Abstract
Objective
To elucidate the physiologic mechanism responsible for the supraphysiologic gonadotropin release from the pituitary induced by GnRH-agonist in GnRH-antagonist-primed female rats.
Design
Controlled experimental intervention.
Setting
Government research facility.
Intervention
Forty oophrectomized rats were randomized into 4 groups of 10 and treated with: Group A) control vehicles; Group B) GnRH-a (leuprolide acetate; 1.7μg/kg BID) on day 4; Group C) GnRH-ant (Nal-Lys; 3mg/kg QD) days 1–4; or D) GnRH-ant (Nal-Lys; 3mg/kg QD) days 1–4 and GnRH-a (1.7μg/kg BID) on day 4.
Main Outcome Measure(s)
Immunohistochemical methods, Northern, and in situ hybridization to quantitate pituitary FSH-β, LH-β and GnRH-R mRNA and receptor protein levels in all treatment groups.
Results
Treatment with GnRH-ant was associated with increased storage of gonadotropin in the pituitary for FSH-β and LH-β, but mRNA levels were unchanged. GnRH-R mRNA decreased following GnRH-a treatment but remained stable in the GnRH-ant-treated groups. Levels of GnRH-R were decreased following GnRH-ant treatment.
Conclusions
These data indicate that the in vivo mechanism responsible for the exaggerated release of gonadotropins in the GnRH-ant primed, GnRH-a treated rat was an increase in releasable gonadotropin pools coupled with a reduction in GnRH-R, but receptor function was preserved.
Keywords: LH-RH, GnRH receptor, ovulation induction, LH surge, OHSS, Ovarian Hyperstimulation syndrome, pituitary
Introduction
GnRH analogs play an important role as treatment options for many reproductive diseases (1). GnRH-agonists (GnRH-a) are commonly used to induce a transient menopausal state for the treatment of estrogen-dependent diseases such as endometriosis and uterine leiomyomata, and in assisted reproductive technologies (ART) during ovulation stimulation (1–3). In the clinical setting of ART where ovarian hyperstimulation syndrome (OHSS) is a concern, GnRH-a has been used to trigger an LH surge in women pre-treated with a GnRH-antagonist (GnRH-ant) (4–8; and references therein). This strategy was appealing because endogenously produced LH has a shorter half-life than hCG (7), and therefore may reduce the risk of developing OHSS (10–13). Quantitatively, this surge of LH is supraphysiologic compared to GnRH-a induced surge in the non GnRH-ant primed female.
There are more than 24 reports of this strategy in the literature (reviewed in ref. 14), and four randomized, controlled studies have examined clinical outcomes after a GnRH-a induced surge in women pretreated with GnRH-ant (7,10,11,13). In all four studies, no statistically significant differences were noted in the number of oocytes retrieved, proportion of metaphase II oocytes, fertilization rate or embryo quality score in GnRH-a compared to hCG treated women (7,10,11,13). Fauser et al. (7) and Engmann et al. (13) reported a comparable clinical pregnancy rate for GnRH-a compared to hCG treatment. In contrast, studies by Humaidan et al. (10) and Kolibianakis et al. (11) were terminated before completion because of the lower pregnancy rate observed in the GnRH-a treatment arm compared to hCG. Of note, only the trials by Fauser et al. (7) and Engmann et al. (13) used luteal supplementation with intramuscular progesterone, a very critical detail for women treated with GnRH analogues (15). Humaidan et al. (10) stopped luteal phase support immediately after a positive pregnancy test was obtained and documented a high early pregnancy loss. Despite the two trials suggesting a reduction in pregnancy (10,11), if the luteal phase was supported, pregnancy rates were comparable (7,13) and the strategy may be effective for patients at risk for OHSS (13,14).
While both GnRH-a and GnRH-ant ultimately reduce gonadotropin levels, the mechanisms of pituitary desensitization differ. Administration of a GnRH-a induces a transient rise in gonadotropins, known as a flare, as GnRH-receptors (GnRH-R) are initially bound and activated. This is followed by a state of pituitary desensitization resulting in a decrease in GnRH receptors, and a diminished response to GnRH stimulation (1, 16–19). In contrast, GnRH-ant reduces gonadotropin levels without producing an initial flare, a characteristic of benefit in many therapeutic applications (20,21). The rat has often been used as a model to dissect in vivo responses to GnRH-a and GnRH-ant at the level of the pituitary (19,22–24). Some studies have reported that GnRH-ant competitively inhibited GnRH receptors (GnRH-R) without reducing GnRH message (24–26). More recent studies showed marked decreases in GnRH-R mRNA and GnRH receptors after treatment with GnRH-ant (19,22,27). In vivo studies clearly show that the antagonistic effects of GnRH-ant may be overcome by GnRH-a leading to a supraphysiologic surge of gonadotropins (28,29), but the mechanism responsible for the supraphysiologic LH surge remains unclear.
The aim of this study was to explore the mechanism responsible for the GnRH-a induced LH surge in ovariectomized (OVX) female rats pre-treated with GnRH-ant. The results suggest that releasable pools of gonadotropin accumulate in the pituitaries of GnRH-ant treated rats and that GnRH-R levels were reduced, but the remaining receptors were sufficient to cause the LH surge upon GnRH-a treatment.
