Abstract
Geometric considerations indicate that the human translational vestibulo-ocular reflex (tVOR) should have substantially different properties than the angular vestibulo-ocular reflex (aVOR). Specifically, tVOR cannot simultaneously stabilize images of distant and near objects on the retina. Most studies make the tacit assumption that tVOR acts to stabilize foveal images even though, in humans, tVOR is reported to compensate for less than 60% of foveal image motion. We have determined that the compensation gain (eye rotational velocity / required eye rotational velocity to maintain foveal target fixation) of tVOR is held steady at ~ 0.6 during viewing of either near or distant targets during vertical (bob) translations in ambient illumination. We postulate that tVOR evolved not to stabilize the image of the target on the fovea, but rather to minimize retinal image motion between objects lying in different depth planes, in order to optimize motion parallax information. Such behavior is optimized when binocular visual cues of both far and distant targets are available in ambient light. Patients with progressive supranuclear palsy or cerebellar ataxia show impaired ability to increase tVOR responses appropriately when they view near targets. In cerebellar patients, impaired ability to adjust tVOR responses to viewing conditions occurs despite intact ability to converge at near. Loss of the ability to adjust tVOR according to viewing conditions appears to represent a distinct disorder of vestibular function.
Keywords: Locomotion, moving platform, motional parallax, PSP, cerebellar ataxia
INTRODUCTION
Thomas Brandt has championed the idea that studies of vestibular reflexes and their disorders should be interpreted from the broader viewpoint of how the organism achieves clear vision and stable balance. For example, disorders such as downbeat nystagmus should not be viewed in isolation, but be thought of as the disturbance of a complex system that normally allows humans to stand, walk erect, and see clearly during locomotion.1,2 Such an approach can be applied to studies of the human linear or translational vestibulo-ocular reflex (tVOR), which has not received as much attention as the rotational or angular vestibulo-ocular reflex (aVOR) until recently, mainly because of methodological limitations. Thus, on the one hand, aVOR is easily tested at the bedside with rapid head rotations and, in the laboratory, using swivel chairs. On the other hand, head-on-body translations are less easy to administer at the bedside, and testing tVOR in the laboratory requires special devices to induce linear accelerations, which are often expensive. And yet, the erect, straight-legged gait of humans induces substantial head translations, especially in the vertical plane (referred to, here, as bob).3 Thus, there is a need for more studies of tVOR.
The geometry of visual requirements of aVOR differ from those of tVOR. Thus, on the one hand, aVOR is required to stabilize images across the whole visual field during head turns, and can achieve this when viewing optical infinity. During viewing of a near target, eye rotations may need to rotate over 30% more than the head rotations that induce them, because the rotational axis of the head is posterior to that of the eyes.4 Thus, under near-viewing conditions, there will be some relative motion between the near image located on the retinal fovea, and far images located on retinal periphery. On the other hand, tVOR is only called into action during near viewing;5 during far viewing, head translations do not induce retinal image slip, and tVOR is not required. During viewing of a near object, eye rotations due to tVOR need to increase substantially, compared with far-viewing, in order to hold the object’s image steady on the fovea. If images of the near target are held steady on the retina, the consequence is that images of the distant surround will move rapidly across the retinal periphery. Here we argue that the human tVOR does not generate eye rotations to stabilize the image of a near target on the fovea but, rather, generates eye rotations to minimize retinal image motion of the near target with respect to image motion of the distant background.6
Prior Studies of tVOR in Humans
Early studies of tVOR suffered from several important drawbacks. First, subjects were often tested in darkness or while viewing a distant target. Second, in some studies, the stimuli applied were low-frequency translations that bore little relationship to the frequency of head perturbations that occur during locomotion. Third, in those cases when higher-frequency or transient head translations were applied during viewing of a near target, no background was visible (e.g., a light-emitting diode in a dark room). In all of these reports, tVOR was found inadequate to provide foveal image stability, and its compensation gain (defined as eye rotational velocity / required eye rotational velocity to maintain foveal target fixation) was less than 0.7. 7–15
Thus, from these studies, it appears that human tVOR cannot achieve the amount of foveal image stability required for clear vision of a near target. Several thoughtful explanation have been advanced to account for the “enigmatic” finding that tVOR compensation gain typically remains ~0.5, irrespective of viewing distance.13,16 First, natural head perturbation that occur during locomotion comprise both rotations and translations, and the combination of responses might somehow be adequate to keep the fovea on target. For example, vertical head translations are combined with pitch movements, such that the naso-occipital axis of the head tends to remain pointed about 1m in front of the subject.16–18 Second, it follows that aVOR gain might be adjusted to aid tVOR responses, depending on the viewing distance of the target. For example, if aVOR was under-compensatory, this might allow pitch head movements to supplement a tVOR that only partially compensated for bob translations. Third, during locomotion while viewing a near target, the amplitude of head movements change, and this might mean that smaller demands are made of tVOR. Finally, central integration of translational and rotational signals from the labyrinths might produce tVOR behavior appropriate for any specific set of demands, such that the overall behavior was more than the sum of its parts. Such an effect would be similar, for example, the way that prior smooth-pursuit movements enhance aVOR.19 As well argued as these suggestions are, we doubt them because in studies of tVOR and aVOR during locomotion on a treadmill, large amounts of retinal image slip (7–14 deg/sec),20 and corresponding oscillopsia,16 indicate that tVOR cannot maintain foveal image stability of near targets.
