Abstract
The progesterone metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP, allopregnanolone), acts in the ventral tegmental area (VTA) to facilitate exploratory, anti-anxiety, and socio-sexual behavior among ovariectomized (OVX), estrogen (E2)-primed rats and gonadally-intact rats with high (proestrus) or low (diestrus) endogenous E2 levels. The extent to which E2 is required for these effects of 3α,5α-THP is not known. OVX rats were primed with systemic 17β-estradiol (10 µg) or oil vehicle and were infused 44 h later with 3α,5α-THP (100 ng) or β-cyclodextrin vehicle to the VTA, substantia nigra (SN), or central grey (CG). Rats were assessed in a battery of exploratory (open field), anxiety (elevated plus maze), social (partner preference, social interaction), and sexual (paced mating) tasks. E2-priming was necessary for 3α,5α-THP infusions to facilitate social interaction and mating and midbrain 3α,5α-THP levels were higher among E2-compared to vehicle-primed rats. Irrespective of E2-priming, rats infused with 3α,5α-THP to the VTA, but not SN or CG, demonstrated increased exploration in an open field, anti-anxiety behavior on an elevated plus maze, and preference for a male. Thus, actions of 3α,5α-THP in the VTA to enhance social and sexual behaviors were reliant on E2 but increases in exploratory and anti-anxiety behavior were not.
Keywords: Allopregnanolone, GABA, Lordosis, Non-genomic, Progesterone
1. Introduction
Progesterone (P4) has requisite, but divergent, actions in the ventromedial hypothalamus (VMH) and midbrain ventral tegmental area (VTA) to mediate lordosis of 17β-estradiol (E2)-primed rodents (Feder, 1984). In the VMH, P4 initiates lordosis of E2-primed rats via actions at intracellular progestin receptors (PRs; Rubin and Barfield, 1980,1983,1984). However, in the VTA, P4 modulates the duration and intensity of lordosis independent of the few PRs localized there (Frye and Gardiner, 1996; Frye et al., 2000a,b; Lonstein and Blaustein, 2004; Luttge and Hughes, 1976; Pleim and DeBold, 1984; Pleim et al., 1991; Ross et al., 1971; Yanase and Gorski, 1976). In the VTA, it is through actions of the P4 metabolite and neurosteroid, 5α-pregnan-3α-ol-20-one (3α,5α-THP; also known as allopregnanolone), at GABAA, NMDA, D1 receptors, and subsequent downstream signal transduction processes that the quality of lordosis can be mediated (Frye, 2001; Frye et al., 2006, 2004a,b; Frye and Vongher, 1999b; Melcangi and Panzica, 2006). In addition to lordosis, actions of 3α,5α-THP in the VTA influence a suite of behaviors that may be important for successful reproduction.
3α,5α-THP can also exert modulatory effects on other motivated behaviors and anxiety behavior, in part, through actions in the hippocampus. Rats in behavioral estrus have levels of 3α,5α-THP in the hippocampus that are sufficient to produce agonist-like actions at GABAA receptors and demonstrate increased anti-anxiety behavior compared to that of diestrous rats, with lower levels of 3α,5α-THP (Frye et al., 2000a,b; Mora et al., 1996; Vinogradova, 1999). Removal of the primary source of endogenous hormones, the ovaries, increases anxiety behavior and replacement with systemic, intra-hippocampal, or intra-amygdala administration of P4 or 3α,5α-THP reverses this effect (Akwa et al., 1999; Frye et al., 2004a,b; Galeeva and Tuohimaa, 2001; Laconi et al., 2001). Further, blocking P4's metabolism to 3α,5α-THP or enhancing 3α,5α-THP biosynthesis in the hippocampus, respectively increases and decreases anxiety behaviors (Bitran et al., 2000; Rhodes and Frye, 2001). Thus, respective actions of 3α,5α-THP in the VTA and hippocampus modulate lordosis and anxiety behavior (Frye et al., 2006, 2000a,b; Bitran et al., 2000).
These separate lines of research begin to converge with 3α,5α-THP influencing the expression of behaviors associated with the appetitive and consummatory aspects of mating. For instance, naturally-receptive female rats have higher endogenous levels of 3α,5α-THP in brain and circulation (Frye et al., 1998a; Frye and Bayon, 1999) and demonstrate more appetitive (exploration, anti-anxiety, social behaviors) and consummatory (lordosis incidence and intensity) behavior than do their non-receptive counterparts (Frye and Rhodes, 2006a). Notably, infusions of 3α,5α-THP to the VTA of E2-primed, ovariectomized (OVX) rats results in facilitation of these appetitive and consummatory behaviors to levels which are akin to that of naturally-receptive rats (Frye and Rhodes, 2006b). We have recently demonstrated that 3α,5α-THP infusions to the VTA of diestrous rats facilitate appetitive and consummatory behavior to levels that are commensurate with naturally-receptive or OVX, E2-primed rats with 3α,5α-THP infusions to the VTA (Frye and Rhodes, 2008). Steroids' effects can be mediated via peripheral- and/or centrally-derived hormone formation (Melcangi and Panzica, 2006). As such, whether 3α,5α-THP mediates these behaviors independent of ovarian E2 is an important question, given that E2 alone can facilitate lordosis (Carter et al., 1987; Kow and Pfaff, 2004), has anti-anxiety effects (Walf and Frye, 2006), and can enhance 3α,5α-THP biosynthesis (Cheng and Karavolas, 1973; Pluchino et al., 2006; Vongher and Frye, 1999). Thus, the extent to which 3α,5α-THP's effects in the VTA to facilitate these reproductively-relevant behaviors are dependent on ovarian E2 is of interest.
The site-specificity for 3α,5α-THP's effects in the VTA to mediate appetitive and consummatory aspects of mating is also of interest. Our investigations have focused on the actions of 3α,5α-THP in the midbrain VTA because of this region's importance for motivational aspects of mating behavior(Yamanouchi and Arai,1982). However, 3α,5α-THP can also have effects on lordosis and cataplexy when infused into the central grey (McCarthy et al.,1995). As such, these studies were designed to test the hypothesis that actions of 3α,5α-THP in the VTA, compared to the central grey (CG) and substantia nigra (SN), may mediate exploratory, anxiety, social and/or sexual behaviors, independent of E2. We predicted that if 3α,5α-THP in the VTA modulates appetitive behaviors, independent of E2, then infusions of 3α,5α-THP to the VTA, but not nearby brain regions, of OVX rats should enhance exploration, anti-anxiety, and/or social behaviors, irrespective of E2-priming.
2. Materials and methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at the University at Albany-SUNY.
2.1. Animals and housing
Adult, intact, Long-Evans female rats (n=94) were bred in the Laboratory Animal Care Facility at The University at Albany. Rats were group-housed (four rats per cage) in polycarbonate cages (45×24×21 cm) in a temperature-controlled room (21±1 °C). Rats were maintained on a 12/12 hour reversed light cycle (lights off 08:00 h) with continuous access to Purina Rat Chow and tap water in their home cages.
2.2. Surgery
Rats were OVX via bilateral flank incisions while under xylazine (12 mg/kg) and ketamine (80 mg/kg) anesthesia at least one week prior to testing. Simultaneous with OVX, rats were stereotaxically implanted with bilateral guide cannulae aimed at the VTA (from bregma: AP=−5.3, ML=±0.4, DV=−7.0), substantia nigra (SN; AP= −5.0, ML=±2.0, DV=−8.0), or central grey (CG; AP=−6.5, ML=±0.5, DV=−5.5). Guide cannulae were modified 23-gauge thin-wall stainless steel needles with 30-gauge removable inserts. Rats were monitored post-surgery and pre-testing for loss of weight, righting response, flank stimulation response, and/or muscle tone (Marshall and Teitelbaum, 1974). All rats gained weight and demonstrated appropriate neurological responses.
2.3. Procedure
In Experiment 1, rats (n=48) were administered either subcutaneous (SC) vehicle (sesame oil, n=24) or 17β-estradiol (10 µg, n=24). The latter is a demonstrated E2-priming regimen to enhance sexual behavior of OVX rats (Frye et al., 1998a). Forty-four hours later, 12 rats in each group received bilateral infusions of either vehicle (β-cyclodextrin) or 3α,5α-THP (100 ng) aimed at the VTA, yielding four experimental groups (SC vehicle+intra-VTA vehicle; SC E2+intra-VTA vehicle; SC vehicle+intra-VTA 3α,5α-THP; SC E2+intra-VTA 3α,5α-THP; n=12/grp). Ten minutes following infusions, rats were behaviorally-tested, as described below. We have previously demonstrated that this 3α,5α-THP infusion regimen to the VTA facilitates lordosis among E2-primed rats (Frye and Rhodes, 2006b; Frye et al., 2004a,b). Immediately following testing, tissues were collected for later steroid measurement.
