Abstract
Background
Because cannabis use is associated with social, physical and psychological problems, it is important to know what causes some individuals to initiate cannabis use and a subset of those to become problematic users. Previous twin studies found evidence for both genetic and environmental influences on vulnerability, but due to considerable variation in the results it is difficult to draw clear conclusions regarding the relative magnitude of these influences.
Method
A systematic literature search identified 28 twin studies on cannabis use initiation and 24 studies on problematic cannabis use. The proportion of total variance accounted for by genes (A), shared environment (C), and unshared environment (E) in (1) initiation of cannabis use and (2) problematic cannabis use was calculated by averaging corresponding A, C, and E estimates across studies from independent cohorts and weighting by sample size.
Results
For cannabis use initiation, A, C, and E estimates were 48%, 25% and 27% in males and 40%, 39% and 21% in females. For problematic cannabis use A, C, and E estimates were 51%, 20% and 29% for males and 59%, 15% and 26% for females. Confidence intervals of these estimates are considerably narrower than those in the source studies.
Conclusions
Our results indicate that vulnerability to both cannabis use initiation and problematic use was significantly influenced by A, C, and E. There was a trend for a greater C and lesser A component for cannabis initiation as compared to problematic use for females.
Keywords: cannabis, genetics, meta-analysis, heritability, twin research
1. Introduction
With about 166 million annual consumers (equivalent to 3.9% of the global population aged 15 to 64), cannabis is the most widely consumed illicit drug worldwide, and by far the illicit drug most commonly consumed by young people (1). Furthermore, a broad estimation suggests that in Europe about 1% of people consume cannabis almost daily, and several European countries have reported an increase in the number of regular or intensive users (2).
Cannabis use can lead to social harms, including accidents, violence and suicide attempts (3), and regular cannabis use can lead to physical or psychological problems, and has been found to interfere with family, school, and work (4–8). Law enforcement, public health costs and loss of productivity and work potential due to health problems are also an economic drain on society (2, 5). According to the United Nations Office on Drugs and Crime (1), cannabis use is among the most common primary reasons for entering drug related treatment. Furthermore, cannabis use often precedes the use of other drugs, which suggests that cannabis may cause further problems as a gateway drug (9–11). However, the exact nature of the association between cannabis use and subsequent other illicit drug use is unclear (12–14).
To deal with the problems associated with cannabis use, it is important to understand what causes some individuals to initiate cannabis use and what causes some of those individuals to become regular users or become dependent on it. Though there may be some completely random events that cause people to vary in their cannabis use (such as changes in availability of the drug), much of the variability is likely to be due both to the nature of the environment they live in and developed in, and to their genetic makeup.
It has long been recognised that risk of cannabis and other substance (ab)use runs in families. Studies aiming to understand the basis of familial risk include family studies, adoption studies, and twin studies. Family studies into cannabis use have shown moderate parent-offspring correlations (ranging between 0.30 and 0.59; (15–18) as well as sibling-sibling correlations (ranging between 0.39 and 0.59 (15, 19)). In a recent study, Merikangas et al. (20) found elevated risks for cannabis use disorders among siblings (odds ratio [OR] = 3.6), offspring (OR = 6.9), and spouses (OR = 4.4) of probands with cannabis use disorders. However, family studies cannot determine whether familial resemblance is due to genetic factors or environmental factors shared between family members. Adoption studies can distinguish genetic and shared environmental factors by comparing the similarity of the adopted child with its adopted parents and with its biological parents. To our knowledge, no adoption study has specifically examined cannabis use, but adoption studies into drug and alcohol use have found that abuse or dependence of adoptees is more related to abuse or dependence of their biological parents than their adoptive parents (21–26), indicating an important role for genetic factors.
Twin studies disentangle familial resemblance into genetic and shared environmental factors by comparing the similarity of identical (monozygotic; MZ) and non-identical (dizygotic; DZ) twins. There have been numerous twin studies into cannabis use, but due to considerable variation in the results it is difficult to draw clear conclusions regarding the relative magnitude of genetic and environmental influences. Estimates of the proportion of variance in cannabis use accounted for by genetic influences (i.e., heritability) range from close to zero (e.g., studies 27, 28) to over 60% (e.g., studies 29, 30, 31). Similarly, estimates of the proportion of variance accounted for by shared environmental factors range from zero (e.g., 30, 32) to 68% (28). Inconsistent results have also been found for problematic cannabis use ((symptoms of) abuse and dependence). Various explanations could be proposed for these inconsistent results, including differences in measurement scales, sample size, and demographic differences (age, sex, nationality, socioeconomic status). In particular, very large sample sizes are required to accurately estimate genetic and shared environmental influences when using dichotomous variables (which is the case in most cannabis use studies). For this reason, many of the individual studies barely had the power to statistically distinguish between genetic and shared environmental influences, and confidence intervals around the estimates were often very wide.
Here we carried out a meta-analysis of existing twin studies in order to provide a more accurate estimate of the magnitude of genetic and environmental influences on cannabis use initiation and problematic cannabis use ((symptoms of) abuse/dependence). Because cannabis use in general is more prevalent among males than females (2), and some twin studies reported sex differences in contributions of genetic and environmental factors (e.g., 33, 34), meta-analyses were carried out separate for males and females, in order to check for sex-differences in cannabis use etiology.
2. Method
2.1 Background information – twin studies and cannabis phenotypes
2.1.1 The twin design
The studies we examined in this meta-analysis are twin studies that apply genetic modelling to determine the sources of individual differences in cannabis use. Below, a short introduction to the classical twin design is provided; further details can be found elsewhere (e.g., 35, 36, 37).
With the classical twin design, trait variance can be partitioned into its genetic and environmental (shared within twin pairs and non-shared) components, by analysing the resemblance in MZ and DZ twin pairs. Additive genetic variance (A) results from the sum of allelic effects within and across multiple genes affecting a trait. Shared environmental variance (C) is due to environmental influences shared within twin pairs, such as the family environment, prenatal influences, parental style, and socio-economic status. Unshared environmental variance (E) results from environmental factors that are not shared within twin pairs (e.g., idiosyncratic events and experiences, unshared peers) and includes measurement error.
Estimates of these genetic and environmental variance components can be obtained because A, C and E each predict different patterns of MZ and DZ twin pair correlations. MZ twins share all their genes, while DZ twins share on average 50% of their genes. Hence, if A were the sole source of variance in a trait, twin correlations of 1.0 for MZ pairs and 0.5 for DZ pairs are expected. If C were the sole source of variance in a trait, a twin correlation of 1.0 for both MZ and DZ pairs is expected, and if E would be the sole source of variance in a trait, a twin correlation of 0.0 for both MZ and DZ pairs is expected.
In reality, individual differences in complex phenotypes result from a combination of these genetic and environmental influences. Using the observed MZ and DZ twin pair correlations, it is possible to estimate standardised A, C and E variance components, which represent the proportion of total variance accounted for by additive genetic, and shared and unshared environmental influences. All A, C, and E estimates reported in this paper refer to standardised variance components. These estimates are obtained by employing maximum-likelihood modelling procedures, which determine the combination of genetic and environmental parameters that best fits the covariance structure of the observed data. In addition, confidence intervals around these estimates can also be calculated. Most reports used in our analyses employed maximum-likelihood modelling procedures using the statistical package MX (38), and others used LISREL (39, 40).
