Abstract
Objectives
The ‘Phenomenology and Course of Pediatric Bipolar Disorders’ study, a National Institute of Mental Health-funded study of child bipolar I disorder (BP-I) begun in 1995, is a prospective follow-up study that included collecting pharmacological and non-drug treatment data.
Methods
There were 115 first-episode subjects who fit full DSM-IV criteria for BP-I, mixed or manic phase, with severity scores in the clinically impaired range, ascertained by consecutive new case ascertainment. Subjects were assessed with the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS), given separately to parents about their children and to children about themselves. All treatment was provided by the subjects’ own community practitioners, exactly as if they had not been in the research study. Thus, families were only seen for research assessments, and research staff was not at all involved in their treatment. Data on type, dose, and duration of pharmacological and non-drug treatment were collected. During follow-up, 93.9% (n = 108) were assessed at each of the nine assessment times.
Results
During the eight years, only 62.6% received any antimanic medication (antipsychotic, anticonvulsant, lithium) at any time. Percents who received non-antimanic medication included 77.4% medication for attention-deficit hyperactivity disorder and 64.3% antidepressants. A total of 67.8% of subjects were taking two or more concurrent medication classes. Subjects ascertained from psychiatric versus pediatric sites received antimanics significantly more frequently (p = 0.006). Earlier recovery during eight-year follow-up was predicted by greater percent of weeks on lithium (p = 0.017).
Conclusions
Given these findings, and the poor prognosis from prospective follow-up of this sample reported elsewhere, there is a need for further research that informs the development of effective treatment strategies.
Keywords: bipolar I disorder, child, follow-up, treatment
Unlike treatment of adults with bipolar I disorder (BP-I), interventions for child BP-I are a far less studied area with a paucity of randomized clinical trials (1-6). Thus, there are scant data to guide treatment of child BP-I in spite of accumulating evidence of marked and progressive increases in the use of antipsychotic medications (7). Against this background, it may be useful to the field to examine community-provided, naturalistic care of children with mania.
The ‘Phenomenology and Course of Pediatric Bipolar Disorders’ study, a National Institute of Mental Health (NIMH)-funded study of child BP-I begun in 1995, is a prospective follow-up study that included collecting pharmacological and non-drug treatment data.
In this study, all treatment was provided by the subjects’ own community practitioners, exactly as if they had not been in the research study. Thus, families were only seen for research assessments and research staff was not at all involved in the treatment.
Methods
Subject ascertainment
Subjects were participants in the ongoing, NIMH-funded ‘Phenomenology and Course of Pediatric Bipolar Disorders’ study (8) who were obtained between 1995 and 1998 by consecutive new case ascertainment from designated outpatient child psychiatric and pediatric sites, as detailed in Geller et al. (9).
Inclusion and exclusion criteria
Inclusion criteria for child BP-I subjects were: (i) age 7-16 years, (ii) male and female gender, (iii) good physical health, (iv) current DSM-IV BP-I (manic or mixed phase) for ≥ 2 weeks with one or both cardinal symptoms of mania (i.e., elation and/or grandiosity), and (v) a Children's Global Assessment Scale (CGAS) score ≤ 60, which indicates definite clinical impairment (10, 11).
Exclusion criteria for child BP-I subjects were: (i) IQ < 70, (ii) adopted status, (iii) pervasive developmental disorders, schizophrenia, epilepsy or other major medical or neurological disorder, (iv) baseline substance dependency, (v) pregnancy, and (vi) manic symptoms only while on medications that may induce manic symptoms [e.g., antidepressant, medication for attention-deficit hyperactivity disorder (ADHD)]. Although substance dependency and pregnancy were exclusions at baseline, subjects who developed substance use disorders (SUD) or became pregnant during follow-up were continued in the study. There were no family psychopathology exclusions. All parents were fluent in English and competent to participate in the interviews.