Rats in Experiment 2 (n=46) were either E2-primed (n=38) and received infusions of 3α,5α-THP to the VTA (n=10), SN (n=11), or CG (n=9) or vehicle (β-cyclodextrin, n=2 VTA, n=2 SN, or n=2 CG) or no infusions (n=2) to serve as E2-primed controls (n=8). The remaining rats (n=8) received neither E2, nor 3α,5α-THP, and served as vehicle controls (but were administered SC oil vehicle). We have previously infused β-cyclodextrin to VTA, SN, or CG and found no behavioral differences in the battery of tasks described below or neuroendocrine differences in brain or plasma (Frye and Rhodes, 2006b, 2008), nor were behavioral or neuroendocrine differences observed among vehicle-infused rats with cannulae aimed at VTA, SN, or CG in the present study. Thus, vehicle-infused rats were combined to form one group yielding a 5-group experiment (SC E2+intra-VTA 3α,5α-THP, n=10; SC E2+intra-SN 3α,5α-THP, n=11; SC E2+intra-CG 3α,5α-THP, n=9; SC E2+vehicle-infused control, n=8; SC vehicle non-infused control, n=8). Following testing, tissues were collected for radio-immunoassay or histological site analyses.
2.4. Behavioral testing
Rats in each experiment were tested through the following battery in the order described below. Testing apparatus were brightly-lit from above with three fluorescent bulbs. Rats were tested in a single room, in a sequential manner, with no breaks between tasks (other than time needed to clean apparatus and move rats from one task to the next). Although prior test exposure may influence performance in subsequent tasks, previous reports comparing males tested in a similar battery of anxiety tasks versus individual anxiety tasks did not reveal differences on behavioral and/or endocrine (5α-reduced androgens) measures (Edinger and Frye, 2005). Further, we have tested females in a single task, multiple tasks, or the full battery described below and have found that only paced mating is associated with neuroendocrine differences which is the last task in the testing sequence (Frye and Rhodes, 2006a; Frye et al., 2007).
Behavioral testing was performed by 1 of 3 observers (98% concordance), who were blind to experimental conditions. All data were collected using the automated ANY-Maze data collection program (Stoelting Co., Wheat Dale, IL) and also hand-scoring with stopwatches. There was a 96% concordance rating between these two methods of data collection. The automated data were used for final analyses.
2.4.1. Open field
The open field (76×57×35 cm) has a 48-square grid floor (6×8 squares, 9.5 cm/side) with an overhead light illuminating the central squares (all but the 24 perimeter squares were considered central). The number of peripheral and central squares entered was recorded during a five-minute test period (Blizard et al., 1975; Frye et al., 2000a,b; McCarthy et al., 1995). The number of central square entries is an index of exploratory and anti-anxiety behavior.
2.4.2. Elevated plus maze
The elevated plus maze consisted of four arms, 49 cm long and 10 cm wide, elevated 50 cm off the ground. Two arms are enclosed by walls 30 cm high while the others are exposed. The number of entries into, and the amount of time spent on, the open or closed arms were recorded during a five-minute test (Dunn et al., 1998; File, 1990; Frye et al., 2000a,b). Open arm time is an index of exploratory and anti-anxiety behavior.
2.4.3. Partner preference
Experimental rats were placed in the center of an open field which contained an OVX stimulus female and an intact stimulus male in opposite corners. Stimulus rats were enclosed in corners by Plexiglass compartments that were permeated with small holes so that experimental rats could exchange visual and olfactory information with stimulus rats without physical contact. Time spent in proximity (within a body's length) to stimulus animals was recorded in a five-minute test. Preference for a stimulus male versus a stimulus female is considered a social choice (Frye et al., 1998a).
2.4.4. Social interaction
An experimental rat and an OVX conspecific were placed in opposite corners of an open field. The total duration of time that the experimental rat engaged the stimulus rat by crawling over or under, sniffing, following with contact, genital investigation, tumbling, boxing or grooming was recorded during a five-minute test. The duration of social interaction is considered a measure of anti-anxiety behavior (File, 1980; Frye et al., 2000a,b).
2.4.5. Paced mating
Paced mating was carried out per previously reported procedures (Erskine, 1985; Frye and Erskine, 1990). Paced mating tests were conducted in a chamber (37.5×75×30 cm), which was equally divided by a partition that had a small (5 cm in diameter) hole in the bottom center, to allow females free access to both sides of the chamber, but prevented the stimulus male from moving between sides. Females were placed in the side of the chamber opposite the stimulus male and behaviorally-tested for an entire ejaculatory series. Frequency of mounts, intromissions, and ejaculations were recorded, as well as the frequency (lordosis quotient) and intensity of lordosis (lordosis rating), quantified by rating female dorsiflexion during lordosis on a scale of 0–3 (Hardy and DeBold, 1973) in response to these contacts. The frequency of proceptive (hopping, darting, ear-wiggling) and aggressive behaviors (vocalizing, attack) in response to sexual contacts and the percentage of times the experimental female left the compartment containing the male (% exits) following sexual contacts were also recorded.
2.5. Tissue collection for radioimmunoassay
Trunk blood and whole brains were collected from most rats for later measurement of circulating and central E2, P4, dihydroprogesterone (DHP), and 3α,5α-THP. Midbrain, hippocampus, striatum, and cortex were dissected for Experiment 1, and these sites as well as remaining subcortical tissue (interbrain) were dissected for Experiment 2. In Experiment 2, some brains were used for histological examination of spread of infusions to the VTA, SN, or CG.
2.6. Radioimmunoassay for steroid hormones
Levels of E2 and progestins were measured using radioimmunoassay per previous methods (Frye et al., 1996, 1998a,b). Sample tube concentrations were calculated using the logit–log method of Rodbard and Hutt (1974). The intra-assay and inter-assay coefficients of variance for each assay were: E2 0.09 and 0.10, P4 0.12 and 0.13, DHP 0.12 and 0.14, and 3α,5α-THP 0.13 and 0.15.
2.7. Verification of infusion site
Because of the need to assess hormone levels via radioimmunoassay, all brains could not be verified by histological analysis. As such, rats from each of the VTA (n=2), SN (n=2), and CG (n=2) experimental groups in Experiment 2 were infused with a 1% cresyl violet solution and then had tissues fixed for histological analyses. Briefly, rats were deeply anesthetized with an overdose of sodium pentobarbital (150 mg/kg or to effect) and then exsanguinated with 0.9% saline followed by intracardial perfusion with 10% formalin, as previously described (Frye and Walf, 2002; Rhodes and Frye, 2001). Frozen brains were sliced on a cryostat to locate infusion site by light microscopy.
2.8. Statistical analyses
In Experiment 1, effects of systemic E2-priming and intra-VTA 3α,5α-THP infusions on behavioral and endocrine outcomes were analyzed using two-way analyses of variance (ANOVAs). Correlation analyses were carried out following significant main effects to determine whether steroid hormone levels in specific brain areas examined contributed to performance in individual tasks. In Experiment 2, effects of no infusions or 3α,5α-THP infusions to the VTA, SN, or CG on endocrine and behavioral outcomes were analyzed using one-way ANOVAs. The alpha level for statistical significance was P<0.05. Trends towards significance were reported when P<0.10. Where appropriate, Fisher's PLSD post hoc test was used to determine group differences.