It is assumed that the shared environmental variance component estimated in twin studies is generalisable to the general population. Studies including twins and their siblings make it possible to distinguish between general shared environmental influences and a special twin environment. Studies including siblings have not identified a significant twin environment effect for cannabis use (31, 34, 41).
Most cannabis use phenotypes are measured as dichotomous variables (i.e. cannabis users versus non-users) which can be analysed by using a threshold model (42). This model assumes that there is an underlying continuum of liability which is normally distributed in the population. Upon this normal distribution, a threshold delimits affected versus unaffected cases. The variation in liability can be analysed in the same way as the variance for continuous variables.
2.1.2 Phenotypes: initiation of cannabis use and problematic use
The various twin studies into cannabis use have used different phenotypes (observable characteristics, traits, or behaviours) such as initiation, use in the last year, regular use, symptoms of abuse, or dependence to full diagnosis of abuse or dependence. In this meta-analysis we examine two cannabis-related phenotypes: initiation of cannabis use and problematic cannabis use.
Initiation of cannabis use is also often referred to as lifetime cannabis use or ‘ever used cannabis’. The core aspect is that it makes a distinction between individuals who have tried cannabis at least once in their lifetime versus those who have not. Hence, this phenotype is a dichotomous variable.
The other phenotype we examine, problematic cannabis use, is less consistently defined. Different definitions of problematic cannabis use can be found in the literature, ranging from symptoms of abuse to a full dependence diagnosis. Most studies use abuse and dependence criteria according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR (8)). According to the DSM-IV-TR (8, page 197) substance abuse is characterised by ‘a maladaptive pattern of substance use manifested by recurrent and significant adverse consequences related to the repeated use of substances’. Substance dependence is a more advanced state of drug abuse, the essential feature of which is ‘a cluster of cognitive, behavioural and physiological symptoms indicating that the individual continues use of the substance despite significant substance-related problems. There is a pattern of repeated self-administration that can result in tolerance, withdrawal, and compulsive drug-taking behaviour’ (8, page 199). Withdrawal symptoms do not have to be met for a cannabis dependence diagnosis (8), though this has been subject to debate (43).
In the present analysis, problematic use is operationally defined as having one or more of the symptoms of abuse or dependence during lifetime. We did not limit our focus to studies that use full abuse or dependence diagnosis only, because we are interested in vulnerability to problematic cannabis use or addiction. Because of this broad definition we incorporated studies using phenotypes like ‘abuse or dependence’, ‘one or more abuse or dependence symptoms’ and ‘abuse’. All studies incorporated in the meta-analyses have analysed problematic use as a dichotomous measure.
2.2 Data collection: literature search and study inclusion criteria
Selection of relevant twin studies on cannabis use and problematic use for this study started with a search of the electronic databases PubMed (http://www.ncbi.nlm.nih.gov/entrez) and ISI web of Knowledge (http://apps.isiknowledge.com) by using the following keywords: heritability/heredity/twin and cannabis/marijuana/hashish. No restrictions regarding date range were specified. Abstracts of these search results (N = 122) were examined and relevant articles were retrieved for review. Three additional studies identified from reference lists from these studies and one manuscript in press (44, results obtained by personal communication) were also added.
Subsequently, unsuitable studies were excluded from the analysis based on two main criteria. First, only studies specifically examining cannabis use were included, and those examining related phenotypes like general drug use were left out. Second, only studies that used twin samples and applied genetic modelling to investigate the genetics of cannabis involvement were included. This procedure identified 28 twin studies on lifetime cannabis use and 24 studies on problematic cannabis use. For the purpose of the meta-analysis only studies using independent samples could be used. Some studies measured slightly different phenotypes in a same cohort or some authors examined more than one dependent measure concerning problematic cannabis use within one study, and some authors used a (sub)sample of the same cohort. In these cases only one of the reports was included in the meta-analysis, with a preference for reports with the largest sample, separate parameter estimates for each sex, the most suitable measure of cannabis use, and estimates based on univariate models as opposed to multivariate models. Table 1 and 2 show overviews of the available studies on cannabis use initiation and problematic use, respectively.
Table 1.
Overview of twin studies into lifetime cannabis use. Studies in bold are used in the present meta-analysis.
| Cohort | Reference | Country | Age | Measure | Prevalence (in %) | Sex | MZ (pairs) | DZ (pairs) | A (in %) | C (in %) | E (in %) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agrawal et al., 2008a (29) | Australia | 24–36 | Use prior to age 17 | 60.2 (lifetime use) | M F OS |
494 698 |
395 513 661 |
72 (64–80) | - | 28 (20–36) |
| 1 | Agrawal et al., 2007 (47) | Australia | 24–36 | Experimentation | M: 69 F: 53 |
