Abstract
Objective
To examine the treatment impact of lamotrigine on the neurocognitive profile of patients with pediatric bipolar disorder (PBD).
Method
Healthy controls (HC) (n = 24; mean age = 12.4 ± 3.3 years) and unmedicated PBD patients with manic, mixed or hypomanic episodes (n = 34; mean age = 13 ± 3.1 years) were matched for IQ, age, sex, race, and socioeconomic status. A neurocognitive battery was administered at baseline and again after 14 weeks, during which PBD patients were treated with lamotrigine.
Results
Clinical symptoms improved with treatment in the patient group with significant change from baseline to follow-up on the Young Mania Rating Scale (p < 0.001) and the Children's Depression Rating Scale–Revised (p < 0.001). Global neurocognitive function improved with lamotrigine in PBD patients over time relative to that in HC, although overall performance remained impaired. Working memory and verbal memory significantly improved with treatment in patients, and deficits in these domains were no longer significantly impaired relative to HC at follow-up. Executive function significantly improved with treatment in the patient group, but still lagged behind HC at follow-up. Performance on attention tests did not improve with treatment.
Conclusions
There appears to be significant improvement in cognitive abilities in PBD patients treated with lamotrigine that is most prominent in the areas of working memory and verbal memory and that occurs along with mood stabilization.
Keywords: attention, bipolar, cognition, executive functioning, lamotrigine, verbal memory, working memory
In young patients receiving mood stabilizers for pediatric bipolar disorder (1-3), the impact of pharmacotherapy on cognitive impairment may be positive and enhance outcome, or may be adverse and impede learning and academic achievement (4, 5). Therefore, in addition to mood stabilization, a primary consideration in choosing a mood stabilizer for patients with pediatric bipolar disorder (PBD) is the effect on cognitive function. There is a growing body of literature supporting lamotrigine's superiority over other mood stabilizers in not exacerbating or causing cognitive dysfunction (6-10) or resulting in marked cognitive improvement (11, 12). However, almost all of these studies of lamotrigine's effects on cognition were conducted in adults with bipolar disorder (11, 12), healthy adult volunteers (6-10), or patients with epilepsy (13). To date, there are no published longitudinal studies of cognitive function in response to mood-stabilizing medications in patients with PBD.
Clinical studies in adult bipolar I disorder have evaluated the neurocognitive effects of lamotrigine as monotherapy or as an adjuvant therapy at 100-200 mg dose based on a self-report cognitive scale [the Medical Outcomes Study Cognitive (MOS-Cog) Scale] (14, 15). The self-reported cognitive improvement was superior for lamotrigine, compared to lithium or placebo (14), as has been shown with lamotrigine monotherapy without concomitant antipsychotic medication (15). This improvement has been observed regardless of mood polarity at treatment initiation (14). Two open trials compared lamotrigine with other mood stabilizers using paper and pencil neuropsychological tests (11) or a computerized battery (12). Further, rank-order analysis of cognitive flexibility and complex attention measured in a cross-sectional study conducted on 18-70 year old patients with bipolar disorder who were taking one of five different antiepileptic drugs or lithium showed that lamotrigine is superior, followed in order, by carbamazepine, lithium, oxcarbazepine, and topiramate. In fact, patients on topiramate showed deterioration in cognitive flexibility and complex attention (12). In summary, findings in patients with adult bipolar disorder collectively indicate that lamotrigine may be an attractive choice in not worsening cognition as is seen with some anticonvulsants, and may even possibly improve it. There is one double-blind, placebo-controlled, crossover trial of lamotrigine in a pediatric study in which epilepsy patients showed no significant impairments relative to placebo on sustained attention, verbal and nonverbal recognition, and working memory (13). Limitations of these studies include the use of self-report measures of cognition, naturalistic study designs, concomitant medications, and small samples. Further, none of the clinical studies, including those in adult bipolar disorder, estimated how practice effects might affect retest performance by examining healthy controls (HC) in parallel over time with the clinical population.
The current study was conducted with the aim of evaluating the neurocognitive effects of lamotrigine in patients with PBD. A healthy comparison group was included to evaluate practice effects with the test battery. We hypothesized that patients with PBD would show cognitive impairment at baseline and improvement in working memory, executive function, verbal memory, and attention after treatment with lamotrigine.
