapy. HIV DNA remains elevated in dementia but not nondementia cases.
These findings have important implications for HAART-era cognitive impairment. Although published data indicate that mild cognitive impairment remains prevalent in patients successfully treated with HAART, few investigators have identified HIV-specific mechanistic links or markers, leading some to hypothesize that the cognitive findings represent inactive disease. However, HIV DNA in circulating monocytes remains elevated in some individuals despite HAART, and the inability to clear this peripheral reservoir is more frequent among individuals with dementia, suggesting that the HAART-era cognitive impairment may, at least in part, be due to ongoing injury. Given that monocytes likely traffic to the CNS resulting in inflammation and viral seeding, it is plausible that HIV infection of these monocytes may contribute to CNS injury, even in the era of HAART.
MONOCYTE HIV DNA AND OTHER CHRONIC COMPLICATIONS OF HIV
The role of monocyte HIV DNA in other HIV-associated complications is less clear. Given the highly proinflammatory nature of HIV-infected activated monocytes and the presence of macrophages in various tissues, it is reasonable to postulate a role of HIV DNA in association with other chronic complications of HIV. High levels of HIV DNA have been hypothesized to play a potential role in unintentional weight loss, as well as in lipoatrophy [47, 48]. High levels of macrophages are found within subcutaneous fat tissue of HIV-infected patients with HIV-associated lipoatrophy [49]. Moreover, high levels in fat tissue of proinflammatory cytokines (TNF, IL-6, IL-8, IL-12, IL-18) are found in adipose tissue that correlate significantly with adipose tissue macrophage content [49], suggesting that the inflammatory cytokines originated from these macrophages. High rates of metabolic dysregulation, including insulin resistance, characterize HIV-infected individuals on HAART [50]. In the general population, monocyte/macrophage and their proinflammatory cytokines have been demonstrated to play significant pathogenic roles in obesity and in insulin resistance-related disease processes, as well as in vascular activation and inflammation [51,52,53]. Thus, it is likely that monocyte/macrophage-mediated immune activation and inflammation increase risk for such complications in the HIV-infected population.
The transmigration of monocytes into the arterial vessel wall initiates the development of atherosclerosis. Such transmigration is enhanced by the inflammation associated with HIV infection, much of which may originate from monocyte/macrophages [54]. Once in the arterial vessel wall, monocytes are transformed into lipid-laden macrophages or “foam cells,” which form the lipid-rich core of atheromatous plaques. HIV-infected macrophages may have a role in the development of foam cells since HIV impairs ATP binding cassette transporter A1 (ABCA1)-dependent cholesterol efflux from macrophages [55]. This condition is likely to be highly atherogenic as the resulting deficit in reverse cholesterol transport can be expected to greatly enhance the accumulation of cholesterol within these cells and increase the development of foam cells. The cholesterol efflux impairment in HIV-infected macrophages and the known persistence of these long-lived HIV-infected monocyte/macrophage pools despite HAART could partially explain the increase in cardiovascular disease seen in the era of HAART [56].
POTENTIAL APPROACHES TO MODULATING THE MAGNITUDE OF HIV DNA IN PERIPHERAL RESERVOIRS
No validated therapeutic approaches to eradication of the peripheral HIV DNA reservoir exist. Control of HIV viremia may diminish this peripheral intracellular reservoir over time [57,58,59,60] and eradication may be potentially modifiable with early and aggressive treatment with HAART [61,62,63]. Recently, a group from France noted that the mean HIV DNA burden is substantially lower in individuals treated during primary infection compared with those initiating treatment during the chronic phase of disease [64]. It is not known, however, if both lymphocyte and monocyte HIV reservoirs are equally affected. Since it is increasingly recognized that the earliest events in HIV infection may set the stage for disease course, it is intriguing to hypothesize that early and intensive treatment may limit the magnitude of the monocyte reservoir.
Although necessarily speculative, other treatment approaches may exist within the confines of currently available therapies. Antiretroviral choice that may be considered “monocyte-directed” deserve evaluation. CCR5 antagonists may have some enhanced efficacy as part of ‘monocyte-directed therapy.’ Both CCR5 and CXCR4 may serve as chemokine receptors for entry of HIV into lymphocytes while CCR5 is the major co-receptor used for HIV entry into macrophages and into brain microglia cells [65]. To date, clinical studies have not demonstrated the efficacy of this “monocyte directed” approach.