Here we draw on evidence from our prior studies to support the hypotheses that (1) tVOR evolved to minimize retinal motion of a near foveal image with respect to motion of more distant images in the visual environment; (2) although the behavior of tVOR is influenced by a variety of factors, an important determinant is relative motion of near and far objects (motion parallax); (3) patients with certain neurological disorders that lead to imbalance and falls have lost the ability to adjust tVOR appropriately to viewing distance.6,21,22 The goal of this paper is to bring together results from these studies into a more general interpretation of human tVOR function.
METHODS
In our prior studies, 20 healthy humans (8 female, age range 25–72 years, median 55 years), 9 patients with progressive supranuclear palsy (PSP) (4 women, age range 61 – 75 years, median 68), and 8 patients with cerebellar ataxia (5 female, age range 27–79, median 57 years) served as subjects. Full details of subjects and methods have been previously described.6,21,22 Most experiments were performed in ambient light, so that natural visual cues, such as motion parallax and relative size, were available. All subjects gave informed consent in accordance with the Declaration of Helsinki and the Institutional Review Board of the Cleveland VA Medical Center.
Vestibular Stimuli were applied as subjects sat in a chair on a Moog 6DOF2000E electric motion platform (East Aurora, New York) that could move with six degrees of freedom. Belts were used to secure the subject’s torso and a snugly fitting skate-board helmet was used to stabilize the subject’s head. We applied bob translations at 2 Hz (typical amplitude ± 1.5 cm) to test tVOR and yaw rotations at 1.0 Hz (typical amplitude ± 5°) to test aVOR. We also applied combined bob at 2 Hz and yaw rotation at 1 Hz. Visual Stimuli: Small visual targets consisted of: (1) a laser spot projected on a wall at a distance of 2m (“far target”); (2) a “near target” (reflective ball, diameter 1 cm) suspended at a distance of ~17 cm in front of their left eye. Background visual stimuli consisted of: (1) the experimental room and its contents, which provided many visual cues of different sizes and distances; (2) an array of horizontal stripes, displayed on a large flat screen at 1.5 m, which subtended 50 degrees horizontally and 30 degrees vertically in an otherwise dark room. To investigate the effects of vergence on tVOR, subjects viewed small targets binocularly at 2m, 40 cm and 17 cm, first directly, and then with a 15- or 10-diopter base-out prism placed before the right eye (prism power selection was based on ability to fuse the visual stimulus). To determine the dependence of tVOR on visual motion inputs, we switched off the room lights for periods of 2–4 seconds, as subjects attempted to fix upon the remembered location of the near target, which they had previously viewed. Subjects also viewed the near target under conditions of strobe illumination, in order to minimize retinal image slip information. Strobe illumination was achieved using an array of bright light-emitting diodes, which flashed at 4 Hz, with a 30 ms flash duration. Three-dimensional eye rotations were measured using the magnetic search coil technique (CNC Engineering, Seattle, WA). Linear and rotational movements of the chair frame and subject’s head were monitored by an infrared reflection system (Vicon Motion Systems, Los Angeles, CA) that allowed head and coil frame movements to be measured with a resolution of 2 mm and 0.1°. Coil signals were digitized and converted to 3-D rotation vectors in degrees.23 Positive values correspond to leftward, downward, and clockwise rotations from the subject’s viewpoint, and divergence. Position signals from the infrared reflection system were used to calculate “ideal eye rotations” to hold gaze (corresponding to the line of sight) on the visual target.6 Using Fourier transforms of eye and head velocity, we measured gain of aVOR as eye-in-head rotational velocity / head rotational velocity, and responsivity of tVOR as eye rotational velocity / head translational acceleration (expressed as deg*sec/m). We also calculated compensation gain: eye rotational velocity / required eye rotational velocity to maintain foveal fixation of the visual target (far or near), which allowed us to relate measured responses to the ideal response (1.0) if the goal of tVOR is to hold the eye on target.