M F |
487 696 |
387 506 |
48 (46–50) 44 (41–49) |
22 (12–41) 28 (26–35) |
30 (28–31) 28 (20–29) |
| 1 | Lynskey et al., 2007 (70) | Australia | 24–36 | Lifetime use | Not reported | M/F | 6265 individuals | 60 (45–72) | 9 (1–22) | 32 (26–36) | |
| 1 | Lynskey et al., 2002 (33) | Australia | 24–36 | Lifetime use |
M: 68.8 F: 53.2 |
M F OS |
487 699 |
387 507 655 |
67 (9–75) 45 (18–70) |
0 (0–50) 26 (3–49) |
33 (25–43) 29 (23–37) |
| 2 | Agrawal et al., 2005 (49) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Cannabis use | 48–53 | M F |
702 556 |
489 378 |
56 56 |
26 28 |
18 16 |
| 2 | Agrawal et al., 2004a (71) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Early use, before age 18 | M: 52.9 F: 46.6 (lifetime use) |
M F |
1196 MM pairs 934 FF pairs |
16 40 |
52 38 |
32 22 |
|
| 2 | Agrawal et al., 2004b (72) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Ever used | M: 54 F: 48 |
M F |
2953 individuals 2132 individuals |
36 (17–41) | 35 (16–39) | 29 (25–34) | |
| 2 | Agrawal et al., 2004c (57) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Lifetime use | M: ~53 F: ~48 |
M F |
2632 individuals 1943 individuals |
23 46 |
45 29 |
32 25 |
|
| 2 | Agrawal et al., 2004d (53) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Lifetime use | M: 54.0 F: 47.6 |
M F |
702 556 |
489 378 |
27 (9–54) 29 (7–55) |
42 (40–48) 50 (49–69) |
31 (30–36) 21 (17–26) |
| 2 | Gillespie et al., 2009 (50) | US, Virginia cohort 1 | 24–62 (at wave 3) | Initiation | 54 | M | 1772 individuals | 27 | 42 | 3 | |
| 2 | Karkowski et al., 2000 (73) | US, Virginia cohort 1 | M = 30.1 ± 7.6 | Lifetime use | Not reported | F | 499 | 327 | 59 (44–73) | 19 (9–31) | 21 |
| 2 | Kendler et al., 2005 (74) | US, Virginia cohort 1 | F: 36.6 ± 8.1 M/OS twins: 36.8 ± 9.1 |
Lifetime use | M: 39–63 F: 33–55 (differs per age group) |
M F |
880/1445/584 pairs 485/1149/283 pairs (wave 1/2/3) |
41 (19–63) | 29 (9–48) | 30 (25–36) | |
| 2 | Kendler et al., 2003 (54) | US, Virginia cohort 1 | 20–58 | Lifetime use | 54.0 | M | 704 | 492 | 35 | 35 | 30 |
| 2 | Kendler et al., 2000 (75) | US, Virginia cohort 1 | 20–58 | Lifetime use |
MZ: 50.4 DZ: 55.9 |
M | 708 | 490 | 33 (5–60) | 34 (10–58) | 33 (26–40) |
| 2 | Kendler et al., 1999 (76) | US, Virginia cohort 1 | 37.7 ± 7.5 | Lifetime use | 48.7 | F | 1934 individuals | 46 (18–77) | 29 (0–54) | 25 (20–32) | |
| 2 | Kendler et al., 1998 (77) | US, Virginia cohort 1 | 22–62 | Lifetime use |
MZ: 46.0 DZ: 52.8 |
F | 485 | 335 | 40 (10–72) | 35 (6–60) | 25 (18–34) |
| 2 | Neale et al., 2006 (78) | US, Virginia cohort 1 | Not reported | Lifetime use | Not reported | F | 499 | 327 | 48 | 28 | 25 |
| 3 | Maes et al., 1999 (28) | US, Virginia cohort 2 | 8–16 | Lifetime use |
M: 0.0–12.6 F: 0.0–10.9 (differs per age) |
M F OS |
300 389 |
184 187 295 |
22 (0–78) | 68 (15–93) | 9 (2–27) |
| 4 | Lessem et al., 2006 (79) | US, Add Health | Wave 1: M = 16.2 ± 1.7 Wave 2: M = 16.7 ± 1.6 |
Ever used | 35 | M/F | 4846/4413 (Pairs per wave, includes sibling pairs) | 21 (0–51) | 57 (32–76) | 22 (10–42) | |
| 4 | Miles et al., 2001 (80) | US, Add Health | 13–21 | Ever used | 35 |
M F OS |
144 145 |
131 114 204 |
31 (1–61) | 47 (21–69) | 22 (14–34) |
| 5 | McGue et al., 2000 (27) | US, Minnesota cohort 2 | 17 | Ever used |
M: 20.4 F: 24.0 |
M F |
188 223 |
101 114 |
26 13 |
56 61 |
18 26 |
| 6 | Rhee et al., 2003 (34) | US, Colorado | 12–19 | Initiation |
M: 27.2 F: 27.1 |
M F OS |
159 186 |
113 101 123 ** 306 siblings 74 adoptive |
39 (2–81) 72 (29–.95) |
44 (7–73) 24 (2–65) |
17 (7–34) 4 (1–12) |
| 7 | Fowler et al., 2007 (51) | UK | 11–19 | Initiation | 24 | M/F | 461 | 714 | 35 (5–63) | 47 (24–71) | 18 (10–36) |
| 7 | Shelton et al., 2007 (81) | UK |
Time 1: 5–13 Time 2: 11– 20 |
Initiation |
M: 22 F: 21 |
M F OS |
177 248 |
132 189 342 |
35 (5–63) | 47 (24–71) | 18 (10–36) |
| 8 | Grant et al., 2006 (68) | US, VETR | 36–55 | Ever used | ~40 | M | 1583 | 1255 | 60 | 8 | 32 |
| 8 | Tsuang et al., 1999 (82) | US, VETR | 36–55 | Transition from exposure to initiation | 47.2 (lifetime use) | M | 1874 | 1498 | 44 (22–60) | 10 (0–28) | 46 (39–53) |
| 9 | Vink et al. (submitted) (44) | the Netherlands | 21–40 | Cannabis initiation |
M: 36.2 F: 24.7 |
M F OS |
158 422 211 |
98 205 |
44 (16–74) | 31 (4–55) | 24 (17–33) |
A = additive genetic variance, C = shared environmental variance, E = unshared environmental variance
Table 2.
Overview of twin studies into problematic cannabis use. Studies in bold are used in the present meta-analysis.
| Cohort | Reference | Country | Age | Measure | Prevalence (%) | Sex | MZ (pairs) | DZ (pairs) | A (in %) | C (in %) | E (in %) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agrawal et al., 2007 (47) | Australia | 24–36 | Abuse/Dependence problems |
M: 28 F: 15 |
M F |
487 696 |
387 506 |
68 (65–69) 55 (30–63) |
14 (12–14) 16 (11–19) |
18 (16–19) 29 (20–31) |
| 1 | Lynskey et al., 2002 (33) | Australia | 24–36 | Lifetime dependence; 2 or more symptoms | M: 15.1 F: 7.8 |
M F |
487 699 |
387 507 655 |
56 (23–77) 21 (0–67) |
13 (0–40) 39 (0–65) |
31 (21–45) 40 (27–54) |
| 2 | Agrawal et al., 2005 (49) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Abuse/Dependence | 8–19 | M F |
702 556 |
489 378 |
31 36 |
0 0 |
69 64 |
| 2 | Agrawal et al., 2004c (57) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Abuse/dependence | M: ~18 F: ~8 |
M F |
2632 MM individuals 1943 FF individuals |
76 69 |
- - |
24 31 |
|
| 2 | Agrawal et al., 2004d (53) | US, Virginia cohort 1 | M 20–58 F 21–62 |
Abuse/dependence | M: 18.0 F: 7.5 |
M F |
702 556 |
489 378 |
76 48 (47–56) |
0 28 (0–31) |
24 24 (18–26) |
| 2 | Gillespie et al., 2009 (50) | US, Virginia cohort 1 | 24–62 (at wave 3) | Symptoms of abuse | Not reported | M | 1772 twins | 35 | 34 | 31 | |