Some have noted that NRTIs are more effective in eradicating virus in monocytes/macrophages than in CD4+ cells, owing to the quiescent nature of monocytes/macrophages and a low endogenous nucleotide pool, enabling the triphosphate forms of the drugs to more successfully compete against the endogenous pool for binding to the HIV reverse transcriptase [66]. Of the newer nucleoside/nucleotide reverse transcriptase inhibitors, tenofovir has potent anti-HIV activity in monocytes, greater than that observed in CD4 lymphocytes [66]. This has been attributed to its monophosphorylated structure, reducing the requirement for intracellular phosphorylation to its active triphosphate form. Consequently, tenofovir has been proposed for post-exposure prophylaxis, due to its enhanced anti-HIV effects in tissue-laden monocyte-derived cells (Langerhan cells). It should be noted that tenofovir does not effectively cross the blood-brain barrier, a factor that may raise some concern with the use of tenofovir in individuals with HIV-associated cognitive dysfunction [67]. However, it has also been postulated that the efficacy of NRTIs in the CNS may be greater than suggested by BBB dynamics and CSF concentrations because of the previously mentioned enhanced intracellular activity in monocytes/macrophages and the critical role of these cells in the CNS [66]. In vitro studies suggest that protease inhibitors (PI), in general, have decreased potency against monocytes [66]. PI concentrations needed to inhibit monocyte HIV RNA production exceed that commonly seen in HIV treatment, although ritonavir boosting appears to overcome much of this issue [66].
Other “monocyte directed” therapeutic approaches are under investigation, including polyamine biosynthesis inhibitors, a class of drugs shown in vitro to selectively kill activated monocytes from patients with HIV dementia and advanced HIV disease [68]. Facilitation of antiretroviral medication carriage across the blood-brain barrier by use of medication containing nanoparticles loaded into bone marrow macrophages has successfully been explored in an HIV encephalitis rodent model [69, 70].
Unlike T-lymphocytes, HIV infected macrophages do not undergo cell death, even when exposed to toxic conditions, thus serving as long-living viral reservoirs in various tissues [66]. Studies of molecular and cellular mechanisms involved in this cytoprotective effect in primary human macrophages indicate that the P13K/Akt pathway is a key contributor to this effect. P13K/Akt inhibitors were recently shown in a cell model to reverse key cellular events typically observed in cell survival activation and result in reduced HIV production [71]. Finally, murine studies have been performed using erythrocytes loaded with a new heterodinucleotide (3TCpPMPA) comprising the drugs lamivudine and tenofovir and modified to increase their phagocytosis by macrophages in an effort to more effectively deliver these antiretroviral medications into macrophages [72].
SUMMARY
Detectable levels of HIV DNA in circulating monocytes are seen in a subset of individuals on HAART. The inability of potent antiretroviral therapy to successfully eradicate HIV from this peripheral reservoir is associated with dementia and may, at least in part, account for the continued prevalence of neurocognitive impairment in HIV-infected individuals in the HAART era. Recent studies have led to be a better understanding of the unique characteristics and importance of this cellular reservoir in the pathogenesis of HIV dementia. Because effective eradication of this reservoir is unlikely to occur with use of antiretroviral therapy as currently practiced, continued research into novel therapeutic approaches to eradication of this cellular reservoir is desperately needed.
ACKNOWLEDGMENTS
The work presented was supported, in part, by National Institutes of Health awards U54NS43049 (C. S.), R21MH072388 (V. V.), K23AG032872 (V. V.), R01NS061696 (V. V.), R01NS053345 (B. T. S.), P20RR011091 (J. Hedges, Univ. of Hawaii), 3P50AG023501 (B. Miller, UCSF), and U19MH081835 (M. McGrath, UCSF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
Dr. Valcour is a consultant for GlaxoSmithKline, Abbott, and Merck; Dr. Shikuma receives HIV research and training funding support from Merck, Gilead, and Pfizer, and is a consultant and on the speakers bureau for GlaxoSmithKine.