RESULTS
Does tVOR improve its performance under natural test conditions?
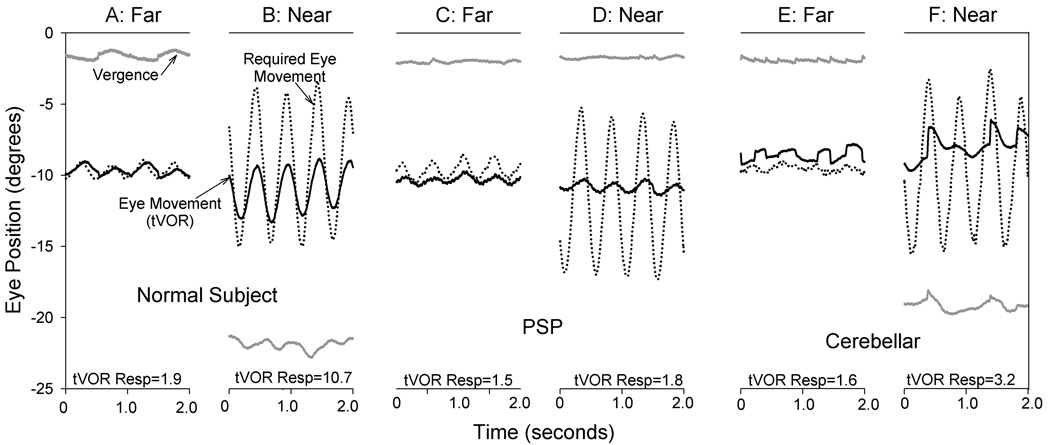
Representative responses tVOR of a normal subject during bob at 2 Hz in ambient illumination are shown in Figure 1A,B. Note that vertical eye movements generated by tVOR increase during viewing the near target, but they are smaller than the “required eye movements” calculated based on the subject’s head movements. Normal subjects made infrequent saccades during tVOR under our test conditions, even though the image of the near target cannot have been held on the fovea.
Figure 1.
Representative records from a healthy normal subject (A,B), a patient with PSP (C,D), and a patient with cerebellar ataxia (E,F). At the bottom of each panel, tVOR responsivity (Resp, in degrees*seconds/meter) is stated. Note that, except for vergence (gray lines), individual traces have been offset in position to aid clarity of display. Positive values indicate downward and divergence movement. Note how tVOR (vertical eye rotation) increased during near viewing (17 cm) compared with far viewing (2 m) for the normal subject, but remained less than the eye movement required to keep the fovea (line of sight) pointed at the visual target (dotted lines). The PSP patient showed inability to converge at near, and tVOR did not increase. The patient with cerebellar ataxia was able to converge at near, but could not substantially increase tVOR responsivity. Required eye rotations were computed from measured head movements.6
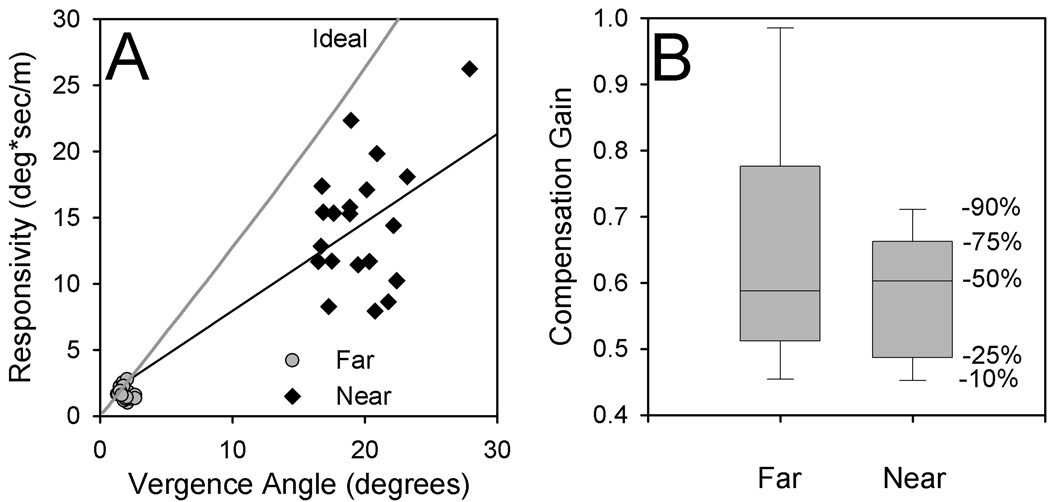
As predicted by geometric considerations, the responsivity of tVOR increased substantially from far (2m) to near (17 cm) viewing – by a factor of almost nine in our group of 20 subjects (Fig. 2A). Applying translation and rotations in combination produced a small increase in tVOR responsivity.6 However, median