Methods
This was a 14-week, single-site, prospective, open-label, outpatient treatment trial of lamotrigine for manic, mixed and hypomanic episodes of PBD. IQ and demographically matched HC were studied in parallel on the cognitive measures. Given that this was a study of a pediatric population, healthy volunteers were not given lamotrigine for ethical reasons. This study was approved by the University of Illinois at Chicago (UIC) Institutional Review Board. Parents gave written permission and children gave assent to participate in this trial.
Subjects
Subjects were screened at our UIC Pediatric Mood Disorders Program Clinic to determine if they qualified for the study according to the inclusion and exclusion criteria as follows. Inclusion criteria for patients were: (i) a DSM-IV (16) diagnosis of bipolar I disorder, mixed or manic episode or bipolar II disorder, hypomanic episode; (ii) 8-18 years of age; (iii) a baseline score of >15 on the Young Mania Rating Scale (YMRS) (17); and (iv) being medication free or currently clinically unstable on medications, justifying discontinuation of the ineffective therapy. To participate, patients receiving active treatment were required to consent to discontinuation of their current medications at study entry. The washout period consisted of tapering their previous medications over one week prior to study entry, except for those who received aripiprazole or fluoxetine, which required a four-week washout period. Exclusion criteria included: (i) active substance abuse, measured through urine drug screen; (ii) serious medical problems; (iii) a history of allergy to lamotrigine; and (iv) the presence of another DSM-IV Axis I diagnosis that required psychopharmacologic treatment. The study sample included 58 youth aged 12.8 ± 3.14 years at the time of recruitment. There were 34 subjects with PBD and 24 HC. Subject groups were matched for age, gender, race, and estimated intellectual potential (Table 1).
Table 1.
Demographic and clinical characteristics of healthy control (HC) subjects and pediatric bipolar disorder (PBD) patients at baseline and at 14-week follow-up
| Baseline | Follow-up | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | PBD | HC | PBD | ||||||||
| Mean | SD | Mean | SD | F(df) | Mean | SD | Mean | SD | F(df) | ||
| Age (years) | 12.4 | 3.3 | 13.0 | 3.1 | F(1,56) = 0.5 | ||||||
| SES | 1.1 | 0.1 | 2.9 | 0.1 | F(1,56) = 3.5 | ||||||
| YMRS | 1.0 | 1.8 | 19.7 | 9.2 | F(1,56) = 95.1a | 0.7 | 1.6 | 4.2 | 4.1 | F(1,56) = 18.2a | |
| CDRS-R | 19.0 | 2.1 | 51.1 | 10.8 | F(1,56) = 158.2a | 18.6 | 1.2 | 24.9 | 4.1 | F(1,56) = 29.2a | |
| WASI | 110.3 | 14.8 | 104.2 | 14.3 | F(1,56) = 2.3 | ||||||
| WRAT | 106.5 | 9.5 | 103.1 | 11.3 | F(1,56) = 1.4 | ||||||
| n | % | n | % | χ2 | df | ||||||
| Sex | 1.5 | 1 | |||||||||
| Male | 15 | 62.5 | 18 | 52.9 | |||||||
| Female | 9 | 37.5 | 16 | 47.1 | |||||||
| Race | 1.7 | 1 | |||||||||
| Caucasian | 13 | 54.2 | 20 | 58.8 | |||||||
| Other | 11 | 45.8 | 14 | 41.2 | |||||||
| Handedness | F(1,56) = 0.1 | ||||||||||
| Right | 23 | 95.8 | 32 | 94.1 | |||||||
| Left | 1 | 4.6 | 2 | 5.9 | |||||||
YMRS and CDRS-R pre-/post-treatment comparison is relevant in patient group, where patients received active lamotrigine treatment, while HC did not receive any treatment. SES = socioeconomic status; YMRS = Young Mania Rating Scale; CDRS-R = Child Depression Rating Scale-Revised; WASI = Wechsler Abbreviated Scale of Intelligence; WRAT = Wide Range Achievement Test.
p < 0.001.