Footnotes
Abbreviations: ABCH1=ATP binding casette transporter A1, HAART=highly active antiretroviral therapy, HAD=HIV-associated dementia, MND=mild neurocongintive disorder
References
- McArthur J C, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–150. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott M P, Marder K, Schifitto G, Selnes O A, McArthur J C, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida J A, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- McArthur J C, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Robertson K R, Smurzynski M, Parsons T D, Wu K, Bosch R J, Wu J, McArthur J C, Collier A C, Evans S R, Ellis R J. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Lorenzini P. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol. 2005;11:265–273. doi: 10.1080/13550280590952790. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker J T, Brew B J, Byrd D A, Cherner M, Clifford D B, Cinque P, Epstein L G, Goodkin K, Gisslen M, Grant I, Heaton R K, Joseph J, Marder K, Marra C M, McArthur J C, Nunn M, Price R W, Pulliam L, Robertson K R, Sacktor N, Valcour V, Wojna V E. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H. The neuropathology of HIV-associated brain injury. Gendelman I, HG, Everall I, Lipton S, Swindells S, editors. New York: Oxford University; The Neurology of AIDS. (2nd ed) 2005 [Google Scholar]
- Williams K C, Hickey W F. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Glass J D, Fedor H, Wesselingh S L, McArthur J C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Anderson E, Zink W, Xiong H, Gendelman H E. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31:S43–S54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- Lawrence D M, Major E O. HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 2002;4:301–308. doi: 10.1016/s1286-4579(02)01542-3. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden G A, Lipton S A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kanmogne G D, Schall K, Leibhart J, Knipe B, Gendelman H E, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk A E, Capini C, L'Heureux D, Régulier E G, Richardson M W, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Williams K C, Corey S, Westmoreland S V, Pauley D, Knight H, deBakker C, Alvarez X, Lackner A A. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D T, Woodman S E, Weiss, J M. Mechanisms of leukocyte trafficking into the CNS. J Neurovirol. 2000;6:S82–S85. [PubMed] [Google Scholar]
- Passlick B, Flieger D, Ziegler-Heitbrock H W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi H M, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Clarke J A, McGrath M S, Moore D, McGuire D. Monokine products as predictors of AIDS dementia. AIDS. 1996;10:1495–1500. doi: 10.1097/00002030-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath M S. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano R F. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10:525–531. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio F A, Yassine-Diab B, Boucher G, Boulassel M R, Ghattas G, Brenchley J M, Schacker T W, Hill B J, Douek D C, Routy J P, Haddad E K, Sékaly R P. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath M J, Pruett J E, Cohn Z A. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci USA. 1989;86:675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich E A, Chen I S, Zack J A, Leonard M L, O'Brien W A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser B, Burger H, Campbell P, Donelan S, Mladenovic J. HIV type 1 RNA expression in bone marrows of patients with a spectrum of disease. AIDS Res Hum Retroviruses. 1996;12:1551–1558. doi: 10.1089/aid.1996.12.1551. [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Zhu T, Muthui D, Holte S. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahner I, Kearns K, Coutinho S, Leonard E H, Kohn D B. Infection of human marrow stroma by human immunodeficiency virus-1 (HIV-1) is both required and sufficient for HIV-1-induced hematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood. 1997;90:1787–1798. [PubMed] [Google Scholar]
- Re M C, Bon I, Monari P, Gorini R, Schiavone P, Gibellini D, La Placa M. Drug failure during HIV-1 treatment. New perspectives in monitoring drug resistance. New Microbiol. 2003;26:405–413. [PubMed] [Google Scholar]