3. Results
3.1. Experiment 1—estrogen effects on 3α,5α-THP-facilitated behavior
3.1.1. Neuroendocrine endpoints
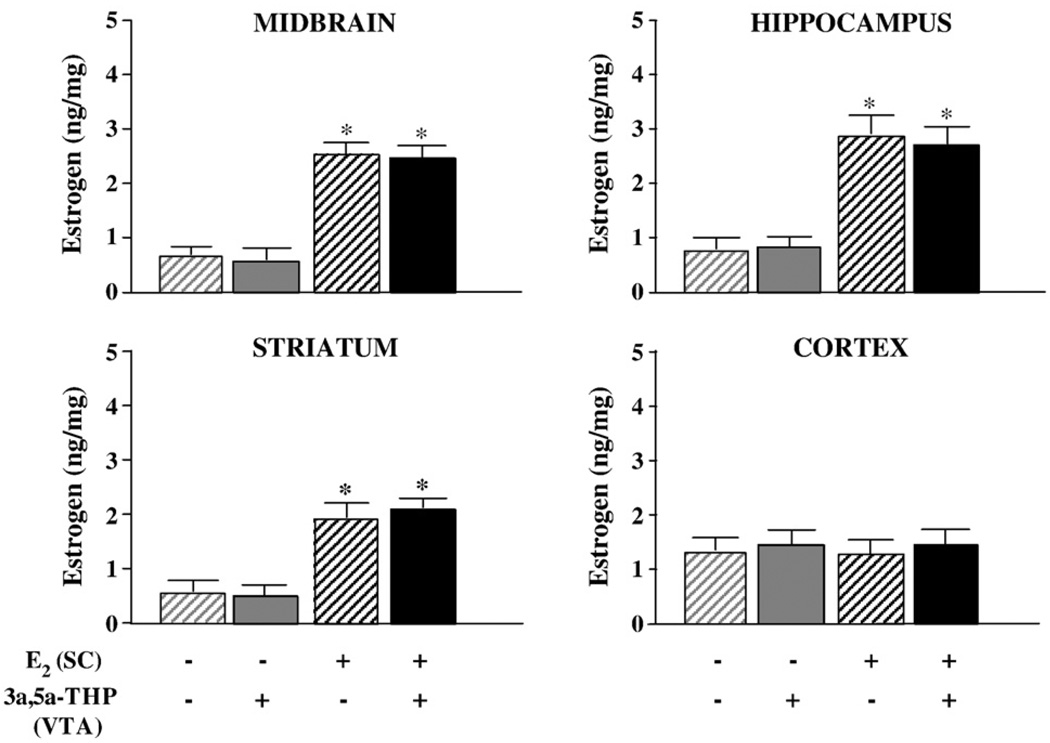
E2-priming significantly increased E2 concentrations in serum (P<0.05; Table 1, left column), midbrain (P<0.05), hippocampus (P<0.05), striatum (P<0.05), and cortex (P<0.05), but 3α,5α-THP infusions to the VTA did not alter E2 levels in brain (Fig. 1) or serum (Table 1, left column). Progesterone (Table 2, top) and DHP (Table 2, bottom) levels were not influenced by E2-priming, or 3α,5α-THP infusions to the VTA, in midbrain, hippocampus, striatum, cortex, or serum (Table 1, middle columns).
Table 1.
Depicts serum E2, P4, DHP, and 3α,5α-THP levels of ovariectomized rats administered subcutaneous (SC) vehicle+Intra-VTA vehicle, SC vehicle+Intra-VTA 3α,5α-THP, SC E2+Intra-VTA vehicle, or SC E2+ Intra-VTA 3α,5α-THP
| Experimental condition | Circulating concentrations |
|||
|---|---|---|---|---|
| E2 (pg/ml) |
P4 (ng/ml) |
DHP (ng/ml) |
3α,5α-THP (ng/ml) |
|
| SC vehicle+Intra-VTA vehicle | 2.0±0.1 | 0.8±0.1 | 1.4±0.1 | 1.5±0.6 |
| SC vehicle+Intra-VTA 3α,5α-THP | 3.1±0.5 | 1.4±0.4 | 1.4±0.8 | 0.6±0.7 |
| SC E2+Intra-VTA vehicle | 29.3±4.0a | 1.4±0.3 | 1.6±0.2 | 1.3±0.9 |
| SC E2+Intra-VTA 3α,5α-THP | 24.2±3.1a | 0.8±0.1 | 1.9±1.5 | 1.3±1.0 |
Indicates E2 enhancement (F1,44=12.14, P<0.05).
Fig. 1.
Mean (±SEM) levels of E2 in midbrain (F1,44=81.89; top left), hippocampus (F1,44=16.97; top right), striatum (F1,44=31.17; bottom left), and cortex (F1,44=9.02; bottom right) of ovariectomized vehicle- (gray) or E2-primed (black) rats infused with β-cyclodextrin vehicle (striped bars) or 3α,5α-THP (solid bars) to the VTA. * indicates E2-primed rats had significantly higher levels of E2 than did vehicle-primed rats (P<0.05).
Table 2.
Levels of P4 (top) and DHP (bottom) in the midbrain, hippocampus, striatum, and cortex of ovariectomized rats administered subcutaneous (SC) vehicle+Intra-VTA vehicle, SC E2+ Intra-VTA vehicle, SC vehicle+Intra-VTA 3α,5α-THP, or SC E2+Intra-VTA 3α,5α-THP
| Experimental condition | Central concentrations |
|||
|---|---|---|---|---|
| Midbrain | Hippocampus | Striatum | Cortex | |
| P4 (ng/g) | ||||
| SC vehicle+Intra-VTA vehicle | 2.1±0.2 | 1.3±0.2 | 1.6±0.2 | 1.3±0.2 |
| SC E2+Intra-VTA vehicle | 1.7±0.1 | 1.1±0.2 | 1.2±0.1 | 2.0±0.2 |
| SC vehicle+Intra-VTA 3α,5α-THP | 1.5±0.1 | 1.2±0.1 | 1.3±0.1 | 1.7±0.1 |
| SC E2+Intra-VTA 3α,5α-THP | 2.2±0.1 | 1.6±0.1 | 1.6±0.1 | 1.9±0.2 |
| DHP (ng/g) | ||||
| SC vehicle+Intra-VTA vehicle | 1.9±0.7 | 0.6±0.1 | 0.6±0.2 | 1.5±0.2 |
| SC E2+Intra-VTA vehicle | 2.6±0.9 | 1.8±0.9 | 1.8±0.4 | 0.9±0.1 |
| SC vehicle+Intra-VTA 3α,5α-THP | 2.3±0.6 | 0.5±0.1 | 1.0±0.7 | 1.4±0.5 |
| SC E2+Intra-VTA 3α,5α-THP | 1.3±0.3 | 1.9±0.8 | 0.8±0.2 | 1.5±0.9 |
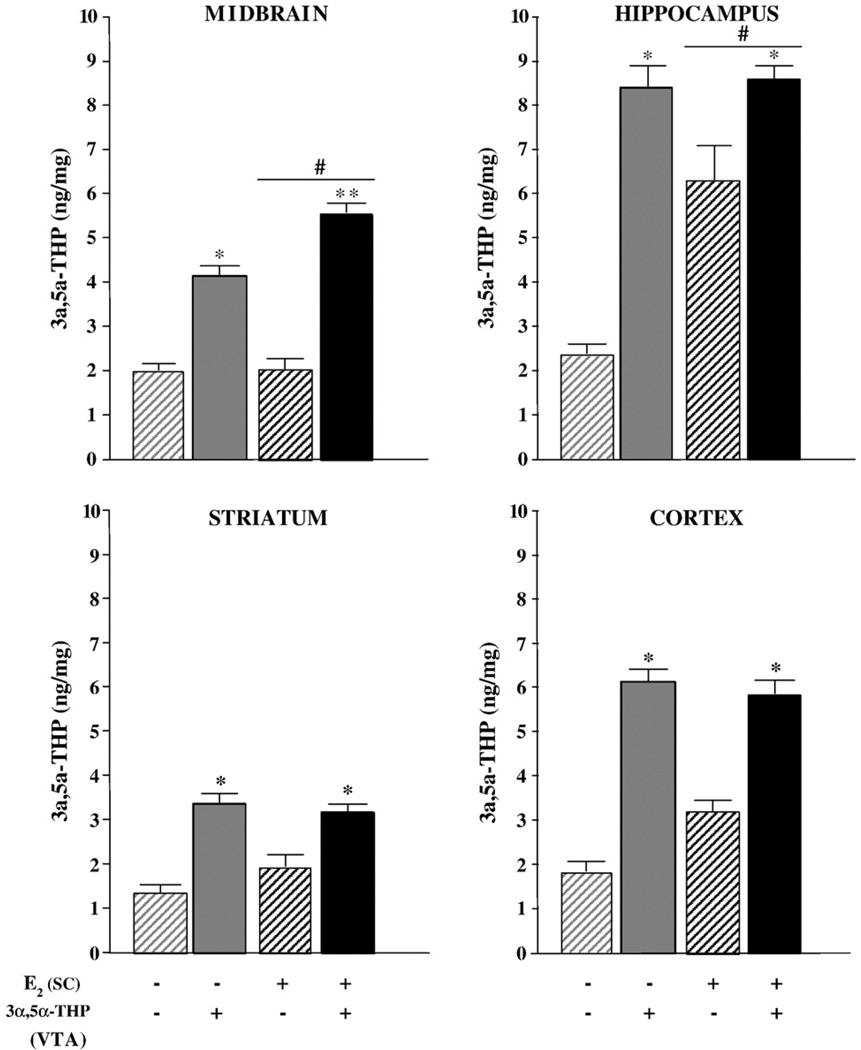
3α,5α-THP infusions to the VTA significantly increased levels of 3α,5α-THP in midbrain (P<0.05), hippocampus (P<0.05), striatum (P<0.05), and cortex (P<0.05; Fig. 2). In the hippocampus, E2-priming tended to increase 3α,5α-THP concentrations of rats that received vehicle infusions (P<0.10). In the midbrain, there was also an interaction between E2-priming and 3α,5α-THP infusions (P<0.05), which was due to 3α,5α-THP infusions increasing midbrain 3α,5α-THP levels more among E2, compared to vehicle-primed, rats. Neither E2-priming, nor 3α,5α-THP infusions to the VTA, altered serum levels of 3α,5α-THP (Table 1, right column).
