Abstract
Sex differences in incidence and severity of some stress-related, neuropsychiatric disorders are often reported to favor men, suggesting that women may be more vulnerable to aberrant hypothalamic-pituitary-adrenal (HPA) axis responses to stress. In this review, we discuss several investigations that we, and others, have conducted assessing salivary cortisol as a measure of HPA function. We have examined basal cortisol among healthy men and women and also following acute exposure to stressors. Among healthy participants, men had higher basal cortisol levels than did women. In response to acute stressors, such as carbon dioxide or noise, respectively, cortisol levels were comparable between men and women or higher among women. We have also examined cortisol levels among those with problem eating, gambling, or post traumatic stress disorder (PTSD). Women with restrained eating habits have higher basal cortisol levels than do women without restrained eating habits. Pathological gamblers have more aberrant stress response to gambling stimuli than do recreational gamblers, and these effects are more prominent among men than women. Men who have motor-vehicle accident related PTSD, demonstrate more aberrant cortisol function, than do their female counterparts. Although these sex differences in cortisol seem to vary with type of stress exposure and/or pathophysiological status of the individual, other hormones may influence cortisol response. To address this, cortisol levels among boys and girls with different stress-related experiences, will be the subject of future investigation.
Keywords: Cortisol, Gender Differences, Hypothalamic-Pituitary-Adrenal Axis, Hypothalamic-Pituitary-Gonadal Axis, Panic Attack
Introduction
Individuals respond to stressors in a variety of ways. Understanding how factors such as sex and/or hormone status mitigates stress response may shed light on how these factors can influence pathophysiological states. First, neuropsychiatric disorders, such as anxiety, depression, and posttraumatic stress disorder (PTSD), are stress-related and influenced by sex and gonadal hormones (Arborelius et al., 1999; Boyer, 2000; Cameron and Nesse, 1988; Esch et al., 2002; Gold and Chrousos, 2002; Kasckow et al., 2001; McEwen, 2002; Rasmusson et al., 2001; Steckler et al., 1999; Young, 1998). Second, stress also modulates engagement in, and effects of, rewarding and/or addictive behavior, and there are salient sex biases associated with these behaviors (Koob and Le Moal, 2008; Lynch, 2006; Lynch et al., 2002; Pendergast, 1994; Sinha, 2001, 2008; van Etten et al., 1999, 2001). In particular, males tend to be more susceptible to engagement in drug abuse whereas females tend to be more labile with greater peaks in abuse behavior. Understanding these pathophysiological states is not only important for revealing the etiology of the disorders but is also crucial for elucidating possible mechanisms of the normative state, which may be influenced by interactions between adrenal and gonadal hormones. Research to identify the extent to which sex- and/or adrenal-hormones can influence response to acute, chronic, and/or pathophysiological stressors is a critical step in understanding treatment and prevention of health risks associated with various types of stress.
Although the extant literature examines how experiential factors, such as the history of stress-related disorder, may influence stress reactivity, here we consider how sex may contribute to stress responses across a variety of healthy and/or pathophysiological states. Our laboratory has investigated sex and/or hormonal differences in various behaviors using animal models, and has worked to elucidate the role that neurosteroids may play in mitigating these effects. We, and others, have found that one mechanism that precipitates neurosteroid biosynthesis to levels that exert effects on behavior is the activation of the hypothalamic-pituitary-adrenal (HPA) stress axis. We have been collaborating with clinical investigators to assess the role that HPA activation may play in healthy, subclinical, and clinical human samples and the following review summarizes a decade of this work.
Salivary Cortisol as a Research Approach to Investigate Sex Differences
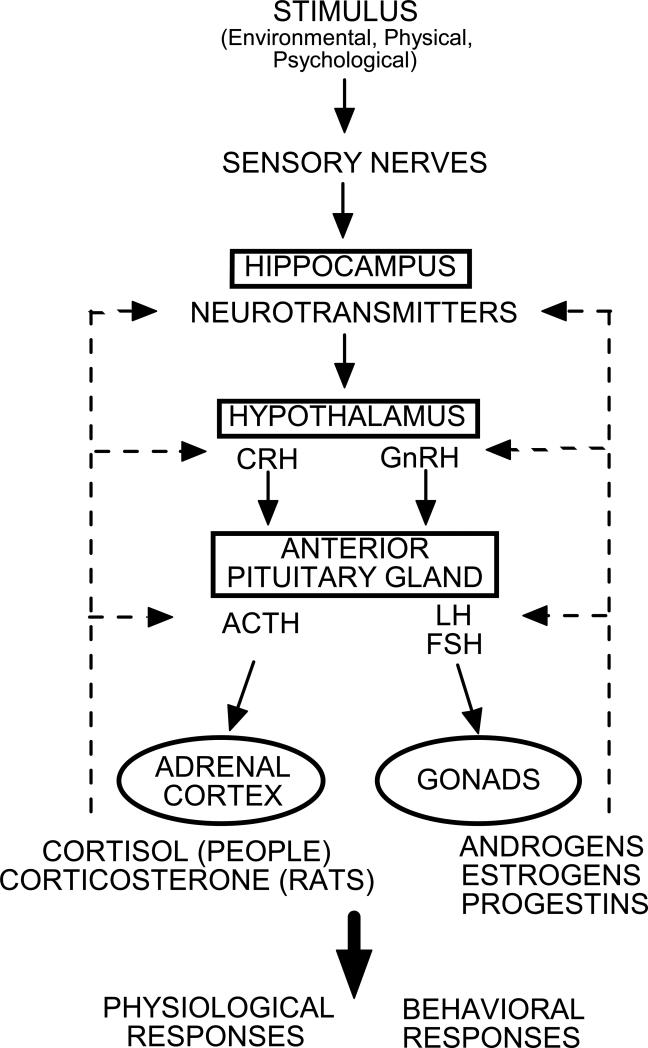
Sex differences in vulnerability to stress are dependent on many factors including the type of stressor utilized as well as the endogenous hormonal status of the individuals examined. While some studies indicate that differences in neurobiological stress response favor men (Gallucci et al., 1993; Jezova et al., 1996), there have been many reports demonstrating the opposite effect (Kajante and Philips, 2006; Kudielka et al., 2009), and some reporting no differences between the sexes (Earle et al., 1999; Owens et al., 1993). Unlike, investigations of people, studies using rodent models typically find females to have increased stress axis activity compared to males (Critchlow et al., 1963; Handa et al., 1994; Kitay, 1961). Thus, activation of the HPA stress axis is a sexually-divergent trait but this relationship may be more difficult to parse out in humans. As depicted in Figure 1, stress elicits production of corticotropin releasing hormone (CRH) from paraventricular hypothalamus, which acts to mediate pulsatile release of adrenocorticotropic hormone (ACTH) from anterior pituitary into circulation. Actions of ACTH at adrenal glands promote release of glucocorticoids (primarily cortisol in humans and corticosterone in most rodents) which can act in the brain to produce some of the psychological effects that are associated with stress (such as enhanced memory consolidation and perception of arousal; Abercrombie et al, 2005; Buchanan and Lovallo, 2001; Roozendaal, 2000) and attenuate CRH/ACTH production in a negative feedback loop. The hippocampus is included in this depiction of the HPA, as stress hormones can have profound effects on physiological, behavioral, and neuroendocrine responses associated with this and other limbic regions (Conrad 2005, 2006; Herman et al., 2005; Weinstock, 2008). Cortisol levels in plasma have been shown to correlate well with cortisol concentrations in saliva among infants (Calixto et al., 2002), children (Schwartz et al., 1998), and adults (Aardal and Holm, 1995; Cadore et al., 2008; Kahn et al., 1988). In the following investigations we have used salivary cortisol as a biomarker for HPA function.
Figure 1.
Depicts hypothalamic-pituitary-adrenal/gonadal axes. ACTH = adrenocorticotropic hormone; CRH = corticotropin releasing hormone; FSH = follicle stimulating hormone; GnRH = gonadotropin releasing hormone; LH = lutenizing hormone.
Acute vs. Chronic Activation of the Hypothalamic-Pituitary-Adrenal Axis
Acute stressors typically produce rapid enhancement in glucocorticoid levels and do not affect the basal (non-stress) activity of the HPA axis (Assenmacher et al., 1995). This glucocorticoid response to an acute stressor is typically considered “adaptive”. Acute increases in glucocorticoids enhance arousal and energy mobilization (the brain stimulates neurotransmitter release, the muscles increase protein metabolism, the adipose tissue mobilizes lipids, and the liver increases glycogen synthesis) so that “fight-or-flight” behavioral responses can be subserved (Axelrod and Reisine, 1984; Sie and Fishman, 1964; Munck et al., 1984). Acute release of adrenal steroids also enhances memory consolidation of stressful events, which may facilitate similar “adaptive” responses in future situations (Buchanan and Lovallo, 2001; McEwen, 2007; Roozendaal, 2000). The physiological, psychological, and behavioral consequences of acute stress are, thus, likely to be adaptive to an individual in the short run. However, sex differences in response to acute stress may influence responding to chronic or unrelenting stress. One aspect of this review will focus on the role that sex and environment may play on acute stress response and implications for pathological states associated with acute and chronic stress, such as panic attack.