compensation gain increased only from 0.52 at far to 0.59 at near (Fig. 2B), even for combined rotation-translation. Thus, although the brain seemed well able to increase eye velocity during near viewing, compensation gain was consistently inadequate to maintain foveal fixation, especially at near. We next considered to what visual demands tVOR could be responding.
Figure 2.
Summary of tVOR responses to bob at 2.0 Hz of 20 normal subjects.6 (A) Responsivity plotted as a function of vergence angle. Note that during near viewing (diamonds, corresponding to larger vergence angles), responsivity increases substantially compared with far viewing (circles). (B) Box plots, showing percentiles for compensation gain values during far and near viewing; these were similar, despite the large changes of responsivity shown in (A).
What factors determine tVOR behavior?
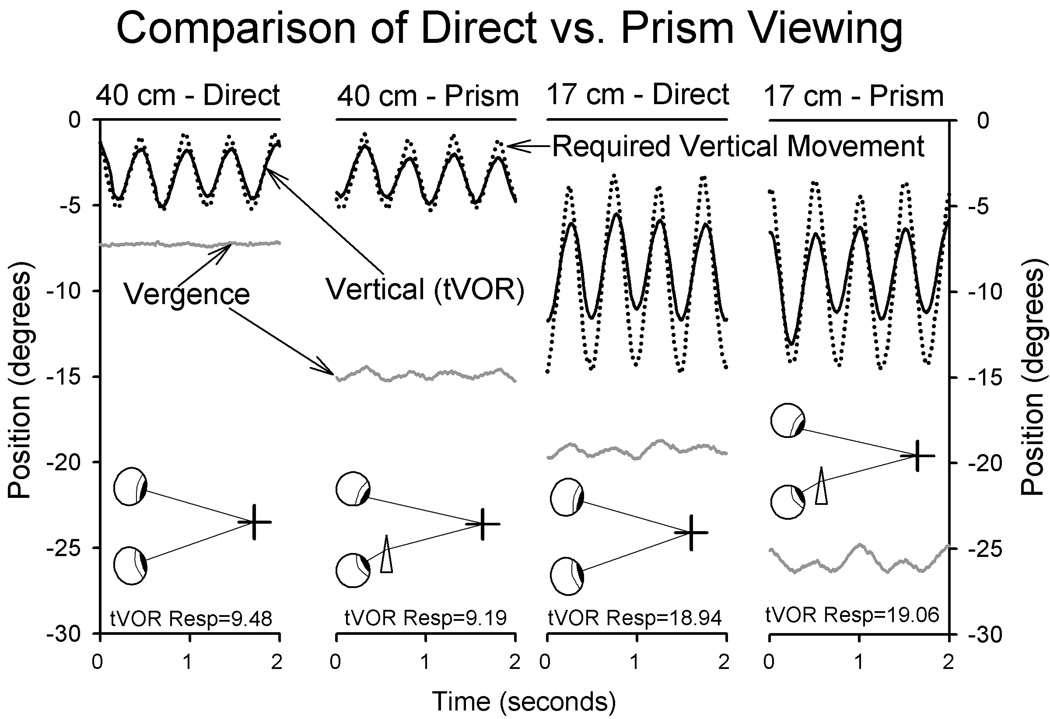
A widely accepted tenet is that tVOR responses are determined by vergence angle,9,12,24 although some investigators have suggested that a number of factors may contribute.25 We found that tVOR responses decreased during monocular viewing,6 but wondered whether this decline was due to decreased convergence, or lack of binocular responses. To address this question, we compared responses during binocular viewing, of targets at three distances (17 cm, 40 cm, 200 cm) either directly or with a base-out prisms in front of the subject’s right eye. Representative data are shown in Figure 3. It is evident that for either of the viewing distances, tVOR behavior is similar even though vergence angle is different. This was a consistent finding in all six of subjects.6 This finding should not be interpreted to mean that vergence effort does not influence tVOR behavior, but rather that binocular visual stimuli appear to be more important under ambient illumination.