| 2 | Kendler et al., 2007 (83) | US, Virginia cohort 1 | F 36.3 (8.2) M 37.0 (9.1) |
Number of abuse/dependence symptoms | M: 20.5 F: 9.4 (at least one symptom) |
M F |
1666 i 1151 individuals |
1269 779 individuals |
71 | - | 29 |
| 2 | Kendler et al., 2003 (54) | US, Virginia cohort 1 | 20–58 | Lifetime abuse/dependence | 18.3 | M | 704 | 492 | 73 | 1 | 26 |
| 2 | Kendler et al., 2000 (75) | US, Virginia cohort 1 | 20–58 | Abuse |
MZ: 16.6 DZ: 20.1 |
M | 708 | 490 | 76 (42–82) | 1 (0–31) | 23 (17–33) |
| 2 | Kendler et al., 1999 (76) | US, Virginia cohort 1 | 37.7 ± 7.5 | Lifetime use | 7.9 | F | 1934 individuals | 47 | 19 | 34 | |
| 2 | Kendler et al., 1998 (77) | US, Virginia cohort 1 | 22–62 | Lifetime Abuse |
MZ: 7.8 DZ: 7.6 |
F | 485 | 335 | 72 (56–84) | - | 28 (16–44) |
| 2 | Neale et al., 2006 (78) | US, Virginia cohort 1 | Not reported | Abuse | Not reported | F | 499 | 327 | 48 | 30 | 33 |
| 3 | van den Bree et al., 1998 (67) | US, Minnesota cohort 1* | 15–63 | Abuse and or dependence | Treatment sample |
M F |
56 38 |
66 28 |
68 53 |
24 0 |
8 47 |
| 4 | McGue et al., 2000 (27) | US, Minnesota cohort 2 | 17 years old | Lifetime Abuse/dependence |
M: 7.1 F: 6.7 |
M F |
188 223 |
101 114 |
54 6 |
27 68 |
19 26 |
| 5 | Rhee et al., 2003 (34) | US, Colorado | 12–19 | One or more abuse/dependence symptoms | M: 13.7 F: 12.7 |
M F |
159 186 |
113 101 123 DOS 306 siblings 74 adoptive |
34 (0–67) | 36 (10–60) | 30 (16–48) |
| 5 | Young et al., 2006 (31) | US, Colorado | 12–18 | One or more abuse/dependence symptoms |
M: 0.8–28.8 F: 0.0–22.9 (age 12 to 18) |
M/F |
645 MZ, 702 DZ 1945 males, 1799 females including siblings |
55 (29–80) | 24 (2–44) | 21 (13–33) | |
| 6 | Fu et al., 2002 (84) | US, VETR | 33–52 | Lifetime dependence | 6.6 | M | 1868 | 1492 | 50 | 13 | 37 |
| 6 | Grant et al., 2006 (68) | US, VETR | 36–55 | Lifetime abuse/dependence | ~8 | M | 1583 | 1255 | 39 | 20 | 40 |
| 6 | True et al., 1999 (85) | US, VETR | 33–52 | 3 or more lifetime symptoms of abuse/dependence | 6.7 | M | 1856 | 1479 | 43.9 | 21.3 | 35.8 |
| 6 | Tsuang et al., 1999 (82) | US, VETR | 36–55 | Transition to abuse and dependence | 7.2 (abuse/dependence) | M | 1874 | 1498 | 22 (0–49) | 9 (0–40) | 69 (51–88) |
| 6 | Tsuang et al., 1998 (48) | US, VETR | 36–55 | Lifetime abuse or dependence | 7.2 | M | 1874 | 1498 | 33 | 29 | 38 |
| 6 | Tsuang, et al., 1996 (86) | US, VETR | 36–55 | Lifetime abuse or dependence | 7.2 | M | 1874 | 1498 | 33 | 29 | 38 |
| 6 | Xian et al., 2008 (55) | US, VETR | 33–55 | Lifetime dependence diagnosis | 6.6 | M | 1857 | 1482 | 38 | 26 | 35 |
| 7 | Kendler et al., 2006b (30) | Norway | Mean = 28.2 (3.9) | Symptoms of abuse or dependence | 1.6 |
M F |
220 448 |
117 263 338 |
75 (34–89) | 0 (0–34) | 23 (11–47) |
A = additive genetic variance, C = shared environmental variance, E = unshared environmental variance
In the end, we used nine independent cohorts for males and eight for females for the meta-analysis of cannabis use initiation. For the meta-analysis of problematic cannabis use we used seven samples for males and six for females.
2.3 Meta-analysis
We meta-analysed the standardised variance components for the two phenotypes by calculating the weighted average genetic (A), shared environmental, (C) and unshared environmental (E) estimates. An explanation of this method can be found in Li et al. (45) and Sutton et al. (46). Briefly, to estimate the weighted mean, the male/female parameter estimates for each cohort were weighted by the number of males/females in the sample. In some cases the reports we used did not report separate parameter estimates for each sex (because they did not differ significantly). In these cases we used the equated estimates for both sexes. Calculations were done in Microsoft Office Excel 2007. Estimates were made separately for each sex and phenotype (cannabis use initiation and problematic cannabis use). We also calculated the 95% confidence interval (CI) around each estimate, calculated from the variance in the sample of source studies.
3. Results
The twin studies we identified from the literature search, including information about the cohort, sample sizes, measure used, and A, C, and E estimates, are presented in Table 1 (cannabis use initiation) and Table 2 (problematic cannabis use). The studies selected for the meta-analysis are shown in bold. One cohort (US, Vietnam Era Twin Registry [VETR]) only used male participants, so we could not include this cohort into our meta-analyses for females.
All cohorts are from Western countries - over half of them from US samples; other data were obtained in Australia, the UK, the Netherlands and Norway. Table 3 shows the results of the meta-analysis.
Table 3.
Parameter estimates (in % of variance explained) for A (additive genetic variance), C (shared environmental variance), and E (unshared environmental variance) for initiation of cannabis use and problematic cannabis use as obtained from meta-analysis. Estimates are presented separately for males and females.
| Initiation of cannabis use | Problematic cannabis use | |||||
|---|---|---|---|---|---|---|
| A | C | E | A | C | E | |
| Males | 47.6 (38.8–56.4) | 25.1 (11.5–38.7) | 27.2 (18.2–36.2) | 51.4 (37.9–64.9) | 19.8 (11.3–28.3) | 28.8 (22.2–35.3) |
| Females | 39.6 (30.0–49.2) | 39.0 (28.6–49.4) | 21.2 (15.5–26.9) | 58.5 (44.3–72.7) | 15.2 (0.6–29.8) | 26.3 (23.1–29.5) |
3.1 Initiation of cannabis use
For both sexes, individual differences in cannabis use initiation are due moderately to genetic, shared environmental as well as unshared environmental influences. Although the confidence intervals for male and female estimates overlap, additive genetic influences are somewhat stronger for males, while the shared environment plays a greater role in females.