Ongoing (chronic) stressors are characterized by overactivation of the HPA axis (compared to acute stress; Chrousos and Gold, 1992; Gold et al., 1988). There may not be differences in the basal levels of glucocorticoids, but stress-induced increases in glucocorticoids may occur with a less robust stimulus, be greater, and persist for a longer time than occurs with acute stressors. This dysfunctional state of the HPA axis can result in persistently decreased basal glucocorticoid levels, as can be seen in PTSD and atypical depression (Ehlert et al., 2001; Mason, 1986; Stratakis and Chrousos, 1995; Yehuda et al, 1990, 1991, 1993, 1995a,b) or basal glucocorticoid levels can be elevated in response to HPA dysregulation, as can be observed in melancholic depression, obesity, and panic and anxiety disorders (Arborelius et al., 1999; Bjorntorp, 1995; Bjorntrop et al., 2000; Bjorntorp and Rosmond, 2000; Board et al., 1957; Gold et al., 1988; Stratakis and Chrousos, 1995). The factors that may promote one HPA response (hypo- or hyperactivity) when confronted with pathological stress are not well-understood but are likely complex and dependent on interactions between organizational, activational, and epigenetic effects (McEwen, 2002, 2007). Chronic stress can dysregulate a variety of normal, peripheral, physiological functions, including cardiovascular, hepatic, immune, and digestive processes in addition to promoting central reductions in neurogenesis and neuron efficiency (McEwen, 2007). The type of stressors that are generally considered pathophysiological may be initially similar to chronic or traumatic stress, but then become pathophysiological because the stress response (HPA activity) and psychological recurrences of trauma are altered and unremitting even when the source of the stressor is removed (Sapolsky, 1992). Stress influences so many physiological systems (central, immune, metabolic, and cardiovascular) that chronic HPA reactivity can have profound negative effects on health. Chronic stress contributes to neurodegeneration of the hippocampus, development of cognitive and affective disorders, cardiovascular disease, immune deficiency and/or autoimmune disorders (Chorot and Sandin, 1994; McEwen, 2000; Torpy and Chrousos, 1996; Young, 1998). Contributors associated with the shift from adaptive responses to acute stress to maladaptive responses to chronic stress are not well-understood. Thus, the latter half of this review will address pathological states associated with more moderate and chronic HPA arousal such as that observed in addiction and posttraumatic stress disorder (PTSD), respectively.
Acute Stress in Men and Women by Induction of Panic Attack Symptoms
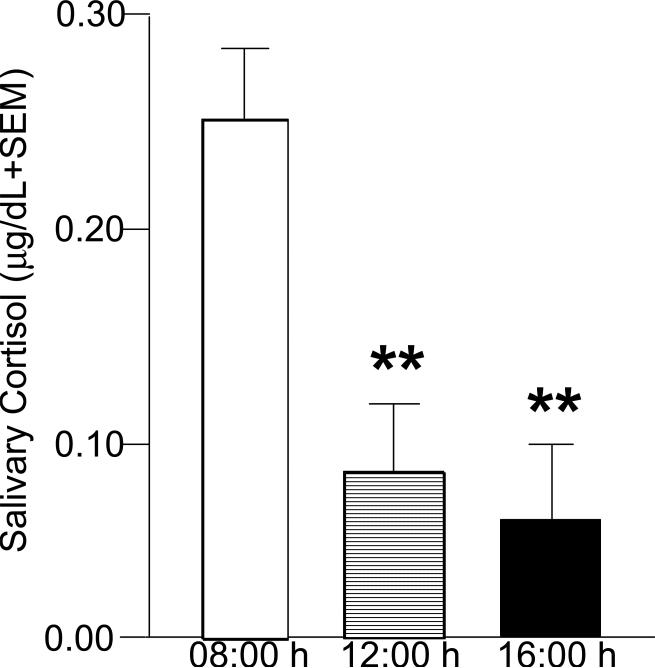
Panic attacks, which occur in both nonclinical and clinically-anxious populations, are time-limited, highly aversive, abrupt neurobiological events. They are often accompanied by wide-ranging negative physiological, psychological, behavioral, and health-related consequences (Barlow, 2001; Gater et al., 1998; Kessler et al., 1994; Lewinsohn et al., 1998; Patel et al. 1999; Pearson 1995). A model of acute stress/panic is exposure to carbon dioxide (CO2)-enriched air, which produces autonomic and psychological effects that are analogous to panic attacks (Forsyth et al., 2000a,b). In this acute stress paradigm, men and women with no known history of psychopathology are exposed to a single 20 second inhalation of 20% CO2-enriched air or normal room air (control condition). This CO2 exposure paradigm has been used successfully in the past to evoke, in both fear learning and biological challenge contexts, psychological and behavioral responses analogous to panicogenic arousal (Forsyth et al., 2000a,b). This procedure is capable of supporting aversive fear learning to exteroceptive and interoceptive cues (Acheson et al., 2007). CO2 inhalation results in refractory elevations in plasma cortisol (Argyropoulos et al., 2002; Coplan et al., 2002; Sasaki et al., 1996) with no acute or long-term health risk or subsequent vulnerability for later panic attacks or anxiety (Harrington et al, 1996; Prenoveau et al., 2006). CO2 exposure is an effective paradigm to examine acute stress-induced changes in nonclinical and clinical populations without inducing actual panic attack (Beck et al., 1996; Bouton et al., 2001; Forsyth and Eifert, 1998; Forsyth et al., 2000a; Fyer et al., 1987; Griez, and van den Hout, 1986; Lejuez et al., 1998; Rapee et al., 1992; Schmidt et al., 1996; van den Hout and Griez, 1984; Zvolensky et al., 1999). We and others have seen that exposure to CO2 increases salivary cortisol (Bandelow et al., 2000; Belgorodsky et al., 2005; van Duinen et al., 2005) but diurnal rhythmicity remains with a 3-fold increase in the morning (0800 h) compared to mid-day (1400 h) exposure, and a 1-fold decrease in the afternoon (1600 h) compared to mid-day exposure (Fig. 2; Murray et al., 1999).
Figure 2.
Depicts salivary cortisol levels of laboratory personnel (4 males and 7 females) following 20% CO2-exposure at 08:00, 14:00, and 16:00 h. * indicates significantly different from 08:00 h sampling, p < 0.05.
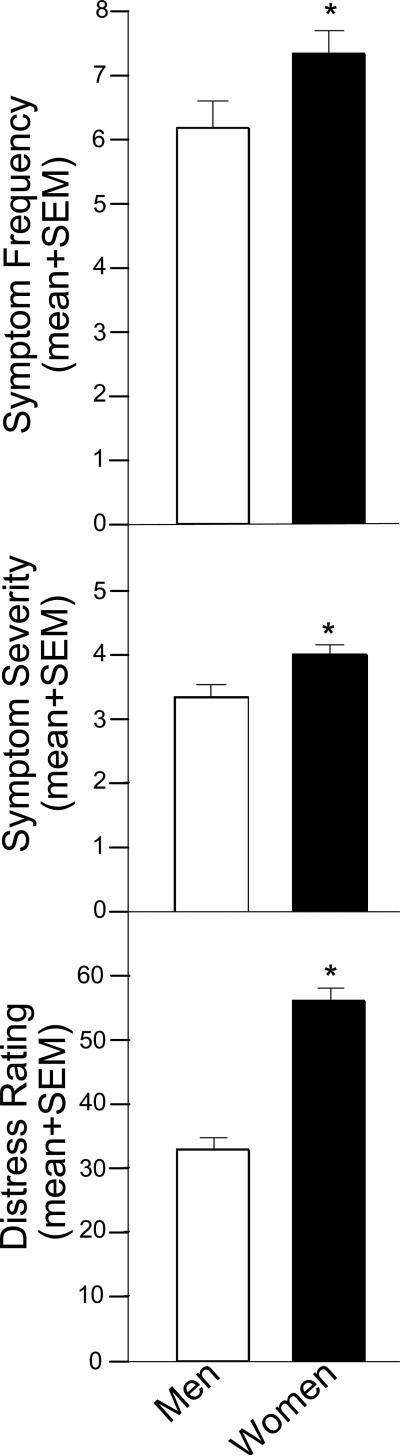
Women are affected more often by panic than are men (Barlow, 2001; Gater et al., 1998; Kessler et al., 1994; Lewinsohn et al., 1998; Patel et al., 1999; Pearson, 1999). However, sex differences in cortisol response to a psychological or physical stressor are not always reported to discriminate between males and females, despite sex differences in self-reported stress, emotional response, and/or discomfort (Kelly et al., 2008; van Stegeren et al., 2008). Our collaborator, Dr. John Forsyth, has found that following CO2 exposure, women consistently report a greater frequency (Fig. 3, top) and greater severity (Fig. 3, middle) of panic symptoms relative to their male counterparts. These women also report a greater distress rating following CO2 compared to men (Fig. 3, bottom). However, it must be noted that both men and women reported panic attack symptoms, but no participants in either group had an experience meeting diagnostic criteria for panic attack. In addition to these findings, we have observed that men (n=19) who were given instruction to elicit a coping response had decreased salivary cortisol compared to women (n=29) or those not given any instructions to counter CO2 when tested in the afternoon (approximately 12:00 h; Murray et al., 1999). Together, these data support the notion that a sex-specific perception, and/or coping skill-set, may be important in the neuroendocrine response to acute stress (see also Kelly and Forsyth, 2007). While, women demonstrated a greater stress response than men in this study, there are many investigations utilizing validated stressors that report the opposite effect (reviewed in Kudielka et al., 2009). It remains an open and interesting question if this paradigm, which induces panic-like symptoms, is sex-biased, as others have discussed in reference to pharmacological stressors (Kudielka et al., 2009).
Figure 3.
Undergraduate women (n=19) exposed to 20% CO2 report increased frequency (top) and severity (middle) of panic attack symptoms and report more distress (bottom) than do undergraduate men (n=29) exposed to 20% CO2. * indicates significant difference, p < 0.05.
Investigations such as this begin to elucidate the behavioral and physiological aspects of stress that may be mitigated by sex under experimental conditions. It is rare to have the opportunity to investigate how sex may influence these factors in the native environment under non-experimental conditions. We have also examined acute stress responses in individuals that are not in an experimental setting.