Materials and Methods
Animal Treatment and Collection of Tissue
All studies were conducted in accordance with Federal guidelines and the study protocol was reviewed and approved by the NIH Animal Care and Use Committee. Ten days after ovariectomy of 8 week old Sprague-Dawley rats, animals were randomized and placed into 4 treatment groups consisting of 10 animals each. The experimental design consisted of 4 consecutive days of treatment followed by sacrifice on the morning of day 5 (Figure 1). Treatment groups were as follows: The control group (Group A) was treated with both vehicles and received a daily morning subcutaneous injection of corn oil days 1–4 (Sigma Chemical Company, St. Louis, Mo.) and two subcutaneous injections of saline given 12 hours apart on day 4; (Group B) the GnRH-a group received 2 doses of leuprolide acetate (1.7μg/kg) diluted in saline (TAP Pharmaceuticals) 12 hours apart on day 4; (Group C) the GnRH-ant group received a daily morning subcutaneous injection of Nal-Lys Antide (3mg/kg) dissolved in corn oil (Organon) days 1–4; and (Group D) the combined treatment group received four consecutive daily morning injections of GnRH-ant (Antide) and two doses of GnRH-a (leuprolide acetate) given 12 hours apart on day 4 at the same doses as in group B and C. All animals were sacrificed by CO2 asphyxiation on Day 5, twelve hours after the last GnRH agonist or saline treatment. The pituitaries of 4 to 5 animals from each group were fixed in 10% buffered formalin for 24 hours, paraffin-embedded, sectioned to a thickness of 7μm, and placed on sialanized slides for immunohistochemical studies of LH and FSH protein expression or for in situ hybridization of GnRH receptor expression. The remaining pituitaries from each group were snap frozen in liquid nitrogen and stored at −70C until processed either for RNA isolation to analyze steady state levels of mRNA for LH, FSH, and GnRH receptor by Northern hybridization or for cell membrane isolation for hormone binding studies to determine free GnRH receptor binding sites.
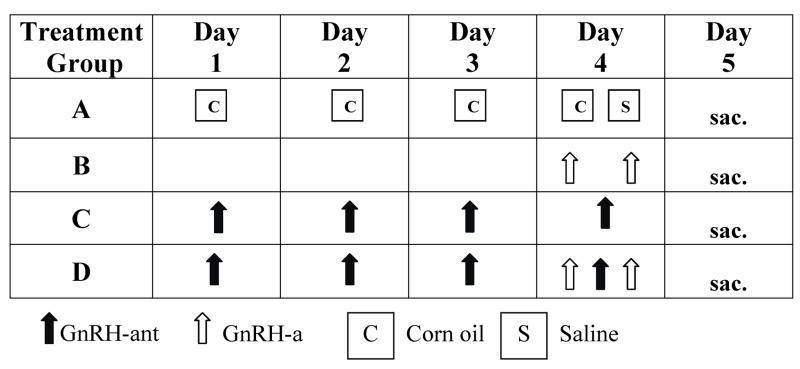
Figure 1.
Diagram of the experimental protocol. Eight-week-old ovariectomized Sprague-Dawley rats were randomized to 4 treatment groups as follows: the control Group A was treated with both vehicles and received a daily morning subcutaneous injection of corn oil on days 1–4 and two subcutaneous injections of saline given 12 hours apart on day 4; Group B received 2 doses of GnRH-a, leuprolide acetate (1.7μg/kg) diluted in saline 12 hours apart on day 4; Group C received subcutaneous injections of 3mg/kg GnRH-ant (Nal-Lys; Antide) dissolved in corn oil daily on days 1–4; and Group D received daily morning injections of GnRH-ant on days 1–4 and 2 doses of GnRH-a on day 4 given 12 hours apart. All animals were sacrificed on the fifth day, 12 hours after the second dose of leuprolide acetate. Black up-arrows indicate GnRH-ant, white up-arrows indicate GnRH-a and boxes represent vehicles, corn oil (C) and saline (S).
RNA Isolation, Northern and in situ Hybridizations
Total RNA was isolated from pituitaries by homogenization of frozen tissue in Tri-ReagentJ (Sigman Chemical Company, Cinncinnati, Ohio) using a Polytron (Brinkman Instruments, Westbury, NY) and following manufacturer’s specifications. RNA samples were suspended in diethylpyrocarbonate (DEPC) treated water and RNA concentration determined by absorbance at 260 nm. RNA was fractionated by electrophoresis in a formaldehyde-1% agarose gel, stained with ethidium bromide, transferred to a nylon membrane, and baked under vacuum at 80EC for 1 hour. The blots were used to evaluate the steady state levels of mRNA for the beta subunits of LH and FSH and GnRH-receptor. This was done by hybridization of the blots at 60 degrees C overnight in 0.4M sodium phosphate pH 7.2, 7% SDS, 1% bovine serum albumin and 0.02M EDTA with 32P-labeled probes generated by random priming of purified cDNA fragments specific for the beta subunits of rat LH, 350 bp fragment in pGEM-2; Chin et al. (30) and FSH, 1.0 Kb fragment in pGEM-2; Gharib et al. (31), and the rat GnRH receptor, nucleotides 1–360; accession #X76635.Gb-Ro. A fragment of the rat GnRH receptor cDNA was cloned in our laboratory by polymerase chain reaction techniques into the BamHI and Xb1 sites of pGEM4Z (Promega, Madison, WI) based on the sequences provided by Kaiser et al. (32). Following hybridization the blots were washed sequentially three times for 15 min each in 2XSSC - 0.1%SDS then three times for 15 min each in 0.2XSSC - 0.1% SDS at 60 degrees C and exposed to X-ray film. The relative optical density of the products was measured using a PDI J densitometer and software (Huntington Station, NY) and normalized to the ribosomal RNAs.