Assessment and treatment procedures
All patients underwent a standard clinical assessment consisting of a diagnostic interview with the patient and family. In addition, each child and the parent or legal guardian were interviewed using the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (18). The WASH-U-KSADS interviews were completed by MNP or TM, both board-certified child psychiatrists, and a doctoral-level nurse practitioner in child psychiatry (JAC). Live diagnostic interviews of 10 cases were independently coded by the three researchers to establish inter-rater reliability. By Cohen's kappa, reliability of diagnoses was 0.94 between the raters. Clinical information from all sources was combined and further discussed to resolve any diagnostic disagreement in a weekly consensus conference involving the treating clinicians (MNP, JAC, and TM). Acute symptoms were treated during the eight-week lamotrigine titration phase using second-generation antipsychotics (SGA), i.e., risperidone, aripiprazole, quetiapine, or ziprasidone. After the SGA therapy in the first four weeks, patients were slowly withdrawn over 2-4 weeks, after which lamotrigine monotherapy at 200 mg per day was administered for a six-week period.
Statistical analyses plan
Statistical analyses were carried out on four main neurocognitive domains at baseline and after 14 weeks of follow-up for the two groups (Table 2): Attention [tests: Trail Making A (19), Continuous Performance Task (CPT) (20)]; Executive Function [tests: Trail Making B (19); Cog Set Shifting, Controlled Oral Word Association (COWA) (21)]; Working Memory [tests: Digit Span (22), Spatial Span (23)], and Verbal Memory [test: California Verbal Learning Test (CVLT) (24)]. All test data, both at baseline and follow-up, were standardized (i.e., converted to z-scores) relative to performance of the HC group at baseline, in order to provide a standard metric for comparison across neurocognitive domains. Extreme z-score values were truncated to +/- 3.0 to avoid their potential excessive impact on statistical analyses. Standardized summary scores were calculated for executive function, attention, working memory, and verbal memory by combining normalized z-scores from tests assessing each of the four cognitive domains. Internal consistencies of scores comprising each of the neurocognitive domains were previously documented (5). We computed a global index of neurocognitive functioning by averaging the means of the four domain scores for each subject.
Table 2.
Performance of patients with pediatric bipolar disorder (PBD) and healthy controls (HC) on individual neuropsychological tests organized by cognitive domains of interest (raw scores)a
| Neuropsychological domain | HC baseline | PBD baseline | HC follow-up | PBD follow-up |
|---|---|---|---|---|
| Attention | ||||
| Trail Making Test: Part A time | 35.57 ± 15.17 | 41.35 ± 14.58 | 35.87 ± 14.65 | 38.74 ± 16.87 |
| Penn Continuous Performance Test | ||||
| True positives | 33.78 ± 2.10 | 31.82 ± 4.67 | 33.42 ± 1.68 | 32.03 ± 5.10 |
| False positives | 5.44 ± 3.47 | 11.85 ± 10.65 | 5.16 ± 3.92 | 9.31 ± 7.95 |
| Domain scoreb | 0.00 ± 0.64 | -0.69 ± 1.15 | -0.04 ± 0.58 | -0.38 ± 0.89 |
| Working memory | ||||
| WMS-III: Digit Span (raw) | 16.21 ± 4.37 | 14.18 ± 3.60 | 16.29 ± 4.32 | 14.71 ± 3.20 |
| WMS-III: Spatial Span (raw) | 15.75 ± 4.10 | 12.85 ± 4.14 | 14.96 ± 3.62 | 13.88 ± 3.69 |
| Domain scoreb | 0.00 ± 0.86 | -0.86 ± 0.79 | -0.09 ± 0.83 | -0.47 ± 0.70 |
| Executive function | ||||
| Cogtest Set Shifting Test | ||||
| Total errors | 12.94 ± 10.26 | 40.70 ± 37.97 | 8.60 ± 7.00 | 23.83 ± 22.48 |
| Trail Making Test: Part B time | ||||
| Controlled Oral Word Association | 76.36 ± 24.01 | 108.47 ± 52.80 | 78.85 ± 24.91 | 97.24 ± 44.80 |