Stress Response Among Laboratory Workers in the Native Environment
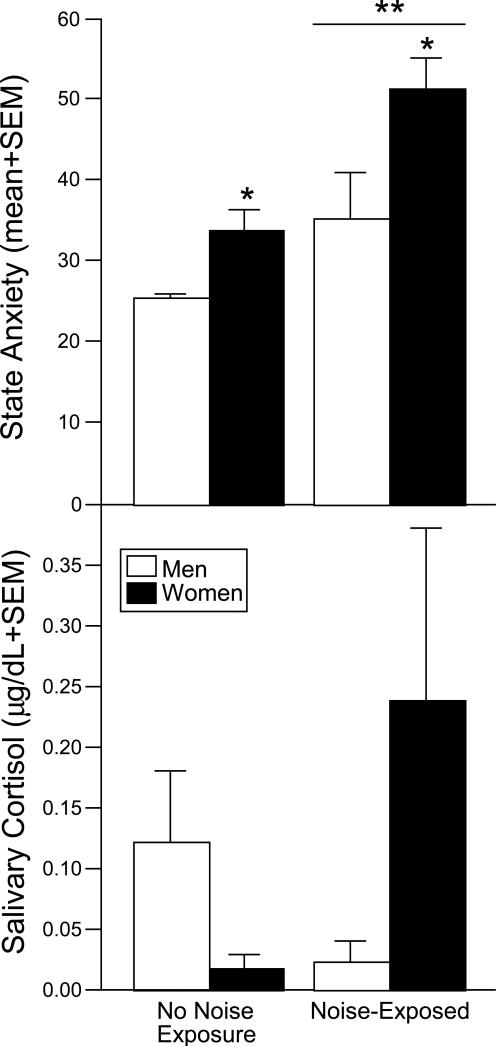
Our laboratory is situated in a new Life Sciences Research Building on The University at Albany-SUNY campus and infrequent construction is still necessary to conduct at times. Given the impact that auditory stimuli can have on behavioral outcomes of people and animals under laboratory conditions, routine construction is coordinated so as not to occur when experiments are being conducted. However, we have recently experienced an unanticipated situation wherein workers (6 male and 9 female observations) in our behavioral laboratory were exposed to an unexpected, intermittent construction noise every 2-4 minutes for a period of approximately 4 hours from 09:00 to 13:00 h. Other workers in our group (3 male and 7 female observations) were in the biochemistry laboratory and were not exposed to this spontaneous, noise stimulus. All workers, in the behavior and biochemistry laboratories, were administered the Spielberger's State-Trait Anxiety Inventory (STAI) at 12:00, 15:00, and 18:00 h, and saliva samples were collected at each of these timepoints. As depicted in Figure 4 (top), mean State anxiety scores were significantly higher among workers exposed to the unpredictable construction noise compared to those who were not. In both groups, women had greater State anxiety scores. No differences were observed in Trait anxiety scores (data not shown). Further, salivary cortisol concentrations appeared to be affected by sex and noise exposure, albeit these differences were not significant (Fig. 4, bottom). Men had higher salivary cortisol than women in the control condition (as we and others have observed is the case at basal concentrations in healthy populations). Among the noise-exposed group, women had a much greater salivary cortisol response than did men. These are consistent with our observations that healthy women may be more responsive to an acute stressor, such as CO2-exposure, than healthy men, and extend them to illustrate that sex differences in response to acute stress can be seen in a native, non-experimental situation. Thus, sex differences observed under these circumstances may be mitigated by environmental conditions associated with testing. The extent to which these factors may influence (sub)clinical disorders that are known to be associated with HPA dysfunction is also of interest.
Figure 4.
Depicts State Anxiety on the Spielberger State-Trait Anxiety Inventory (top) and salivary cortisol (bottom) among male and female laboratory workers that were exposed to intermittent construction noise (6 males and 9 females) or not (3 males and 7 females). * indicates significant main effect for women to score higher than men; ** indicates significant main effect for noise-exposed group to score higher than non-exposed group, p < 0.05.
Salivary Cortisol and Restrained Eating in the Native Environment
A common, sex-biased, stress-related disorder among college students is disordered eating, or aberrant eating/dieting behavior. With our collaborator, Dr. Drew Anderson, we have found that among non-obese, undergraduate college women, those identified by questionnaires (Herman and Polivy, 1980) as demonstrating restrained eating (n=50; characterized by chronic, unsuccessful dieting and fluctuating weight; Anderson et al., 2002; Gorman and Allison 1995; Heatherton et al., 1988) had significantly higher salivary cortisol levels (0.39 ± 0.02 μg/dl) than did non-afflicted college women (n=24) when assessed between 09:00 and 11:00 h (0.17 ± 0.01 μg/dl; see also Anderson et al., 2002). We have also observed that women who exhibit disordered eating or alcohol abuse demonstrate similarly high degrees of impulsivity and attitudes permissive of social deviance (Benjamin and Wulfert, 2005). As well, this impulsivity can be associated with involvement in illicit substance use and poor academic performance (Wulfert et al., 2002). Given these implications, the extent to which HPA dysregulation is associated with sex-differences in addictive behavior was of interest. We assessed HPA response in pathological gambling, as this presents a situation wherein physiological responses to addiction can be studied independent of a drug stimulus that can have confounding psychoactive effects.
HPA Activation in Recreational and Pathological Gamblers in the Laboratory Setting
Neuroendocrine factors appear to play a role in impulsivity associated with motivated behavior. Pathological gamblers can exhibit levels of impulsivity that exceed those observed in non-gambling controls, recreational gamblers, and individuals with substance use addictions that include alcohol and cocaine (Blaszczynski et al., 1997; Castellani and Rugle, 1995; Steel and Blaszczynski, 1998). Moreover, impulsivity has been related to severity of gambling as measured by the South Oaks Gambling Screen (SOGS) among gambling men (Steel and Blaszczynski, 1998; Vitaro et al., 1997) but some studies do not report a clear relationship with HPA function (Krueger et al., 2005). Other investigations have found that plasma cortisol and heart rate are significantly increased among male blackjack players at the onset of engaging in blackjack gambling in a casino when playing with their own money compared to a control condition when they were playing for non-monetary points (Krueger et al., 2005; Meyer et al., 2000). In these studies, both plasma cortisol and heart rate remained elevated for the duration of 60 or 90 minutes of play. As well, investigation in an aboriginal community in which everyone gambles following receipt of weekly pay, demonstrated that urinary cortisol is greater on days when gambling is most intense compared to days when it is minimized, and this effect was more prominent among men than among women (Schmitt et al., 1998). These data suggest a role for HPA arousal in response to gambling when in the casino environment.
In experimental settings, strong evidence support the involvement of prefrontal processes in decision-making that could lead to the addiction process (Bechara et al., 2000a,b; Cavedini et al., 2002; Clarke, 2004). The extent to which HPA status is a causative factor in such behavior or the result of engagement in addictive behavior remains unclear. We have observed that the magnitude of reduction in pathological gambling symptoms with therapeutic intervention is positively correlated with reduction in heart rate to gambling cues (Freidenberg et al., 2002) suggesting a role for autonomic arousal in the addiction process.
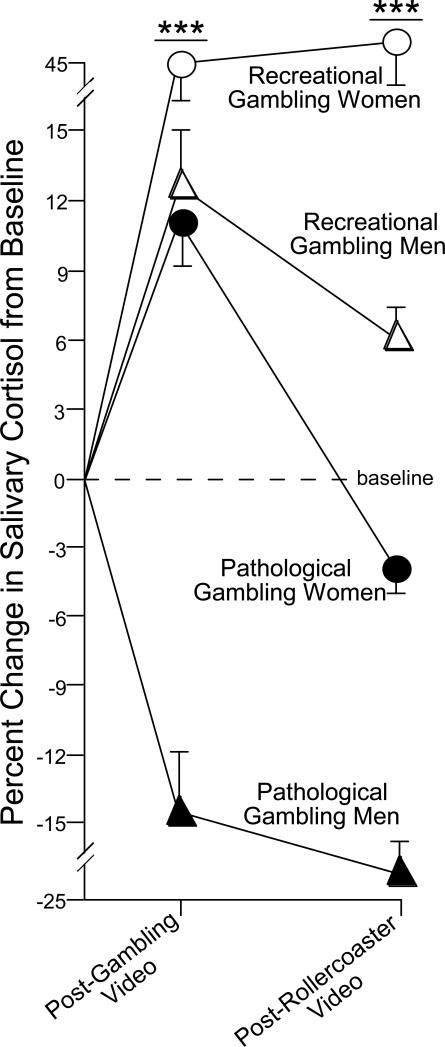
To begin to elucidate the role of HPA and sex on pathological gambling in a laboratory setting, we compared salivary cortisol levels of men and women who were recreational or pathological gamblers following exposure to gambling cues (Paris et al., 2009). It is notable that brief exposure to drug cues can readily reinstate craving for an addiction among human addicts and can reinstate a previously extinguished addiction among animals. Saliva samples were collected at baseline, following visual scenes that depicted engagement in gambling, and after a visual scene that depicted a neutral, stimulating event (a rollercoaster ride). We anticipated that pathological gamblers would have an attenuated HPA response following exposure to gambling cues compared to recreational gamblers. Participants each viewed gambling scenes depicting a gambling win and a gambling loss (in a counterbalanced manner). Whereas no differences emerged in basal levels of salivary cortisol among recreational or pathological gamblers, following the gambling stimuli, recreational gamblers had significantly elevated cortisol levels compared to pathological gamblers, who did not demonstrate a significant cortisol response to the stimuli. Moreover, pathological gambling men demonstrated a depression in cortisol concentrations from baseline after exposure to gambling footage, whereas all other groups demonstrated an elevation (Fig. 5). These data suggest that hypo-arousal of the HPA axis is associated with pathological gambling and that men may be particularly vulnerable. Similarly, others have found that lower concentrations of salivary cortisol were associated with riskier choices and monetary loss in the Iowa gambling task, whereas higher salivary cortisol indicated less risky choices and monetary gain (van Honk et al., 2003). As observed in some chronic stress-related pathologies, repeated exposure to the stressor may be associated with dysregulation of the stress response, wherein the stimuli no longer elicits HPA activation. In animal models, cortisol administration is rewarding. Thus, pathological-gamblers may have a hypocortisolemic response to gambling stimuli in the laboratory environment and this may play a role in the addiction process as the stimuli no longer elicits the prior degree of rewarding stress-related arousal.