For in situ hybridization, dewaxed slides were pretreated with 0.2N HCL for 30 min at room temperature, digested with 10ug/ml proteinase-K in 0.1M Tris-HCL (pH7.4)-0.05M EDTA for 15 min at 37 degrees C, and then treated with 0.1M triethanolamine-0.25% acetic anhydride for 5 min and 0.1M Tris-glycine (pH7.4) for 30 min at room temperature. The sections were prehybridized at 50 degrees C for 1 h in 2XSSC, 10mMDTT, 5X Denhardt’s solution, 100ug/ml of both salmon sperm DNA and yeast tRNA, and 50% formamide. The slides were hybridized overnight at 50 degrees C in the same medium with 10% dextran sulfate and digoxigenin-labeled sense or anti-sense riboprobes to rat GnRH receptor prepared by transcription with SP6 or T7 RNA polymerases from the appropriate linearized GnRH cDNA plasmid (Genius IV kit; Boehringer-Mannheim). Following hybridization, the slides were washed twice in 1XSSC for 10 min, digested with RNases (2.8ug/ml RNase-A and 0.3ug/ml RNase-T1 in 10mM Tris-HCL pH7.4), washed twice more in 1XSSC at 55 degrees C for 20min, and then twice in 0.1XSSC at 55 degrees C for 30min. The sections were equilibrated in Tris buffered saline with Tween-20 (TBST: 0.01 M Tris-HCL pH7.6, 0.9% NaCL, 0.05% Tween-20) and incubated with anti-digoxigenin alkaline-phosphatase antibody (Boehringer-Mannheim) according to manufacturerer’s directions, washed with TBST, and visualized using BCIP/NBT as a substrate.
Immunohistochemistry and Quantitation
Detection of FSH and LH was performed with guinea pig antibodies (1:250–1:500 dilution) specific to their respective beta subunits obtained from the National Pituitary Hormones & Antisera Center (Torrance, California). In addition, a commercially available rabbit antibody against FSH beta subunit was used at a 1:50 dilution (Biomeda, Foster City, CA). Unless otherwise stated, all incubations were performed at room temperature. Prior to immunostaining, the deparaffinized tissue sections were subjected to antigen retrieval using a pressure cooker in 0.01M citrate buffer pH 6.0 followed by incubation in 3% H2O2 in methanol for 10 min to block endogenous peroxidase activity. The slides were then washed thoroughly and equilibrated in Tris buffered saline with Tween-20 (TBST: 0.01 M Tris-HCL pH7.6, 0.9% NaCL, 0.05% Tween-20). Nonspecific sites were blocked by incubation for 20 min with 1% normal serum from a Vectastain ABC elite kit for guinea pig primary antisera (Vector laboratories, Burlingame, CA) diluted in TBST and were incubated overnight at 4 degrees C with the primary antiserum (1:100 dilution). The slides were washed in TBST twice for 10 minutes each and then incubated with the appropriate biotinylated secondary antibody and peroxidase-conjugated streptavidin as suggested by the manufacturer (Vectastain ABC Elite kit, Vector laboratories, Burlingame, CA). FSH and LH were immunolocalized using 2,2-diaminobenzidine as a substrate. To ensure consistency in immunohistochemical detection of the gonadotropins, the specimens were cut on the same day, placed on the same lot of sialanized slides, and stained simultaneously in one experimental run. The entire staining procedure was repeated at least two times using the same or different antisera when available. Background staining was estimated by substituting nonimmune serum for the primary antisera where the staining intensity was found to be negligible (data not shown).
The relative intensity of the immunoreaction was estimated by measurement of the diaminobenzidine peroxidase product in pituitaries from the various treatment groups using light microscopic image analysis consisting of digital Pro-ImageJ software (Media Cybernetics, Silver Spring, MD), a color CD-camera, and an OLYMPUS microscope (Opelco, Sterling VA). The system was first set to measure linear optical density with the larger values corresponding to more intense staining followed by calibration so that immunoreactive cells in all samples would fall within the linear range. At least four random areas were selected from each pituitary (4–6 pituitaries/group) using 400X magnification. All immunostained gonadotropes within the field were manually traced to obtain the relative area and optical density values. Greater than 300 cells were traced and quantified for each treatment group. The mean and standard error were determined for the gonadotrope cell area and relative optical density of staining for either FSH or LH. Statistical significance between two groups for parametric data was assessed by the Student’s t test and multiple group comparisons were analyzed using one-way analysis of variance, with Duncan’s multiple range test applied to determine significant differences between groups. Results are expressed as the mean ± SD, and statistical significance was assumed at p<0.05.
GnRH receptor binding assays
4 to 6 frozen pituitaries from each treatment group were thawed and homogenized in assay buffer (0.025M Tris-HCL pH7.4, 0.01M MgCl2, and 0.1% BSA) using 1 ml buffer/pituitary. One hundred microliters of cell homogenate were used for each binding point according to the method of Nett et al. (33), in the presence of [125I]-Buserelin (Hoescht-Roussel Pharmaceuticals, Frankfurt, Germany). Nonspecific binding was assessed in the presence of 5 μg/tube of unlabeled GnRH analog. Measurements were repeated three times and the coefficient of variance was calculated. Results are expressed as the mean ± SD, and statistical significance was accepted at p<0.05.