Rationales for these inclusion and exclusion criteria were as follows. As the first NIMH study in the field, a lower age of 7 was selected for credibility of interview assessments, and an upper age of 16 was selected so that subjects would still be teenagers at the two-year follow-up (12). The mania duration of ≥ 2 weeks was similar to conservative duration in multiple nosological schemas and was longer than the DSM-IV duration criterion, for credibility in a new field. Current BP-I, manic or mixed episodes, was required at baseline, because this was a phenomenology study of child mania. Subjects were required to have one or both of the cardinal symptoms of mania (i.e., elated mood or grandiose behaviors) to avoid diagnosing mania only by symptoms that overlapped with those for ADHD (i.e., distractibility, hyperactivity). The cardinal symptom approach was analogous to the DSM-IV requirement of depressed mood and/or anhedonia for a diagnosis of major depressive disorder. A score of ≤ 60 on the CGAS was chosen to ensure clinical impairment (10, 11). Subjects could not be adopted because of concurrent family and molecular genetic studies (e.g., 13, 14). SUD and pregnancy were exclusion criteria at baseline to avoid confounding the diagnosis of child BP-I with mental status effects of substance use or gestational state. The SUD and pregnancy exclusions, however, did not alter entrance into the study, likely due to the young age of the subjects. Pregnancy and drug screens were only done when there was suspicion.
Assessment
Experienced, blinded, research nurses separately assessed parents about their children and children about themselves with the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (15, 16). The WASH-U-KSADS is a semistructured interview with excellent reliability for mania symptoms, mood diagnoses, daily cycling, and time frames (kappas 0.82–1.00) (16). In order to avoid bias, different raters were used for the parent and child within each family (17). Research nurses were trained to inter-rater reliability prior to the start of the study and were recalibrated annually (16,18). There are no skip-outs on the WASH-U-KSADS except for impossible circumstances; e.g., circadian quality of mood is not asked if no abnormal mood state was elicited. Time frames for children's ratings were established by using birthdays, holidays, start of school, end of school, and whether present in earlier grades (e.g., if subject is in third grade, was it there in second grade?) as anchor points. Severity ratings for items on the WASH-U-KSADS were as follows: 1 = no pathology, 2 = doubtful pathology, 3 = mild with no impairment (e.g., child with tics who is not teased or ashamed), ≥ 4 = clinically significant pathology (e.g., child with tics refuses to go to school). Mania symptoms needed to be rated ≥ 4 to count toward a mania diagnosis. The WASH-U-KSADS narrative next to each rating is a part of the assessment tool, and narratives must justify the rating with respect to onset, offset, frequency, duration, intensity, and specific examples. Examples of the developmental manifestations of child mania phenomenology have been published (19). Responses from parent and child were combined by using the most severe, in accordance with the methods described by Bird et al. (20).
The CGAS was completed by the same research nurses who administered the WASH-U-KSADS. On the CGAS, 0 = worst functioning, 100 = best functioning, and ≤ 60 is definite clinical impairment (11). During follow-up, subjects who were aged ≥ 19.0 were given the Global Assessment of Functioning (GAS), the adult counterpart of the CGAS.
The Hollingshead Four Point Index (21) was used to obtain a measure of socioeconomic status. The Duke Pubertal Status Questionnaire (22) was administered to subjects aged ≥ 10.0 years old at baseline and each follow-up assessment until ≥ Tanner Stage III was obtained.
After each follow-up assessment consensus conferences were held to establish DSM-IV consensus diagnoses. During consensus conferences all assessment instruments, videotapes of interviews, school reports, agency records, and pediatrician records were reviewed.
This study was approved by the Human Studies Committee at Washington University in St. Louis. At baseline, subjects signed assent forms and parents signed consent forms. Subjects who reached age 18.0 during follow-up were reconsented.
Psychosis, mixed mania, and rapid cycling
Psychosis was defined as pathologic delusions or hallucinations that did not occur only hypnagogically or hypnopompically. The WASH-U-KSADS has sections for assessing delusions and hallucinations. In addition, the highest rating on the WASH-U-KSADS items of grandiosity, hopelessness, hypochondriasis, and guilt signify delusions in these areas.
Mixed mania was defined as DSM-IV mania and major depressive disorder (MDD) in overlapping time periods. All overlapping weeks for which there was a diagnosis of DSM-IV mania and a diagnosis of DSM-IV MDD were considered weeks of mixed mania.
Historically, rapid cycling was defined as four or more episodes per year. This pattern, however, is uncommon in child BP-I (9). The more common pattern in children is ultradian cycling, i.e., multiple mood switches during a day, every or almost every day during an episode. To address the confusion in terminology, Geller et al. (23) proposed definitions for episode and cycle that are relevant across the age span, based on the work of Kramlinger and Post (24). Episode refers to onset to offset of full criteria from DSM-IV BP-I, and cycle refers to mood switches within an episode. Ultradian cycling is not simply a labile mood. Rather, a cycle needed to be at least four hours per day with sufficient symptoms that were significantly impairing (severity rating of 4 or higher) to support a manic or depressed episode (23).