Fig. 2.
Mean (±SEM) levels of 3α,5α-THP in midbrain (F1,44=77.35; top left), hippocampus (F1,44=20.52; top right), striatum (F1,44=14.42; bottom left), and cortex (F1,44=4.28; bottom right) of ovariectomized vehicle- (gray) or E2-primed (black) rats infused with β-cyclodextrin vehicle (striped bars) or 3α,5α-THP (solid bars) to the VTA. * indicates 3α,5α-THP-infused rats had significantly higher levels of 3α,5α-THP than did vehicle–vehicle controls (P<0.05). ** indicates E2-primed rats infused with 3α,5α-THP had significantly higher 3α,5α-THP levels than did vehicle-primed rats infused with 3α,5α-THP (F1,44=6.72, P<0.05). # indicates tendency for E2-primed rats to have higher levels of 3α,5α-THP than vehicle-primed rats (F1,44=3.42, P<0.10).
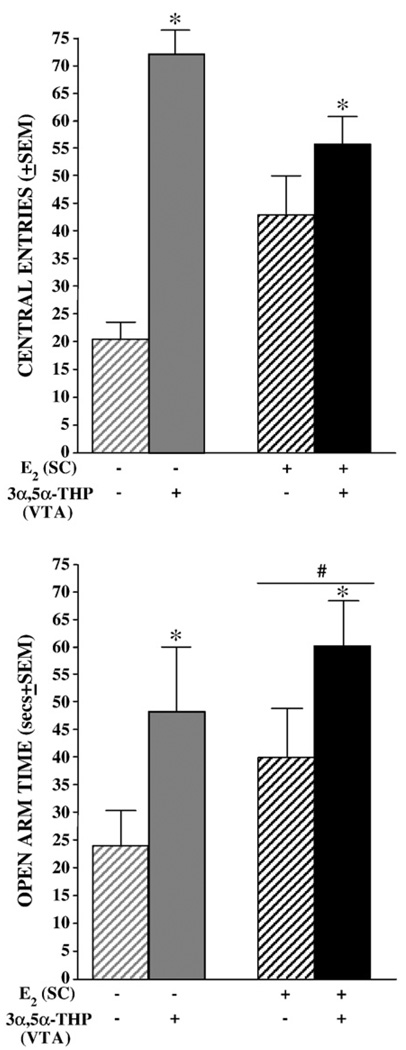
3.1.2. Open field
3α,5α-THP infusions to the VTA significantly increased the number of central entries in the open field (P=0.05). There was an apparent, albeit non-significant, effect of E2-priming alone to increase central entries (Fig. 3, top). Levels of 3α,5α-THP in the hippocampus [r(48)= 0.43, P<0.05] and striatum [r(48)=0.38, P<0.05] were positively correlated with central square entries.
Fig. 3.
Mean (±SEM) central square entries in the open field (top) and open arm time (bottom) of ovariectomized vehicle- (gray) or E2-primed (black) rats infused with β-cyclodextrin vehicle (striped bars) or 3α,5α-THP (solid bars) to the VTA. * indicates 3α,5α-THP -infused rats entered significantly more central squares (F1,44=14.09, P<0.05) and spent more time on open arms (F1,44=11.35, P<0.05) than did vehicle-vehicle controls. # indicates tendency for E2-primed rats to spend more time on open arms compared to vehicle-primed rats (F1,44=1.96, P<0.10).
3.1.3. Elevated plus maze
3α,5α-THP infusions to the VTA (P<0.05) significantly, and E2-priming tended to (P<0.10), increase open arm time in the elevated plus maze (Fig. 3, bottom). 3α,5α-THP levels in the hippocampus were positively correlated with time spent on the open arms of the elevated plus maze [r(48)=0.41, P<0.05].
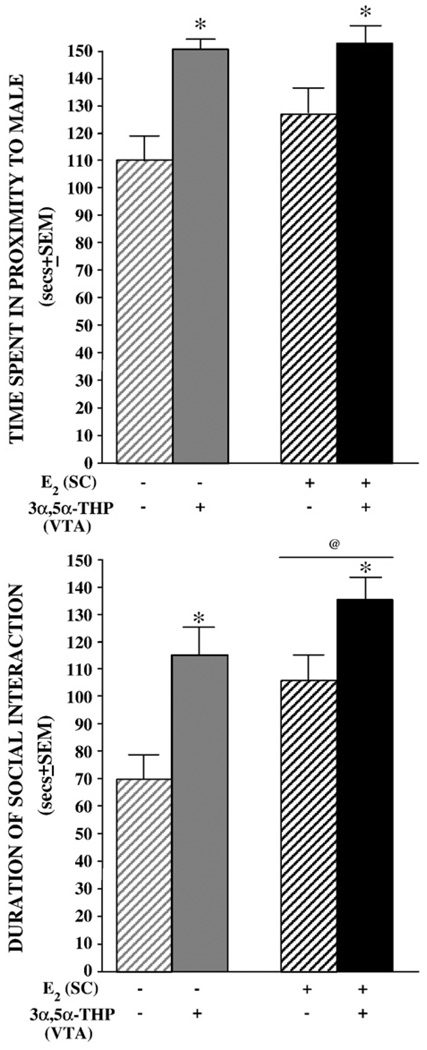
3.1.4. Partner preference
3α,5α-THP infusions to the VTA (P<0.05), but not E2-priming, significantly increased time spent in close proximity to a stimulus male (Fig. 4, top).
Fig. 4.
Mean (±SEM) time spent in close proximity to a stimulus male (top) and in social interaction with a conspecific (bottom) of ovariectomized vehicle- (gray) or E2-primed (black) rats infused with β-cyclodextrin vehicle (striped bars) or 3α,5α-THP (solid bars) to the VTA. * indicates rats infused with 3α,5α-THP spent significantly more time in close proximity to a male (F1,44=10.99, P<0.05) and in social interaction (F1,44=19.49, P<0.05) than did vehicle–vehicle controls. @ indicates E2-primed rats spent significantly more time in social interaction with a conspecific than did vehicle-primed rats (F1,44=6.90, P<0.05).
3.1.5. Social interaction
Systemic E2-priming (P<0.05) and 3α,5α-THP infusions to the VTA (P<0.05) significantly increased time spent in social interaction with a conspecific (Fig. 4, bottom). E2 levels in midbrain [r(48)=0.43, P<0.05] and hippocampus [r(48)=0.54, P<0.05] were positively correlated with social interaction with a conspecific.
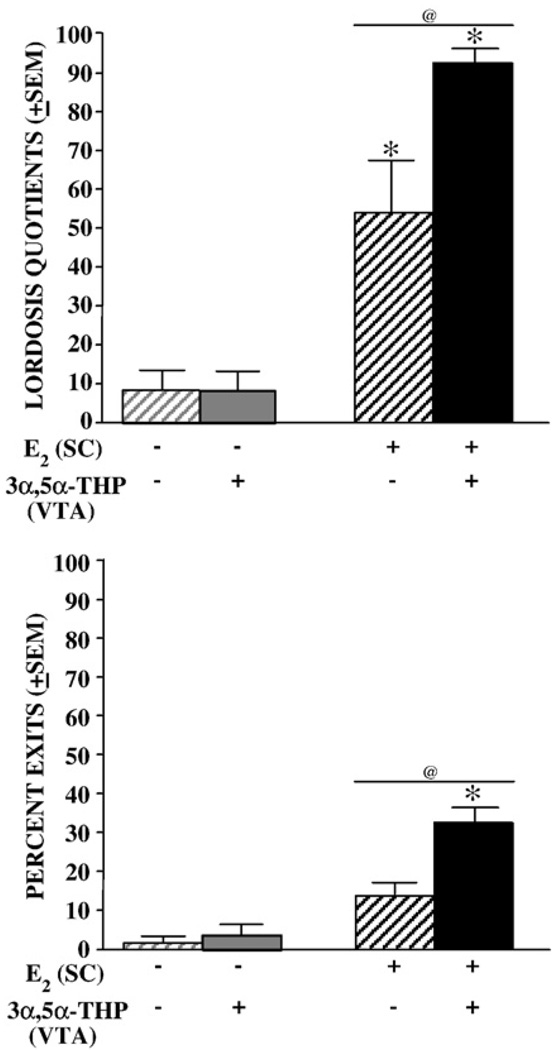
3.1.6. Lordosis quotient
E2-priming (P<0.05), 3α,5α-THP infusions to the VTA (P<0.05), and their interaction (P<0.05; Fig. 5, top) influenced lordosis quotients, such that infusions of 3α,5α-THP produced higher lordosis quotients in E2-versus vehicle-primed rats. E2 levels in midbrain [r(48)=0.63, P<0.05], hippocampus [r(48)=0.50, P<0.05], and striatum [r(48)=0.48, P<0.05] were positively correlated with lordosis quotients. 3α,5α-THP levels in midbrain [r(48)=0.32, P<0.05] were also positively correlated with lordosis quotients.