Results
The response of pituitary gonadotropes to GnRH-a and GnRH-ant was examined in 40 sexually mature female rats 10 days post ovariectomy (Figure 1). Ovariectomized (OVX) rats were divided into four groups. Group D was intended to mimic the clinical use of GnRH-a in GnRH-ant primed women. The control group (A) only received vehicle injections of corn oil and saline. Group B was treated with 2 doses of GnRH-a, leuprolide acetate (1.7 μg/kg) given 12 hours apart on day 4. Group C received 3mg/kg of GnRH-ant (Nal-Lys; Antide) daily for four consecutive days. Group D was given 3mg/kg GnRH-ant (Nal-Lys; Antide) daily for 4 days and 2 doses GnRH-a, leuprolide acetate (1. μg/kg) on day 4. All animals were sacrificed on the fifth day, 12 hours after the second dose of GnRH-a.
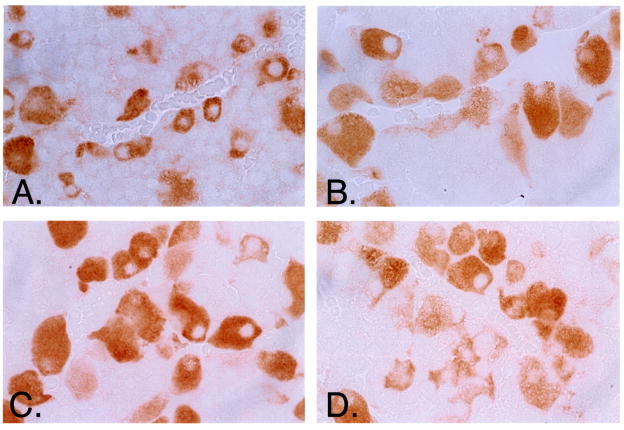
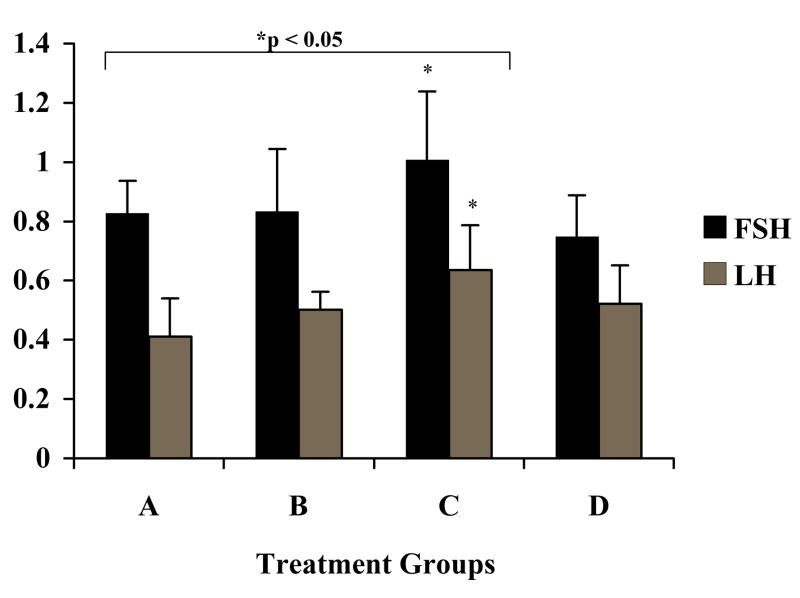
Immunohistochemical staining was performed on 4–6 pituitaries from each treatment group to analyze intracellular pools of gonadotropin. A representative staining of pituitary sections (Figure 2A) from the four different treatment groups demonstrated increased FSH-β staining in GnRH-ant treated animals (Groups C and D). Pituitary sections stained for LH-β revealed similar findings in GnRH-ant treated animals (not shown). The intensity of the staining for LH-β and FSH-β was quantified using a computer based density quantification software program (Image Pro Plus ®). Quantitative analysis showed animals treated with GnRH-ant alone had a statistically significant increase in staining for both LH-β and FSH-β (Figure 2B).
Figure 2.
Figure 2A. Immunohistochemical staining for gonadotropins. Pituitary sections stained for FSH-β (A-D) from OVX rats after treatment with: GnRH-a alone (B); GnRH-ant alone (C); both (D); or neither (A). In all samples antibody-antigen interaction was visualized as brown staining. FSH-β staining was visibly increased after treatment with GnRH-ant alone (C). (A–D) Original magnification, X 400.
Figure 2B. Quantification of Immunohistochemical staining for FSH and LH using computer-based density quantification software. The relative intensity of the immunoreaction was estimated by measurement of the diaminobenzidine peroxidase product from pituitaries of all treatment groups using light microscopic image analysis as described in materials and methods. Groups demonstrated statistically significant increased pituitary pools of FSH-β and LH-β in rats treated with GnRH-ant alone compared to control (p < 0.05). Dark bars represent FSH. Light bars represent LH. Y-axis=optical density units. Error bars=standard deviation. Significance, compared to the control group, was assumed at p < 0.05 (all asterisked values).