Treatment
The ‘Phenomenology and Course of Pediatric Bipolar Disorders’ study was a natural history study of child BP-I. Therefore, both pharmacological and non-drug treatment was provided by participants’ own clinicians in the community and not in any way by the research nurses who conducted the assessments. Diagnostic feedback was provided to treating physicians, but the research site was not involved in treatment choices in any way. Pharmacological and non-drug treatment was documented at each follow-up assessment using the Treatment Documentation Form, which included medication type, total daily dose, and weeks of treatment. Blood levels were not available. Non-pharmacological treatment was documented by treatment type and duration in weeks.
For analysis, data reduction of the medication section was necessary, because subjects had been on a total of 50 different psychotropic medications between baseline and the eight-year follow-up. Medications were separated into six classes of drugs: medication for ADHD, antidepressants, antipsychotics, anticonvulsants, lithium, and anxiolytics. In addition, there was a category of any antimanic medication, which included antipsychotics, anticonvulsants, and lithium. The medication for ADHD class of drugs included amphetamine, methylphenidate, atomoxetine, modafinil, pemoline, clonidine, and guanfacine. The anxiolytic class of drugs included alprazolam, busiprone, clonazepam, diazepam, and lorazepam. Non-pharmacological treatment was separated into five types: individual, family, group, self-help, and other. Only drug or non-drug modalities used by at least 10 subjects were included in the analyses.
Follow-up methods
All baseline instruments were administered to parents about their children and to children about themselves at the 6-, 12-, and 18-month, and 2-, 3-, 4-, 5-, 6-, and 8-year follow-up times. Subjects from two control groups (ADHD, hyperactive or combined types, and healthy control groups) were randomly mixed in with the child BP-I subjects, allowing raters to be blind to diagnostic status.
Statistical analyses
Estimates of the rates of recovery from mania and relapse after recovery were made using the life table method (8). Percent of weeks subjects were on medication from each of the six medication classes, percent of weeks subjects were on medication from two medication classes, and percent of weeks subjects were in the various types of non-pharmacological treatment were calculated. These variables were then examined as potential predictors of recovery and relapse in univariate Cox proportional hazards models. Only treatments used by ≥ 10 subjects were analyzed in these models. Significant predictors at the p < 0.05 level were examined in multivariate models. For the recovery models, the potential predictors were percent of weeks between baseline and recovery (or end of follow-up for unrecovered subjects) on psychotropic medication or in non-pharmacological treatment. For the relapse models, the potential predictors were percent of weeks between recovery and relapse (or end of follow-up for unrelapsed subjects) on psychotropic medication or in non-pharmacological treatment.
As noted in the Methods section, child BP-I subjects were ascertained from child psychiatry or pediatric sites. Comparisons of medication classes during eight-year follow-up were compared between subjects obtained from psychiatric sites and those obtained from pediatric sites using Chi-square tests. Differences in non-treatment variables by ascertainment site have been published (25).
The Bonferroni method of correcting for multiple comparisons was used for comparisons of rates of medication use in subjects ascertained at psychiatric versus pediatric sites. For these eight comparisons, the Bonferroni-corrected p-value was p = 0.006, which ensured an overall significance level of p = 0.05 for the group of tests.
All analyses were conducted using SAS v8.2 software.
Results
Characteristics of the 115 child BP-I subjects at baseline are shown in Table 1. During follow-up, 93.9% (n = 108) of the subjects were assessed at each of the nine follow-up times. All subjects were outpatients. Results of diagnostic outcome have been presented elsewhere (8).
Table 1.