- Marcello A. Latency: the hidden HIV-1 challenge. Retrovirology. 2006;3:7. doi: 10.1186/1742-4690-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A, Ellery P, Maslin C L, Naim E, Heinlein A C, Ryan C E, Paukovics G, Hocking J, Sonza S, Crowe S M. Normal CD16 expression and phagocytosis of Mycobacterium avium complex by monocytes from a current cohort of HIV-1-infected patients. J Infect Dis. 2006;193:693–697. doi: 10.1086/500367. [DOI] [PubMed] [Google Scholar]
- Ellery P J, Tippett E, Chiu Y L, Paukovics G, Cameron P U, Solomon P, Lewin S R, Gorry P R, Jaworowski A, Greene W C, Sonza S, Crowe S M. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–1164. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- Gibellini D, Borderi M, De Crignis E, Cicola R, Cimatti L, Vitone F, Chiodo F, Re M C. HIV-1 DNA load analysis in peripheral blood lymphocytes and monocytes from naive and HAART-treated individuals. J Infect. 2008;56:219–225. doi: 10.1016/j.jinf.2008.01.001. [DOI] [PubMed] [Google Scholar]
- McDermott J L, Giri A A, Martini I, Bono M, Giacomini M, Campelli A, Tagliaferro L, Cara A, Varnier O E. Level of human immunodeficiency virus DNA in peripheral blood mononuclear cells correlates with efficacy of antiretroviral therapy. J Clin Microbiol. 1999;37:2361–2365. doi: 10.1128/jcm.37.7.2361-2365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin I, Caumont A, Garrigue I, Merel P, Schrive M H, Fleury H, Dupon M, Pellegrin J L, Ragnaud J M. Predictive value of provirus load and DNA human immunodeficiency virus genotype for successful abacavir-based simplified therapy. J Infect Dis. 2003;187:38–46. doi: 10.1086/345860. [DOI] [PubMed] [Google Scholar]
- Rouzioux C, Hubert J B, Burgard M, Deveau C, Goujard C, Bary M, Séréni D, Viard J P, Delfraissy J F, Meyer L, SEROCO Cohort Study Group Early levels of HIV-1 DNA in peripheral blood mononuclear cells are predictive of disease progression independently of HIV-1 RNA levels and CD4+ T cell counts. J Infect Dis. 2005;192:46–55. doi: 10.1086/430610. [DOI] [PubMed] [Google Scholar]
- Goujard C, Bonarek M, Meyer L, Bonnet F, Chaix M L, Deveau C, Sinet M, Galimand J, Delfraissy J F, Venet A, Rouzioux C, Morlat P, Agence Nationale de Recherche sur le Sida PRIMO Study Group CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis. 2006;42:709–715. doi: 10.1086/500213. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Paul R, Williams A, Shikuma C, Watters M, Grove J, Valcour V. HIV proviral DNA associated with decreased neuropsychological function. J Neuropsychiatry Clin Neurosci. 2007;19:157–163. doi: 10.1176/appi.neuropsych.19.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Shikuma C, Ratto-Kim S, Valcour V. Alexandria, VA, USA: Foundation for Retrovirology and Human Health; HIV DNA in PBMC and monocytes associated with dementia (Abstract). 14th Conference on Retroviruses and Opportunistic Infections. Los Angeles, California. 2007 [Google Scholar]
- Valcour V G, Shiramizu B T, Sithinamsuwan P. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 Cohort Study. Neurology. 2009;72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma C M, Valcour V G, Ratto-Kim S. HIV-associated wasting in the era of highly active antiretroviral therapy: a syndrome of residual HIV infection in monocytes and macrophages? Clin Infect Dis. 2005;40:1846–1848. doi: 10.1086/430376. [DOI] [PubMed] [Google Scholar]
- Shikuma C, McGrath M, Shagrun L, Chow C, Gerschenson M. HIV-infected monocytes and macrophages in adipose tissue contribute to the development of lipoatrophy (Abstract) Antiviral Therapy. 2005;10:1. [Google Scholar]
- Hammond E, James I, McKinnon E, Pace C, Metcalf C, Mallal S. Assessing the contribution of ART, HIV and host factors to adipose tissue changes occurring in HIV-infected individuals: risk profile for lipoatrophy (Abstract) Antiviral Therapy. 2005;10:2. [Google Scholar]