Figure 5.
Men (n=15/group) and women (n=6/group) with gambling pathology demonstrate significantly attenuated salivary cortisol in response to gambling cues compared to recreational gamblers. As well, pathological gambling men demonstrate a hypocortisolemic response to gambling cues that is greater than all other groups (adapted from Paris et al., 2009). *** indicates significant interaction wherein pathological gamblers are different from recreational gamblers at all timepoints and pathological gambling men are different from all other groups, p < 0.05.
Stress Response and Engagement in Gambling Behavior in the Native Environment
We have observed that HPA arousal is attenuated among pathological gamblers in a laboratory setting when exposed to gambling cues, but whether engaging in gambling in the native environment would differentially influence HPA function was of interest. Engagement in motivated behaviors in the native situation presents a unique circumstance under which HPA-related effects on behavior can be observed. A reciprocal relationship between pharmacological addiction and HPA activation may exist, such that stress and HPA activation promote the likelihood of use (Goeders 1997, 2002a,b), and use alters HPA arousal. Indeed, research in animal models indicates that early stress experiences alter HPA response throughout life such that HPA hormones can be down-regulated while ligand targets can be upregulated and these effects are sex-dependent with males attenuating and females hyper-activating HPA response (Bosch et al., 2007; Mabandla et al., 2007; Ordyan and Pivina, 2004). As well, reinforcing properties of hedonic stimuli can be enhanced by early stress (Kippin et al., 2007). Similarly, human research indicates that negative life events in adolescence (such as loss of a parent, parental divorce, abuse, etc.), trauma, and certain socio-cultural stressors are all associated with increased vulnerability to engagement in addictive processes (reviewed in Sinha, 2008) and enhancement of cortisol can promote pharmacological addiction (Elman et al., 2003; Sinha et al., 2000). Some of these effects may be related to glucocorticoid actions to enhance central dopamine secretion (Barrot et al., 2001). These data support the notion that HPA activation can influence addiction.
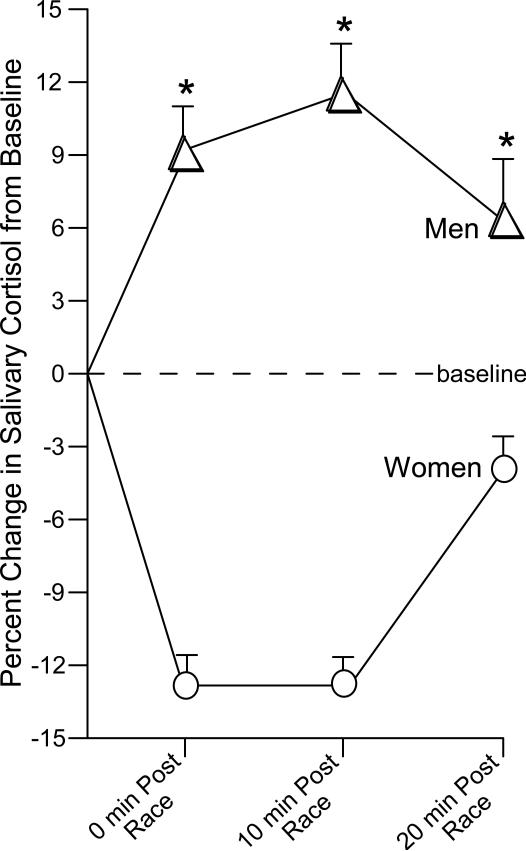
The environment may provide salient cues to elicit HPA response that may be associated with motivated and addictive processes. To investigate the role of HPA involvement on sex differences and gambling in the native environment, we assessed salivary cortisol in men and women gamblers at an off-track-betting (OTB) establishment (Franco et al., 2009). All participants engaged in gambling at the OTB at least twice per week. Participants placed a $2 bet of their own money on a horse-race to win and had saliva sampled before the race, immediately following the race, 10-min post-race, and 20-min post-race. Among OTB bettors, we found that men had significantly higher salivary cortisol concentrations at all sampling times compared to women. By 20 minutes post-race salivary cortisol was resolving among both men and women (Fig. 6). Absolute values of salivary cortisol were also significantly higher among men compared to women (Franco et al., 2009). Thus, among OTB bettors engaging in gambling in the natural environment, men have a greater and more sustained HPA response to gambling than do women. These data support the notion that gambling men may have an increased cortisol response when gambling in the native environment that is not as readily observed among women. However, it is a notable drawback that non-gambling controls were not included in either gambling experiment as men have been found to demonstrate a greater anticipatory stress response than women (Kirschbaum et al., 1992; Kudielka et al., 2009). Future experiments will seek to assess the role that anticipatory appraisal plays in the observed findings.
Figure 6.
Off-track betting men (n=21) demonstrate a significantly greater cortisol response to gambling on a horse race than do off-track betting women (n=11; adapted from Franco et al., 2009). * indicates significant main effect of sex, p < 0.05.
These data may be important for addictive processes apart from gambling. There is evidence that sex differences exist in problem and pathological gambling as in pharmacological addiction wherein men may be more vulnerable (Wilson et al., 2006; Blanco et al., 2006; Westermeyer and Boedicker, 2000). Pathological gambling and substance abuse are often co-morbid (Lesieur et al., 1986) and share many characteristics in pathology. In support, assessments of personality are similar among pathological gamblers and substance abusers (McCormick et al., 1987; Ramirez et al., 1988). Engaging in gambling activates substrates that are also associated with natural or drug-induced reward (Bechara, 2001; Kalenscher et al., 2006; Goodman, 2008; Reuter et al., 2005). In addition, therapeutics with efficacy treating substance addictions can reduce pathological gambling (Kim et al., 2001; Grant et al., 2006a,b, 2008). Engaging in gambling also elicits similar physiological responses as drugs of abuse. For instance, as is observed in drug use, there are autonomic effects of gambling. Autonomic arousal, measured by elevation in heart rate, has been demonstrated in response to many types of gambling stimuli including blackjack (Anderson and Brown 1984; Krueger et al., 2005; Meyer et al., 2000), poker machines (Coulombe et al., 1992; Dickerson et al., 1992; Leary and Dickerson 1985), slot machines (Carroll and Huxley 1994; Griffiths 1993), and horse race betting (Coventry and Norman 1997). Further, greater stakes elicit a greater autonomic response (Anderson and Brown 1984). These autonomic responses are indicative of HPA arousal. Thus, stress factors may underlie aspects of the general addiction process.
Chronic Stress in Posttraumatic Stress Disorder
We have found that, among healthy individuals in the described studies, HPA activity is greater among women than men in response to acute stressors. When considering a pathological state, such as gambling addiction, this sex difference may be reversed and this reflects the behavioral phenotype wherein men may be more vulnerable to pathology. It is also important to understand the influence that sex may have in cases of extreme pathology caused by stress.
Stress is a cause of PTSD, which results in impaired daily and HPA function (Aardal-Eriksson et al., 2001; Vanitallie, 2002). It has been observed that people with PTSD for 20+ years have lower basal urinary cortisol compared to controls (Mason et al., 1986; Yehuda et al., 1990, 1991, 1993, 1995a,b). Levels of urinary or serum cortisol, proximate to a motor vehicle accident (MVA), are lower among people with intrusive thoughts of the MVA, or that are later diagnosed with PTSD, than are those without such symptomology (Delahanty et al, 2000; McFarlane et al., 1997). These results suggest that altered HPA axis activity associated with extreme stress may lead to the development of “overconsolidation” of memories and thereby contribute to the development of PTSD (Pitman, 1989; Pitman et al., 1991). Van der Vegt et al. (2009) report a bifurcated effect of traumatic stress on cortisol among individuals maltreated during childhood such that individuals with histories of severe maltreatment exhibited lower cortisol levels and a flatter diurnal variation, whereas individuals who had experienced moderate maltreatment had higher levels and a steeper diurnal pattern.
More women than men are diagnosed with PTSD (Aardal-Eriksson et al., 2001; Breslau et al., 1997; Vanitallie, 2002). Examinations of sex effects on development of PTSD reveal that the majority of those diagnosed with PTSD are women (Breslau et al., 1991, 1997, 1998, 2004). However, when considering the influence of sex on pathophysiological state, investigations of PTSD have suffered inherent sex-specific confounds. That is, sex differences in stress-related pathologies are often subject to a priori categorization related to trauma experiences that are more prevalent in men vs. women (e.g., combat vs. sexual assault). These trauma experiences have different characteristic consequences (Buck and Walker, 1982; Frayne et al., 1999; Hofmann et al., 2003; Lang et al., 2003) and may also be superimposed on characteristics of traumatized individuals that confound apparent gender effects. We have examined men and women suffering from PTSD as a result of MVA-related trauma as this is a more gender-neutral stimulus.