Figure 3.
Representative records comparing direct versus prism viewing for a single subject. Note how tVOR is larger during viewing the target at 17cm versus 40 cm, but is unaffected by vergence angle. Plotting conventions are similar to Fig. 1.
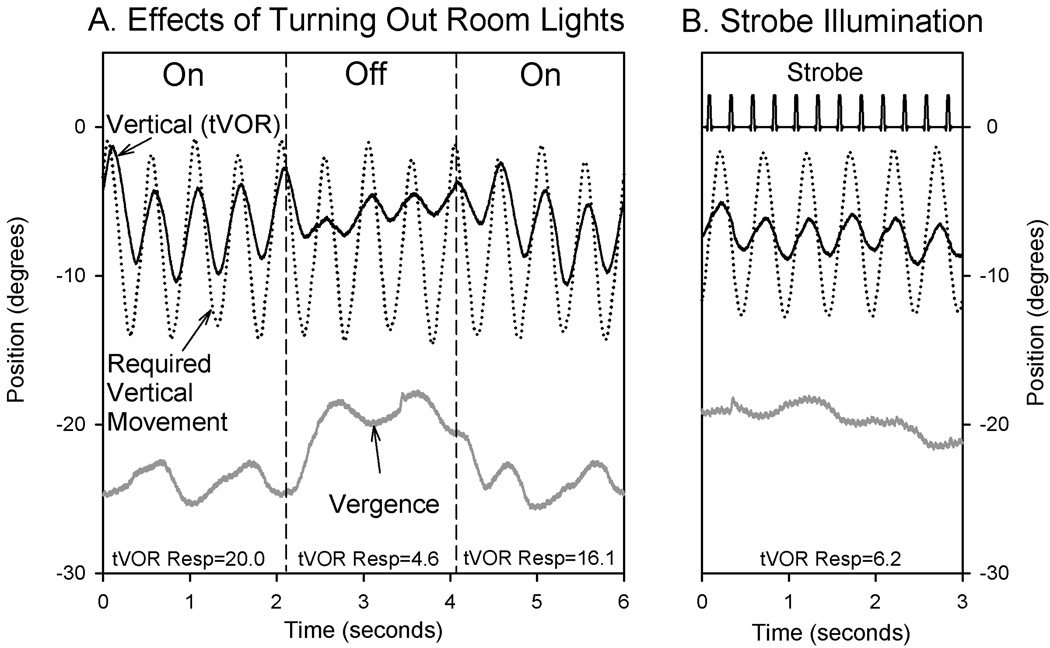
Not only monocular viewing, but also transient darkness or strobe illumination caused tVOR to decline;6 representative records are shown in Figure 4. However, it seems unlikely that visual tracking eye movements, such as smooth pursuit, are responsible for several reasons. First, the phase lag of tVOR for our 2 Hz stimuli was consistently about ~19 degrees, irrespective of viewing distance of the target; this is much smaller that the phase lag values of smooth tracking of a large visual target moving at 2 Hz (median lag of ~58 degrees).6 Second, tVOR can be suppressed during viewing of a head-fixed target (such as a mirror image) much better than can be accounted for by smooth pursuit.26
Figure 4.
