Abstract
Objective
To identify predictors of Alzheimer’s disease (AD) versus frontotemporal lobar degeneration pathology in primary progressive aphasia (PPA), and determine whether the AD pathology is atypically distributed to fit the aphasic phenotype.
Methods
Neuropsychological and neuropathological analyses of 23 consecutive PPA autopsies. All had qualitative determination of neurofibrillary tangle (NFT) density. Additional quantitation was done in four of the PPA/AD cases and four AD cases with the typical amnestic dementia of the Alzheimer type.
Results
The sample contained mostly logopenic, agrammatic, and mixed forms of PPA. All six agrammatics had frontotemporal lobar degeneration (five of six with tauopathy). Seven of the 11 logopenics had AD. In logopenics, lower memory scores increased the probability of AD, but there were exceptions. The PPA/AD group showed predominance of entorhinal NFT typical of the amnestic dementia of the Alzheimer type. In the small subgroup examined quantitatively, neocortical NFTs were more numerous in the left hemisphere of PPA/AD. However, the asymmetry was low and inconsistent. Neuritic plaques did not display consistent asymmetry. Apolipoprotein E4, a major risk factor for typical AD, did not predict AD pathology in PPA.
Interpretation
Subtyping PPA helps to predict AD versus frontotemporal lobar degeneration pathology at the group level. However, our results and the literature also indicate that no clinical predictor is completely reliable in individual patients. The inconsistent concordance of NFT distribution with the asymmetric atrophy and the nonamnestic phenotype also raises the possibility that the AD markers encountered at autopsy in PPA may not always reflect the nature of the initiating neurodegenerative process.
The relation of disease markers to dementia phenotypes has been investigated most productively in Alzheimer’s disease (AD). In most AD cases, neurofibrillary tangles (NFTs) emerge and reach their greatest densities in hippocampoentorhinal areas, regions critical for episodic memory.1 Significant correlations exist between entorhinal NFT density and memory scores,2 and between NFT density and regional neuronal loss.3 Shrinkage of the hippocampoentorhinal region is therefore one of the earliest markers of AD,4 and a progressive amnesia is its most typical clinical correlate.5 Autopsy series have also shown that AD pathology is the single most common cause of late-onset progressive memory loss,6 so the term dementia of the Alzheimer type (DAT) has become a generic clinical designation for all amnestic dementias. Less frequently, AD pathology can also lead to syndromes where frontal or parietal dysfunction predominates.7,8 Such atypical phenotypes become associated with equally atypical distributions of NFT in a way that further supports the principles of clinicopathological concordance in this disease.
The primary progressive aphasia (PPA) syndrome is entirely different from that of DAT. It is diagnosed when a language impairment (ie, aphasia), caused by a progressive neurodegenerative disease, constitutes the most salient (ie, primary) aspect of the clinical picture. Memory for recent events is initially preserved, although memory scores obtained on verbally mediated tests may be abnormal. This selectivity of the language deficit is matched by an asymmetric and selective atrophy of the language network in the dominant (usually left) hemisphere.9 In keeping with these clinical differences from DAT, the majority of PPA autopsies have shown the neuropathology of frontotemporal lobar degeneration (FTLD) rather than AD.10–14 Nonetheless, many autopsies from patients with PPA have also shown the amyloid plaques and NFTs of AD. Whether the distribution of AD markers in these aphasic cases differs from patterns seen in the amnestic phenotype of DAT remains poorly understood.
Because accurate diagnosis influences therapy, there has been considerable interest in identifying clinical predictors of FTLD versus AD pathology in PPA. An autopsy series on 38 cases found that fluent/semantic and nonfluent forms of PPA have an equal likelihood (approximately 30%) of having AD pathology,11 whereas a subsequent series from the same group, based on 53 cases of PPA, found AD pathology in 44% of the nonfluent but in only 10% of the fluent/ semantic patients.6 The prediction has been made that a “logopenic” variant of PPA, characterized by frequent word-finding pauses without major grammar or comprehension impairments, is more likely to have AD pathology.15 However, this prediction has not yet been tested in autopsy series. Although the language disorder dominates the early stages of PPA, lesser memory impairments can also arise and have been found to predict AD pathology.16,17 Not all autopsy series, however, confirm this conclusion.11
This report includes 23 consecutive, unselected autopsies on patients with the clinical syndrome of PPA. One goal was to explore factors that could predict AD versus FTLD pathology. Another was to determine whether AD pathology in the aphasic dementia of PPA had a different distribution than in the amnestic dementia of DAT.
Patients and Methods
Patients
Patients fulfilling criteria for PPA18,19 were included and subtyped as logopenic, agrammatic, or semantic based on a modification of guidelines that Gorno-Tempini and colleagues outlined.15,20 Although agrammatic patients were also disfluent, we required the presence of prominent syntax errors (not just low fluency) for inclusion in this subgroup. For example, Patient 20 had sent the following e-mail to her daughter: “I will come my house in your car and drive my car into Chicago…You will back get your car and my car park in my driveway. Love, Mom.” At her initial interview, 2 years after onset, Patient 23 gave the following account of her problem: “I have to force myself to tell people about understand me. Words come out wrong the way.” Patient 4 was the only one classified as semantic based on the constellation of preserved fluency and syntax but abnormal language comprehension. Patients with intact syntax and comprehension but frequent word-finding pauses and variable fluency were classified as logopenic. Patients with agrammatism and also comprehension deficits of comparable magnitude, or whose language output was too limited for specific characterization, were designated as “mixed” in Table 1. Demographic data were analyzed using independent samples t tests. Fisher’s exact test was used to examine group differences in categoric data.
Table 1.