Baseline characteristics of child bipolar I disorder subjects (n = 115)
| Mean | SD | |
|---|---|---|
| Age | 11.1 | 2.6 |
| Onset age of first (baseline) mania (years) | 8.3 | 3.7 |
| Duration first (index) mania episode (weeks) | 142.7 | 139.4 |
| Age at 8-year follow-up assessment | 18.1 | 2.9 |
| CGAS score | 43.5 | 8.0 |
| n |
% |
|
|---|---|---|
| Sex | ||
| Male | 77 | 67.0 |
| Female | 38 | 33.0 |
| Pubertal status | ||
| Prepubertal | 58 | 50.4 |
| Pubertal | 57 | 49.6 |
| Race | ||
| Caucasian | 101 | 87.8 |
| Other | 14 | 12.2 |
| Socioeconomic status | ||
| Class I (highest) | 34 | 29.6 |
| Class II | 53 | 46.1 |
| Class III | 22 | 19.1 |
| Class IV | 5 | 4.3 |
| Class V (lowest) | 1 | 0.9 |
| Psychosis | 70 | 60.9 |
| Mixed mania | 62 | 53.9 |
| Ultradian cycling | 90 | 78.3 |
| Comorbid ADHD | 102 | 88.7 |
| Comorbid ODD/CD | 87 | 75.7 |
CGAS = Children's Global Assessment Scale; ADHD = attention-deficit hyperactivity disorder; ODD/CD = oppositional defiant disorder/conduct disorder.
Rates of psychotropic medication use by class and of non-pharmacological treatment by type are shown in Table 2. Of note, only 62.6% of subjects with child BP-I received antimanic medication between baseline and the eight-year follow-up assessment. Seven (6.1%) subjects did not receive any psychotropic medication during follow-up, and 10 (8.7%) did not receive any non-pharmacological treatment. Percent of weeks during eight-year follow-up spent on psychotropic medication (by class) and in non-pharmacological therapy (by type) are also presented in Table 2. Only treatments used by ≥ 10 subjects are shown in the table.
Table 2.
Percent of weeks during eight-year follow-up on community-prescribed psychotropic medication and non-pharmacological treatment in 115 child bipolar I disorder subjects
| % weeks during 8 years |
|||
|---|---|---|---|
| Medication class | n (%) subjects | Mean | SD |
| Medication for ADHD | 89 (77.4) | 49.6 | 32.5 |
| Antidepressant | 74 (64.3) | 31.1 | 30.5 |
| Antimanic | 72 (62.6) | 48.1 | 34.1 |
| Antipsychotic | 59 (51.3) | 45.6 | 33.1 |
| Anticonvulsant | 48 (41.7) | 32.0 | 30.4 |
| Lithium | 37 (32.2) | 21.0 | 23.3 |
| Anxiolytic | 12 (10.4) | 16.8 | 16.1 |
| Non-pharmacological treatment |
|
|
|
|---|---|---|---|
| Individual | 103 (89.6) | 12.8 | 12.9 |
| Family | 64 (55.7) | 4.8 | 6.3 |
| Group | 41 (35.7) | 13.6 | 12.8 |
The ratio of percent of weeks of antipsychotic to anticonvulsant use was 1.43, of antipsychotic to lithium use was 2.17, and of anticonvulsant to lithium use was 1.52. ADHD = attention-deficit hyperactivity disorder.
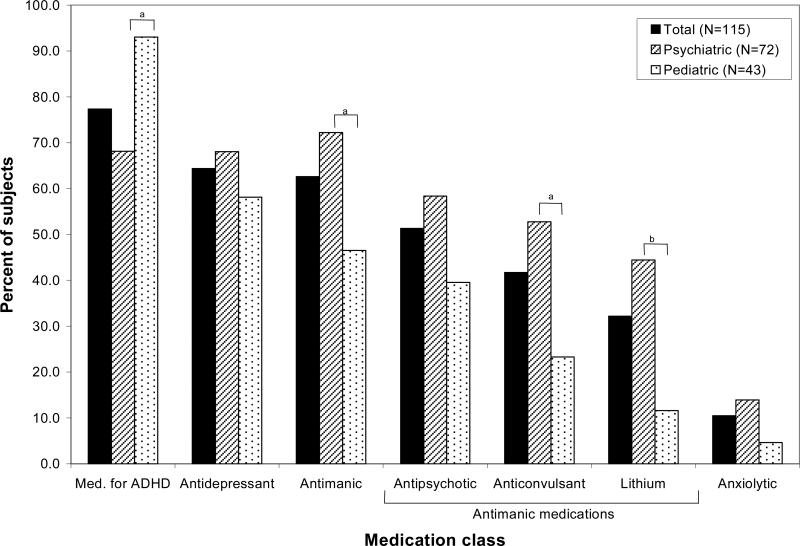
Of the 115 child BP-I subjects, 72 (62.6%) were obtained at psychiatric sites, and 43 (37.4%) were ascertained at pediatric sites. Figure 1 shows rates of psychotropic medication use by class in subjects ascertained at psychiatric sites versus pediatric sites. Subjects obtained from psychiatric venues had significantly higher rates of antimanic (χ2 = 7.6, p = 0.006), anticonvulsant (χ2 = 9.6, p = 0.002), and lithium (χ2 = 13.3, p < 0.001) use between baseline and eight-year follow-up compared to subjects obtained at pediatric venues. Subjects obtained from pediatric facilities had a significantly higher rate of medication for ADHD use during eight-year follow-up compared to subjects from psychiatric facilities (χ2 = 9.6, p = 0.002).