Representative records of the effects of illumination on tVOR from a single subject. (A) Switching to darkness. Note how vergence angle and tVOR declined when the room lights were turned off (first vertical dashed line), and then increased when lights were turned on again. (B) Strobe illumination (flashes lasting 30 ms at 4 Hz) are indicated by spikes in strobe channel. Note that tVOR and vergence are decreased compared with viewing in ambient light (first part of A). Plotting conventions are similar to Fig. 1.
A hypothesis to account for tVOR behavior
Taken together, these findings led us to postulate that relative motion of the near target with respect to the distant background (motion parallax) was an important determinant of tVOR behavior. Since discrimination of relative motion is better at lower velocities of retinal image motion,27,28 we postulated that tVOR responses are set to minimize retinal image speed for both the target and the visual background.6,26 A prediction of this hypothesis is that motion of the visual background should influence tVOR behavior while the near visual target remains constant. When subjects fixed on a near earth-fixed target against a background of horizontal stripes on a large flat screen that moved vertically at a different frequency (2.1 Hz) than the platform (2.0 Hz), tVOR showed consistent changes in behavior.26 Thus, as relative motion of the background increased, tVOR decreased (increasing slip speed of the foveal image) and thereby tended to equalize retinal image slip of the foveal target with respect to the background. Under natural conditions, a changing relationship between image slip of a near target and the background occurs as the subject travels forward through the visual environment.
Thus, our current hypothesis is that the responsiveness of tVOR is adjusted as a continuous function of retinal image motion of near target versus distant background, with the goal of minimizing relative motion of one with respect to the other. In fact, for objects lying at greater distance than about 1 meter, tVOR compensation gain of 0.6 is all that is required to reduce retinal image motion below 5 degrees/second, to permit clear vision.6
What insights into tVOR are provided by evidence from patients with neurological disease?
Disorders of aVOR, due to a wide range of neurological disorders, have been the subject of a large literature.2,29 Much less is known about how neurological disease affects tVOR. Based on our knowledge of aVOR, it might be expected that cerebellar and brainstem disease could lead to abnormalities of tVOR. For example, skew deviation and ocular tilt reaction that occurs with both brainstem and cerebellar disorders is conceptualized as a central imbalance of otolithic pathways.30 We postulated that neurological disorders that commonly lead to falls, presumably by disruption of otolith-spinal reflexes, would also cause abnormal otolith-ocular reflexes. Accordingly, we studied patients with two disorders: (1) PSP, which causes falls early in its course; (2) the cerebellar ataxias.
Our main findings are summarized by the representative records in Figure 1. In patients with PSP, the responsivity of tVOR fails to increase during viewing of a near target during bob translation (Fig. 1C,D); note that these patients cannot converge.21 In patients with cerebellar ataxia, tVOR also fails to increase during near viewing, but such patients can converge (Fig. 1E,F);22 a similar result has previously been reported with translation along the interaural axis.31 Thus, failure to converge seems unlikely to be the fundamental problem in PSP for two reasons: (1) Under ambient illumination, normal subjects show modulation of tVOR with viewing distance, not vergence angle (Figure 3). (2) Cerebellar patients can converge but cannot increase their tVOR responsivity during near viewing (Figure 1EF). Furthermore, vestibular-evoked myogenic potentials (VEMPS), in which a loud click is used to stimulate the saccular otolithic organ, are impaired in PSP.21 Thus, it seems that a specific class of vestibular disorders consists of loss of ability to increase tVOR responsivity appropriately during viewing of a near target. More work, including development of animal models, seems necessary to understand more fully the pathophysiology of these findings.
Conclusions
The present work has shown that tVOR seems ill-suited to achieve stabilization of foveal images, and although this remains possible, no prior study has clearly demonstrated it. Further, geometric considerations indicate that if tVOR did stabilize foveal images of a near target, such behavior would be detrimental to clear vision of the more distant surround, particularly in erect humans, with their bouncy form of walking. To govern tVOR behavior, it appears that the brain uses a range of visual cues to estimate the relative distances of near targets and their surround, including motion parallax, which is now known to be encoded in cortical visual area MT.32 Diseases affecting regions of the brain from cerebral cortex to brainstem may cause disorders of tVOR that have yet to be defined.