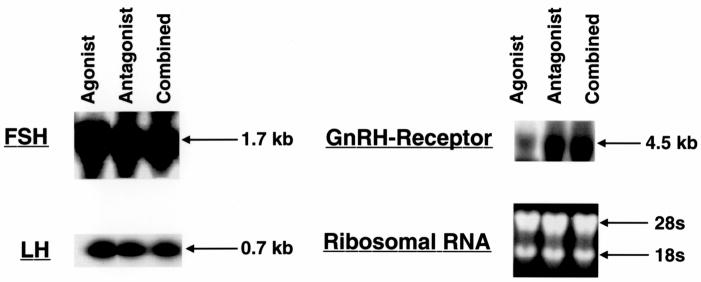
To determine whether the increased pools of FSH and LH were associated with increased transcription, steady state levels of RNA were harvested from the pituitaries of all treatment groups and probed with cDNA corresponding to either FSH-β or LH-β. We observed no change in steady state levels of transcripts encoding either FSH-β or LH-β in all treatment groups (Figure 3, left panel). This finding, coupled with the immunohistochemical staining of the pituitaries, suggests that the increase in FSH and LH protein in the pituitary resulted from an increase in the storage of gonadotropin, and not increased transcription.
Figure 3.
Northern Blot Analysis. RNA harvested from OVX rats following treatment with GnRH-a (Group B), GnRH-ant (Group C) or both (Group D) were probed with cDNA as described in the methods. Ribosomal RNA was used as a control for loading. Levels of steady state FSH and LH transcripts remained constant regardless of treatment group. Steady state level GnRH-R transcripts were reduced following GnRH-a treatment (Group B).
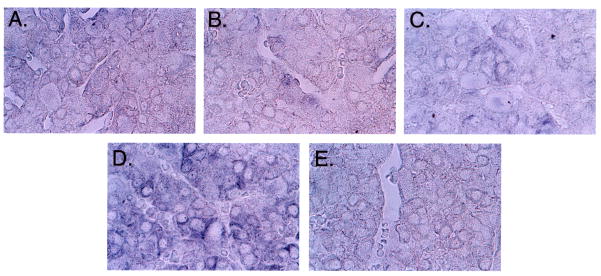
Next, we examined GnRH receptor (GnRH-R) mRNA in all treatment groups. Despite equal loading, GnRH-R transcripts were reduced in GnRH-a treated animals, however no change was observed in GnRH-ant only, or combined treatment groups (Figure 3, right panel). To corroborate these findings, in situ hybridization for GnRH-R was performed on pituitaries from all treatment groups (Figure 4). Consistent with the Northern blot analysis, no change in GnRH-R mRNA was apparent in pituitaries harvested from the two GnRH-ant treated groups (Figure 4, C–D), but a reduction in signal was noted in the GnRH-a treated group (Figure 4, B). As expected, staining was localized to gonadotropes and was most pronounced in the combination group (Figure 4, D). As a control, sense riboprobe showed no staining (Figure 4, E). These observations indicate that steady state levels of mRNA encoding the GnRH-R were not reduced in GnRH-ant treated rats, in contrast to rats treated with GnRH-a alone.
Figure 4.
In situ hybridization of pituitaries from treatment groups (A-D) using a riboprobe generated from a cDNA encoding GnRH-R. Compared to the control group (A), GnRH-R transcript levels showed a reduction in staining in the GnRH-a treatment group (B). No reduction was observed in the GnRH-ant treatment group (C) or the combination group (D). Sense riboprobe (E) served as control.
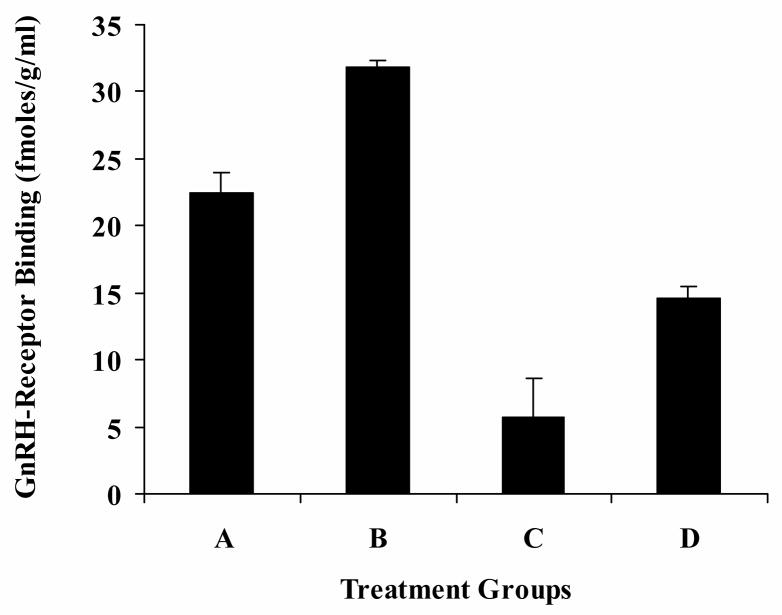
To determine whether there was a change in the level of GnRH-R protein in the pituitaries among the four treatment groups we used a receptor-binding protein assay. Pituitary homogenates were prepared from each of the four treatment groups and receptor levels were assessed using binding of I125-labeled Buserelin (33). The level of GnRH-R (fmoles/gram) was increased after treatment with GnRH-a, but was reduced in pituitaries harvested from animals that received GnRH-ant (Figure 5). In pituitaries harvested from rats treated with both GnRH analogs, receptor levels were decreased compared to the control although not to the extent of those treated with GnRH-ant alone.
Figure 5.
Levels of GnRH-R protein in pituitary homogenates from treatment groups AD. The level of GnRH-R increased following treatment with GnRH-a (Group B) and decreased in both the GnRH-ant and combination groups (Groups C and D). Y-axis=GnRH-R binding in fentamolar/gram of pituitary lysate. Error bars=standard deviation. Significance was assumed at p < 0.05 for all treatment groups compared to the control.