- Schambelan M, Benson C A, Carr A, Currier J S, Dubé M P, Gerber J G, Grinspoon S K, Grunfeld C, Kotler D P, Mulligan K, Powderly W G, Saag M S, International AIDS Society-USA Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr. 2002;31:257–275. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes G T, Yang Q, Chou C J, Sole J, Nichols A, Ross J S, Tartaglia L A, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S P, McCann D, Desai M, Rosenbaum M, Leibel R L, Ferrante A W., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- Crowe S M, Westhorpe C L, Mukhamedova N, Jaworowski A, Sviridov D, Bukrinsky M. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010 doi: 10.1189/jlb.0809580. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujawar Z, Rose H, Morrow M P, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein J M, Bobryshev Y V, Bukrinsky M, Sviridov D. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4:e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M, Sviridov D. HIV and cardiovascular disease: contribution of HIV-infected macrophages to development of atherosclerosis. PLoS Med. 2007;4:e43. doi: 10.1371/journal.pmed.0040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitone F, Gibellini D, Schiavone P, Re M C. Quantitative DNA proviral detection in HIV-1 patients treated with antiretroviral therapy. J Clin Virol. 2005;33:194–200. doi: 10.1016/j.jcv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Khiri H, Halfon P. Impact of immune interventions on proviral HIV-1 DNA decay in patients receiving highly active antiretroviral therapy. HIV Med. 2001;2:189–194. doi: 10.1046/j.1468-1293.2001.00065.x. [DOI] [PubMed] [Google Scholar]
- Ngo-Giang-Huong N, Deveau C, Da Silva I, Pellegrin I, Venet A, Harzic M, Sinet M, Delfraissy J F, Meyer L, Goujard C, Rouzioux C, French PRIMO Cohort Study Group Proviral HIV-1 DNA in subjects followed since primary HIV-1 infection who suppress plasma viral load after one year of highly active antiretroviral therapy. AIDS. 2001;15:665–673. doi: 10.1097/00002030-200104130-00001. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re M C, Vitone F, Bon I, Schiavone P, Gibellini D. Meaning of DNA detection during the follow-up of HIV-1 infected patients: a brief review. New Microbiol. 2006;29:81–88. [PubMed] [Google Scholar]
- Hocquloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Mélard A, Viard J-P, Le Moal G, Rouzioux C. Better HIV DNA depletion and CD4 restoration with HAART initiated at the time of primary HIV infection than with HAART started during chronic HIV infection. Abstract #515. 16th Conference of Retroviruses and Opportunistic Infections. Montreal, CA, CROI, Alexandria, VA [Google Scholar]
- Albright A V, Shieh J T, Itoh T, Lee B, Pleasure D, O'Connor M J, Doms R W, González-Scaranoet F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquaro S, Svicher V, Schols D, Pollicita M, Antinori A, Balzarini J, Federico Perno C. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006;80:1103–1110. doi: 10.1189/jlb.0606376. [DOI] [PubMed] [Google Scholar]
- Anthonypillai C, Gibbs J E, Thomas S A. The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 2006;3:1. doi: 10.1186/1743-8454-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M H K, Kim W, Williams K. Develoment of macrophage targeted therapy for treatment of HIV associated neurological disease: results of two SIV associated encephalitis clinical studies with PA001. Sydney, Australia: 2007 [Google Scholar]
- Dou H, Kingsley J D, Mosley R L, Gelbard H A, Gendelman H E. Neuroprotective strategies for HIV-1 associated dementia. Neurotox Res. 2004;6:503–521. doi: 10.1007/BF03033447. [DOI] [PubMed] [Google Scholar]
- Dou H, Grotepas C B, McMillan J M, Destache CJ, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman H E. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of NeuroAIDS. J Immunol. 2009;183:661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh P, Bradel-Tretheway B, Monteiro-Filho C M, Planelles V, Maggirwar S B, Dewhurst S, Kim B. Akt inhibitors as an HIV-1 infected macrophage-specific anti-viral therapy. Retrovirology. 2008;5:11. doi: 10.1186/1742-4690-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Franchetti P, Pierige F, Serafini S, Magnani M, Balestra E, Perno C F. Inhibition of HIV-1 replication in macrophages by a heterodinucleotide of lamivudine and tenofovir. J Antimicrob Chemother. 2007;59:666–675. doi: 10.1093/jac/dkm011. [DOI] [PubMed] [Google Scholar]