Acknowledgments
Supported by NASA/NSBRI NA00208, US Department of Veterans Affairs, NIH grant EY06717, and the Evenor Armington Fund. We are grateful to Drs. Gary Paige, Harold Bedell, John Stahl, David Zee, Stefano Ramat, and Matthew Thurtell for helpful discussions.
References
- 1.Büchele W, Brandt T, Degner D. Ataxia and oscillopsia in downbeat-nystagmus vertigo syndrome. Adv Oto-Rhino-Laryngol. 1983;30:291–297. doi: 10.1159/000407661. [DOI] [PubMed] [Google Scholar]
- 2.Brandt T. Its multisensory syndromes. London: Springer-Verlag; 1999. Vertigo. [Google Scholar]
- 3.Massaad F, Lejeune TM, Detrembleur C. The up and down bobbing of human walking: a compromise between muscle work and efficiency. J Physiol. 2007;582:789–799. doi: 10.1113/jphysiol.2007.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viirre E, Tweed D, Milner K, Vilis T. A reexamination of the gain of the vestibuloocular reflex. Journal of Neurophysiology. 1986;56:439–450. doi: 10.1152/jn.1986.56.2.439. [DOI] [PubMed] [Google Scholar]
- 5.Angelaki DE. Eyes on target: what neurons must do for the vestibuloocular reflex during linear motion. J. Neurophysiol. 2004;92:20–35. doi: 10.1152/jn.00047.2004. [DOI] [PubMed] [Google Scholar]
- 6.Liao K, Walker MF, Joshi A, Reschke MF, Leigh RJ. Vestibulo-ocular responses to vertical translation in normal human subjects. Exp Brain Res. 2008;185:553–562. doi: 10.1007/s00221-007-1181-z. [DOI] [PubMed] [Google Scholar]
- 7.Bronstein AM, Gresty MA. Short latency compensatory eye movement responses to transient linear head acceleration: a specific function of the otolith-ocular reflex. Exp. Brain Res. 1988;71:406–410. doi: 10.1007/BF00247500. [DOI] [PubMed] [Google Scholar]
- 8.Israël I, Berthoz A. Contribution of the otoliths to the calculation of linear displacement. J Neurophysiol. 1989;62:247–263. doi: 10.1152/jn.1989.62.1.247. [DOI] [PubMed] [Google Scholar]
- 9.Paige GD. The influence of target distance on eye movement responses during vertical linear motion. Exp Brain Res. 1989;77:585–593. doi: 10.1007/BF00249611. [DOI] [PubMed] [Google Scholar]
- 10.Busettini C, Miles FA, Schwarz U, Carl JR. Human ocular responses to translation of the observer and of the scene: dependence on viewing distance. Exp Brain Res. 1994;100:484–494. doi: 10.1007/BF02738407. [DOI] [PubMed] [Google Scholar]
- 11.Gianna CC, Gresty MA, Bronstein AM. The human linear vestibulo-ocular reflex to transient accelerations: visual modulation of suppression and enhancement. J. Vestib. Res. 2000;10:227–238. [PubMed] [Google Scholar]
- 12.Paige GD, Telford L, Seidman SH, Barnes GR. Human vestibuloocular reflex and its interactions with vision and fixation distance during linear and angular head movement. J Neurophysiol. 1998;80:2391–2404. doi: 10.1152/jn.1998.80.5.2391. [DOI] [PubMed] [Google Scholar]
- 13.Ramat S, Zee DS. Ocular motor responses to abrupt interaural head translation in normal humans. J. Neurophysiol. 2003;90:887–902. doi: 10.1152/jn.01121.2002. [DOI] [PubMed] [Google Scholar]
- 14.Ramat S, Straumann D, Zee DS. The interaural translational VOR: suppression, enhancement and cognitive control. J. Neurophysiol. 2005;94:2391–2402. doi: 10.1152/jn.01328.2004. [DOI] [PubMed] [Google Scholar]
- 15.Tian JR, Mokuno E, Demer JL. Vestibulo-ocular reflex to transient surge translation: complex geometric response ablated by normal aging. J Neurophysiol. 2006;95:2042–2054. doi: 10.1152/jn.00635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore ST, Hirasaki E, Cohen B, Raphan T. Effect of viewing distance on the generation of vertical eye movements during locomotion. Exp. Brain Res. 1999;129:347–361. doi: 10.1007/s002210050903. [DOI] [PubMed] [Google Scholar]