Characteristics of the 23 Cases
| Case No. |
Early Problems of Memory/ Behavior |
Neuro- pathology |
Aphasia Subtype |
Asymmetry | ATAC | TDP-43 | Braak Stage |
Onset Age (yr)/Sex |
Age at Death (yr) |
ApoE | Imaging |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No/No | AD | Mixed | No | No | No | 6 | 58/M | 73 | 3, 4 | MRI = atrophy, most prominent L temporal lobe |
| 2 | No/No | AD | Logopenic | sm (F, T, P) L>R |
Multiple clusters |
No | 6 | 48/F | 59 | 3, 3 | SPECT = decreased perfusion R hemisphere |
| 3* | No/No | AD | Mixed | a (T) L>R | No | No | 6 | 51/M | 72 | 3, 3 | CT = L perisylvian atrophy |
| 4* | No/No | AD | Semantic | No | No | No | 6 | 70/F | 76 | 4, 4 | MRI = small cerebellar infarct, otherwise unremarkable |
| 5* | No/No | AD | Logopenic | a (T) L>R | No | No | 6 | 67/M | 73 | 3, 3 | MRI = nonspecific subcortical changes; SPECT = normal |
| 6 | No/No | AD | Logopenic | sm (F) L>R | No | No | 6 | 71/M | 78 | 3, 4 | SPECT = L temporoparietal hypoperfusion |
| 7 | No/No | AD | Mixed | a (H, T, P) L>R |
Multiple clusters |
No | 6 | 59/M | 71 | 3, 4 | MRI = unremarkable |
| 8 | No/No | AD | Logopenic | sm (F, T, P) L>R |
Single cluster |
No | 6 | 71/F | 78 | 3, 3 | MRI = L perisylvian atrophy |
| 9* | No/No | AD | Logopenic | a, nlg (T) L>R |
Single cluster |
ND | 5 | 47/M | 70 | 3, 3 | PET = L perisylvian and parietofrontal hypometabolism |
| 10 | No/No | AD | Logopenic | a, sm (F, T, P) L>R |
Single cluster |
No | 6 | 58/F | 68 | 3, 3 | PET = L temporoparietal hypometabolism |
| 11 | No/No | AD | Logopenic | ?** | ND | ND | 6 | 80/M | 87 | ND | MRI = atrophy L temporal pole and bilateral frontal |
| 12 | No/No | FTLD-U | Logopenic | ?** | Yes | 0 | 53/M | 62 | 3, 3 | MRI = L perisylvian atrophy; PET = L temp hypoperfusion |
|
| 13 | No/No | FTLD-U | Agrammatic | a (F, T, P) L>R |
Yes | 2 | 56/F | 62 | 3, 3 | SPECT = L frontotemporal hypoperfusion |
|
| 14 | No/No | FTLD-U | Logopenic | No | Yes | 2 | 63/F | 69 | 3, 4 | MRI = unremarkable |
|
| 15 | No/No | FTLD-U (MND) |
Mixed | No | ND | Yes | 2 | 65/F | 69 | 3, 4 | MRI = symmetric; SPECT = L temporal hypoperfusion |
| 16 | No/No | FTLD-U (MND) |
Logopenic | a, nlg (T) L>R |
ND | Yes | 2 | 58/M | 65 | 3, 3 | MRI = L perisylvian and inferotemporal atrophy; SPECT = patchy hypoperfusion mostly L |
| 17 | No/No | FTLD-T (Pick) |
Agrammatic | a, nlg (F, T, P) L>R |
ND | No | 0 | 45/F | 60 | 2, 3 | MRI = atrophy L frontotemporal |
| 18 | No/No | FTLD-T | Logopenic | No | ND | ND | 0 | 56/M | 70 | 3, 4 | EEG = left slowing; SPECT = L frontotemporal hypoperfusion |
| 19 | No/No | FTLD-T (CBD) |
Agrammatic | ?** | ND | No | 0 | 56/M | 66 | 3, 3 | MRI = L perisylvian atrophy |
| 20 | No/No | FTLD-T (CBD) |
Agrammatic | No | ND | No | 1 | 59/F | 65 | 3, 3 | MRI = unremarkable; PET = mild hypometabolism L parietal |
| 21 | No/No | FTLD-T (CBD) |
Mixed | sm, nlg (F) L>R |
No | 0 | 67/M | 78 | 3, 3 | MRI = L perisylvian and temporal/parietal atrophy |
|
| 22 | No/No | FTLD-T | Agrammatic | No | ND | ND | 0 | 71/M | 77 | 2, 3 | MRI = unremarkable; EEG = L frontotemporal slowing |
| 23 | No/No | FTLD-T (CBD) |
Agrammatic | ?** | ND | ND | 0 | 57/F | 67 | 2, 4 | MRI = L temporal atrophy; SPECT = L hypometabolism |
All brains were evaluated grossly for atrophy, and microscopically with hematoxylin and eosin, thioflavin-S, and the Gallyas stain. Additional procedures included thioflavine-S histofluorescence, Gallyas stain for argyrophilia, and immunohistochemistry for tau (AT8; Pierce-Endogen, Rockford, IL), β amyloid (4G8; Signet, Dedham, MA), α-synuclein (LB509; Zymed-Invitrogen, Carlsbad, CA), ubiquitin (polyclonal; DAKO, Carpinteria, CA), and TDP-43 (polyclonal; ProteinTech, Chicago, IL). Clinical details of Cases 9 and 17 were reported previously.23
Cases where stereological neurofibrillary tangle (NFT) quantitation was done.
Asymmetry cannot be determined because only one hemisphere was available.
a = atrophy determined by gross examination of brain surface; AD = Alzheimer’s disease; ApoE = apolipoprotein E; ATAC = argyrophilic thorny astrocyte clusters; CBD = corticobasal degeneration-type tauopathy; CT = computed tomography; EEG = electroencephalogram; F = inferior frontal gyrus; FTLD-T = frontotemporal lobar degeneration with tau-positive inclusions; FTLD-U = frontotemporal lobar degeneration with ubiquinated inclusions; H = hippocampus; MND = motor neuron disease type; MRI = magnetic resonance imaging; ND = not done; nlg = neuronal loss and gliosis; no = none found; P = inferior parietal lobule; PET = positron emission tomography; sm = superficial cortical layer microvacuolation; SPECT = single-photon emission computed tomography; T = superior temporal gyrus.