Discussion
We were unable to identify previous reports in the literature that described the mechanism responsible for the supraphysiologic LH surge induced by GnRH-a in GnRH-ant primed females. We found increased staining for LH-β and FSH-β in pituitaries of rats during GnRH-ant treatment, accompanied by reduced, but functional GnRH-R that could be activated to effect release of gonadotropin pools upon GnRH-a treatment. We confirmed depletion of gonadotropin pools in the pituitary (i.e., release) by immunohistochemistry (Figures 2A and 2B).
Studies conducted in a primate model demonstrated that a GnRH-a caused a supraphysiological release of gonadotropins in animals pretreated with GnRH-ant (34). Subsequently, this strategy has been used in controlled ovarian hyperstimulation (COH) with a single dose of GnRH-a to trigger ovulation and induce final oocyte maturation (7, 10, 11, 13, 14, 34–36). This strategy is of clinical relevance, since it may reduce the risk of ovarian hyperstimulation syndrome (13,14).
Previous studies have focused on the mechanism of action of individual gonadotrope analogs upon the pituitary. The results we obtained in the GnRH-ant only treatment group (Group C) were consistent with prior reports (23,27). These reports also showed the level of GnRH receptor protein decreased while GnRH-R mRNA remained stable after GnRH-ant (23,27). Additional studies extended these findings and showed that a single injection of GnRH-ant significantly reduced the number of membrane receptors for LH-RH (GnRH-R) in a time dependent manner after 10 days of treatment (19) and 30 days after depot injection (37). Notably, an earlier study showed that the inhibitory effects of GnRH-ant resulted from reduced occupancy of the GnRH-R binding sites as well as sustained reduction in membrane receptors (38).
In contrast, animals treated exclusively with GnRH-a (Group B) demonstrated a marked decrease in GnRH-R mRNA and an increase in receptor protein compared to the control 12 hours after treatment (Figure 5). The increase GnRH-R protein likely results from an initial flare response to GnRH-a since the pituitaries were collected within 12 hours of treatment. In support of our findings, Horvath et al. (27) showed a decrease in GnRH-R mRNA after 10 days of a microcapsule GnRH-a treatment and an increase in receptor protein after 10 days of daily injection. Murase et al. (39) reported similar results with two other GnRH agonists at multiple time points. GnRH agonists have been reported to cause a rapid decrease in GnRH-R mRNA in the rat pituitary as early as 2 hours after treatment and a decrease in LH mRNA after 48 hours and FSH mRNA after 3 hours (22,39). In our current study, the two-dose regimen of GnRH-a (Groups B and D) was sufficient to reduce the levels of GnRH-R mRNA, but not FSH and LH mRNA, a result likely explained by the termination of our study 12 hours after GnRH-a.
Increased intracellular staining for LH and FSH after treatment with GnRH-ant, without appreciable alterations in mRNA concentration, suggests that GnRH antagonists stimulate intracellular storage of FSH and LH within pituitary gonadotropes. Given the short delay between agonist treatment and necropsy (12 hours) we interpret the finding of decreased intracellular pools of gonadotropins in the combined treatment group (Group D, Figures 2A and 2B) to indicate that gonadotropes have released storage pools of gonadotropin in response to GnRH-a treatment. These results support the exaggerated release of gonadotropins caused by GnRH-a in GnRH-ant primed females. This observation is consistent with preservation of intact signaling despite reduced GnRH receptors and indicates receptor function.
One limitation of the current study is that experiments were performed on castrated sexually mature female rats ten days after ovariectomy to eliminate estrogen’s effect upon control of gonadotropin in vivo, at the level of the pituitary. Pituitary secretion of gonadotropins has been shown to vary depending upon the length of time following oophorectomy in the rat and other species (4,40). Our results in ovariectomized female rats may not be applicable to intact rats since there is a rise in follistatin and follistatin mRNAs following gonadectomy (41–43). Despite limitations inherent in the model, our approach mirrors that of previous studies in which OVX rats were used for the expected higher levels of GnRH in the pituitary (19,23).
In conclusion, these findings suggest the mechanism of supraphysiologic release of gonadotropin in GnRH-ant primed female is that some GnRH receptors, albeit reduced in amount, remain active and responsive to pharmacologic doses of GnRH-a which in turn stimulates the release of large storage of pools of gonadotropins that accumulate during GnRH-ant treatment.
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the Reproductive Biology and Medicine Branch, NICHD, NIH.