| Letters (mean C) | 12.30 ± 8.10 | 9.06 ± 3.72 | 10.82 ± 4.29 | 10.14 ± 3.99 |
| Letters (mean F) | 11.61 ± 4.90 | 9.00 ± 4.20 | 11.05 ± 4.32 | 9.62 ± 4.11 |
| Letters (mean L) | 10.57 ± 2.86 | 8.65 ± 3.36 | 10.00 ± 3.96 | 10.07 ± 3.94 |
| Categories (mean animal) | 18.48 ± 5.75 | 16.81 ± 5.27 | 19.73 ± 4.98 | 19.55 ± 6.33 |
| Categories (mean fruit) | 17.65 ± 7.37 | 14.23 ± 4.71 | 15.86 ± 4.85 | 15.66 ± 4.36 |
| Domain scoreb | 0.02 ± 0.73 | -1.00 ± 1.04 | 0.04 ± 0.55 | -0.53 ± 0.76 |
| Verbal memory | ||||
| CVLT: Total Trials 1-5 (raw) | 57.04 ± 7.18 | 44.06 ± 10.35 | 57.42 ± 9.04 | 50.09 ± 12.11 |
| CVLT: Short Delay Free Recall | 12.25 ± 1.40 | 9.47 ± 2.36 | 12.50 ± 1.67 | 11.58 ± 2.95 |
| CVLT: Long Delay Free Recall | 12.44 ± 1.53 | 10.00 ± 3.03 | 12.95 ± 1.33 | 11.76 ± 3.18 |
| Domain scoreb | 0.03 ± 0.63 | -1.09 ± 0.83 | 0.15 ± 0.99 | -0.41 ± 1.18 |
| Global functioningb | 0.01 ± 0.74 | -0.91 ± 0.53 | 0.02 ± 0.66 | -0.45 ± 0.50 |
Domain scores are normalized to baseline data from the healthy control subjects.
Indicates composite z-scores referenced to baseline values of healthy control subjects for tests in each domain.
CVLT = California Verbal Learning Test–Second Edition; WMS-III = Wechsler Memory Scale–Third Edition.
Results
Demographic and clinical features
Repeated measures analyses of variance (ANOVA) revealed that for the PBD patients, mean YMRS scores improved significantly from baseline (19.7 ± 9.2) to follow-up (4.2 ± 4.1) [F(1,33) = 102.9, p < 0.001]. Also, the PBD mean score for the Child Depression Rating Scale-Revised (CDRS-R) (25) improved significantly from baseline (51.1 ± 10.8) to follow-up (24.9 ± 4.1) [F(1,33) = 148.2, p < 0.001] (Table 1). For the HC group, there were no significant differences (F < 1) between YMRS scores at baseline (1.00 ± 1.8) and follow-up (0.67 ± 1.6), or between CDRS-R scores at baseline (19.0 ± 2.1) and follow-up (18.60 ± 1.2) (Table 1).
Neurocognitive function
Our goal was to initially examine differences in global cognitive function between the study groups at baseline, and then test for differences between the PBD and HC groups in their change in test performance over the 14-week study period, with subsequent step-down cognitive domain-wise comparisons of PBD and HC scores.
Omnibus test of cognitive functioning in PBD versus HC group at baseline versus follow-up
We compared PBD and HC group scores at baseline and follow-up, using a two-way repeated measures ANOVA on the global index of neurocognitive functioning (Table 2). There was a significant interaction of group by testing time [F(1,56) = 12.6, p < 0.001] on the global index of neurocognitive functioning, indicating an improvement in performance at retest in lamotrigine treated patients that exceeded practice effects in HC. Step-down comparisons of this effect revealed that PBD scores were significantly worse than HC scores both at baseline (PBD = -0.9; HC = -0.01) [F(1,56) = 27.2, p < 0.001] and at follow-up (PBD = -0.5; HC = -0.02) [F(1,56) = 8.6, p < 0.005]. Further, global neurocognitive function improved significantly from baseline to follow-up in the PBD group [F(1,56) = 30.9, p < 0.001], but not in the HC group (F < 1).
Domain-specific analyses
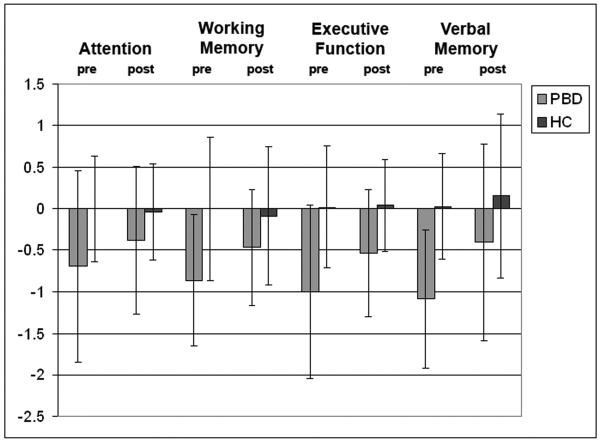
Neuropsychological performance for each of the four neurocognitive domains in the PBD compared to the HC group is illustrated in Figure 1 for both time-points. Given the significant group by testing time interaction obtained for the global index of neurocognitive functioning, we proceeded to carry out step-down analyses using two-way ANOVAs to examine group changes over time within each neurocognitive domain (Table 2).