All specimens were evaluated grossly for atrophy. Microscopic examination was conducted in sections from language-related areas such as the inferior frontal gyrus (IFG), the superior temporal gyrus (STG), and the inferior parietal lobule stained with hematoxylin and eosin, thioflavin-S, and multiple antibodies for the diagnosis of AD and FTLD.21,22 In the FTLD group, those with tau-positive inclusions (including Pick- and corticobasal degeneration–type) were designated FTLD-T, and those with tau-negative but TDP-43 and ubiquitin containing inclusions (including motor neuron disease–type) were designated FTLD-U. Moreover, sections from the middle frontal gyrus, STG, and IFG were examined with Gallyas stains for thorny astrocyte clusters.17
Standardized Consortium to Establish a Registry for Alzheimer’s Disease criteria were always used to rate NFT and neuritic plaque density in entorhinal and language-related neocortical areas. In addition, four of the PPA/AD cases (Cases 3–5 and 9) had sufficient numbers of serially cut thioflavin-S sections from both hemispheres to allow stereological quantitation in the entorhinal cortex, IFG, inferior parietal lobule, and STG. In this subset (Cases 3–5 and 9 in Table 1), the Fractionator method from the Stereoinvestigator software (Microbrightfield, Williston, VT) was used to count NFTs throughout the section thickness. Data were entered into the software to adjust section thickness for dehydration and to account for intersection distance so that the counts could be expressed as NFTs per cubic millimeter. The findings were compared with counts obtained with the same method in a group of four additional cases from our brain bank who had typical amnestic DAT and the pathology of AD. These four DAT/AD comparison cases were chosen by section availability, comparable disease severity (Clinical Dementia Rating of 3) at the last examination before death, and age at death (see Table 3). The counting was done on 13 to 22 slides in each of these 8 cases by an investigator blinded to clinical diagnosis and then analyzed with analysis of variance.
Table 3.
Distribution of Neuropathological Markers
| Cases | Age at Death (yr) |
NFT Neocortex | NFT Entorhinal |
Neuritic Plaques Neocortex |
||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||||
| PPA/AD Case 1 | 73 | F | +++ | ++ | +++ | F | +++ | +++ |
| T | +++ | +++ | T | +++ | +++ | |||
| P | +++ | +++ | P | +++ | +++ | |||
| PPA/AD Case 2 | 59 | F | +++ | +++ | +++ | F | +++ | +++ |
| T | +++ | +++ | T | +++ | +++ | |||
| P | +++ | +++ | P | +++ | +++ | |||
| PPA/AD Case 3 | 72 | F, T, P | 2.73 | 1.70 | 30.96 | F | ++ | +++ |
| T | +++ | +++ | ||||||
| P | ++ | ++ | ||||||
| PPA/AD Case 4 | 76 | F, T, P | 6.67 | 2.75 | 50.39 | F | +++ | +++ |
| T | +++ | +++ | ||||||
| P | +++ | +++ | ||||||
| PPA/AD Case 5 | 73 | F, T, P | 3.82 | 1.64 | 21.84 | F | +++ | +++ |
| T | +++ | +++ | ||||||
| P | +++ | +++ | ||||||
| PPA/AD Case 6 | 78 | F | +++ | +++ | +++ | F | +++ | +++ |
| T | +++ | +++ | T | +++ | +++ | |||
| P | +++ | +++ | P | +++ | +++ | |||
| PPA/AD Case 7 | 71 | F | +++ | ++ | +++ | F | +++ | ++ |
| T | +++ | +++ | T | +++ | +++ | |||
| P | +++ | +++ | P | +++ | +++ | |||
| PPA/AD Case 8 | 78 | F | +++ | +++ | +++ | F | +++ | ++ |
| T | +++ | +++ | T | +++ | +++ | |||
| P | +++ | +++ | P | +++ | +++ | |||
| PPA/AD Case 9 | 70 | F, T, P | 1.55 | 1.44 | 29.95 | F | + | ++ |
| T | +++ | +++ | ||||||
| P | +++ | +++ | ||||||
| PPA/AD Case 10 | 68 | F | +++ | +++ | +++ | F | +++ | +++ |
| T | +++ | +++ | T | +++ | +++ | |||
| P | +++ | +++ | P | +++ | +++ | |||
| DAT/AD Case 1 | 72 | F, T, P | 3.93 | 3.38 | 35.02 | |||
| DAT/AD Case 2 | 79 | F, T, P | 1.52 | 0.78 | 39.43 | |||
| DAT/AD Case 3 | 58 | F, T, P | 5.23 | 4.75 | 20.83 | |||
| DAT/AD Case 4 | 78 | F, T, P | 1.39 | 2.59 | 23.85 | |||
In the 10 primary progressive aphasia/Alzheimer’s disease (PPA/AD) cases where tissue was available, neurofibrillary tangles (NFT) and neuritic plaques were assessed qualitatively and rated as sparse (+), moderate (++), or frequent (+++) in the inferior frontal gyrus (F), inferior parietal lobule (P), and superior temporal gyrus (T) according to established Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)–based neuropathological criteria.55 NFT numbers in Cases 3, 4, 5, and 9 and in the four dementia of the Alzheimer type (DAT)/AD cases are given per cubic millimeter. These were the only cases where multiple serially cut sections through the relevant areas were available in thioflavin-S–stained preparations suitable for stereology. Quantitative neocortical counts reflect the average derived from the inferior frontal gyrus, superior temporal gyrus, and inferior parietal lobule sections (F, T, P). The entorhinal densities reflect an average of the two sides. Within the set of stereologically analyzed cases, the qualitative ratings (data not shown) showed asymmetry only in Case 3 and only in the inferior frontal gyrus (++ in the right, +++ in the left). Although the stereologically determined neocortical NFT counts appear low, they represent densities per cubic milllimeter and are all in the high +++ range of qualitative assessment.
Results
There were 11 AD and 12 FTLD cases. In the FTLD group, five had FTLD-U and seven had FTLD-T. Abnormal TDP-43 immunohistochemistry was detected in all FTLD-U cases but not in FTLD-T or AD cases. PPA/AD cases had Braak stage 6 neurofibrillary degeneration, with the exception of Case 9 who had a Braak stage of 5. Most (7/11) logopenics had AD, and all 6 agrammatics had FTLD, 5 with tauopathy (FTLD-T) (see Table 1).