Our collaborator, Dr. Edward Blanchard, has shown that PTSD can occur following an MVA (Barton et al., 1996; Blanchard et al., 1994, 1995a,b, 1996). Those with acute stress disorder due to an MVA are at high risk (60 to 80%) for converting to PTSD within 6 months (Harvey and Bryant, 1998). In our study, men and women who were referred by medical professionals or recruited by local media coverage and advertising were invited to The Center for Stress and Anxiety Disorders at The University at Albany-SUNY for an assessment of PTSD. Participants were diagnosed with PTSD via the Clinician Administered Post-Traumatic Stress Disorder Scale (Blake et al., 1998; Weathers et al., 2001; Weathers and Litz 1994). Within a week of the assessment, participants underwent re-exposure therapy for their MVA and saliva samples were collected at 14:00, 18:00, and 22:00 h.
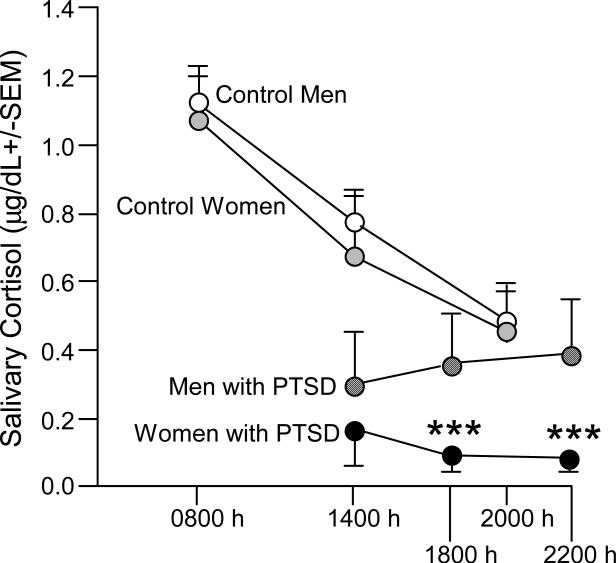
Among men and women with MVA-related PTSD, men had higher levels of salivary cortisol than did women and demonstrated atypical diurnal variation whereas women still demonstrated some reduction in cortisol concentrations across testing times, albeit these were not typical (Fig. 7; Freidenberg et al., 2009). However, compared to reference values from non-afflicted controls, both men and women with MVA-related PTSD were hypocortisolemic. These data support the notion that men and women with PTSD may present with perturbation of the HPA axis, and that men, compared to women, may have a more aberrant response when suffering from MVA-related PTSD. Past and recent investigations have also found that among men and women with MVA-related PTSD, cortisol levels are depressed (Delahanty et al., 2000) and males may have greater HPA arousal than women (Hawk et al., 2000; Shalev et al., 2008). Furthermore, examinations of MVA-related PTSD in children and adolescents have demonstrated that urinary cortisol predicts the degree of PTSD, with a larger effect among boys (Delahanty et al., 2005). These data support the notion that hormonally-mediated organizational sex differences may underlie some of the observed effects in HPA aberration associated with MVA-related PTSD.
Figure 7.
Men (n=3) and women (n=6) with posttraumatic stress disorder (PTSD) from exposure to motor vehicle accidents (MVA) have lower salivary cortisol than do non-afflicted historical controls (11 males and 13 females). As well, diurnal cortisol rhythmicity is attenuated among those with MVA-PTSD (adapted from Freidenberg et al., 2009). *** indicates significant interaction wherein women with PTSD have lower cortisol at 18:00 and 22:00 h, but not at 14:00 h, than do men with PTSD, p < 0.05.
Role of Hypothalamic-Pituitary-Gonadal Axis
An important question is what processes may underlie these sex differences in cortisol response. Sexual differentiation is initiated by early organizational effects of androgens and estrogens in humans and animals. Throughout life, production of steroids (primarily androgens from the testes of males and estrogens/progestogens from the ovaries of females) will have activational effects to alter physiological and psychological processes. Secretion of gonadotropin releasing hormone from hypothalamus acts on anterior pituitary to secrete lutenizing hormone and follicle stimulating hormone into circulation. Gonadotropins mediate gonadal release of sex steroids which can alter stress response in the HPA axis (Fig. 1). For example, in rats, estrous cycle differences can alter stress response. During the proestrous phase of the rat estrous cycle, when eastradiol (E2) levels peak, female rats have increased stress responsiveness compared to females in other stages of the estrous cycle (Audrain et al., 1978; Carey et al., 1995; Critchlow et al., 1963; Shors et al., 1998; Viau and Meaney, 1991). Removal of the ovaries, the primary source of E2, attenuates elevated stress response among female rats (Audrain et al., 1978; Kitay, 1961) and replacement with E2 and/or progestogens to ovariectomized rats reinstates this (Burgess and Handa, 1992; Dayas et al., 2000; Kitay, 1961; Patchev and Almeida, 1996; Viau and Meaney, 1991). Evidence for such effects are observed in women wherein salivary cortisol is observed to fluctuate across the menstrual cycle with greatest levels occurring during the menstrual phase (days 1-5 of the cycle), when endogenous estrogens and progestogens are at nadir (McCormick and Teillon, 2001). Further, progesterone elevations in the menstrual cycle have been associated with enhancement of plasma cortisol in response to a physical stressor (Roca et al., 2003). When considering sex differences, we must consider both the “organizing” effects of gonadal hormones during development (usually defined as sex), as well as the “activating” effects of gonadal hormones on behavior and physiology (typically defined as diurnal phase, time of day, in males and cycle phase in females) of the hypothalamic-pituitary-gonadal (HPG) axis. One of our goals is to examine the physiological and behavioral response to stressors as a function of both the organizational and activational effects of gonadal hormones. Throughout many of the aforementioned investigations, we were not able to control for cyclical variations in women's menstrual cycles. The extent to which observed sex differences are attributable to activational vs. organizational effects of sex hormones remains unknown.
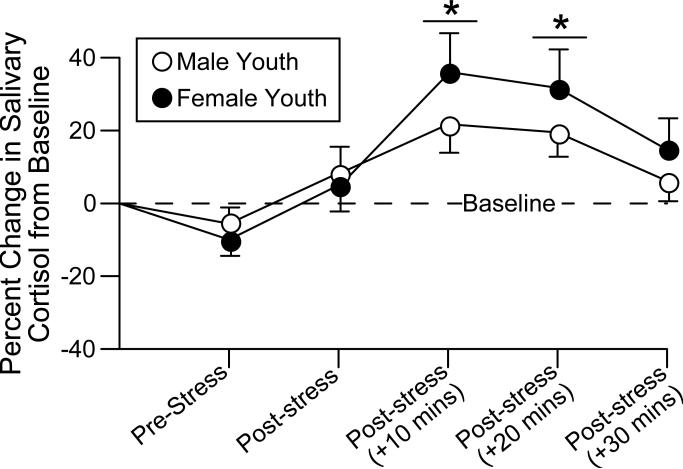
To begin to address this, one situation that can be considered is whether there are sex differences in stress responses among boys and girls. Our collaborator, Dr. Elana Gordis, has assessed salivary cortisol in maltreated and comparison male and female youth (age 9-14). In a pilot sub-sample selected from the larger study sample, youth provided 2 saliva samples before and 4 saliva samples after a psychological stressor (a modified version of the Trier Social Stress Test; Kirschbaum et al., 1993). Results suggest that maltreated youth overall demonstrated a hypocortisolemic response compared to age-matched control youth (Gordis et al., 2008), and that HPA aberration in combination with lower adrenergic responses may predict parent-reported aggressive behavior (Gordis et al., 2006). Preliminary analyses with the full sample of 454 youths (Trickett et al., 2006) indicate effects of cortisol response to the acute stressor, in addition to the main effects of maltreatment, with females showing lower overall levels but greater percent change from baseline in response to the stressor. Together, these data suggest that there are effects of maltreatment on cortisol response, but also that sex differences in cortisol response to stress may be present in youth as well as adulthood. These data may have important implications for development of cortico-limbic and/or executive functioning processes. Figure 8 displays the percent change from baseline salivary cortisol for male (n=201) and female (n=235) youth across the 6 samples. Following stressor, females have a consistent, albeit non-significant elevation in cortisol compared to males. This difference may be mediated by organizational as opposed to activational factors.
Figure 8.
Female youth (n=235) have a greater and more sustained salivary cortisol response to a psychological stressor than do male youth (n=201). * indicates significant main effect for cortisol to be greater compared to baseline, p < 0.05.
Conclusion
Together our data and the findings of others reveal that HPA function can influence, and be influenced by, pathophysiological state and this may have implications for neuropsychiatric disorders (such as panic attack, disordered eating, and PTSD) as well as development of pathological engagement in reward behavior such as drug use and gambling. Further, these data elucidate the nature of sex differences when observing situations wherein sex biases are minimized or are atypical. The sex-specific neuroendocrine factors that mediate HPA responsiveness in these situations will be the target of future investigation. As well, work in animal models will be aimed to elucidate the extent to which sex-differences in stress-response are due to genetics, organizational hormone effects, or epigenetic phenomena.
Acknowledgements
We would like to thank Jennifer Courtney for providing information on the history and background of posttraumatic stress disorder. These investigations were supported by funding from The University at Albany-SUNY Faculty Research Award Program, the National Institute of Mental Health (MH06769801), and the National Institute of Child Health and Human Development (K23HD041428, R01HD039129).