Fig. 5.
Mean (±SEM) lordosis quotients (top) and percentage of exits (bottom) of ovariectomized vehicle- (gray) or E2-primed (black) rats infused with β-cyclodextrin vehicle (striped bars) or 3α,5α-THP (solid bars) to the VTA. * indicates 3α,5α-THP -infused rats had significantly higher lordosis quotients (F1,44=5.25,P<0.05; top) and a greater percentage of exits following sexual contacts (F1,44=8.06, P<0.05; bottom) than did vehicle–vehicle controls. @ indicates E2-primed rats had significantly higher lordosis quotients (F1,44=69.44, P<0.05; top) and a significantly greater percentage of exits (F1,44=34.70, P<0.05; bottom) following contacts than did vehicle-primed rats.
3.1.7. Lordosis rating
3α,5α-THP infusions to the VTA (P<0.05), but not E2-priming, significantly increased lordosis ratings and interacted such that infusions of 3α,5α-THP produced higher lordosis ratings in E2-compared to vehicle-primed rats (P<0.05; Table 3, left column]. 3α,5α-THP levels in midbrain [r(48)=0.52, P<0.05] were positively correlated with lordosis ratings.
Table 3.
Depicts lordosis ratings, proceptivity and aggression quotients of ovariectomized rats administered SC vehicle+Intra-VTA vehicle, SC E2+Intra-VTA vehicle, SC vehicle+Intra-VTA 3α,5α-THP, or SC E2+Intra-VTA 3α,5α-THP
| Experimental condition | Lordosis rating |
Proceptivity quotient |
Aggression quotient |
|---|---|---|---|
| SC vehicle+Intra-VTA vehicle | 0.3±0.1 | 7±6 | 18±5 |
| SC E2+Intra-VTA vehicle | 0.7±0.2 | 3±3 | 24±9 |
| SC vehicle+Intra-VTA 3α,5α-THP | 0.2±0.1 | 27±12 | 5±2* |
| SC E2+Intra-VTA 3α,5α-THP | 2.7±0.1** | 71±5** | 5±2* |
Indicates significant reduction in aggression among 3α,5α-THP-infused rats compared to vehicle-infused rats (F1,44=7.17, P<0.05).
Indicates significant interaction for 3α,5α-THP to enhance lordosis ratings (F1,44=4.29, P<0.05) and proceptivity (F1,44=9.88, P<0.05) to a greater degree among E2 primed rats than in vehicle-primed rats.
3.1.8. Proceptivity quotient
E2-priming (P<0.05), 3α,5α-THP infusions to the VTA (P<0.05), and their interaction (P<0.05) increased proceptivity quotients, such that 3α,5α-THP infusions produced higher proceptivity quotients in E2-versus vehicle-primed rats (Table 3, middle column). E2 and 3α,5α-THP levels in midbrain [E2: r(48)=0.33, P<0.05; 3α,5α-THP: r(48)=0.64, P<0.05] and hippocampus [E2: r(48)=0.33, P<0.05; 3α,5α-THP: r(48)=0.30, P<0.05] were positively associated with proceptivity quotients.
3.1.9. Aggression quotient
3α,5α-THP infusions to the VTA (P<0.05, Table 3, right column), but not E2-priming, decreased aggression quotients. 3α,5α-THP levels in midbrain [r(48)=0.39, P<0.05], hippocampus [r(48)=0.46, P<0.05], and striatum [r(48)=0.46, P<0.05] were negatively correlated with aggression quotients.
3.1.10. Percent exits
E2-priming (P<0.05), and 3α,5α-THP infusions to the VTA (P<0.05), increased the percentage of exits following sexual contacts and had interactive effects, such that 3α,5α-THP infusions produced greater increases in the percentage of exits following intromissions in E2- over vehicle-primed rats (Fig. 5, bottom). E2 [r(48)=0.57, P<0.05] and 3α,5α-THP [r(48)=0.28, P<0.05] levels in midbrain, and E2 in hippocampus [r(48)=0.49, P<0.05] and striatum [r(48)=0.48, P<0.05] were positively associated with percent exits.
3.2. Experiment 2—effects of estrogen on 3α,5α-THP infusion to the VTA, SN, or CG
Examination of infusion of site indicated that the protocol utilized in the present experiments was successful at differentially delivering 3α,5α-THP to the VTA, SN, or CG (as previously reported; Frye and Rhodes, 2008). Commensurate with this, infusions of 3α,5α-THP to the SN or CG produced different effects on behavior and endocrine measures than did infusions of 3α,5α-THP to the VTA.
3.2.1. Neuroendocrine endpoints
As in Experiment 1, Table 4 depicts E2, but not vehicle, administration increased E2 concentrations in serum (P<0.05), midbrain (P<0.05), hippocampus (P<0.05), striatum (P<0.05), and interbrain (P<0.05). Neither P4 nor DHP levels in midbrain, hippocampus, striatum, cortex, interbrain, or serum were different among groups (Table 4).
Table 4.
Depicts E2, P4, and DHP concentrations in serum, midbrain, hippocampus, striatum, cortex, and interbrain of vehicle- (n=8) or E2-primed (n=8) rats that received no infusions and E2-primed rats that received 3α,5α-THP infusions to the VTA (n=9), SN (n=10), or CG (n=8)
| Experimental condition |
Serum | Midbrain | Hippocampus | Striatum | Cortex | Interbrain |
|---|---|---|---|---|---|---|
| E2 (ng/ml/g) | ||||||
| Vehicle control | 2.8±0.6 | 1.2±0.1 | 1.6±0.3 | 1.0±0.2 | 1.2±0.2 | 1.2±0.2 |
| E2 control | 27.8±3.8* | 2.6±0.3* | 3.2±0.5* | 2.1±0.2* | 1.5±0.1 | 3.7±0.4* |
| VTA 3α,5α-THP | 27.6±3.5* | 2.7±0.4* | 3.5±0.4* | 2.1±0.3* | 1.2±0.3 | 3.3±0.2* |
| SN 3α,5α-THP | 27.7±2.1* | 2.6±0.3* | 3.7±0.4* | 2.5±0.3* | 1.5±0.1 | 3.3±0.4* |
| CG 3α,5α-THP | 29.1±3.3* | 2.7±0.5* | 3.3±0.3* | 2.3±0.2* | 1.7±0.2 | 3.3±0.4* |
| P4 (ng/ml/g) | ||||||
| Vehicle control | 1.6±0.4 | 1.5±0.1 | 1.9±0.2 | 1.7±0.6 | 1.9±0.3 | 1.9±0.3 |
| E2 control | 1.3±0.4 | 1.6±0.1 | 1.8±0.2 | 1.6±0.1 | 1.9±0.2 | 1.9±0.2 |
| VTA 3α,5α-THP | 1.4±0.4 | 1.7±0.2 | 1.5±0.2 | 1.7±0.4 | 1.9±0.2 | 1.9±0.2 |
| SN 3α,5α-THP | 1.8±0.3 | 1.8±0.5 | 1.8±0.3 | 2.0±0.4 | 1.7±0.2 | 1.8±0.3 |
| CG 3α,5α-THP | 1.4±0.4 | 1.8±0.2 | 1.8±0.3 | 1.8±0.5 | 1.9±0.2 | 1.9±0.3 |
| DHP (ng/ml/g) | ||||||
| Vehicle control | 3.2±0.5 | 1.5±0.1 | 1.5±0.1 | 1.5±0.1 | 1.3±0.4 | 1.6±0.3 |
| E2 control | 3.4±0.5 | 1.7±0.1 | 1.8±0.3 | 1.7±0.1 | 2.1±0.6 | 1.4±0.3 |
| VTA 3α,5α-THP | 2.7±0.4 | 1.5±0.1 | 1.6±0.4 | 1.6±0.1 | 2.0±0.4 | 1.6±0.3 |
| SN 3α,5α-THP | 3.2±0.2 | 1.6±0.1 | 1.5±0.1 | 1.7±0.1 | 1.8±0.3 | 1.9±0.3 |
| CG 3α,5α-THP | 3.3±0.4 | 1.7±0.1 | 1.6±0.2 | 1.8±0.2 | 1.7±0.4 | 1.5±0.4 |
Indicates significant E2 enhancement in serum (F4,38=14.19, P<0.05), midbrain (F4,38=3.24, P<0.05), hippocampus F4,38=4.54, P<0.05), striatum [F4,38=4.52, P<0.05), and interbrain (F4,38=8.85, P<0.05).