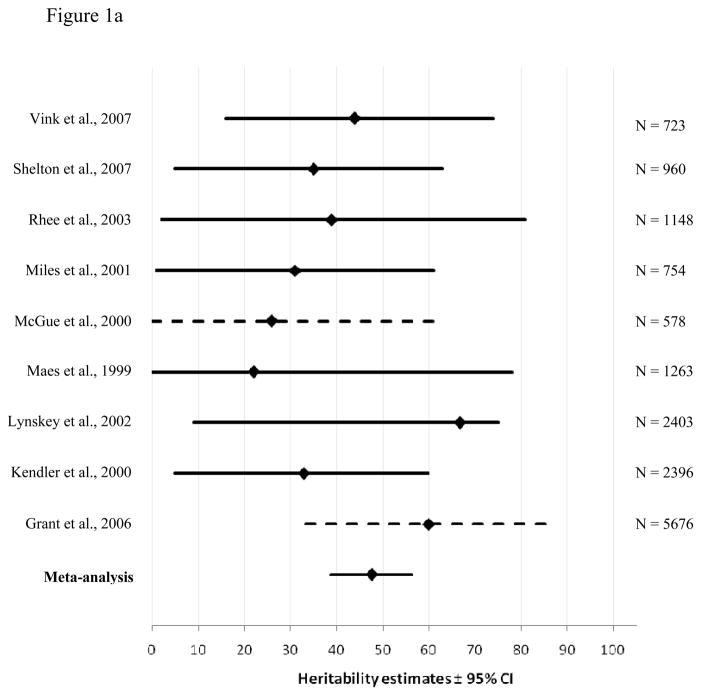
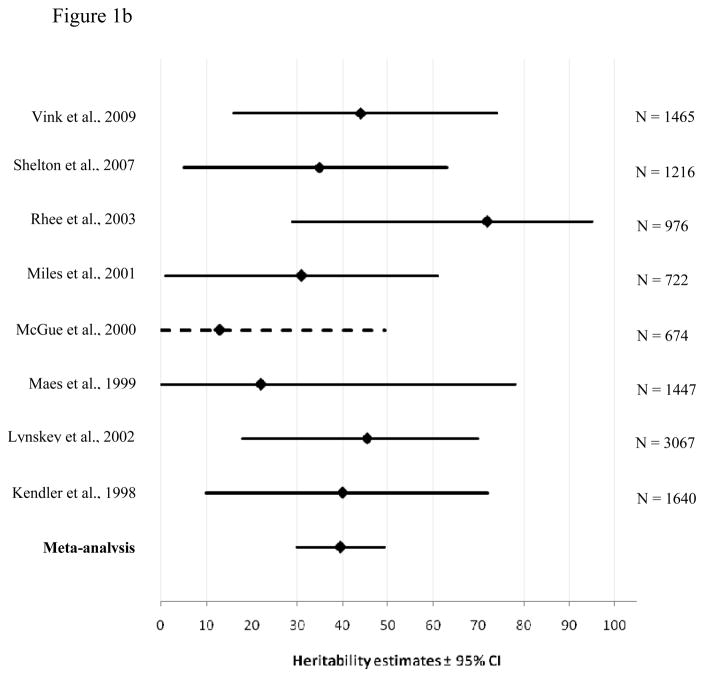
Figure 1a and 1b display the results of the meta-analysis for genetic contributions to cannabis use initiation for males and females, respectively. The horizontal lines represent the 95% confidence intervals around the heritability estimates (diamonds) from the different cohorts. When confidence intervals were not reported by the source studies, they were estimated (dotted lines), based on a logarithmic curve regression of the confidence intervals and sample size from the other studies. The bottom line shows the results of our meta-analysis, displaying the narrower confidence interval of the estimates as compared to the intervals from the source studies. As can be seen in the Figure, the point estimates from the meta-analyses fall within all confidence intervals from the source studies, suggesting the source studies are homogenous.
Figure 1.
Heritability estimates (i.e. proportion of variance accounted for by genetic influences (A)) and 95% confidence intervals for the studies used in the meta-analysis of cannabis use initiation for males (1a) and females (1b). The bottom line shows the weighted A estimate and 95% confidence intervals estimated in the present meta-analysis. Dotted lines show confidence intervals estimated by a logarithmic curve regression on the sample sizes.
3.2 Problematic cannabis use
According to our meta-analysis, more than half of the individual differences in problematic cannabis use is due to genetic variance, while shared environmental influences and unique environmental influences have substantially lower contributions. The A estimate is higher for females than for males, but again, confidence intervals overlap.
Compared to those on cannabis use initiation, genetic influences on problematic use are higher while shared environmental influences are lower for females. The most notable difference is the C effect for females, which explains only 15% of the variance for problematic use but almost 40% for initiation of cannabis use.
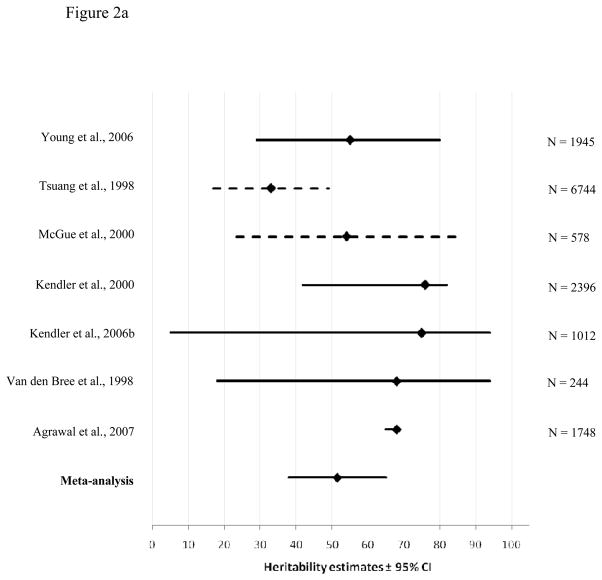
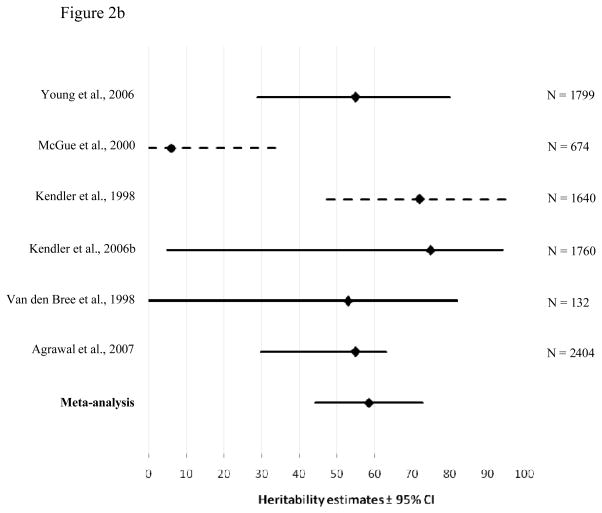
Figure 2a and 2b represent the meta-analyses for genetic contributions to problematic cannabis use for males and females, respectively. Again, the bottom line shows the results of our meta-analysis. The point estimates from the meta-analyses generally fall within the reported confidence intervals of the estimates from the source studies, suggesting reasonable homogeneity of studies. Exceptions were Agrawal et al. (47), where the confidence intervals are particularly narrow, and McGue et al. (27) and Tsuang et al. (48), where confidence intervals were not reported but estimated by us.
Figure 2.
Heritability estimates (i.e. proportion of variance accounted for by genetic influences (A)) and 95% confidence intervals for the studies used in the meta-analysis of problematic cannabis use for males (2a) and females (2b). The bottom line shows the weighted A estimate and 95% confidence intervals estimated in the present meta-analysis. Dotted lines show confidence intervals estimated by a logarithmic curve regression on the sample sizes.
4. Discussion
Results of twin studies investigating the extent to which cannabis use vulnerability is due to genetic and environmental influences have been inconsistent. We carried out the first meta-analysis of twin studies into cannabis use, in order to obtain more accurate estimates of the relative magnitude of genetic and environmental influences on cannabis use initiation and problematic use. Results for cannabis use initiation showed significant A, C, and E influences accounting for 48%, 25% and 27% of the variance in males, and 40%, 39% and 21% of the variance in females. The corresponding A, C, and E estimates for problematic cannabis use were 51%, 20% and 29% for males and 59%, 15% and 26% for females, all of which were significant. Confidence intervals for these estimates were considerably narrower than those in the source studies.