References
- Aardal E, Holm AC. Cortisol in saliva--reference ranges and relation to cortisol in serum. Eur. J. Clin. Chem. Clin. Biochem. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- Aardal-Eriksson E, Eriksson TE, Thorell LH. Salivary cortisol, posttraumatic stress symptoms, and general health in the acute phase and during 9-month follow-up. Biol. Psychiatry. 2001;50:986–993. doi: 10.1016/s0006-3223(01)01253-7. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Kalin NH, Davidson RJ. Acute cortisol elevations cause heightened arousal ratings of objectively nonarousing stimuli. Emotion. 2005;5:354–359. doi: 10.1037/1528-3542.5.3.354. [DOI] [PubMed] [Google Scholar]
- Acheson DT, Forsyth JP, Prenoveau JM, Bouton ME. Interoceptive fear conditioning as a learning model of panic disorder: an experimental evaluation using 20% CO2-enriched air in a non-clinical sample. Behav. Res. Ther. 2007;45:2280–2294. doi: 10.1016/j.brat.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Argyropoulos SV, Bailey JE, Hood SD, Kendrick AH, Rich AS, Laszlo G, Nash JR, Lightman SL, Nutt DJ. Inhalation of 35% CO2 results in activation of the HPA axis in healthy volunteers. Psychoneuroendocrinology. 2002;27:715–729. doi: 10.1016/s0306-4530(01)00075-0. [DOI] [PubMed] [Google Scholar]
- Anderson G, Brown RI. Real and laboratory gambling, sensation seeking and arousal. Br. J. Psychol. 1984;75:401–410. doi: 10.1111/j.2044-8295.1984.tb01910.x. [DOI] [PubMed] [Google Scholar]
- Anderson DA, Shapiro JR, Lundgren JD, Spataro LE, Frye CA. Self-reported dietary restraint is associated with elevated levels of salivary corstisol. Appetite. 2002;38:13–17. doi: 10.1006/appe.2001.0459. [DOI] [PubMed] [Google Scholar]
- Assenmacher I, Barbanel G, Gaillet S, Givalois L, Ixart G, Malaval F, Mekaouche M, Siaud P, Szafarczyk A. Central regulation of ACTH release in stress. Ann. N. Y. Acad. Sci. 1995;771:41–54. doi: 10.1111/j.1749-6632.1995.tb44669.x. [DOI] [PubMed] [Google Scholar]
- Audrain M, Beraud G, Lescoat G, Feliot J, Maniey J. Effect of estrus cycle, ovariectomy and lactation on variations of basal corticosterone levels or agression stress levels in rats C. R. Seances. Soc. Biol. Fil. 1978;172:33–37. [PubMed] [Google Scholar]
- Axelrod J, Reisine TD. Stress Hormones: Their Interaction and Regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Wedekind D, Pauls J, Broocks A, Hajak G, Rüther E. Salivary cortisol in panic attacks. Am. J. Psychiatry. 2000;157:454–456. doi: 10.1176/appi.ajp.157.3.454. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Clinical Handbook of Psychological Disorders. 3rd edition Guilford Publications; New York: 2001. [Google Scholar]
- Barrot M, Abrous DN, Marinelli M, Rougé-Pont F, Le Moal M, Piazza PV. Influence of glucocorticoids on dopaminergic transmission in the rat dorsolateral striatum. Eur. J. Neurosci. 2001;13:812–818. doi: 10.1046/j.1460-9568.2001.01434.x. [DOI] [PubMed] [Google Scholar]
- Barton KA, Blanchard EB, Hickling EJ. Antecedents and consequences of acute stress disorder among motor vehicle accident victims. Behav. Res. Ther. 1996;34:805–813. doi: 10.1016/0005-7967(96)00027-7. [DOI] [PubMed] [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Semin. Clin. Neuropsychiatry. 2001;6:205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000a;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000b;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beck JG, Shipherd JC, Zebb BJ. Fearful responding to repeated CO2 inhalation: a preliminary investigation. Behav. Res. Ther. 1996;34:609–620. doi: 10.1016/0005-7967(96)00039-3. [DOI] [PubMed] [Google Scholar]
- Belgorodsky A, Knyazhansky L, Loewenthal U, Arbelle J, Cohen H, Benjamin J. Effects of the cortisol synthesis inhibitor metyrapone on the response to carbon dioxide challenge in panic disorder. Depress. Anxiety. 2005;21:143–148. doi: 10.1002/da.20062. [DOI] [PubMed] [Google Scholar]
- Benjamin L, Wulfert E. Dispositional correlates of addictive behaviors in college women: binge eating and heavy drinking. Eat. Behav. 2005;6:197–209. doi: 10.1016/j.eatbeh.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Endocrine abnormalities of obesity. Metabolism. 1995;44:21–23. doi: 10.1016/0026-0495(95)90315-1. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Holm G, Rosmond R, Folkow B. Hypertension and the metabolic syndrome: closely related central origin? Blood. Press. 2000;9:71–82. doi: 10.1080/08037050050151762. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Klauminzer G, Charney D, Keane TM. Clinician-Administered PTSD Scale (CAPS) National Center for Post-Traumatic Stress Disorder, Behavioral Science Division; Boston, MA: 1998. [Google Scholar]
- Blanchard EB, Hickling EJ, Mitnick N, Taylor AE, Loos WR, Buckley TC. The impact of severity of physical injury and perception of life threat in the development of post-traumatic stress disorder in motor vehicle accident victims. Behav. Res. Ther. 1995a;33:529–534. doi: 10.1016/0005-7967(94)00079-y. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos W. Psychiatric morbidity associated with motor vehicle accidents. J. Nerv. Ment. Dis. 1995b;183:495–504. doi: 10.1097/00005053-199508000-00001. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Gerardi RJ. Psychological morbidity associated with motor vehicle accidents. Behav. Res. Ther. 1994;32:283–290. doi: 10.1016/0005-7967(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Forneris CA, Jaccard J. Who develops PTSD from motor vehicle accidents? Behav. Res. Ther. 1996;34:1–10. doi: 10.1016/0005-7967(95)00058-6. [DOI] [PubMed] [Google Scholar]
- Blanco C, Hasin DS, Petry N, Stinson FS, Grant BF. Sex differences in subclinical and DSM-IV pathological gambling: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol. Med. 2006;36:943–953. doi: 10.1017/S0033291706007410. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Steel Z, McConaghy N. Impulsivity in pathological gambling: The antisocial impulsivist. Addiction. 1997;92:75–87. [PubMed] [Google Scholar]
- Board F, Wadeson R, Persky H. Depressive affect and endocrine function in humans: the last two decades of research. Psychother. Psychosom. 1957;78:114–150. [Google Scholar]
- Bosch OJ, Müsch W, Bredewold R, Slattery DA, Neumann ID. Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: implications for postpartum mood disorder. Psychoneuroendocrinology. 2007;32:267–278. doi: 10.1016/j.psyneuen.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol. Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Boyer P. Do anxiety and depression have a common pathophysiological mechanism? Acta. Psychiatr. Scand. Suppl. 2000;406:24–29. [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch. Gen. Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch. Gen. Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch. Gen. Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Poisson LM, Schultz LR, Lucia VC. Estimating post-traumatic stress disorder in the community: lifetime perspective and the impact of typical traumatic events. Psychol. Med. 2004;34:889–898. doi: 10.1017/s0033291703001612. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Buck OD, Walker JI. Posttraumatic stress disorder in Vietnam veterans: a review. South. Med. J. 1982;75:704–706. doi: 10.1097/00007611-198206000-00019. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Cadore E, Lhullier F, Brentano M, Silva E, Ambrosini M, Spinelli R, Silva R, Kruel L. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J. Sports. Sci. 2008;26:1067–1072. doi: 10.1080/02640410801919526. [DOI] [PubMed] [Google Scholar]
- Calixto C, Martinez FE, Jorge SM, Moreira AC, Martinelli CE., Jr. Correlation between plasma and salivary cortisol levels in preterm infants. J. Pediatr. 2002;140:116–118. doi: 10.1067/mpd.2002.120765. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Nesse RM. Systemic hormonal and physiological abnormalities in anxiety disorders. Psychoneuroendocrinology. 1988;13:287–307. doi: 10.1016/0306-4530(88)90054-6. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J. Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carroll D, Huxley JAA. Cognitive, dispositional, and psychophysiological correlates of dependent slot machine gambling in young people. J. Appl. Sociol. Psychol. 1994;24:1070–1083. [Google Scholar]
- Castellani B, Rugle L. A comparison of pathological gamblers to alcoholics and cocaine misusers on impulsivity, sensation seeking, and craving. Int. J. Addict. 1995;30:275–289. doi: 10.3109/10826089509048726. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D'Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biol. Psychiatry. 2002;51:334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- Chorot P, Sandin B. Life events and stress reactivity as predictors of cancer, coronary heart disease and anxiety disorders. Int. J. Psychosom. 1994;41:34–40. [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress system disorders: overview of behavioral and physical homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Clarke D. Impulsiveness, locus of control, motivation and problem gambling. J. Gambl. Stud. 2004;20:319–345. doi: 10.1007/s10899-004-4578-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD. The relationship between acute gluccocorticois levels and hippocampal function depends upon task aversiveness and memory processing stage. Nonlinearity. Biol. Toxicol. Med. 2005;3:57–78. doi: 10.2201/nonlin.003.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cogn. Neurosci. Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Moreau D, Chaput F, Martinez JM, Hoven CW, Mandell DJ, Gorman JM, Pine DS. Salivary cortisol concentrations before and after carbon-dioxide inhalations in children. Biol. Psychiatry. 2002;51:326–333. doi: 10.1016/s0006-3223(01)01250-1. [DOI] [PubMed] [Google Scholar]
- Coulombe A, Ladouceur R, Desharnais R, Jobin J. Erroneous perceptions and arousal among regular and occasional video poker players. J. Gambling. Stud. 1992;8:235–244. doi: 10.1007/BF01014651. [DOI] [PubMed] [Google Scholar]