The authors would like to thank Dr. Terry Nett of Colorado State University for the GnRH-R analogs and Drs. Gharib and Chin for providing the cDNA clones for beta-subunits of rat FSH and LH. Drs. Prabir Chakraborty and Anitha Nair provided essential assistance and expertise. Maggie Cisar provided technical assistance and support and performed several of the experiments. The authors also thank Drs. Barbara Stegmann and Alan DeCherney for guidance and statistical support, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schally AV. Luteinizing hormone-releasing hormone analogs: their impact on the control of tumorigenesis. Peptides. 1999;20:1247–1262. doi: 10.1016/s0196-9781(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 2.Filicori M. Gonadotropin-releasing hormone agonists. A guide to use and selection. Drugs. 1994;48:41–58. doi: 10.2165/00003495-199448010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Olivennes F. The use of gonadotropin-releasing hormone antagonists in ovarian stimulation. Clin Obstet Gynecol. 2006;49:12–22. doi: 10.1097/01.grf.0000197520.53682.32. [DOI] [PubMed] [Google Scholar]
- 4.Illions EH, Scott RT, Carey KD, Navot D. Evaluation of the impact of concurrent gonadotropin-releasing hormone (GnRH) antagonist administration on GnRH agonist-induced gonadotrope desensitization. Fert Steril. 1995;64:848–854. doi: 10.1016/s0015-0282(16)57864-9. [DOI] [PubMed] [Google Scholar]
- 5.Olivennes F, Fanchin R, Bouchard P, Taieb J, Frydman R. Triggering of ovulation by a gonadotropin-releasing hormone (GnRH) agonist in patients pretreated with a GnRH antagonist. Fertil Steril. 1996;66:151–153. doi: 10.1016/s0015-0282(16)58404-0. [DOI] [PubMed] [Google Scholar]
- 6.Itskovitz-Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report: short communication. Hum Reprod. 2000;15:1965–1968. doi: 10.1093/humrep/15.9.1965. [DOI] [PubMed] [Google Scholar]
- 7.Fauser BC, De Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during varian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:709–715. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 8.Orvieto R, Zagatsky I, Yulzari-Roll V, La Marca A, Fisch B. Substituting human chorionic gonadotropin by gonadoropin-releasing hormone agonist to trigger final follicular maturation, during controlled ovarian hyperstimultion, results in less inflammation. Gynecol Endocrinol. 2006;22:437–440. doi: 10.1080/09513590600881339. [DOI] [PubMed] [Google Scholar]
- 9.Reissmann T, Felberbaum R, Diedrich K, Engel J, Comaru-Schally AM, Schally AV. Development and applications of luteinizing hormone-releasing hormone atagonists in the treatment of infertility: an overview. Hum Reprod. 1995;10:1974–1981. doi: 10.1093/oxfordjournals.humrep.a136219. [DOI] [PubMed] [Google Scholar]
- 10.Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grondahl ML, Westergaard L, et al. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2005;20:1213–1220. doi: 10.1093/humrep/deh765. [DOI] [PubMed] [Google Scholar]
- 11.Kolibianakis EM, Schultz-Mosgau A, Schroer A, van Steirteghem A, Devroey P, Diedrich K, et al. A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocye maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Hum Reprod. 2005;20:2887–2892. doi: 10.1093/humrep/dei150. [DOI] [PubMed] [Google Scholar]
- 12.Griesinger G, von Otte S, Schroer A, Ludwig AK, Diedrich K, Al-Hasani S, et al. Elective cryopreservation of all pronuclear oocytes after GnRH agonist triggering of final oocyte maturation in patients at risk of developing OHSS: a prospective, observational proof-of-concept study. Hum Reprod. 2007;22:1348–1352. doi: 10.1093/humrep/dem006. [DOI] [PubMed] [Google Scholar]
- 13.Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89:84–91. doi: 10.1016/j.fertnstert.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta analysis. Hum Reprod. 2006;12:159–168. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- 15.Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88:4186–4192. doi: 10.1210/jc.2002-021953. [DOI] [PubMed] [Google Scholar]
- 16.Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;325:93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 17.Mason DR, Arora KK, Mertz LM, Catt KJ. Homologous down-regulation of gonadotropin-releasing hormone receptor sites and messenger ribonucleic acid transcripts in alpha T3–1 cells. Endocrinology. 1994;135:1165–70. doi: 10.1210/endo.135.3.8070359. [DOI] [PubMed] [Google Scholar]
- 18.Scheele F, Hompes PG, Lambalk CB, Schoute E, Broekmans FJ, Schoemaker J. The GnRH challenge test: a quantitative measure of pituitary desensitization during GnRH agonist administration. Clin Endocrinol. 1996;44:581–586. doi: 10.1046/j.1365-2265.1996.730551.x. [DOI] [PubMed] [Google Scholar]
- 19.Halmos G, Schally A. Changes in subcellular distribution of pituitary receptors for luteinizing hormone-releasing hormone (LH-RH) after treatment with the LH-RH antagonist cetrorelix. Proc Natl Acad Sci. 2002;99:961–965. doi: 10.1073/pnas.012598399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huirne JA, Lambalk CB. Gonadotropin-releasing-hormone antagonists. Lancet. 2001;358:1793–1803. doi: 10.1016/S0140-6736(01)06797-6. [DOI] [PubMed] [Google Scholar]
- 21.Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod. 2006;12:333–340. doi: 10.1093/humupd/dml001. [DOI] [PubMed] [Google Scholar]