Our findings thus indicate that vulnerability to both cannabis use initiation and problem use is substantially heritable. Twin studies that analysed both phenotypes in one model have revealed that part of these genetic factors overlap between cannabis use initiation and problematic use (49–51). This implies that vulnerability to initiate cannabis use is partly due to the same set of genes as vulnerability to progress cannabis use.
For females, the relative genetic contribution was lower, and the shared environmental contribution higher, for initiation of cannabis use as compared to problematic use, in accordance with Agrawal & Lynskey (43). This may be because the initial stages of the process of cannabis use are more sensitive to environmental factors, like drug availability and use by peers (52), whereas the likelihood of dependence is more influenced by biological factors such as individual differences in physical response to the drug.
Genetic factors influencing cannabis use overlap with those influencing use of other illicit drugs, although there are also specific genetic factors influencing use of each particular drug (31, 48, 53–55). The general genetic vulnerability to drug use could be related genes underlying personality characteristics such as novelty seeking (56–59), to biochemical attributes (60), or to psychiatric vulnerability(61, 62).
By means of genetic linkage and association studies it could be possible to identify some of the specific genetic variants that influence cannabis use. However, cannabis use phenotypes are likely to be polygenic, with each gene accounting for only a small proportion of the variance, as seems to be the case for other complex phenotypes (63). For substance use disorders in general, genome wide association studies have found dozens of genes that could contribute to vulnerability (64). Many of these gene variants are likely to alter specification and maintenance of neuronal connections (64). Genes identified as affecting vulnerability to drug use problems could be potential targets for pharmaceutical drugs aiming to modify addictions.
The magnitude of C and E contributions to both cannabis use initiation and problematic use indicates that environmental factors (which are often modifiable) also play a substantial role. In a longitudinal twin study, Korhonen et al. (65) identified some of the environmental factors that predict cannabis as well as other illicit drug use. They found that paternal drinking behaviour was a significant familial predictor (although this could also be a manifestation of their shared genetic vulnerability). Other predictors they mention are early smoking onset, drinking to intoxication, having peers who smoke cigarettes or have acquaintances with drug experience, and aggressive behaviours among males (65). Scherrer et al. (66) also found that perceptions of substance use among siblings, friends and school peers are strongly associated with cannabis abuse/dependence in young adults.
Prevention and intervention programs should focus on identifying and modifying these risk factors. Thus, programs focussing on not just the individual, but also their family and peer groups, could be beneficial. Also, parents should be aware of the role they could play in preventing use of cannabis and other drugs by their children. Peers and parents can probably also serve as protective factors for cannabis use. Chabrol et al. (52) found that the number of peers opposed to cannabis use as well as student’s negative expectations of cannabis use were protective factors. Our findings that C influences are more important to cannabis use initiation for females than for males suggest that females may be more sensitive to prevention and intervention programs.
Although the parameter estimates from this meta-analysis have narrower confidence intervals than any of the source studies, their precision is still limited for several reasons. Firstly, despite the large number of twin studies into cannabis use phenotypes, they were only based on a low number of independent cohorts; our variance components estimates are based on six to nine cohorts. Also, all cohorts are from Western countries, with over half of them from the US, so the results are not necessarily generalisable to different populations. Additional and more varied cohorts would increase the generalisability as well as the precision of the estimates. On the other hand, the countries from which the different samples are drawn have different policies toward cannabis use, which might influence the reported genetic and environmental estimates. For example, in countries with a more liberal cannabis policy, like the Netherlands, cannabis is more easily available. Therefore, the relative contribution of environmental effects could be smaller and that of genetic effects larger. However, such a difference is not supported by the findings from Vink et al (44), who reported A, C, and E estimates for initiation for the Dutch sample that fit very well within the confidence intervals of our weighted averages.
Another limitation is that the twin studies we analysed differed regarding the composition of the sample, the phenotypic measures, and the statistical method used. These inconsistencies between the studies are likely to be partly responsible for their inconsistent results. By combining the studies into one analysis we did not acknowledge possible differences between different samples and methods. For example, two source cohorts did not use population based samples. Firstly, in the meta-analysis for problematic use, we included a study using a treatment sample (67). Because of the small sample size of this study and the fact that the reported variance components were relatively consistent with those from the other source studies, this one study should not have strongly biased our results.
Secondly, for both variables we incorporated a male cohort of twins where both twins served the US army during the Vietnam Era (48, 68). Genetic estimates based on this cohort are relatively high for cannabis initiation and relatively low for problematic cannabis use. Because the sample size of the source study is quite large, if the etiology of cannabis use in Vietnam veterans is different from that in the general population, the inclusion of this study could bias our results.
Also, in our analyses we combined studies using samples of varying age ranges, while results of earlier studies have suggested that from adolescence to adulthood the effect of shared environment gradually declined, while genetic influences gradually increased (69). Younger individuals might have limited access to marijuana and experience less peer pressure. However, because of the low number of independent samples, it was not possible to meaningfully distinguish between different age groups or to do other sub-analyses. The inclusion of adolescents and adults in one analysis might have modestly influenced our results, but estimates from the adolescent samples did not differ markedly from those from adult samples, so the effect would be small. Compared to the results of our analyses, heritability estimates for cannabis use initiation were relatively low and shared environmental estimates relatively high for some adolescent samples (27, 28). However, Rhee et al. (34) found a very high A estimate of 72%, and a relatively low C estimate for adolescent females.
Overall, our meta-analyses, by aggregating the results of a number of previous twin studies, provided more robust estimates of the genetic and environmental influences on cannabis initiation and problematic use. Because our analyses average estimates over samples of different sizes and demographic makeup, our findings are likely to be more generalisable than the source studies. Our results indicate that A, C and E factors each contribute significantly to vulnerability to both cannabis use initiation and problematic use. This confirms that cannabis problems do not have a single or simple cause, and suggests that both genetic and environmental factors are potential targets for treatment and prevention measures.
Acknowledgments
KJHV is supported by DA018267 from the National Institute on Drug Abuse to M.T.L. JMV is supported by NWO (VENI 451-06-004). SEM is supported by an Australian NHMRC Sidney Sax Fellowship (443036).
We thank Arpana Agrawal for advice regarding the Virginia twin sample, and Marianne van den Bree for providing additional results from their study not reported in the published text.
Footnotes
Declaration of interest
None
References
- 1.United Nations Office on Drugs and Crime [UNODC] 2008 World Drug Report. 2008 Available from: http://www.unodc.org/documents/wdr/WDR_2008/WDR_2008_eng_web.pdf. Archived by WebCite at http://www.webcitation.org/5guBGlv8A.