- Coventry KR, Norman AC. Arousal, sensation seeking and frequency of gambling in off-course horse racing bettors. Br. J. Psychol. 1997;88:671–681. doi: 10.1111/j.2044-8295.1997.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am. J. Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Xu Y, Buller KM, Day TA. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J. Neuroendocrinol. 2000;12:784–794. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol. Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dickerson M, Hinchy J, England SL, Fabre J, Cunningham R. On the determinants of persistent gambling behavior. I. High-frequency poker machine players. Br. J. Psychol. 1992;83:237–248. doi: 10.1111/j.2044-8295.1992.tb02438.x. [DOI] [PubMed] [Google Scholar]
- van Duinen MA, Schruers KR, Maes M, Griez EJ. CO2 challenge results in hypothalamic-pituitary-adrenal activation in healthy volunteers. J. Psychopharmacol. 2005;19:243–247. doi: 10.1177/0269881105051527. [DOI] [PubMed] [Google Scholar]
- Earle TL, Linden W, Weinberg J. Differential effects of harassment on cardiovascular and salivary cortisol stress reactivity and recovery in women and men. J. Psychosom. Res. 1999;46:125–141. doi: 10.1016/s0022-3999(98)00075-0. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol. Psychol. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Elman I, Lukas SE, Karlsgodt KH, Gasic GP, Breiter HC. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol. Bull. 2003;37:84–89. [PubMed] [Google Scholar]
- Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinol. Lett. 2002;23:199–208. [PubMed] [Google Scholar]
- Forsyth JP, Eifert GH. Systemic alarms in fear conditioning--I: A reappraisal of what is being conditioned. Behav. Ther. 1996;27:441–462. [Google Scholar]
- Forsyth JP, Daleiden E, Chorpita BF. Response primacy in fear conditioning: Disentangling the contributions of the UCS vs. the UCR. The. Psyc. Rec. 2000a;50:17–33. [Google Scholar]
- Forsyth JP, Eifert GH, Canna MA. Evoking analogue subtypes of panic attacks in a nonclinical population using carbon dioxide-enriched air. Behav. Res. Ther. 2000b;38:559–572. doi: 10.1016/s0005-7967(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Franco C, Paris JJ, Frye CA, Wulfert E. Gender differences in cortisol response to gambling: an exploratory study. Physiol. Behav. Special Issue: Conversations in the Discipline. 2009 In Revision. [Google Scholar]
- Frayne SM, Seaver MR, Loveland S, Christiansen CL, Spiro A, 3rd, Parker VA, Skinner KM. Burden of medical illness in women with depression and posttraumatic stress disorder. Arch. Intern. Med. 2004;164:1306–1312. doi: 10.1001/archinte.164.12.1306. [DOI] [PubMed] [Google Scholar]
- Freidenberg BM, Gusmano R, Frye CA, Hickling EJ, Blanchard EB, Bremner JD. Women with PTSD have lower basal salivary cortisol levels later in the day than do their male counterparts. Physiol. Behav. 2009 doi: 10.1016/j.physbeh.2009.06.002. doi:10.1016/j.physbeh.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg BM, Blanchard EB, Wulfert E, Malta LS. Changes in physiological arousal to gambling cues among participants in motivationally enhanced cognitive-behavior therapy for pathological gambling: a preliminary study. Appl. Psychophysiol. Biofeedback. 2002;27:251–260. doi: 10.1023/a:1021057217447. [DOI] [PubMed] [Google Scholar]
- Fyer MR, Uy J, Martinez J, Goetz R, Klein DF, Fyer A, Liebowitz MR, Gorman J. CO2 challenge of patients with panic disorder. Am. J. Psychiatry. 1987;144:1080–1082. doi: 10.1176/ajp.144.8.1080. [DOI] [PubMed] [Google Scholar]
- Gallucci WT, Baum A, Laue L, Rabin DS, Chrousos GP, Gold PW, Kling MA. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health. Psychol. 1993;12:420–425. doi: 10.1037//0278-6133.12.5.420. [DOI] [PubMed] [Google Scholar]
- Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Report from the world health organization collaborative study on psychological problems in general health care. Arch. Gen. Psychiatry. 1998;55:405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J. Pharmacol. Exp. Ther. 2002a;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002b;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol. Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin F, Chrousos GP. Clinical and biochemical manifestation of depression: relationship to the neurobiology of stress, Part 2. N. Engl. J. Med. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Goodman A. Neurobiology of addiction. An integrative review. Biochem. Pharmacol. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary α-amylase-cortisol asymmetry in maltreated youth. Horm. Behav. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gorman BS, Allison DB. Measures of restrained eating. In: Allison DB, editor. Handbook of assessment methods for eating behaviors and weight-related problems. Sage; Newbury Park, CA: 1995. pp. 149–184. [Google Scholar]
- Grant JE, Brewer JA, Potenza MN. The neurobiology of substance and behavioral addictions. CNS. Spectr. 2006a;11:924–930. doi: 10.1017/s109285290001511x. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Hollander E, Potenza MN. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology. 2008;200:521–527. doi: 10.1007/s00213-008-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Hollander E, Cunningham-Williams R, Nurminen T, Smits G, Kallio A. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am. J. Psychiatry. 2006b;163:303–312. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- Griez E, van den Hout M. CO2 inhalation in the treatment of panic attacks. Behav. Res. Ther. 1986;24:145–50. doi: 10.1016/0005-7967(86)90085-9. [DOI] [PubMed] [Google Scholar]
- Griffiths M. Tolerance in gambling: An objective measure using the psychophysiological analysis of male fruit machine gamblers. Addict. Behav. 1993;18:365–372. doi: 10.1016/0306-4603(93)90038-b. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Harrington PJ, Schmidt NB, Telch MJ. Prospective evaluation of panic potentiation following 35% CO2 challenge in nonclinical subjects. Am. J. Psychiatry. 1996;153:823–825. doi: 10.1176/ajp.153.6.823. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Bryant RA. The relationship between acute stress disorder and posttraumatic stress disorder: a prospective evaluation of motor vehicle accident survivors. J. Consult. Clin. Psychol. 1998;66:507–512. doi: 10.1037//0022-006x.66.3.507. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Dougall AL, Ursano RJ, Baum A. Urinary catecholamines and cortisol in recent-onset posttraumatic stress disorder after motor vehicle accidents. Psychosom. Med. 2000;62:423–434. doi: 10.1097/00006842-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Herman CP, Polivy J, King GA, McGree ST. The (Mis)measurement of restraint: An analysis of conceptual and psychometric issues. J. Abnorm. Psychol. 1988;97:19–28. doi: 10.1037//0021-843x.97.1.19. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. Restrained eating. In: Stunkard AJ, editor. Obesity. Saunders; New York: 1980. pp. 208–225. [Google Scholar]
- Hofmann SG, Litz BT, Weathers FW. Social anxiety, depression, and PTSD in Vietnam veterans. J. Anxiety. Disord. 2003;17:573–582. doi: 10.1016/s0887-6185(02)00227-x. [DOI] [PubMed] [Google Scholar]
- Jezova D, Jurankova E, Mosnarova A, Kriska M, Skultetyova I. Neuroendocrine response during stress with relation to gender differences. Acta. Neurobiol. Exp. (Warsz) 1996;56:779–785. doi: 10.55782/ane-1996-1183. [DOI] [PubMed] [Google Scholar]
- Kahn JP, Rubinow DR, Davis CL, Kling M, Post RM. Salivary cortisol: a practical method for evaluation of adrenal function. Biol. Psychiatry. 1988;23:335–349. doi: 10.1016/0006-3223(88)90284-3. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kalenscher T, Ohmann T, Güntürkün O. The neuroscience of impulsive and self-controlled decisions. Int. J. Psychophysiol. 2006;62:203–211. doi: 10.1016/j.ijpsycho.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Kasckow JW, Baker D, Geracioti TD., Jr. Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides. 2001;22:845–851. doi: 10.1016/s0196-9781(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Forsyth JP. Sex differences in response to an observational fear conditioning procedure. Behav. Ther. 2007;38:340–349. doi: 10.1016/j.beth.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J. Behav. Ther. Exp. Psychiatry. 2008;39:87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12 month prevalence of DSM III R psychiatric disorders in the United States. Arch. Gen. Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J. Clin. Endocrinol. Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Szumlinski KK, Kapasova Z, Rezner B, See RE. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology. 2008;33:769–782. doi: 10.1038/sj.npp.1301447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol. Psychiatry. 2001;49:914–921. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- Kitay JL. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger TH, Schedlowski M, Meyer G. Cortisol and heart rate measures during casino gambling in relation to impulsivity. Neuropsychobiology. 2005;52:206–211. doi: 10.1159/000089004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Rodgers CS, Laffaye C, Satz LE, Dresselhaus TR, Stein MB. Sexual trauma, posttraumatic stress disorder, and health behavior. Behav. Med. 2003;28:150–158. doi: 10.1080/08964280309596053. [DOI] [PubMed] [Google Scholar]
- Leary K, Dickerson M. Levels of arousal in high- and low-frequency gamblers. Behav. Res. Ther. 1985;23:635–640. doi: 10.1016/0005-7967(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Forsyth JP, Eifert GH. Devices and methods for administering carbon dioxide-enriched air in experimental and clinical settings. J. Behav. Ther. Exp. Psychiatry. 1998;29:239–248. doi: 10.1016/s0005-7916(98)00018-4. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB, Zoppa RM. Alcoholism, drug abuse, and gambling. Alcohol. Clin. Exp. Res. 1986;10:33–38. doi: 10.1111/j.1530-0277.1986.tb05610.x. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB. Gender differences in anxiety disorders and anxiety symptoms in adolescents. J. Abnorm. Psych. 1998;107:109–117. doi: 10.1037//0021-843x.107.1.109. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp. Clin. Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Owens JF, Stoney CM, Matthews KA. Menopausal status influences ambulatory blood pressure levels and blood pressure changes during mental stress. Circulation. 1993;88:2794–2802. doi: 10.1161/01.cir.88.6.2794. [DOI] [PubMed] [Google Scholar]