- 22.Lerrant Y, Kottler ML, Bergametti F, Moumni M, Blumberg-Tick J, Counis R. Expression of gonadotropin-releasing hormone (GnRH) receptor gene is altered by GnRH agonist desensitization in a manner similar to that of gonadotropin beta-subunit genes in normal and castrated rat pituitary. Endocrinol. 1995;136:2803–2808. doi: 10.1210/endo.136.7.7789305. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs M, Schally AV. Comparison of mechanisms of action of luteinizing hormone-releasing (LHRH) antagonist cetrorelix and LHRH agonist triptorelin on the gene expression of pituitary LHRH receptors in rats. Proc Natl Acad Sci USA. 2001;98:12197–12202. doi: 10.1073/pnas.211442598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth C, Hegemann F, Hildebrandt J, Balzer I, Witt A, Wuttke W, et al. Pituitary and Gonadal Effects of GnRH (gonadotropin releasing hormone) analogs in two peripubertal female rat models. Pediatr Res. 2004;55:126–133. doi: 10.1203/01.PDR.0000100463.84334.3F. [DOI] [PubMed] [Google Scholar]
- 25.Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15:462–499. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- 27.Horvath JE, Bajo AM, Schally AV, Kovacs M, Herbert F, Groot K. Effects of long-term treatment with the luteinizing hormone-releasing hormone (LHRH) agonist decapeptyl and the LHRH antagonist Cetrorelix on the levels of pituitary LHRH receptors and their mRNA expression in rats. Proc Natl Acad Sci. 2002;99:15048–15053. doi: 10.1073/pnas.232579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leal JA, Gordon K, Williams RF, Danforth DR, Roh SI, Hodgen GD. Probing Studies on Multiple Dose Effects of Antide (NAL-LYS) GnRH Antagonist in Ovariectomized Monkeys. Contraception. 1989;40:623–633. doi: 10.1016/0010-7824(89)90134-0. [DOI] [PubMed] [Google Scholar]
- 29.Scott RT, Illions EH, Carey KD, Navot D. Gonadotropin-releasing hormone antagonist administration enhances gonadotrope responsiveness at doses inadequate to suppress immunoassayable gonadotropin levels. Fertil Steril. 1994;64:1069–1071. [PubMed] [Google Scholar]
- 30.Chin WW, Godine JE, Klein DR, Chang AS, Tan LK, Habener JF. Nucleotide sequence of the cDNA encoding the precursor of the beta-subunit of rat lutropin. Proc Natl Acad Sci USA. 1983;80:4649–4653. doi: 10.1073/pnas.80.15.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gharib SD, Roy A, Wierman ME, Chin WW. Isolation and characterization of the gene encoding the b-subunit of rat follicle-stimulating hormone. DNA. 1989;8:339–349. doi: 10.1089/dna.1.1989.8.339. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser UB, Zhao D, Cardona GR, Chin WW. Isolation and characterization of cDNAs encoding the rat pituitary gonadotropin-releasing hormone receptor. Biochem Biophys Res Commun. 1992;189:1645–1652. doi: 10.1016/0006-291x(92)90266-n. [DOI] [PubMed] [Google Scholar]
- 33.Nett TM, Crowder ME, Moss GE, Duello TM. GnRH-receptor interaction.v. down-regulation of pituitary receptors for GnRH in ovariectomized ewes by infusion of homologous hormone. Biol Reprod. 1981;24:1145–1155. [PubMed] [Google Scholar]
- 34.Ron-El R, Raziel A, Schachter M, Strassburger D, Kasterstein E, Friedler S. Induction of ovulation after GnRH antagonists. Hum Reprod Update. 2000;6:318–321. doi: 10.1093/humupd/6.4.318. [DOI] [PubMed] [Google Scholar]
- 35.Kol S, Itskovitz-Eldor J. Severe OHSS: yes, there is a strategy to prevent it! Hum Reprod. 2000;15:2266–2267. doi: 10.1093/humrep/15.11.2266. [DOI] [PubMed] [Google Scholar]
- 36.de Jong D, Van Hooren EG, Macklon NS, Mannaerts BM, Fauser BC. Pregnancy and birth after GnRH agonist for induction of final oocyte maturation in a woman undergoing ovarian stimulation for ICSI, using a GnRH antagonist (Orgalutran/Antagon) to prevent a premature LH surge: a case report. J Assist Reprod Genet. 2001;18:30–33. doi: 10.1023/A:1026498629324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horvath JE, Toller GL, Schally AV, Bajo AM, Groot K. Effects of long-term treatment with low doses of the LHRH antagonist Cetrorelix on pituitary receptors for LHRH and gonadal axis in male and female rats. Proc Natl Acad Sci. 2004;101:4996–5001. doi: 10.1073/pnas.0400605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halmos G, Schally AV, Pinski J, Vadillo-Buenfil M, Groot K. Down-regulation of pituitary receptors for luteinizing hormone-releasing hormone (LH-RH) in rats for LH-RH antagonist cetrorelix. Proc Natl Acad Sci. 1996;93:2398–2402. doi: 10.1073/pnas.93.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murase M, Uemura T, Gao M, Inada M, Funabash T, Hirahara F. GnRH antagonist-induced down-regulation of the mRNA expression of pituitary receptors: comparisons with GnRH agonist effects. Endocr J. 2005;52:131–137. doi: 10.1507/endocrj.52.131. [DOI] [PubMed] [Google Scholar]
- 40.Roth C, Schricker M, Lakomek M, Strege A, Heiden I, Luft H, et al. Autoregulation of the gonadotropin-releasing hormone (GnRH) system during puberty: effects of antagonistic versus agonistic GnRH analogs in a female rat model. J Endocrinol. 2001;169:361–371. doi: 10.1677/joe.0.1690361. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133:931–934. doi: 10.1210/endo.133.2.8393779. [DOI] [PubMed] [Google Scholar]
- 42.Depaolo LV, Mercado M, Guo Y, Ling N. Increased follistatin (activin-binding protein) gene expression in rat anterior pituitary tissue after ovariectomy may be mediated by pituitary activin. Endocrinology. 1993;132:2221–2227. doi: 10.1210/endo.132.5.8477666. [DOI] [PubMed] [Google Scholar]
- 43.Kirk SE, Dalkin AC, Yasin M, Haisenleder DJ, Marshall JC. Gonadotropin-releasing hormone pulse frequency regulates expression of pituitary follistatin messenger ribonuceic acid: a mechanism for differential gonadotrope function. Endocrinol. 1994;135:876–880. doi: 10.1210/endo.135.3.8070381. [DOI] [PubMed] [Google Scholar]