Clinical, Demographic, and Imaging Data
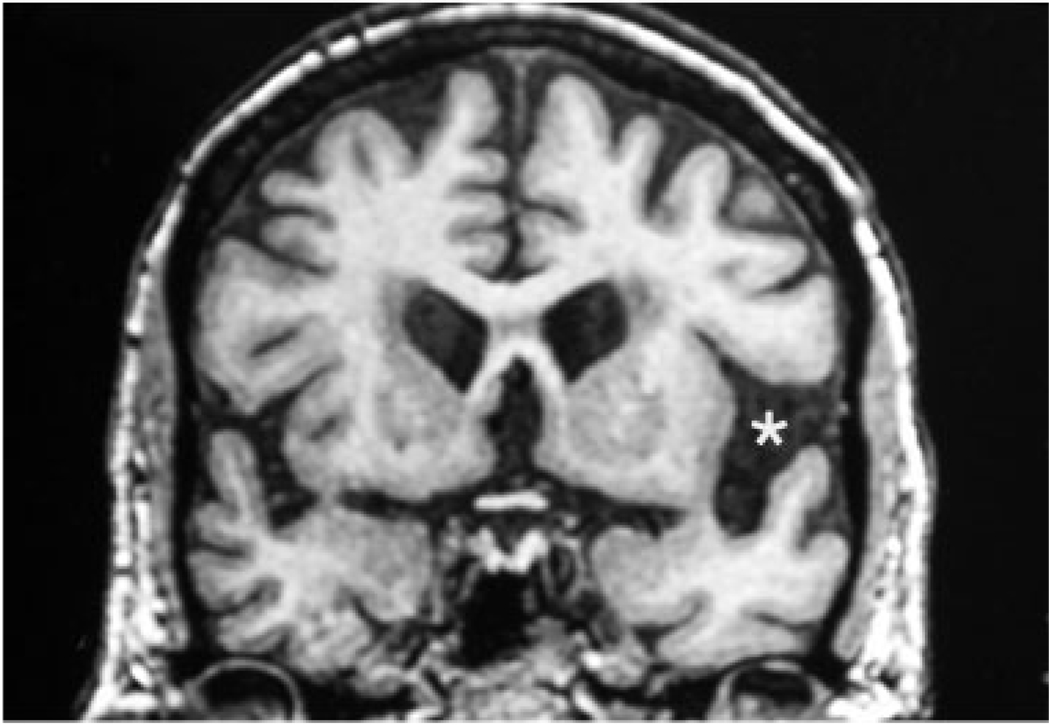
The AD and FTLD groups did not differ significantly in sex, duration of illness, or family history of dementia. Age at death was significantly younger (67.4 ± 5.7 vs 73.2 ± 7.0 years; p = 0.04) for the FTLD group. Although mean age of onset was not significantly different, symptom onset at 70 years or older occurred in four AD but only one FTLD case. Neuroimaging in both groups showed more atrophy, hypoperfusion, and hypometabolism in the left hemisphere, especially in the perisylvian areas (Fig).
Fig. 1.
Coronal magnetic resonance imaging (MRI) scan of Case 8 done 1 year after onset. The left perisylvian cistern is wider (asterisk), indicating more atrophy on that side. Postmortem examination also showed asymmetry, with greater microvacuolation in the left neocortex. However, there were no asymmetries of neurofibrillary tangle (NFT) distribution. Visual inspection of the MRI also showed that perisylvian atrophy was more severe than medial temporal atrophy. However, the NFT density did not show a perisylvian over entorhinal predominance. This patient illustrates the inconsistency of correlation between atrophy patterns and NFT distribution in primary progressive aphasia/Alzheimer’s disease (PPA/AD).
At initial disease stages, aphasia was the most salient feature; no significant behavioral abnormalities were present; and episodic memory for events was preserved as established by history, clinical examination, and chart review. In 20 of the cases, additional standardized testing of memory, naming, and fluency was done (Table 2). Results were converted to Z-scores, taking into account age and education, to facilitate comparisons of impairment severity across tests. Years of disease duration at the time of testing was not significantly different in the two groups (3.3 ± 1.5 years for AD vs 3.7 ± 1.7 years in FTLD). In each case where both domains had been tested, performance on at least one of the three language tests was worse than the performance on the memory tests, confirming the greater impairment of language even beyond the initial stages of the disease. In the logopenic group, cases with the lowest memory scores (Cases 5, 8, 10, 11) had AD. However, there were also two logopenic cases (Cases 2 and 9) with normal memory who turned out to have AD, and one with abnormal scores, albeit of lesser severity (Case 12), who had FTLD. Naming and lexicosemantic fluency scores overlapped in the two groups, indicating equivalent aphasia severity. Word and sentence comprehension was assessed during the neurological and speech/language examination of all cases. Only the “mixed” and “semantic” cases had comprehension deficits. Word comprehension scores on the Boston Diagnostic Aphasia Examination and Western Aphasia Battery were available for 18 cases. Word comprehension accuracy scores in the agrammatics and logopenics within this group was 85% or better.
Table 2.