Fig. 1.
Group differences in neurocognitive functions in the pediatric bipolar disorder (PBD) and healthy control (HC) groups at baseline and follow-up. All test data, both at baseline and follow-up, were standardized relative to performance of the HC group at baseline.
With regard to the Working Memory domain, a significant interaction of group by testing time [F(1,56) = 7.1, p < 0.01] indicated that the PBD patients improved more at retest than HC. Although PBD performance was impaired relative to HC at baseline, [F(1,56) = 15.2, p < 0.001] with a large effect size (Cohen's d = 1.03), their performance impairment was no longer significant at follow-up [F(1,56) = 3.5, p < 0.07] (Cohen's d = 0.49). PBD scores improved significantly from baseline to follow-up [F(1,56) = 12.87, p < 0.001], whereas HC scores did not change over time [F(1,56) = 0.46, p < 0.50].
In the case of the Executive Function domain, the significant interaction of group by testing time [F(1,56) = 6.3, p < 0.01] indicated that patients improved more at retest than HC subjects. PBD scores were significantly worse than the HC scores both at baseline [F(1,56) = 17.2, p < 0.001] and at follow-up [F(1,56) = 10.1, p < 0.01], even though PBD scores improved significantly from baseline to follow-up [F(1,56) = 17.2, p < 0.001]. HC scores did not improve over time [F(1,56) = 0.3, p < 0.6].
For the Verbal Memory domain, there was also a significant interaction of group by testing time [F(1,56) = 4.7, p < 0.03], indicating a greater improvement in the PBD patients at retest. PBD scores were impaired relative to the HC scores at baseline [F(1,56) = 31.2, p < 0.001], with a large effect size (Cohen's d = 1.54). While there was a trend for this effect at follow-up, it was no longer statistically significant [F(1,56) = 3.6, p < 0.06] and the effect size was much smaller (Cohen's d = 0.51). PBD scores improved with treatment over time [F(1,56) = 16.3, p < 0.001], while HC scores did not [F(1,56) = 0.3, p < 0.6].
Finally, for the Attention domain, the change in performance in PBD patients treated with lamotrigine was not greater than that seen in HC [F(1,56) = 2.2, p < 0.14]. A main effect of group showed that the PBD group performed overall worse than the HC group independent of testing time [F(1,56) = 6.41, p < 0.01].
Correlation between symptom response and change in neurocognitive performance
Based on the significant interaction between global neurocognitive performance and testing time in the PBD group, Pearson correlation analyses (two-tailed) were conducted to investigate the relationship between clinical improvement with treatment on YMRS and CDRS-R scores and improvement in neurocognitive functioning. For each subject, improvement was measured in terms of the difference between baseline and follow-up scores. There was a significant positive correlation between improvement on YMRS scores and the global index of neurocognitive functioning (r = 0.36, p < 0.04), but this correlation was not significant for any specific neurocognitive domain. Patient improvement on CDRS-R scores did not correlate with the global index of neurocognitive functioning or individual domain scores. Symptom severity on YMRS and CDRS-R at baseline was examined to see if it predicted neurocognitive outcome. A significant correlation was found only between baseline YMRS scores and the improvement on global index of neurocognitive function (r = 0.38, p < 0.03).
In order to examine whether the improvement in symptoms led to the change in neurocognitive domains in patient group, we carried out separate ANCOVAs for each neurocognitive domain at baseline and follow-up with improvement in YMRS and CDRS-R scores as covariates. No significant results (p > 0.05) were found in relation to the YMRS or CDRS-R improvement, suggesting that the change in any of the individual neurocognitive domains was not dependent on symptomatic improvement.