Fig 1.
Percent of child bipolar I disorder subjects (n = 115) on various community-prescribed medication classes during eight-year follow-up by psychiatric or pediatric ascertainment site. ADHD = attention-deficit hyperactivity disorder. ap < 0.006; bp < 0.001.
Polypharmacy was evident, in that 67.8% of subjects were taking medication from two or more medication classes concurrently during eight-year follow-up. The most frequent combinations of medication classes, occurring in over 35% of subjects, were antimanic with medication for ADHD (43.5%), antidepressant with medication for ADHD (43.5%), and antimanic with antidepressant (39.1%).
As previously reported (8), 101 (87.8%) of subjects recovered from mania during eight-year follow-up. The life table estimate of recovery was 95.2% (8). In a multivariate Cox model of recovery from mania through eight-year follow-up, greater percent of weeks on lithium (χ2 = 5.7, p = 0.017) and fewer percent of weeks in group treatment (χ2 = 5.4, p = 0.020) were significantly associated with earlier recovery, controlling for baseline age, sex, and variables that were significant in univariate Cox models (percent of weeks of concurrent antidepressant and antimanic use and percent of weeks of concurrent antidepressant and antipsychotic use).
Of the 101 subjects who recovered from mania during eight-year follow-up, 74 (73.3%) relapsed to mania. The life table estimate of relapse after recovery was 77.8% (8). In a multivariate Cox model of relapse to mania through eight-year follow-up, fewer percent of weeks of concurrent antidepressant and antipsychotic use (χ2 = 5.4, p = 0.021) and greater percent of weeks in group treatment (χ2 = 6.6, p = 0.010) were significantly associated with earlier relapse, controlling for baseline age and sex (no other variables were significant in univariate Cox models).
Discussion
Treatment findings are consistent with those at baseline and at six months regarding relatively low use of antimanic medications given by subjects’ own community practitioners (18, 25), as shown in Table 3. This finding was unexpected based on epidemiological findings of markedly increased prescriptions of antipsychotic medications during the same time frame as this study (e.g., 7). Speculations on why there was relatively low use of psychotropic medications in the ‘Phenomenology’ sample include the following. One possibility, given the generous use of non-antimanic medications, is that community practitioners were under-recognizing child BP-I. Support for this conjecture was provided by Tillman et al. (25), who showed that antimanic medications were prescribed significantly more frequently by psychiatric compared to pediatric physicians, who likely would be less informed about child BP-I. The large shift of care to primary caregivers over the time frame of ‘Phenomenology’ also bolsters the likelihood of physicians less knowledgeable about child BP-I being responsible for treatment. If these factors related to primary caregivers are operative, education programs to teach primary physicians about when to refer for psychiatric consultation would be in order.
Table 3.
Percent of subjects on community-prescribed antimanic medication by assessment time
The finding that greater weeks on lithium was associated with earlier recovery is consistent with one double-blind, placebo-controlled trial (1), but not another (6).
Given recent data from adult BP-I subjects on the possible increase of manic episodes with use of antidepressants (26), the relatively high percentage of child BP-I subjects on antidepressants may be of concern. Greater dissemination about data supporting antidepressant properties of lithium (e.g., 27) and antipsychotics (e.g., 28) might be warranted.