Characteristics of the Twenty Cases Who Had Standardized Memory and/or Language Tests
| Case No. |
Diagnosis (years to test)a |
Aphasia Subtype |
Verbal Memory |
Nonverbal Memory |
Object Naming |
Lexical Fluency |
Category Fluency |
|---|---|---|---|---|---|---|---|
| 2 | AD (4) | Logopenic | 0.5 (WST-W) | 0.5 (WST-S) | ND | ND | ND |
| 4 | AD (2) | Semantic | −3.8 | ND | −7.5 | −2.7 | −2.9 |
| 5 | AD (3) | Logopenic | −3.5 | −2.3 | −10.5 | −2.2 | −4.1 |
| 6 | AD (2) | Logopenic | −0.7 | −1.1 | 0.5 | −0.4 | −2.4 |
| 7 | AD (6) | Mixed | −2.3 | −3.7 | −19.4 | −2.4 | −2.4 |
| 8 | AD (2) | Logopenic | −3.2 | −1.6 | −4.2 | ND | −1.9 |
| 9 | AD (5) | Logopenic | 0.97 (Rey AVLT) |
ND | −2.8 | −0.9 | −0.9 |
| 10 | AD (4) | Logopenic | ND | −3.2 | −18.9 | ND | ND |
| 11 | AD (2) | Logopenic | −2.9 | ND | −5.4 | −2.9 | −2.8 |
| 12 | FTLD-U (4) | Logopenic | −1.6 | 0.3 | −15.5 | −2.9 | −1.8 |
| 13 | FTLD-U (2) | Agrammatic | −0.5 | −0.3 | −1.5 | −3.0 | −1.6 |
| 14 | FTLD-U (2) | Logopenic | −1.2 | −0.1 | −9 | −2.2 | −2.4 |
| 15 | FTLD-U (2) | Mixed | −3.5 (WRMT- W) |
−3.4 (WRMT-F) | −7.3 | ND | ND |
| 16 | FTLD-U (4) | Logopenic | 1.8 (WMS-R) | ND | −7.6 | −1.6 | −1.7 |
| 17 | FTLD-T (6) | Agrammatic | 1.0 (Rey AVLT) |
ND | 0.5 | −1.8 | −0.4 |
| 19 | FTLD-T (4) | Agrammatic | −0.5 | 0.6 | 0.4 | −1.5 | 1.5 |
| 20 | FTLD-T (4) | Agrammatic | 0.6 | −1.8 | −0.6 (BDAE) |
ND | −2.0 |
| 21 | FTLD-T (7) | Mixed | −3.5 | ND | −7.0 | ND | −3.8 |
| 22 | FTLD-T (2) | Agrammatic | 0.5 (WST-W) | 0.5 (WST-S) | 0.7 | ND | −0.5 |
| 23 | FTLD-T (4) | Agrammatic | −1.2 | −0.7 | −14.9 | −2.4 | −3.0 |
All performance data are given as Z-scores (a Z-score of 1 indicates performance 1 standard deviation [SD] better than average and a Z-score of −1 indicates performance 1 SD below average). Unless indicated otherwise, verbal memory was tested by the delayed Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) word list recall,47 nonverbal memory by the delayed Visual Reproduction Subtest of a version of the Wechsler Memory Scale,48 Object Naming by the Boston Naming Test,49 Lexical Fluency by the FAS test,50 and category fluency by the generation of animal names.47 Other tests that were used in individual cases include Boston Diagnostic Aphasia Examination (BDAE)51; Rey Auditory Verbal Learning Test (Rey AVLT), 30-minute delayed recall condition52; Logical memory of the Wechsler Memory Scale-Revised (WMS-R), 30-minute delayed recall; Warrington Recognition Memory Test for Faces (WRMT-F)53; Warrington Recognition Memory Test for Words (WRMT-W)53; the 3-word/3-shape test for shapes (WST-S), 30-minute delay condition54; the 3-word/3-shape test for words (WST-W), 30-minute delayed-recall condition.54
Numbers in parentheses indicate the number of years elapsed between symptom onset and the date of the examination corresponding to the listed scores. A number 2, for example, indicates that 2 years had elapsed since symptom onset and that the testing was done in the third year of disease.
AD = Alzheimer’s disease; ND = test not done; FTLD-U = frontotemporal lobar degeneration with ubiquinated inclusions; FTLD-T = frontotemporal lobar degeneration with tau-positive inclusions.
Apolipoprotein Genotyping and Neuropathology
The apolipoprotein E4 allele frequency did not differentiate the AD group from the FTLD group (see Table 1). At postmortem examination, asymmetrically greater left hemisphere atrophy, neuronal loss, microvacuolation, and gliosis were seen in 4 of the 9 PPA/FTLD cases and 8 of the 10 PPA/AD cases where both hemispheres were available (see Table 1). However, standard Consortium to Establish a Registry for Alzheimer’s Disease–based qualitative neuropathological assessment showed leftward asymmetry in only three of the PPA/AD cases (Cases 1, 3 and 7). In these three cases, NFT densities on the left were slightly higher than those on the right (+++ vs ++) in the IFG but not the STG or inferior parietal lobule. (Table 3). Standard neuropathological assessments of NFT density did not show any atypical preponderance of neocortical over entorhinal NFT density.
The stereological NFT quantitation of the four PPA/AD cases (Cases 3–5 and 9) and the four DAT/AD cases is shown in Table 3. The NFT counts in these cases were calculated per cubic millimeter of tissue. The similar number of entorhinal NFTs (33.37 ± 6.7 in PPA vs 29.96 ± 4.50 in DAT; p = 0.67) indicates that the two groups had comparable levels of AD disease severity. Despite the small number of cases, the PPA/AD group had more neocortical NFT in the left than the right (3.71 ± 0.79 vs 1.90 ± 0.79; p < 0.005), and this left-right asymmetry was greater than in the DAT/AD group (1.81 ± 0.41 vs −0.06 ± 0.46; p < 0.03). The degree of asymmetry in this small subset of four PPA/AD cases was variable. Case 9, for example, had almost none. Furthermore, the magnitude of asymmetry in the other three cases was small enough that it was detected by qualitative grading of NFT density only in the IFG of Case 3, as noted earlier. The NFT counts in language-related left-hemisphere neocortex were not significantly different in the two groups (3.69 ± 1.10 in PPA vs 2.96 ± 0.96 in DAT; p = 0.63). In both groups, NFTs were at least an order of magnitude more numerous in entorhinal than in language areas (p < 0.0001). No consistent neocortical asymmetry was seen in neuritic plaques. In 10 of the 11 PPA/AD cases, tissue was examined for argyrophilic thorny astrocyte clusters (ATAC). None was seen in five cases, a single cluster in three, and multiple clusters in two (see Table 1). None of the four DAT/AD cases had ATAC.