Discussion
This is the first study examining the neurocognitive outcome after lamotrigine treatment in PBD. The central findings indicate that there is significant improvement in neurocognitive functioning over 14 weeks within the PBD group in general cognitive performance, and in working memory, executive function, and verbal memory domains after lamotrigine treatment. Although patients were impaired in all domains prior to treatment, impairments in working memory and verbal memory relative to HC were no longer significant, albeit by a small margin, after treatment. Improvement on executive function in patients did not reach the level of performance seen in HC at follow-up. There was no improvement in attention test performance after lamotrigine treatment within the patient group, and they remained impaired after treatment relative to HC. Improvement in cognitive functions had no significant relation to improvement in clinical ratings of manic symptoms. These results demonstrate independent domain-specific neurocognitive effects of lamotrigine in patients with PBD. Since improvement was observed even relative to change in HC subjects who were matched and retested over a similar time interval, and assuming that practice effects follow the same pattern in both groups, the improvement in PBD neurocognitive scores appears to be due to treatment effects rather than practice effects with the neuropsychological tests.
There are no comparable studies that examined neurocognitive effects of lamotrigine monotherapy in PBD in direct comparison to HC individuals. Enhanced working memory in our PBD patient group is similar to that observed in adult patients with bipolar disorder that received lamotrigine (12). Further, verbal learning and memory that involves learning and recalling new verbal information showed a similar effect trend as working memory in showing improvement to the extent that patients no longer had significant deficits relative to HC.
It may be that lamotrigine has an advantage in either enhancing or not impairing cognitive function over other mood stabilizers by the virtue of its glutamatergic attenuation function (26, 27) and inhibition of voltage-gated sodium (28) and calcium channels (27). In line with our findings, in an adult study of euthymic bipolar patients performing an ‘N-back’ working memory task, greater activation in the left prefrontal cortex and bilateral pregenual anterior cingulate cortex was observed after six weeks of treatment with lamotrigine relative to baseline (29).
Executive function involves complex higher cortical functions such as cognitive flexibility and cognitive processing speed. Despite the improvement within PBD patients after lamotrigine treatment, functioning in this domain continued to lag behind that of HC. In fact, executive function and verbal learning are the two domains that showed no change in development over a three-year follow-up, relative to HC, in our neurocognitive follow-up study (30). These two domains are complex and include multiple dimensions of cognition such as encoding, attention, processing, and execution or recall that appear to be persistent regardless of illness state or medication in pediatric (5, 31-35) or adult bipolar disorder (36-38). It may be that the mood stabilization with lamotrigine is not adequate to treat these deficits. With regard to attention, it did not improve and remained impaired with lamotrigine treatment in this study, similar to previous findings from cross-sectional studies that showed no improvement on attaining mood stability (31, 33, 39). Additional cognitive-enhancing pharmacotherapeutic interventions or rehabilitation may be required to target these specific cognitive deficits. Even though we excluded the patients with comorbid ADHD in this study, these findings illustrate the persistence of inattention as a trait intrinsic to bipolar diathesis. It may be that the future studies, regardless of inclusion or exclusion of specific comorbid diagnoses, must also include a dimensional approach to measuring symptom profiles such as attention and anxiety to determine the impact of treatment on each of these symptoms and the cognitive function.
Our findings demonstrated that symptomatic improvement in manic and hypomanic symptoms over 14 weeks correlated with global neurocognitive improvement. Thus, it seems likely that some of the improvement in cognitive performance may be due to the reduction in affective symptoms that followed initiation of lamotrigine treatment. Our study, in fact, indicated that the cognitive dysfunction is unrelated to the actual improvement in manic or depressive symptoms. Further studies that can examine clinically stable patients switched to lamotrigine are needed to evaluate the degree to which lamotrigine treatment improves cognition independent of its benefit in reducing affective symptoms. In addition to the correlation of psychiatric improvement with cognitive improvement, baseline manic and hypomanic symptom severity also was positively correlated with improvement on overall neurocognitive improvement. These results, consistent with the idea that reducing manic symptom severity contributed to cognitive improvement after lamotrigine, are in contrast to those reported by Khan et al. (14), where the severity of depressive symptoms predicted the most cognitive improvement in adult patients with bipolar disorder. This difference may reflect the fact that bipolar patients included in the current study presented with manic, mixed, or hypomanic symptoms, while patients with depressive episodes were included in the Kahn et al. (14) study.