Controversy about what effect stimulant medications have on the onset and course of child BP-I (e.g., 29-32) supports that clinicians should be vigilant for worsening of mania with the start of stimulants, but does not yet support avoiding these medications in child BP-I. Although theoretically mania should worsen with stimulant use on the basis of increased available dopamine, this was not shown in a controlled, blindly rated prospective study (32). Moreover, in a double-blind, placebo-controlled treatment study, add-on stimulant significantly improved mania symptoms that overlapped with those of ADHD, compared to add-on placebo (33). A chart review, however, that examined the relationship of beginning stimulant medication to onset of mania found earlier mania onset in subjects who received stimulants prior to the onset of mania (31). A caveat to this study is that the stimulants may have been instituted because mania was already present, but only the severe ADHD was diagnosed. Overall, the factors that precipitate mania may not be the same as those that worsen the illness after it has started. Thus, conceivably, stimulants may hasten onset but not worsen the course.
One possible reason for the association of fewer weeks of group therapy with earlier recovery might be that only subjects who were more severely ill received more group therapy.
Finally, the high rate of retention during the eight-year follow-up warrants discussion. The following are the stringent measures that the research site used to ensure a high retention rate. (i) At baseline, there was exhaustive documenting of how to contact family members, friends, and neighbors, and this information was updated at each of the nine assessment times. (ii) Only highly experienced research nurses made the appointments so that any acute circumstance such as an ill parent could be professionally handled. (iii) Calls to establish appointments and the appointments themselves were done in the evenings and on weekends to accommodate when families were not at work or school. When the family preferred, the assessments were done in the home. (iv) The tone and content of phone calls avoided any mention of repeated phone calls or missed appointments, so that guilt or anxiety was not provoked in the participants. The research nurses used a tone that can be described as ‘Sunday brunch,’ meaning it should be in the tone of telling a friend you are preparing eggs instead of waffles. (v) It works best if the mother (in most families there is a mother in the home) handles all calls, because she is usually responsible for family medical care.
Given these findings, and the poor prognosis from prospective follow-up studies of child BP-I (8, 34, 35), there is a need for further research that informs the development of treatment strategies.
Acknowledgement
This research was supported by National Institute of Mental Health grant R01 MH-53063 to BG.
Footnotes
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37:171–178. doi: 10.1097/00004583-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 2.DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45:305–313. doi: 10.1097/01.chi.0000194567.63289.97. [DOI] [PubMed] [Google Scholar]
- 3.Wagner KD, Kowatch RA, Emslie GJ, et al. A double-blind, randomized, placebo-controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Am J Psychiatry. 2006;163:1179–1186. doi: 10.1176/ajp.2006.163.7.1179. [DOI] [PubMed] [Google Scholar]
- 4.Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164:1547–1556. doi: 10.1176/appi.ajp.2007.06111932. [DOI] [PubMed] [Google Scholar]
- 5.Geller B, DelBello MP, editors. Treatment of Bipolar Disorder in Children and Adolescents. Guilford Press; New York: 2008. [Google Scholar]
- 6.Kowatch RA. Pediatric bipolar collaborative trial of lithium versus divalproex.. Presented at the 55th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Chicago, IL. October 28-November 2, 2008. [Google Scholar]
- 7.Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 8.Geller B, Tillman R, Bolhofner K, Zimerman B. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geller B, Zimerman B, Williams M, et al. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer D, Gould MS, Brasic J, et al. A Children's Global Assessment Scale (C-GAS). Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 11.Bird HR, Canino G, Rubio-Stipec M, Ribera JC. Further measures of the psychometric properties of the Children's Global Assessment Scale. Arch Gen Psychiatry. 1987;44:821–824. doi: 10.1001/archpsyc.1987.01800210069011. [DOI] [PubMed] [Google Scholar]
- 12.Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B. Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2002;159:927–933. doi: 10.1176/appi.ajp.159.6.927. [DOI] [PubMed] [Google Scholar]