Case Report of a Primary Progressive Aphasia/Alzheimer’s Disease Patient with Serially Documented Nonamnestic Course
The initial course of Case 9 was reported previously.23,24 A brief account, including his final 14 years, is summarized to illustrate the profound divergence from the DAT phenotype. His first examination at age 52, 5 years after onset, showed word-finding pauses, phonemic paraphasias, and mild anomia (Boston Naming Test score = 48/60). Semantic category fluency was normal. Performance was superior (memory quotient, 124) on the Wechsler Memory Scale (WMS), high average (26/30) on the Hooper Visual Organization Test, and average on the Facial Recognition Test (40/54).
Nine years after onset, the Boston Naming Test score declined from 48 to 32, and category fluency from 16 to 8. Syntax and comprehension remained unremarkable. On the Facial Recognition task, his score improved from 40 to 46, and on the Hooper Visual Organization Test from 26 to 28. Reasoning, tested by Raven’s Progressive Matrices, changed minimally from 47 of 60 to 44 of 60. Orientation on the WMS remained perfect (11/11), design recall remained in the unimpaired range, and the Rey Auditory Verbal Learning Test delayed recall (inadvertently omitted at the 9-year mark) was 13 of 15 at initial testing and 12 of 15 at the 8-year mark.
His son-in-law reported that the patient had behaved appropriately on their plane trip for the last visit to our clinic, 20 years after symptom onset, at a stage where he had no intelligible written or verbal output. On returning from the clinic, the patient led the way to the hotel and prevented his son-in-law from taking a wrong turn. A year later, he became agitated and did not respond to reassurances from family members. He died at the age of 70, 23 years after symptom onset. Autopsy showed AD pathology at the Braak 5 stage. Despite greater neuronal loss and dysfunction in the left hemisphere, shown by imaging and gross inspection of the brain, NFT counts in language cortices showed almost no hemispheric asymmetry (see Table 3).
Discussion
Guidelines for subtyping PPA are still evolving. We subdivided disfluent patients who would have fit Neary and colleagues’25 criteria for progressive nonfluent aphasia into agrammatic and logopenic variants, as Gorno-Tempini and colleagues15 proposed. In our sample of 23 autopsies, all 6 agrammatic cases had FTLD. However, the literature also contains agrammatic cases with AD pathology.26 In keeping with some11 but not all PPA autopsy series,27 we found that the agrammatic subjects were more likely to have FTLD-T than FTLD-U. The logopenic group was more heterogeneous. The majority (7/11) had AD, a result that confirms Gorno-Tempini and colleagues’15 prediction. In the logopenic group, lower memory scores with Z-values around −3 were predictive of AD pathology but were not necessary for this diagnosis. In particular, Case 9, whose memory preservation was rigorously documented by serial testing for many years after aphasia onset, also had AD pathology. The relation of low memory scores to AD pathology in PPA is consistent with Kertesz and colleagues’16 findings. Because one of the logopenic cases with FTLD (Case 12) also had abnormal memory scores (Z-score = −1.6), the predictive value of this marker at the level of individual patients is likely to be relative rather than absolute.
Our sample had only one semantic PPA case. Other series have found a 10 to 30% frequency of AD pathology in patients with characteristics of semantic PPA but have not identified clinical markers that distinguish this subgroup from semantic patients with FTLD.6,11 The evidence from the present sample and othere autopsy series in the literature would therefore advise caution in predicting, on clinical grounds, whether an individual PPA patient will have AD or FTLD pathology. At the group level, however, the agrammatic pattern favors FTLD-T pathology, whereas the logopenic pattern with low memory scores favors AD. Our results show that the predictive value of clinical diagnosis is improved by replacing the progressive nonfluent aphasia designation with designations of “agrammatic PPA” and “logopenic PPA.”
The most important risk factor for “typical” DAT/ AD, the e4 allele of apolipoprotein E, was not a risk factor for AD pathology in PPA, suggesting that the PPA and DAT phenotypes differ with respect to molecular risk factors for AD pathology.28 The use of apolipoprotein E genotyping for predicting AD versus FTLD pathology in PPA is therefore not justified. Age at symptom onset was not significantly different in the two groups, but there were 4 PPA/AD patients with onset at age 70 and older as opposed to one such PPA/ FTLD case. The one significant relationship, clearly of no predictive value, was the age of death, so that only 2 of the 11 FTLD cases died after the age of 70, whereas 8 of the 11 AD cases died after age 70.
The concordance among the temporal course of NFT density, distribution of atrophy, and evolution of clinical features is extensively documented in the amnestic DAT phenotype of AD.29–32 However, AD pathology has also been reported with other clinical patterns such as progressive visuospatial dysfunction. In such patients, the NFT densities in visual areas (BA17, BA18), are up to 25-fold greater than in typical DAT/ AD, and the subiculum of the hippocampoentorhinal complex has fewer tangles than BA18, a reversal of the pattern seen in typical DAT/AD.33,34 Another atypical correlate of AD neuropathology is the frontal lobe syndrome where frontal NFT densities have been 10-fold greater than in typical DAT/AD patients.7 In distinct contrast with DAT/AD, frontal NFT densities were also higher in frontal neocortex than in the hippocampoentorhinal complex of these patients.
Clinically concordant hemispheric asymmetries have also been reported in AD. In one patient, progressive left hemiparesis was associated with an NFT density 10 times greater in the contralateral right somatosensory cortex than in the left.35 In three patients with the typical amnestic onset of DAT, who then experienced development of prominent aphasia and asymmetric left cortical atrophy, NFT densities were also asymmetrically higher in the neocortical regions of the atrophic hemisphere.36 The patients did not meet the criteria for PPA because of the early amnesia. Nonetheless, the prominence of the aphasia was mirrored by the asymmetric density of NFT that fit the pattern of cortical atrophy.