- 17.Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans. I. Normal subjects. Exp Brain Res. 1990;82:97–106. doi: 10.1007/BF00230842. [DOI] [PubMed] [Google Scholar]
- 18.Bloomberg JJ, Reschke MF, Huebner WP, Peters BT. The effects of target distance on eye and head movement during locomotion. Ann NY Acad Sci. 1992;656:699–707. doi: 10.1111/j.1749-6632.1992.tb25247.x. [DOI] [PubMed] [Google Scholar]
- 19.Das VE, Dell'Osso LF, Leigh RJ. Enhancement of the vestibulo-ocular reflex by prior eye movements. J. Neurophysiol. 1999;81:2884–2892. doi: 10.1152/jn.1999.81.6.2884. [DOI] [PubMed] [Google Scholar]
- 20.Crane BT, Demer JL. Human gaze stabilization during natural activities: translation, rotation, magnification, and target distance effects. J Neurophysiol. 1997;78:2129–2144. doi: 10.1152/jn.1997.78.4.2129. [DOI] [PubMed] [Google Scholar]
- 21.Liao K, Wagner J, Joshi A, Estrovich I, Walker MF, Strupp M, Leigh RJ. Why do patients with PSP fall? Evidence for abnormal otolith responses. Neurology. 2008;70:802–809. doi: 10.1212/01.wnl.0000304134.33380.1e. [DOI] [PubMed] [Google Scholar]
- 22.Liao K, Walker MF, Leigh RJ. Abnormal vestibular responses to vertical head motion in cerebellar ataxia. Ann. Neurol. 2008 doi: 10.1002/ana.21407. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Haustein W. Considerations on Listing's Law and the primary position by means of a matrix description of eye position control. Biol Cybern. 1989;60:411–420. doi: 10.1007/BF00204696. [DOI] [PubMed] [Google Scholar]
- 24.Paige GD. Linear vestibulo-ocular reflex (LVOR) and modulation by vergence Acta. Otolaryngol. 1991;481 Suppl:282–286. doi: 10.3109/00016489109131402. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz U, Miles FA. Ocular responses to translation and their dependence on viewing distance. I. Motion of the observer. J Neurophysiol. 1991;66:851–864. doi: 10.1152/jn.1991.66.3.851. [DOI] [PubMed] [Google Scholar]
- 26.Liao K, Walker MF, Joshi A, Reschke MF, Wang Z, Leigh RJ. A reinterpretation of the purpose of the translational vestibulo-ocular reflex in human subjects. Prog. Brain Res. 2008;171 doi: 10.1016/S0079-6123(08)00643-2. In press. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama K. Biological image motion processing: a review. Vision Res. 1985;25:625–660. doi: 10.1016/0042-6989(85)90171-3. [DOI] [PubMed] [Google Scholar]
- 28.Howard IP, Rogers BJ. Depth from Motion Parallax. In: Howard IP, Rogers BJ, editors. Seeing in Depth. volume 2. Toronto: I. Porteus; 2002. pp. 411–443. [Google Scholar]
- 29.Leigh RJ, Zee DS. The Neurology of Eye Movements (Book/DVD) Fourth Edition. New York: Oxford University Press; 2006. [Google Scholar]
- 30.Brandt T, Dieterich M. Vestibular syndromes in the roll plane: topographic diagnosis from brain stem to cortex. Annals of Neurology. 1994;36:337–347. doi: 10.1002/ana.410360304. [DOI] [PubMed] [Google Scholar]
- 31.Wiest G, Tian JR, Baloh RW, Crane BT, Demer JL. Otolith function in cerebellar ataxia due to mutations in the calcium channel gene. CACNA1A Brain. 2001;124:2407–2416. doi: 10.1093/brain/124.12.2407. [DOI] [PubMed] [Google Scholar]
- 32.Nadler JW, Angelaki DE, Deangelis GC. A neural representation of depth from motion parallax in macaque visual cortex. Nature. 2008;452:642–645. doi: 10.1038/nature06814. [DOI] [PMC free article] [PubMed] [Google Scholar]