- 2.European Monitoring Centre for Drugs and Drug Addiction [EMCDDA] Annual report 2008: the state of the drugs problem in Europe. Luxembourg: Office for Official Publications of the European Communities; 2008. Available from: http://www.emcdda.europa.eu/attachements.cfm/att_64227_EN_EMCDDA_AR08_en.pdf. Archived by WebCite at http://www.webcitation.org/5guBKirCX. [Google Scholar]
- 3.Huas C, Hassler C, Choquet M. Has occasional cannabis use among adolescents also to be considered as a risk marker. Eur J Public Health. 2008;18:626–9. doi: 10.1093/eurpub/ckn065. [DOI] [PubMed] [Google Scholar]
- 4.Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–6. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- 5.Hall W, Babor TF. Cannabis use and public health: assessing the burden. Addiction. 2000;95:485–90. doi: 10.1046/j.1360-0443.2000.9544851.x. [DOI] [PubMed] [Google Scholar]
- 6.van Ours JC, Williams J. Why parents worry: Initiation into cannabis use by youth and their educational attainment. J Health Econ. 2009;28:132–42. doi: 10.1016/j.jhealeco.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Fergusson DM, Horwood LJ. Early onset cannabis use and psychosocial adjustment in young adults. Addiction. 1997;92:279–96. [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 9.Kandel DB. Stages in adolescent involvement in drug-use. Science. 1975;190:912–4. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- 10.Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood - further evidence for the gateway theory. Journal of Studies on Alcohol. 1992;53:447–57. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- 11.Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000;95:505–20. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- 12.Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug and Alcohol Review. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland HH, Wiebe RP. Understanding the association between adolescent marijuana use and later serious drug use: Gateway effect or developmental trajectory? Development and Psychopathology. 2008;20:615–32. doi: 10.1017/S0954579408000308. [DOI] [PubMed] [Google Scholar]
- 14.Morral AR, McCaffrey DF, Paddock SM. Reassessing the marijuana gateway effect. Addiction. 2002;97:1493–504. doi: 10.1046/j.1360-0443.2002.00280.x. [DOI] [PubMed] [Google Scholar]
- 15.Brook JS, Whiteman M, Gordon AS, Brook DW. Father’s influence on his daughter’s marijuana use viewed in a mother and peer context. Advances in Alcohol & Substance Abuse. 1985;4:165–90. doi: 10.1300/J251v04n03_08. [DOI] [PubMed] [Google Scholar]
- 16.Gfroerer J. Correlation between drug use by teenagers and drug use by older family members. American Journal of Drug and Alcohol Abuse. 1987;13:95–108. doi: 10.3109/00952998709001502. [DOI] [PubMed] [Google Scholar]
- 17.Johnson GM, Shontz FC, Locke TP. Relationship between adolescent drug-use and parental drug behaviors. Adolescence. 1984;19:295–9. [PubMed] [Google Scholar]
- 18.Meller WH, Rinehart R, Cadoret RJ, Troughton E. Specific familial transmission in substance abuse. International Journal of the Addictions. 1988;23:1029–39. doi: 10.3109/10826088809056183. [DOI] [PubMed] [Google Scholar]
- 19.Brook JS, Whiteman M, Brook DW, Gordon AS. Sibling influences on adolescent drug-use - older brothers on younger brothers. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:958–66. doi: 10.1097/00004583-199111000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Merikangas KR, Li JJ, Stipelman B, Yu K, Fucito L, Swendsen J, et al. The familial aggregation of cannabis use disorders. Addiction. 2009;104:622–9. doi: 10.1111/j.1360-0443.2008.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yates WR, Cadoret RJ, Troughton E, Stewart MA. An adoption study of DSM-IIIR alcohol and drug dependence severity. Drug and Alcohol Dependence. 1996;41:9–15. doi: 10.1016/0376-8716(96)01221-5. [DOI] [PubMed] [Google Scholar]
- 22.Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating 2 genetic pathways to drug-abuse. Archives of General Psychiatry. 1995;52:42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- 23.Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. An adoption study of drug abuse dependency in females. Comprehensive Psychiatry. 1996;37:88–94. doi: 10.1016/s0010-440x(96)90567-2. [DOI] [PubMed] [Google Scholar]
- 24.Cloninger CR, Bohman M, Sigvardson S. Inheritance of alcohol-abuse-cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–8. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 25.Cloninger CR, Bohman M, Sigvardsson S, Knorring ALv. Psychopathology in adopted-out children of alcoholics. The Stockholm adoption study. Recent developments in Alcoholism. 1985;3:37–51. doi: 10.1007/978-1-4615-7715-7_4. [DOI] [PubMed] [Google Scholar]
- 26.Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm adoption study of alcoholism - Confirmatory cross-fostering analysis. Archives of General Psychiatry. 1996;53:681–7. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- 27.McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–7. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: The Virginia Twin Study of Adolescent Behavioral Development. Journal of Studies on Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A, Lynskey MT, Pergadia ML, Bucholz KK, Heath AC, Martin NG, et al. Early cannabis use and DSM-IV nicotine dependence: a twin study. Addiction. 2008a;103:1896–904. doi: 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendler KS, Aggen SH, Tambs K, Reichborn-Kjennerud T. Illicit psychoactive substance use, abuse and dependence in a population-based sample of Norwegian twins. Psychological Medicine. 2006b;36:955–62. doi: 10.1017/S0033291706007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: General or specific? Behavior Genetics. 2006;36:603–15. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 32.Cho HS, Guo G, Iritani BJ, Hallfors DD. Genetic contribution to suicidal behaviors and associated risk factors among adolescents in the US. Prevention Science. 2006;7:303–11. doi: 10.1007/s11121-006-0042-5. [DOI] [PubMed] [Google Scholar]
- 33.Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Slutske WS, et al. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychological Medicine. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- 34.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–64. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 35.Neale MC, Cardon LR. In: Methodology for Genetic Studies of Twins and Families. series NA, editor. Dordrecht: Kluwer; 1992. [Google Scholar]
- 36.Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Lachine I, et al. Theory and practice in quantitative genetics. Twin Research. 2003;6:361–76. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- 37.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3:872–82. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 38.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 7. VCU Box 900126, Richmond VA 23298: Department of Psychiatry; 2006a. [Google Scholar]
- 39.Jöreskog KG, Sörbom D. LISREL 8.30 and PRELIS 2.30 for Windows. Chicage: Scientific Software; 1999. [Google Scholar]
- 40.Boomsma DI, Molenaar PCM. Using LISREL to analyze genetic and environmental covariance structure. Behavior Genetics. 1986;16:237–50. doi: 10.1007/BF01070799. [DOI] [PubMed] [Google Scholar]
- 41.Kendler KS, Neale MC, Thornton LM, Aggen SH, Gilman SE, Kessler RC. Cannabis use in the last year in a US national sample of twin and sibling pairs. Psychological Medicine. 2002;32:551–4. doi: 10.1017/s0033291701004950. [DOI] [PubMed] [Google Scholar]
- 42.Falconer DS. Introduction to quantitative genetics. Harlow, Essex, UK: Longman Scientific and Technical; 1989. [Google Scholar]
- 43.Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101:801–12. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- 44.Vink JM, Wolters L, Neale MC, Boomsma DI. Heritability of cannabis initiation in Dutch adult twins. Addictive Behaviors. doi: 10.1016/j.addbeh.2009.09.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 46.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-analysis in Medical Research. New-York: John Wiley & Sons, Ltd; 2000. [Google Scholar]
- 47.Agrawal A, Lynskey MT, Bucholz KK, Martin NG, Madden PAF, Heath AC. Contrasting models of genetic co-morbidity for cannabis and other illicit drugs in adult Australian twins. Psychological Medicine. 2007;37:49–60. doi: 10.1017/S0033291706009287. [DOI] [PubMed] [Google Scholar]