These examples show that the distribution of NFT in AD can be congruent with functional impairment and cortical atrophy patterns not only in typical DAT but also in atypical clinical presentations, and that this concordance persists until death. If similar concordance existed in PPA/AD, NFT in neocortex would be expected to be more numerous than in entorhinal cortex, and the NFT counts in the left hemisphere would be expected to be greater than counts in the right hemisphere and also greater than counts in analogous neocortices of DAT/AD. However, microscopic analyses in the 10 PPA/AD patients where tissue from both hemispheres was available demonstrated no atypical instance in which neocortical NFTs predominated over entorhinal NFTs. The small subset of four PPA/AD and four DAT/AD cases where the NFTs were stereologically quantitated showed that the PPA/AD group had a significantly greater leftward asymmetry of neocortical NFT in language-related parts of the brain. However, the magnitude of this asymmetry was much lower than in some of the atypical cases mentioned earlier; it was not always detectable by standard Consortium to Establish a Registry for Alzheimer’s Disease ratings of density; and it was inconsistent, as shown by the nearly symmetric NFT distribution in Patient 9, who had a distinctly aphasic profile for many years and a left hemisphere predominance of dysfunction. Furthermore, the NFT counts in language-related left-hemisphere neocortical areas were nearly identical in the two groups despite the distinctly different phenotypes. This inconsistency of clinicopathological concordance in our cases is in keeping with the literature on AD pathology in PPA.
The PPA patient that Engel and Fleming37 reported had asymmetric left perisylvian atrophy and AD neuropathology. However, NFT density was higher in entorhinal cortex than in language-related neocortical areas, and asymmetry was not mentioned. Green and coworkers38 also reported a PPA/AD patient in whom NFTs were maximal in the hippocampoentorhinal complex and without hemispheric asymmetry. One of the three PPA cases that Kempler and investigators39 reported had AD at autopsy, but there was no mention of NFT distribution. The eight “possible” PPA cases with AD pathology that Munoz and colleagues17 reported and the case that Li and coworkers40 reported are also said to have had a “typical” distribution of AD-specific lesions, although no quantitation was reported.17
The Cambridge group reported six cases with “progressive aphasia” and qualitative assessment of AD pathology.41 In three cases, NFT had a pattern indistinguishable from DAT/AD. A fourth case had no plaques and would not appear to fit the diagnosis of AD. Of the remaining two cases, Case PB had more NFTs in temporal than in entorhinal cortex, and Case AS had more NFTs in temporal, parietal, and frontal cortices than in entorhinal cortex. Case AS had been reported previously in a separate publication by the same group.26 Cases PB and AS are thus the only examples of PPA/AD we found in the literature showing greater NFT densities in language- than in memory-related parts of the brain, albeit without evidence of hemispheric asymmetry.
We therefore agree with Munoz and colleagues17 that there is relatively little correspondence between the anatomic distribution of disease markers and clinical features in PPA patients with AD pathology. This contrasts with other atypical AD phenotypes in which NFT distributions become equally atypical in ways that mirror the patterns of atrophy and clinical deficits. Furthermore, a major risk factor for AD, the e4 allele of apolipoprotein E, is not predictive of AD pathology in our PPA sample. The one significant correlate of AD pathology was age at death, an interesting relation in light of the correlation of autopsy age to NFT density, even in nondemented subjects.42,43
The often-quoted statement that PPA can be caused by atypically distributed AD pathology may therefore need to be revised. Although there is agreement that many PPA patients have the neuropathological markers of AD at death, it is not entirely clear that these markers are necessarily responsible for the initial aphasic presentation and the associated asymmetric atrophy of language-related areas in the brain. This question becomes particularly relevant to Case 9, in whom the “aphasia without amnesia” pattern had been documented over many years, in whom imaging and autopsy showed asymmetrically greater left hemisphere abnormalities, but in whom the microscopic examination demonstrated typical AD with no significant asymmetry or neocortical preponderance of NFT.
This evidence raises the possibility that some cases of PPA/AD may reflect a truly atypical presentation of AD, whereas others may have a concomitant process that triggers the distinctive clinical picture, but that subsequently becomes overshadowed by AD markers. As an illustration of this possibility, Amador-Ortiz and coauthors44 reported TDP-43 immunoreactivity characteristic of concomitant FTLD in 23% of their AD cases. Such overlap was not seen in our nine PPA/AD cases where TDP-43 was investigated. Munoz and colleagues17 described ATAC in PPA/AD, but not in DAT/AD, and hypothesized that these clusters are markers “of a pathological process concurrent with AD, and related to the focality of the clinical presentation.” In our sample, only 2 of the 11 PPA/AD cases had multiple ATACs. This is an interesting lead to follow, although it does not appear to provide an answer applicable to all PPA/AD cases.
In the future, cerebrospinal fluid analyses of β-amyloid and phosphotau may help to predict underlying pathology in PPA.45 Positron emission tomography with amyloid and NFT ligands could favor a diagnosis of FTLD when negative. There may also be alternative mechanisms, perhaps based on amyloid oligomers or a special subset of plaques, that would reconcile the role of AD pathology in PPA/AD even in the absence of concordant NFT distributions. However, it is worth pointing out that in vivo amyloid imaging in DAT has not shown a relation between left-hemisphere amyloid binding and language impairment.46 Further clarification of the questions raised by AD pathology in PPA could demonstrate novel principles of clinicopathological correlations and may help to explore the unique molecular fingerprints of the left-hemisphere language network that make it the selective target of atrophy and dysfunction in PPA.
Acknowledgments
This work was supported by the (National Institute on Deafness and Communication Disorders, DC008552, M.M.; Alzheimer Disease Center, National Institute on Aging, AG13854, M.M.).