- Mabandla MV, Dobson B, Johnson S, Kellaway LA, Daniels WM, Russell VA. Development of a mild prenatal stress rat model to study long term effects on neural function and survival. Metab. Brain. Dis. 2008;23:31–42. doi: 10.1007/s11011-007-9049-2. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. J. Nerv. Ment. Dis. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- McCormick RA, Taber J, Kruedelbach N, Russo A. Personality profiles of hospitalized pathological gamblers: the California Personality Inventory. J. Clin. Psychol. 1987;43:521–527. doi: 10.1002/1097-4679(198709)43:5<521::aid-jclp2270430516>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Teillon SM. Menstrual cycle variation in spatial ability: relation to salivary cortisol levels. Horm. Behav. 2001;39:29–38. doi: 10.1006/hbeh.2000.1636. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain. Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology and neuroendocrinology of stress implications for post-traumatic stress disorder from a basic science perspective. Psychiatr. Clin. North. Am. 2002;25:469–494. doi: 10.1016/s0193-953x(01)00009-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McFarlane AC, Atchison M, Yehuda R. The acute stress response following motor vehicle accidents and its relation to PTSD. Ann. N. Y. Acad. Sci. 1997;821:437–441. doi: 10.1111/j.1749-6632.1997.tb48299.x. [DOI] [PubMed] [Google Scholar]
- Meyer G, Hauffa BP, Schedlowski M, Pawlak C, Stadler MA, Exton MS. Casino gambling increases heart rate and salivary cortisol in regular gamblers. Biol. Psychiatry. 2000;48:948–953. doi: 10.1016/s0006-3223(00)00888-x. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Murray L, Canna M, Forsyth J, Frye CA. Effects on salivary cortisol from CO2 stress with relation to sex differences.. Poster presentation at the NorthEast Under/graduate Research Organization for Neuroscience Conference; Hartford, CT. 1999. [Google Scholar]
- Ordyan NE, Pivina SG. Characteristics of the behavior and stress-reactivity of the hypophyseal-adrenal system in prenatally stressed rats. Neurosci. Behav. Physiol. 2004;34:569–574. doi: 10.1023/b:neab.0000028286.83083.73. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Franco C, Sodano R, Frye CA, Wulfert E. Gambling pathology is associated with dampened cortisol response among men and women. Physiol. Behav. 2009 doi: 10.1016/j.physbeh.2009.04.002. doi:10.1016/j.physbeh.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Almeida OF. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J. Neurosci. 1996;16:7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Araya R, de Lima M, Ludermir A, Todd C. Women, poverty and common mental disorders in four restructuring societies. Soc. Sci. Med. 1999;49:1461–1471. doi: 10.1016/s0277-9536(99)00208-7. [DOI] [PubMed] [Google Scholar]
- Pearson V. Goods on which one loses: women and mental health in China. Soc. Sci. Med. 1995;41:1159–1173. doi: 10.1016/0277-9536(94)00424-r. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Editorial: Posttraumatic stress disorder, hormones, and memory. Biol. Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Lowenhagen MJ, Macklin ML, Altman B. Pre-Vietnam contents of posttraumatic stress disorder veterans’ service medical and personnel records. Compr. Psychiatry. 1991;32:416–422. doi: 10.1016/0010-440x(91)90018-8. [DOI] [PubMed] [Google Scholar]
- Prendergast ML. Substance use and abuse among college students: a review of recent literature. J. Am. Coll. Health. 1994;43:99–113. doi: 10.1080/07448481.1994.9939094. [DOI] [PubMed] [Google Scholar]
- Prenoveau JM, Forsyth JP, Kelly MM, Barrios V. Repeated exposure to 20% CO2 challenge and risk for developing panic attacks: a controlled 6- and 12-month follow-up in a nonclinical sample. J. Anxiety. Disord. 2006;20:1158–1167. doi: 10.1016/j.janxdis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Ramirez LF, McCormick RA, Lowy MT. Plasma cortisol and depression in pathological gamblers. Br. J. Psychiatry. 1988;153:684–686. doi: 10.1192/bjp.153.5.684. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Brown TA, Antony MM, Barlow DH. Response to hyperventilation and inhalation of 5.5% carbon dioxide-enriched air across the DSM-III-R anxiety disorders. J. Abnorm. Psychol. 1992;101:538–552. doi: 10.1037//0021-843x.101.3.538. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, Southwick SM, Charney DS. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol. Psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat. Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J. Clin. Endocrinol. Metab. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, the aging brain, and the mechanisms of neuronal death. MIT Press; Cambridge, MA: 1992. [Google Scholar]
- Sasaki I, Akiyoshi J, Sakurai R, Tsutsumi T, Ono H, Yamada K, Fujii I. Carbon dioxide induced panic attack in panic disorder in Japan. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1996;20:1145–1157. doi: 10.1016/s0278-5846(96)00102-9. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Telch MJ, Jaimez TL. Biological challenge manipulation of PCO2 levels: a test of Klein's suffocation alarm theory of panic. J. Abnorm. Psychol. 1996;105:446–454. doi: 10.1037//0021-843x.105.3.446. [DOI] [PubMed] [Google Scholar]
- Schmitt LH, Harrison GA, Spargo RM. Variation in epinephrine and cortisol excretion rates associated with behavior in an Australian Aboriginal community. Am. J. Phys. Anthropol. 1998;106:249–253. doi: 10.1002/(SICI)1096-8644(199806)106:2<249::AID-AJPA10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child. Dev. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Shalev AY, Videlock EJ, Peleg T, Segman R, Pitman RK, Yehuda R. Stress hormones and post-traumatic stress disorder in civilian trauma victims: a longitudinal study. Part I: HPA axis responses. Int. J. Neuropsychopharmacol. 2008;11:365–372. doi: 10.1017/S1461145707008127. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Sie HG, Fishman WH. Glycogen Synthetase: Its Response to Cortisol. Science. 1964;143:816–817. doi: 10.1126/science.143.3608.816. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F, Reul JM. Glucocorticoids and depression. Baillieres. Best. Pract. Res. Clin. Endocrinol. Metab. 1999;13:597–614. doi: 10.1053/beem.1999.0046. [DOI] [PubMed] [Google Scholar]
- Steel Z, Blaszczynski A. Impulsivity, personality disorders and pathological gambling severity. Addiction. 1998;93:895–905. doi: 10.1046/j.1360-0443.1998.93689511.x. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Ann. N. Y. Acad. Sci. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Chrousos GP. The three-way interactions between the hypothalamic-pituitary-adrenal and gonadal axes and the immune system. Baillieres. Clin. Rheumatol. 1996;10:181–198. doi: 10.1016/s0950-3579(96)80014-8. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Gordis EB, Horn J. Resilience and cortisol dysregulation in maltreated young adolescents. In: J. Noll (Chair), Diverse Cortisol Stress Reactivity Patterns and Genotype Risk in Maltreated Children Across Development.. Symposium at the Biennial meeting of the Society for Research on Adolescence; San Francisco, CA. 2006. [Google Scholar]
- Van den Hout MA, Griez E. Panic symptoms after inhalation of carbon dioxide. Br. J. Psychiatry. 1984;144:503–507. doi: 10.1192/bjp.144.5.503. [DOI] [PubMed] [Google Scholar]
- Van der Vegt EJM, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults: A study of international adoptees. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2008.11.004. In Press. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94:1413–1419. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Anthony JC. Male-female differences in transitions from first drug opportunity to first use: searching for subgroup variation by age, race, region, and urban status. J. Womens. Health. Gender-based. Med. 2001;10:797–804. doi: 10.1089/15246090152636550. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14:1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Kindt M. Salivary α-amylase and cortisol responses to different stress tasks: impact of sex. Int. J. Psychophysiol. 2008;69:33–40. doi: 10.1016/j.ijpsycho.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Vanitallie TB. Stress: a risk factor for serious illness. Metabolism. 2002;51:40–45. doi: 10.1053/meta.2002.33191. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Arseneault L, Tremblay RE. Dispositional predictors of problem gambling in male adolescents. Am. J. Psychiatry. 1997;154:1769–1770. doi: 10.1176/ajp.154.12.1769. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JRT. Clinician-Administered PTSD Scale: A review of the first ten years of research. Depress. Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT. Psychometric properties of the Clinician-Administered PTSD Scale, CAPS-I. PTSD. Res. Q. 1994;5:2–6. [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am. J. Drug. Alcohol. Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- Wilson DH, Gilliland J, Ross NA, Derevensky J, Gupta R. Video lottery terminal access and gambling among high school students in Montréal. Can. J. Public. Health. 2006;97:202–206. doi: 10.1007/BF03405585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfert E, Block JA, Santa Ana E, Rodriguez ML, Colsman M. Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. J. Pers. 2002;70:533–552. doi: 10.1111/1467-6494.05013. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J. Nerv. Ment. Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Lowy MT, Southwick SM, Shaffer D, Giller EL. Lymphocyte glucocorticoid receptor number in posttraumatic stress disorder. Am. J. Psychiatry. 1991;148:499–504. doi: 10.1176/ajp.148.4.499. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Mason JW, Giller EL. Glucocorticoid receptor number and cortisol secretion in mood, anxiety, and psychotic disorders. Biol. Psychiatry. 1993;34:18–25. doi: 10.1016/0006-3223(93)90252-9. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL. Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch. Gen. Psychiatry. 1995a;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Byrnes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am. J. Psychiatry. 1995b;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J. Gend. Specif. Med. 1998;1:21–27. [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH, Lejuez CW, McNeil DW. The effects of offset control over 20% carbon-dioxide-enriched air on anxious responding. J. Abnorm. Psychol. 1999;108:624–632. doi: 10.1037//0021-843x.108.4.624. [DOI] [PubMed] [Google Scholar]