- 48.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men - The role of drug-specific and shared vulnerabilities. Archives of General Psychiatry. 1998;55:967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 49.Agrawal A, Neale MC, Jacobson KC, Prescott CA, Kendler KS. Illicit drug use and abuse/dependence: modeling of two-stage variables using the CCC approach. Addictive Behaviors. 2005;30:1043–8. doi: 10.1016/j.addbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction. 2009;104:430–8. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102:413–22. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chabrol H, Chauchard E, Mabila JD, Mantoulan R, Adele A, Rousseau A. Contributions of social influences and expectations of use to cannabis use in high-school students. Addictive Behaviors. 2006;31:2116–9. doi: 10.1016/j.addbeh.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Agrawal A, Neale MC, Prescott CA, Kendler KS. Cannabis and other illicit drugs: Comorbid use and abuse/dependence in males and females. Behavior Genetics. 2004d;34:217–28. doi: 10.1023/B:BEGE.0000017868.07829.45. [DOI] [PubMed] [Google Scholar]
- 54.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journal of Psychiatry. 2003;160:687–95. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 55.Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, et al. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103:1391–8. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]
- 56.Vink JM, Nawijn L, Boomsma DI, Willemsen G. Personality differences in monozygotic twins discordant for cannabis use. Addiction. 2007;102:1942–6. doi: 10.1111/j.1360-0443.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 57.Agrawal A, Jacobson KC, Prescott CA, Kendler KS. A twin study of personality and illicit drug use and abuse/dependence. Twin Research. 2004c;7:72–81. doi: 10.1375/13690520460741462. [DOI] [PubMed] [Google Scholar]
- 58.Jang KL, Livesley WJ, Vernon PA. Heritability of the big five personality dimensions and their facets: A twin study. Journal of Personality. 1996;64:577–91. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 59.Stoel RD, De Geus EJC, Boomsma DI. Genetic analysis of sensation seeking with an extended twin design. Behavior Genetics. 2006;36:229–37. doi: 10.1007/s10519-005-9028-5. [DOI] [PubMed] [Google Scholar]
- 60.Duaux E, Krebs MO, Loo H, Poirier MF. Genetic vulnerability to drug abuse. Eur Psychiat. 2000;15:109–14. doi: 10.1016/s0924-9338(00)00204-2. [DOI] [PubMed] [Google Scholar]
- 61.Caroti E, Fonzi L, Marconi D, Bersani G. Cannabis and depression. Riv Psichiatr. 2007;42:8–16. [Google Scholar]
- 62.Shih RA, Belmonte PL, Zandi PP. A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. International Review of Psychiatry. 2004;16:260–83. doi: 10.1080/09540260400014401. [DOI] [PubMed] [Google Scholar]
- 63.Weiss KM. Tilting at quixotic trait loci (QTL): An evolutionary perspective on genetic causation. Genetics. 2008;179:1741–56. doi: 10.1534/genetics.108.094128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uhl GR, Drgon T, Johnson C, Fatusin OO, Liu QR, Contoreggi C, et al. “Higher order” addiction molecular genetics: Convergent data from genome-wide association in humans and mice. Biochemical Pharmacology. 2008;75:98–111. doi: 10.1016/j.bcp.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korhonen T, Huizink AC, Dick DM, Pulkkinen L, Rose RJ, Kaprio J. Role of individual, peer and family factors in the use of cannabis and other illicit drugs: A longitudinal analysis among Finnish adolescent twins. Drug and Alcohol Dependence. 2008;97:33–43. doi: 10.1016/j.drugalcdep.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scherrer JF, Grant JD, Duncan AE, Pan H, Waterman B, Jacob T, et al. Measured environmental contributions to cannabis abuse/dependence in an offspring of twins design. Addictive Behaviors. 2008;33:1255–66. doi: 10.1016/j.addbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Bree MBM, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug and Alcohol Dependence. 1998;52:231–41. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- 68.Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, et al. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychological Medicine. 2006;36:109–18. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- 69.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lynskey MT, Grant JD, Li L, Nelson EC, Bucholz KK, Madden PAF, et al. Stimulant use and symptoms of abuse/dependence: Epidemiology and associations with cannabis use - A twin study. Drug and Alcohol Dependence. 2007;86:147–53. doi: 10.1016/j.drugalcdep.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 71.Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychological Medicine. 2004a;34:1227–37. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- 72.Agrawal A, Prescott CA, Kendler KS. Forms of cannabis and cocaine: A twin study. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2004b;129B:125–8. doi: 10.1002/ajmg.b.30046. [DOI] [PubMed] [Google Scholar]
- 73.Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. American Journal of Medical Genetics. 2000;96:665–70. [PubMed] [Google Scholar]
- 74.Kendler KS, Gardner C, Jacobson KC, Neale MC, Prescott CA. Genetic and environmental influences on illicit drug use and tobacco use across birth cohorts. Psychological Medicine. 2005;35:1349–56. doi: 10.1017/S0033291705004964. [DOI] [PubMed] [Google Scholar]
- 75.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–9. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 76.Kendler KS, Karkowski LM, Corey LA, Prescott CA, Neale MC. Genetic and environmental risk factors in the aetiology of illicit drug initiation and subsequent misuse in women. British Journal of Psychiatry. 1999;175:351–6. doi: 10.1192/bjp.175.4.351. [DOI] [PubMed] [Google Scholar]
- 77.Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–22. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- 78.Neale MC, Harvey E, Maes HHM, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: Applications to substance use and abuse. Behavior Genetics. 2006b;36:507–24. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- 79.Lessem JM, Hopfer CJ, Haberstick BC, Timberlake D, Ehringer MA, Smolen A, et al. Relationship between adolescent marijuana use and young adult illicit drug use. Behavior Genetics. 2006;36:498–506. doi: 10.1007/s10519-006-9064-9. [DOI] [PubMed] [Google Scholar]
- 80.Miles DR, van den Bree MBM, Gupman AE, Newlin DB, Glantz MD, Pickens RW. A twin study on sensation seeking, risk taking behavior and marijuana use. Drug and Alcohol Dependence. 2001;62:57–68. doi: 10.1016/s0376-8716(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 81.Shelton K, Lifford K, Fowler T, Rice F, Neale M, Harold G, et al. The association between conduct problems and the initiation and progression of marijuana use during adolescence: A genetic analysis across time. Behavior Genetics. 2007;37:314–25. doi: 10.1007/s10519-006-9124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, et al. Genetic and environmental influences on transitions in drug use. Behavior Genetics. 1999;29:473–9. doi: 10.1023/a:1021635223370. [DOI] [PubMed] [Google Scholar]
- 83.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Archives of General Psychiatry. 2007;64:1313–20. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 84.Fu QA, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, et al. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence - Contribution of antisocial personality disorder in men. Archives of General Psychiatry. 2002;59:1125–32. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- 85.True WR, Heath AC, Scherrer JF, Xian H, Lin N, Eisen SA, et al. Interrelationship of genetic and environmental influences on conduct disorder and alcohol and marijuana dependence symptoms. American Journal of Medical Genetics. 1999;88:391–7. doi: 10.1002/(sici)1096-8628(19990820)88:4<391::aid-ajmg17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 86.Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, et al. Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. American Journal of Medical Genetics. 1996;67:473–7. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]