- 13.Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH., Jr Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161:1698–1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- 14.Geller B, Tillman R, Bolhofner K, Zimerman B, Strauss NA, Kaufmann P. Controlled, blindly rated, direct-interview family study of a prepubertal and early-adolescent bipolar I disorder phenotype: morbid risk, age at onset, and comorbidity. Arch Gen Psychiatry. 2006;63:1130–1138. doi: 10.1001/archpsyc.63.10.1130. [DOI] [PubMed] [Google Scholar]
- 15.Geller B, Williams M, Zimerman B, Frazier J. Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) Washington University; St. Louis, MO: 1996. [DOI] [PubMed] [Google Scholar]
- 16.Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Tillman R, Geller B, Craney JL, Bolhofner K, Williams M, Zimerman B. Relationship of parent and child informants to prevalence of mania symptoms in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161:1278–1284. doi: 10.1176/appi.ajp.161.7.1278. [DOI] [PubMed] [Google Scholar]
- 18.Geller B, Zimerman B, Williams M, et al. Six-month stability and outcome of a prepubertal and early adolescent bipolar disorder phenotype. J Child Adolesc Psychopharmacol. 2000;10:165–173. doi: 10.1089/10445460050167278. [DOI] [PubMed] [Google Scholar]
- 19.Geller B, Zimerman B, Williams M, Delbello MP, Frazier J, Beringer L. Phenomenology of prepubertal and early adolescent bipolar disorder: examples of elated mood, grandiose behaviors, decreased need for sleep, racing thoughts and hypersexuality. J Child Adolesc Psychopharmacol. 2002;12:3–9. doi: 10.1089/10445460252943524. [DOI] [PubMed] [Google Scholar]
- 20.Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. J Am Acad Child Adolesc Psychiatry. 1992;31:78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven, CT: 1976. [Google Scholar]
- 22.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 23.Geller B, Tillman R, Bolhofner K. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers, school-aged children, adolescents, and adults. J Child Adolesc Psychopharmacol. 2007;17:217–222. doi: 10.1089/cap.2007.0017. [DOI] [PubMed] [Google Scholar]
- 24.Kramlinger KG, Post RM. Ultra-rapid and ultradian cycling in bipolar affective illness. Br J Psychiatry. 1996;168:314–323. doi: 10.1192/bjp.168.3.314. [DOI] [PubMed] [Google Scholar]
- 25.Tillman R, Geller B, Frazier J, et al. Children with a prepubertal and early adolescent bipolar disorder phenotype from pediatric versus psychiatric facilities. J Am Acad Child Adolesc Psychiatry. 2005;44:776–781. doi: 10.1097/01.chi.0000164588.10200.ed. [DOI] [PubMed] [Google Scholar]
- 26.Ghaemi SN, Wingo AP, Filkowski MA, Baldessarini RJ. Long-term antidepressant treatment in bipolar disorder: meta-analyses of benefits and risks. Acta Psychiatr Scand. 2008;118:347–356. doi: 10.1111/j.1600-0447.2008.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein DF, Gittelman R, Quitkin F, Rifkin A. Diagnosis and Drug Treatment of Psychiatric Disorders: Adults and Children. Williams and Wilkins; Baltimore, MD: 1980. pp. 268–408. [Google Scholar]
- 28.Biederman J, Mick E, Faraone SV, Wozniak J, Spencer T, Pandina G. Risperidone for the treatment of affective symptoms in children with disruptive behavior disorder: a post hoc analysis of data from a 6-week, multicenter, randomized, double-blind, parallel-arm study. Clin Ther. 2006;28:794–800. doi: 10.1016/s0149-2918(06)00132-9. [DOI] [PubMed] [Google Scholar]
- 29.Koehler-Troy C, Strober M, Malenbaum R. Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry. 1986;47:566–567. [PubMed] [Google Scholar]
- 30.Carlson GA, Loney J, Salisbury H, Kramer JR, Arthur C. Stimulant treatment in young boys with symptoms suggesting childhood mania: a report from a longitudinal study. J Child Adolesc Psychopharmacol. 2000;10:175–184. doi: 10.1089/10445460050167287. [DOI] [PubMed] [Google Scholar]
- 31.DelBello MP, Soutullo CA, Hendricks W, Niemeier RT, McElroy SL, Strakowski SM. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3:53–57. doi: 10.1034/j.1399-5618.2001.030201.x. [DOI] [PubMed] [Google Scholar]
- 32.Tillman R, Geller B. Controlled study of switching from attention-deficit/hyperactivity disorder to a prepubertal and early adolescent bipolar I disorder phenotype during 6-year prospective follow-up: rate, risk, and predictors. Dev Psychopathol. 2006;18:1037–1053. doi: 10.1017/S0954579406060512. [DOI] [PubMed] [Google Scholar]
- 33.Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry. 2005;162:58–64. doi: 10.1176/appi.ajp.162.1.58. [DOI] [PubMed] [Google Scholar]
- 34.Biederman J, Faraone SV, Wozniak J, et al. Clinical correlates of bipolar disorder in a large, referred sample of children and adolescents. J Psychiatr Res. 2005;39:611–622. doi: 10.1016/j.jpsychires.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]