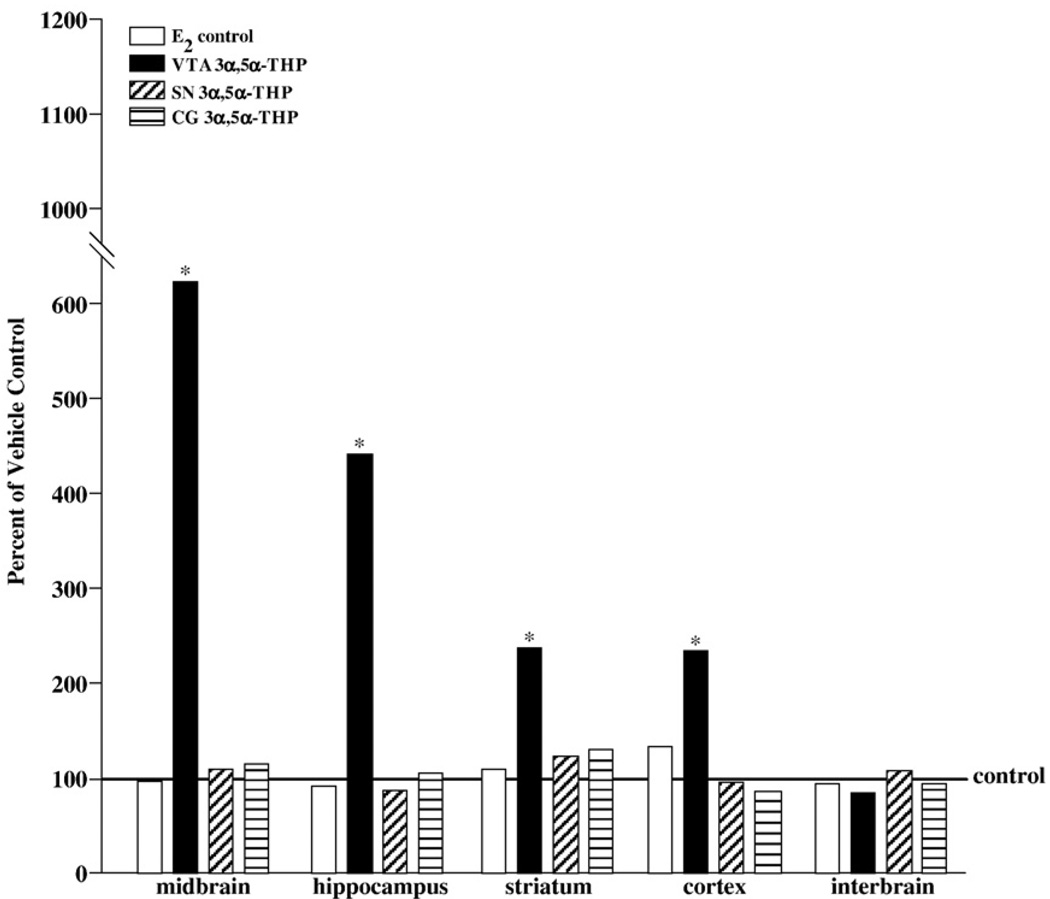
As depicted in Fig. 6, 3α,5α-THP infusions to the VTA, but not the SN or CG, increased concentrations of 3α,5α-THP in midbrain (P<0.05), hippocampus (P<0.05), striatum (P<0.05), and cortex (P<0.05). There were no effects of central 3α,5α-THP infusions on concentrations of 3α,5α-THP in interbrain or serum.
Fig. 6.
Represents percent of vehicle control for 3α,5α-THP concentrations in midbrain, hippocampus, striatum, cortex, and interbrain of E2-primed rats that received no infusions (white bars), or infusions of 3α,5α-THP to the VTA (black bars), SN (diagonally-striped bars), or CG (horizontally-striped bars). * indicates that rats infused with 3α,5α-THP to the VTA had significantly higher levels of 3α,5α-THP in midbrain (F4,38=25.00, P<0.05), hippocampus (F4,38=71.71, P<0.05), striatum (F4,38=6.27, P<0.05), and cortex (F4,38=11.01, P<0.05) compared to all other groups.
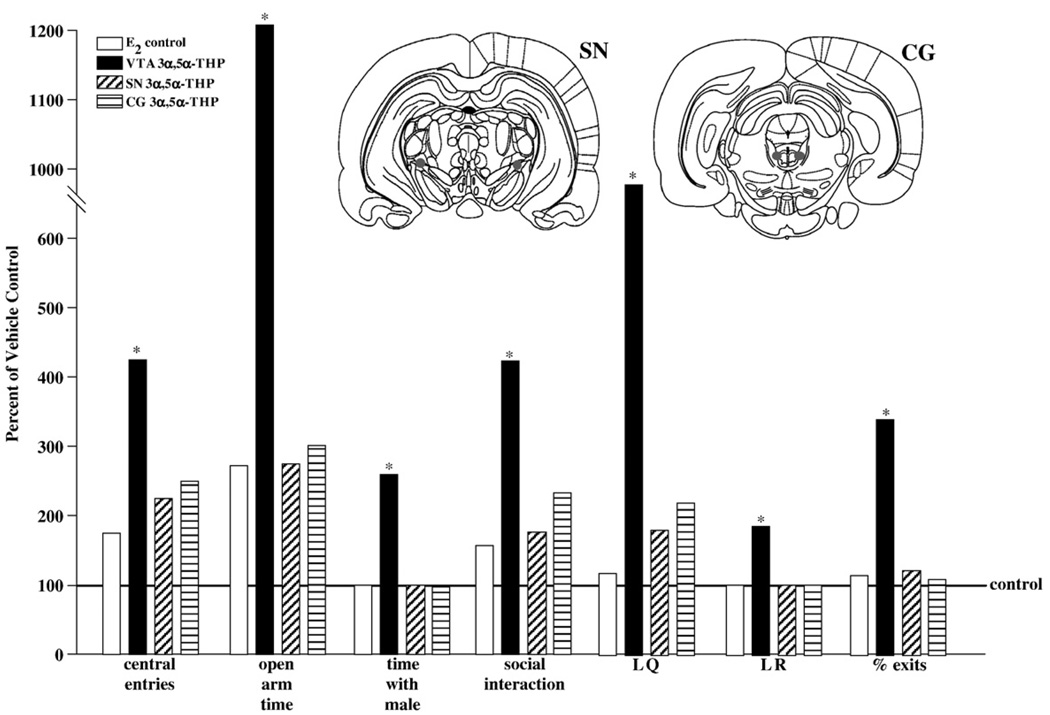
3.2.2. Behavioral endpoints
As depicted in Fig. 7, infusions of 3α,5α-THP to the VTA, but not the SN or CG, significantly increased the number of central square entries in the open field (P<0.05), open arm time on the elevated plus maze (P<0.05), time spent in close proximity to a stimulus male (P<0.05), social interaction with a conspecific (P<0.05), increased lordosis quotients (P<0.05), lordosis ratings (P<0.05), and percentage of exits following mating contacts compared to vehicle or E2-priming alone (P<0.05).
Fig. 7.
Represents percent of vehicle control for central entries, open arm time, time in proximity to male, social interaction, lordosis quotients (LQ) and ratings (LR), and percentage of exits after contacts, of E2-primed rats that received no infusions (white bars), or infusions of 3α,5α-THP to the VTA (black bars), SN (diagonally-striped bars), or CG (horizontally-striped bars). * indicates that rats infused with 3α,5α-THP to the VTA had significantly more central entries (F4,38=5.71, P<0.05), open arm time (F4,38=5.42, P<0.05), time with a male (F4,38=6.37, P<0.05), social interaction (F4,38=6.44, P<0.05), lordosis quotients (F4,38=10.68, P<0.05) and ratings (F4,38=6.44, P<0.05), and percent exits (F4,38=5.22, P<0.05) compared to all other groups. Insets depict spread of 3α,5α-THP infusions when administered to the SN (left) or CG (right).
4. Discussion
In the present studies, infusions of 3α,5α-THP to the VTA (but neither CG nor SN) of OVX rats consistently enhanced appetitive behaviors, such as exploration, anti-anxiety behavior, proximity to a male, social interaction, and anti-aggression, irrespective of E2-priming. Consummatory aspects of mating, such as initiation and intensity of lordosis, as well as pacing of sexual contacts, required systemic E2-priming and were most commensurate with natural-receptivity when both E2 and 3α,5α-THP were administered. 3α,5α-THP infusions aimed at SN or CG did not significantly enhance these aspects of reproduction, although 3α,5α-THP did increase the frequency of lordosis when infused to the CG. As such, these data suggest that actions of 3α,5α-THP in the VTA mediate appetitive aspects of reproduction characterized by exploration, anti-anxiety, and social behavior independent of E2. Alternatively, consummatory behaviors, such as the expression and quality of lordosis, may be modulated by E2 with essential actions of 3α,5α-THP in the VTA required for the culmination of the full mating repertoire. These data are consistent with prior reports indicating that 3α,5α-THP and/or E2 can mediate approach/avoidance behaviors important for mating and extend these findings in several important ways.