References
- 1.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand. 1996;165 suppl:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 2.Guilloz AL, Weintraub S, Mash DC, Mesulam M-M, et al. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 3.Bobinski M, Wegiel J, Wisniewski HM, et al. Neurofibrillary pathology—correlation with hippocampal formation atrophy in Alzheimer disease. Neurobiol Aging. 1996;17:909–919. doi: 10.1016/s0197-4580(97)85095-6. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Slomkowski M, Gracon S, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60:253–260. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKhann G, Drachman DA, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 6.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JK, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 8.Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res. 1997;37:3609–3625. doi: 10.1016/S0042-6989(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 9.Sonty SP, Mesulam M-M, Thompson CK, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- 10.Rossor MN, Revesz T, Lantos PL, Warrington EK. Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain. 2000;123:267–276. doi: 10.1093/brain/123.2.267. [DOI] [PubMed] [Google Scholar]
- 11.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 12.Kertesz A, Hudson L, Mackenzie IRA, Munoz DG. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44:2065–2072. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- 13.Turner RS, Kenyon LC, Trojanowski JQ, et al. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol. 1996;39:166–173. doi: 10.1002/ana.410390205. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam M-M, Weintraub S. Spectrum of primary progressive aphasia. In: MN Rossor., editor. Unusual Dementias. London: Baillière Tindall; 1992. pp. 583–609. [PubMed] [Google Scholar]
- 15.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kertesz A, McMonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 17.Munoz DG, Woulfe J, Kertesz A. Argyrophilic thorny astrocyte clusters in association with Alzheimer’s disease pathology in possible primary progressive aphasia. Acta Neuropathol. 2007;114:347–357. doi: 10.1007/s00401-007-0266-x. [DOI] [PubMed] [Google Scholar]
- 18.Mesulam M-M. Primary progressive aphasia: a language-based dementia. N Engl J Med. 2003;348:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 20.Rogalski E, Mesulam M-M. An update on primary progressive aphasia. Curr Neurol Neurosci Rep. 2007;7:388–392. doi: 10.1007/s11910-007-0060-0. [DOI] [PubMed] [Google Scholar]
- 21.Hyman BT, Trojanowski JQ. Editorial on consensus recommendations for the postmortem diagnosis of Alzheimer’s disease from the National Institute on Aging and the Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 22.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- 24.Chawluk JB, Mesulam MM, Hurtig H, et al. Slowly progressive aphasia without generalized dementia: studies with positron emission tomography. Ann Neurol. 1986;19:68–74. doi: 10.1002/ana.410190112. [DOI] [PubMed] [Google Scholar]
- 25.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 26.Greene JDW, Patterson K, Xuereb J, Hodges JR. Alzheimer disease and nonfluent progressive aphasia. Arch Neurol. 1996;53:1072–1079. doi: 10.1001/archneur.1996.00550100158027. [DOI] [PubMed] [Google Scholar]
- 27.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 28.Mesulam M-M, Johnson N, Grujic Z, Weintraub S. Apolipoprotein E genotypes in primary progressive aphasia. Neurology. 1997;49:51–55. doi: 10.1212/wnl.49.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Arnold SE, Hyman BT, Flory J, et al. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 30.Hyman BT, Damasio AR, Van Hoesen GW, Barnes CL. Alzheimer’s disease: cell specific pathology isolates the hippocampal formation. Science. 1984;298:83–95. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 31.Mesulam M-M. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 32.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 33.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 34.Hof PR, Bouras C, Constantinidis J, Morrison JH. Selective disconnection of specific visual association pathways in cases of Alzheimer’s disease presenting with Balint’s syndrome. J Neuropathol Exp Neurol. 1990;49:168–194. doi: 10.1097/00005072-199003000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Jagust WJ, Davies P, Tiller-Borcich JK, Reed BR. Focal Alzheimer’s disease. Neurology. 1990;40:14–19. doi: 10.1212/wnl.40.1.14. [DOI] [PubMed] [Google Scholar]
- 36.Giannakopoulos P, Hof PR, Bouras C. Alzheimer disease with asymmetric atrophy of the cerebral hemispheres: morphometric analysis of four cases. Acta Neuropathol. 1994;88:440–447. doi: 10.1007/BF00389496. [DOI] [PubMed] [Google Scholar]
- 37.Engel PA, Fleming PD. Primary progressive aphasia, left anterior atrophy, and neurofibrillary hippocampal pathology: observations in an unusual case. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:213–218. [PubMed] [Google Scholar]
- 38.Green J, Morris JC, Sandson J, et al. Progressive aphasia—a precursor of global dementia. Neurology. 1990;40:423–429. doi: 10.1212/wnl.40.3_part_1.423. [DOI] [PubMed] [Google Scholar]
- 39.Kempler D, Metter EJ, Riege WH, et al. Slowly progressive aphasia: three cases with language, memory, CT and PET data. J Neurol Neurosurg Psychiatry. 1990;53:987–993. doi: 10.1136/jnnp.53.11.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li F, Iseki E, Kato M, et al. An autopsy case of Alzheimer’s disease presenting with primary progressive aphasia: a clinicopathological and immunohistochemical study. Neuropathology. 2000;20:239–245. doi: 10.1046/j.1440-1789.2000.00343.x. [DOI] [PubMed] [Google Scholar]
- 41.Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123:484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- 42.Jellinger KA. Alzheimer’s changes in non-demented and demented patients. Acta Neuropathol. 1995;89:112–113. doi: 10.1007/BF00294269. [DOI] [PubMed] [Google Scholar]
- 43.Davis DG, Schmitt A, Wekstein DR, Merkesberry WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Amador-Ortiz C, Lin W-L, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grossman M, Farmer J, Leight S, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer’s disease. Ann Neurol. 2005;57:721–729. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- 46.Nelissen N, Vandenbulcke M, Fannes K, et al. Aβ amyloid deposition in the language system and how the brain responds. Brain. 2007;130:2055–2069. doi: 10.1093/brain/awm133. [DOI] [PubMed] [Google Scholar]
- 47.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 49.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 50.Benton A, Hamsher KdS. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1989. [Google Scholar]
- 51.Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. 3rd ed. Austin, TX: Pro-Ed; 2001. [Google Scholar]
- 52.Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 53.Warrington E. Recognition Memory Test manual. Windsor, United Kingdom: NFER-Nelson; 1984. [Google Scholar]
- 54.Weintraub S, Peavy GM, O’Connor M, et al. Three words -three shapes: a clinical test of memory. J Clin Exp Neuropsychol. 2000;22:267–278. doi: 10.1076/1380-3395(200004)22:2;1-1;FT267. [DOI] [PubMed] [Google Scholar]
- 55.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]