Infusions of 3α,5α-THP to the midbrain VTA enhanced levels of 3α,5α-THP in midbrain, hippocampus, striatum, and cortex but expression of appetitive behaviors correlated more with 3α,5α-THP levels in the hippocampus, than these other regions. Prior reports find that enhancement of 3α,5α-THP in hippocampus increases open arm time on the elevated plus maze and decreases time spent burying in response to shock (Bitran et al., 1999, 2000). Further, blocking P4's metabolism to 3α,5α-THP in the hippocampus of proestrous rats decreases central entries in the open field and open arm time on the elevated plus maze (Rhodes and Frye, 2001). Indeed, the VTA and the hippocampus have very high levels of 3α,5α-THP and greater activity of the metabolism enzymes necessary for 3α,5α-THP formation than do the other brain areas examined in these studies (Frye and Bayon, 1999; Li et al., 1997; Palumbo et al., 1995; Roselli and Snipes, 1984). Together, these data suggest that 3α,5α-THP in the VTA may trigger biosynthesis in these other regions (hippocampus, striatum, cortex), that may help prepare for reproductive experiences by decreasing anxiety and enhancing approach behaviors and evaluation of socially-relevant stimuli.
3α,5α-THP's effects to enhance exploratory, anti-anxiety, social, and reproductive behaviors as well as biosynthesis may be specific to manipulations in the VTA. It should be noted that we have previously observed that intra-VTA infusions of 3α,5α-THP dose-dependently increase 3α,5α-THP in midbrain, hippocampus, striatum, and cortex and dose-dependently enhance each of the behaviors examined in the present report (Frye and Rhodes, 2006b). Further, intra-VTA inhibition of 3α,5α-THP attenuates these enhancements (Frye et al., 2008). In the present study, infusions of 3α,5α-THP to the SN or CG neither enhanced exploratory, anti-anxiety, social, and reproductive behaviors nor increased 3α,5α-THP concentrations in midbrain, hippocampus, striatum, or cortex. These behavioral data are consistent with prior reports that progestins have different patterns of effects in the SN and CG than in the VTA. Enhancing 3α,5α-THP biosynthesis in the VTA, but not the SN, facilitates lordosis of E2-primed rats (Frye and Petralia, 2003). In the VTA, P4 enhances GABAA receptor function of E2-primed rodents; however, in the SN, GABAA receptor function is decreased following P4 administration (Frye, 2001; Schindler et al., 2003). As well, others have found that infusions of 3α,5α-THP to the CG do not increase the ratio of central to total squares entered in the open field but can enhance lordosis (McCarthy et al., 1995). Congruent with this report, we also saw non-significant, but apparent, increases in lordosis quotients following 3α,5α-THP infusions to the CG, although it should be noted that the earlier study utilized a much higher concentration of 3α,5α-THP (250 and 500 ng; McCarthy et al., 1995) than did the present studies (100 ng). Thus, while the SN and the CG are clearly progestin-sensitive, 3α,5α-THP has very different effects in these areas than it does in the VTA.
In addition to 3α,5α-THP, E2 is also a neurosteroid when it is synthesized de novo in brain. Enhancement of E2 biosynthesis and/or bioactivity has been reported in avian and rodent models, in response to mating-related stimuli or stress (Balthazart et al., 2004; Cohen-Parsons and Carter, 1987; Cornil et al., 2005; Wood et al., 2001). However, there was no evidence of E2 biosynthesis in the present studies, which suggests that the behavioral effects observed were due primarily to E2 administration and 3α,5α-THP administration and/or biosynthesis. Notably, E2 alone has also been shown to alter exploratory, anti-anxiety, and reproductive behaviors (Satou and Yamanouchi, 1996; Walf and Frye, 2006). In the present studies, E2-priming was observed to enhance 3α,5α-THP biosynthesis in hippocampus, striatum, and cortex and had a synergistic effect to enhance 3α,5α-THP in midbrain when administered among 3α,5α-THP-infused rats. E2 has been found to increase 3α,5α-THP biosynthesis. Post-menopausal women on E2-based hormonal replacement therapies have significant enhancement of circulatory 3α,5α-THP levels (Pluchino et al., 2006). Circulatory E2 enhances progestin biosynthesis both in vivo in astrocytes and in vitro in hypothalamus and hippocampus (Cheng and Karavolas, 1973; Frye and Vongher, 1999a; Sinchak et al., 2003; Soma et al., 2005). These effects could be due to enhancement of 3α,5α-THP-synthesizing enzymes such as 3β-hydroxysteroid dehydrogenase (which converts pregnenolone to P4; Soma et al., 2005; Micevych et al., 2008) and/or 5α-reductase (which catalyzes P4's conversion to DHP; Cheng and Karavolas, 1973). Thus, E2's effects to enhance exploratory, anxiety, social, and reproductive behaviors may be due, at least in part, to its actions to increase progestin biosynthesis in the hippocampus, which may play a role in preparing for reproductive experiences.
There is evidence in early development and adulthood that 3α,5α-THP can alter behavior, which may be due, in part, to its actions as a homeostatic modulator (Engel and Grant, 2001). Indeed, chronic stress can alter expression of enzymes necessary for neurosteroidogenesis to occur in mice (Agís-Balboa et al., 2007; Dong et al., 2001) and has been demonstrated to reduce central neuroactive steroid concentrations in rats, including 3α,5α-THP (Serra et al., 2000). 3α,5α-THP can also dampen stress-responsiveness in adulthood. In support, administration of systemic 3α,5α-THP reduces ACTH levels in response to intermittent air-puff exposure (Patchev et al., 1996). As well, 3α,5α-THP has similar effects as does corticosterone to block adrenalectomy-induced increases in corticotrophin releasing hormone mRNA (Patchev et al., 1994). Systemically blocking formation of 3α,5α-THP increases stress-induced dopamine release in the cortex of rats (Dazzi et al., 2002). Interestingly, we saw increases in 3α,5α-THP levels in the hippocampus, striatum, and cortex, sites which are distal to the VTA, that could not be accounted for by diffusion of 3α,5α-THP. These increases in 3α,5α-THP in the hippocampus, striatum, and cortex, brain regions that are important for mediating effects of stress, may also play a role in mitigating stressors associated with engaging in exploratory, anti-anxiety, social, and/or reproductive behaviors. Although, we did not assess 3α,5α-THP concentrations in amygdala, a region that is associated with stress, anxiety, hippocampal, and cortical function (Pitkänen et al., 1997), this area is of interest for future investigation. Indeed we have previously seen that E2 and/or P4 infusion to this region can reduce anxiety in rats (Frye and Walf, 2004), whereas, inhibition of 3α,5α-THP formation in this region can reduce anti-anxiety behavior (Walf et al., 2006). The extent to which these behaviors are facilitated by E2, independent of 3α,5α-THP biosynthesis is an interesting question.
In summary, the present data supported our hypothesis that 3α,5α-THP in the VTA influences behaviors other than lordosis. Infusions of 3α,5α-THP to the VTA, but not SN or CG, enhanced lordosis, exploratory, anti-anxiety, approach, and social behaviors. Infusions of 3α,5α-THP to the VTA, but not the SN or CG, also increased 3α,5α-THP concentrations in the hippocampus, striatum, and cortex, as well as midbrain. These data are especially important given that recent reports from clinical and basic research suggest that an underlying factor in the pathophysiology and/or treatment of stress-induced affective and neuropsychiatric disorders may be alterations in production of, or response to, neurosteroids (Grobin et al., 2006; Guidotti and Costa, 1998; Guidotti et al., 2001; Paul and Purdy, 1992; Schmidt et al., 1998; Smith et al., 2003). 3α,5α-THP levels are reduced in cerebrospinal fluid of depressed men, compared to non-depressed men, and are normalized concomitant with depressive symptom relief following treatment with the selective serotonin reuptake inhibitor, fluoxetine (Uzunova et al., 1998). Low levels of plasma 3α,5α-THP are also associated with increased negative symptoms in schizophrenia (Shirayama et al., 2002) and 3α,5α-THP is increased by administration of antipsychotics, such as olanzapine and clozapine (Barbaccia et al., 2001; Frye and Seliga, 2003; Marx et al., 2000). Thus, our findings demonstrating that 3α,5α-THP in the VTA can enhance anti-anxiety, social behaviors and biosynthesis of 3α,5α-THP in the hippocampus, striatum, and cortex, suggests an important role of 3α,5α-THP for mediating approach/avoidance behaviors and social interactions which are typically disrupted in neuropsychiatric disorders.
Acknowledgements
This research was supported by a grant from the National Institute of Mental Health (MH06769801).
References
- Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwa Y, Purdy RH, Koob GF, Britton KT. Abstract The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Abstract Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25:489–497. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology (Berl) 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Auksi T, Casten L. Estrogen and the induction of lordosis in female and male prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:65–73. doi: 10.1016/0018-506x(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Cohen-Parsons M, Carter CS. Males increase serum estrogen and estrogen receptor binding in brain of female voles. Physiol Behav. 1987;39:309–314. doi: 10.1016/0031-9384(87)90227-7. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball G, et al. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzi L, Serra M, Vacca G, Ladu S, Latrofa A, Trapani G, et al. Depletion of cortical allopregnanolone potentiates stress-induced increase in cortical dopamine output. Brain Res. 2002;932:135–139. doi: 10.1016/s0006-8993(02)02290-4. [DOI] [PubMed] [Google Scholar]
- Dong H, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Normura H, et al. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RW, Reed TA, Copeland PD, Frye CA. The nitric oxide synthase inhibitor 7-nitroindazole displays enhanced anxiolytic efficacy without tolerance in rats following subchronic administration. Neuropharmacology. 1998;37:899–904. doi: 10.1016/s0028-3908(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Engel SR, Grant KA. Neurosteroids and behavior. Int Rev Neurobiol. 2001;46:321–348. doi: 10.1016/s0074-7742(01)46067-3. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- Feder HH. Hormones and sexual behavior. Annu Rev Psychol. 1984;35:165–200. doi: 10.1146/annurev.ps.35.020184.001121. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990;5:195–201. [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. J Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90:375–385. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–691. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM. Lordosis of rats is modified by neurosteroidogenic effects of membrane benzodiazepine receptors in the ventral tegmental area. Neuroendocrinology. 2003;77:71–82. doi: 10.1159/000068338. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006a;83:336–347. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006b;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Behav Brain Res. 2008;187:88–99. doi: 10.1016/j.bbr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Olanzapine's effects to reduce fear and anxiety and enhance social interactions coincide with increased progestin concentrations of ovariectomized rats. Psychoneuroendocrinology. 2003;28:657–673. doi: 10.1016/s0306-4530(02)00049-5. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. GABA(A), D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999a;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progestins' rapid facilitation of lordosis when applied to the ventral tegmentum corresponds to efficacy at enhancing GABA(A)receptor activity. J Neuroendocrinol. 1999b;11:829–837. doi: 10.1046/j.1365-2826.1999.00367.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3α-diol and corticosterone. Psychoneuroendocrinology. 1996;21:431–439. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998a;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ, Bayon LE. Finasteride blocks the reduction in ictal activity produced by exogenous estrous cyclicity. J Neuroendocrinol. 1998b;10:291–296. doi: 10.1046/j.1365-2826.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Murphy RE, Platek SM. Anti-sense oligonucleotides, for progestin receptors in the VMH and glutamic acid decarboxylase in the VTA, attenuate progesterone-induced lordosis in hamsters and rats. Behav Brain Res. 2000a;115:55–64. doi: 10.1016/s0166-4328(00)00242-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000b;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004a;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Sumida K. Progestins' actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors. Pharmacol Biochem Behav. 2004b;78:405–418. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5alpha-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Exploratory, anti-anxiety, social, and sexual behaviors of rats in behavioral estrus is attenuated with inhibition of 3α,5α-THP formation in the midbrain ventral tegmental area. Behav Brain Res. 2008;193:269–276. doi: 10.1016/j.bbr.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeeva A, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–47. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Gizerian S, Lieberman JA, Morrow AL. Perinatal allopregnanolone influences prefrontal cortex structure, connectivity and behavior in adult rats. Neuroscience. 2006;138:809–819. doi: 10.1016/j.neuroscience.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3α,5α-tetrahydroprogesterone (allopregnanolone) availability? Biol Psychiatry. 1998;44:865–873. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5α-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Hardy DF, DeBold JF. Effects of repeated testing on sexual behavior of the female rat. J Comp Physiol Psychol. 1973;85:195–202. doi: 10.1037/h0034895. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi MR, Casteller G, Gargiulo PA, Bregonzio C, Cabrera RJ. The anxiolytic effect of allopregnanolone is associated with gonadal hormonal status in female rats. Eur J Pharmacol. 2001;417:111–116. doi: 10.1016/s0014-2999(01)00865-2. [DOI] [PubMed] [Google Scholar]
- Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–318. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Blaustein JD. Immunocytochemical investigation of nuclear progestin receptor expression within dopaminergic neurones of the female rat brain. J Neuroendocrinol. 2004;16:534–543. doi: 10.1111/j.1365-2826.2004.01198.x. [DOI] [PubMed] [Google Scholar]
- Luttge WG, Hughes JR. Intracerebral implantation of progesterone: re-examination of the brain sites responsible for facilitation of sexual receptivity in estrogen-primed ovariectomized rats. Physiol Behav. 1976;17:771–775. doi: 10.1016/0031-9384(76)90038-x. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Teitelbaum P. Further analysis of sensory inattention following lateral hypothalamic damage in rats. J Comp Physiol Psychol. 1974;86:375–395. doi: 10.1037/h0035941. [DOI] [PubMed] [Google Scholar]
- Marx CE, Duncan GE, Gilmore JH, Lieberman JA, Morrow AL. Olanzapine increases allopregnanolone in the rat cerebral cortex. Biol Psychiatry. 2000;47:1000–1004. doi: 10.1016/s0006-3223(99)00305-4. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Horm Behav. 1995;29:279–295. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Panzica GC. Neuroactive steroids: old players in a new game. Neuroscience. 2006;138:733–739. doi: 10.1016/j.neuroscience.2005.10.066. [DOI] [PubMed] [Google Scholar]
- Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev. 2008;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Palumbo MA, Salvestroni C, Gallo R, Guo AL, Genazzani AD, Artini PG, et al. Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endocrinol Investig. 1995;18:853–856. doi: 10.1007/BF03349832. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pleim ET, DeBold JF. The relative effectiveness of progestins for facilitation and inhibition of sexual receptivity in hamsters. Physiol Behav. 1984;32:743–747. doi: 10.1016/0031-9384(84)90188-4. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Luisi M, Lenzi E, Centofanti M, Begliuomini S, Freschi L, et al. Progesterone and progestins: effects on brain, allopregnanolone and β-endorphin. J Steroid Biochem Mol Biol. 2006;102:205–213. doi: 10.1016/j.jsbmb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci. 2001;1:287–296. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- Rodbard D, Hutt DM. Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. In: International Atomic Energy Agency, editor. Symposium on radioimmunoassay and related procedures in medicine; NY: Uniput; 1974. pp. 209–233. [Google Scholar]
- Roselli CE, Snipes CA. Progesterone 5α-reductase in mouse brain. Brain Res. 1984;305:197–202. doi: 10.1016/0006-8993(84)90425-6. [DOI] [PubMed] [Google Scholar]
- Ross J, Claybaugh C, Clemens LG, Gorski RA. Short latency induction of estrous behavior with intracerebral gonadal hormones in overiectomized rats. Endocrinology. 1971;89:32–38. doi: 10.1210/endo-89-1-32. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Priming of estrous responsiveness by implants of 17β-estradiol in the ventromedial hypothalamic nucleus of female rats. Endocrinology. 1980;106:504–509. doi: 10.1210/endo-106-2-504. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus of ovariectomized, estrogen-primed rats inhibits subsequent facilitation of estrous behavior by systemic progesterone. Brain Res. 1984;294:1–8. doi: 10.1016/0006-8993(84)91303-9. [DOI] [PubMed] [Google Scholar]
- Satou M, Yamanouchi K. Inhibitory effect of progesterone on sexual receptivity in female rats: a temporal relationship to estrogen administration. Zoolog Sci. 1996;13:609–613. doi: 10.2108/zsj.13.609. [DOI] [PubMed] [Google Scholar]
- Schindler CJ, Slamberová R, Vathy I. Bicuculline seizure susceptibility and nigral GABAA α1 receptor mRNA is altered in adult prenatally morphine-exposed females. Psychoneuroendocrinology. 2003;28:348–363. doi: 10.1016/s0306-4530(02)00027-6. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58:69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry. 2003;54:757–762. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3β-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova EP. The effect of different stages of the sex cycle on rat behavior in a plus maze. Zh Vyssh Nery Deiat Im I P Pavloya. 1999;49:1039–1045. [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5alpha-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology. 2006;186:302–311. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K, Arai Y. Dissociation between the display of lordosis and soliciting behaviors in female rats with lesions of the dorsomedial pontine tegmentum. Physiol Behav. 1982;28:155–1559. doi: 10.1016/0031-9384(82)90117-2. [DOI] [PubMed] [Google Scholar]
- Yanase M, Gorski RA. The ability of the intracerebral exposure to progesterone on consecutive days to facilitate lordosis behavior: an interaction between progesterone and estrogen. Biol Reprod. 1976;15:544–550. doi: 10.1095/biolreprod15.4.544. [DOI] [PubMed] [Google Scholar]