Conclusions
Lamotrigine treatment did not impair cognition in patients with PBD. Rather, there was an improvement with treatment within the patient group after lamotrigine treatment. Although there was significant improvement, overall cognitive function continued to lag behind HC after 14 weeks of lamotrigine therapy. These findings suggest that lamotrigine treatment may have the potential to reduce cognitive deficits that are a significant source of academic and other functional disabilities in PBD (4).
Acknowledgments
This research was funded by a NARSAD Young Investigator Award, The Marshall Reynolds Foundation, NIH-K23, GlaxoSmithKline, Mr. and Mrs. Walter and Susan Berger, and RR018638.
Footnotes
MNP receives research support, unrelated to this manuscript, from NIMH, NICHD, The Dana Foundation, The American Foundation for Suicide Prevention, NARSAD Independent Investigator Award, Abbott Pharmaceuticals (study medication), and Johnson & Johnson (study medication). JAS has received research support, unrelated to this work, from NIH, GlaxoSmithKline, Johnson & Johnson, and AstraZeneca. AMP, TM, and JAC have no financial relationships to disclose.
References
- 1.McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107–125. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 2.Findling RL, McNamara NK, Stansbrey R, et al. Combination lithium and divalproex sodium in pediatric bipolar symptom re-stabilization. J Am Acad Child Adolesc Psychiatry. 2006;45:142–148. doi: 10.1097/01.chi.0000189135.05060.8a. [DOI] [PubMed] [Google Scholar]
- 3.DelBello MP, Kowatch RA. Pharmacological interventions for bipolar youth: developmental considerations. Dev Psychopathol. 2006;18:1231–1246. doi: 10.1017/S0954579406060597. [DOI] [PubMed] [Google Scholar]
- 4.Pavuluri MN, O';Connor MM, Harral EM, Moss M, Sweeney JA. Impact of neurocognitive function on academic difficulties in pediatric bipolar disorder: a clinical translation. Biol Psychiatry. 2006;60:951–956. doi: 10.1016/j.biopsych.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 6.Meador KJ, Loring DW, Ray PG, et al. Differential cognitive and behavioral effects of carbamazepine and lamotrigine. Neurology. 2001;56:1177–1182. doi: 10.1212/wnl.56.9.1177. [DOI] [PubMed] [Google Scholar]
- 7.Smith ME, Gevins A, McEvoy LK, Meador KJ, Ray PG, Gilliam F. Distinct cognitive neurophysiologic profiles for lamotrigine and topiramate. Epilepsia. 2006;47:695–703. doi: 10.1111/j.1528-1167.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin R, Kuzniecky R, Ho S, et al. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52:321–327. doi: 10.1212/wnl.52.2.321. [DOI] [PubMed] [Google Scholar]
- 9.Aldenkamp AP, Arends J, Bootsma HP, et al. Randomized double-blind parallel-group study comparing cognitive effects of a low-dose lamotrigine with valproate and placebo in healthy volunteers. Epilepsia. 2002;43:19–26. doi: 10.1046/j.1528-1157.2002.29201.x. [DOI] [PubMed] [Google Scholar]
- 10.Sinclair K, Martin RC, Faught E, et al. Tolerability of lamotrigine and carbamazepine in healthy senior adults. Epilepsia. 2000;41(Suppl. 7):255. [Google Scholar]
- 11.Daban C, Martinez-Aran A, Torrent C, et al. Cognitive functioning in bipolar patients receiving lamotrigine: preliminary results. J Clin Psychopharmacol. 2006;26:178–181. doi: 10.1097/01.jcp.0000204332.64390.f3. [DOI] [PubMed] [Google Scholar]
- 12.Gualtieri CT, Johnson LG. Comparative neurocognitive effects of 5 psychotropic anticonvulsants and lithium. MedGenMed. 2006;8:46. [PMC free article] [PubMed] [Google Scholar]
- 13.Pressler RM, Binnie CD, Coleshill SG, Chorley GA, Robinson RO. Effect of lamotrigine on cognition in children with epilepsy. Neurology. 2006;66:1495–1499. doi: 10.1212/01.wnl.0000216273.94142.84. [DOI] [PubMed] [Google Scholar]
- 14.Khan A, Ginsberg LD, Asnis GM, et al. Effect of lamotrigine on cognitive complaints in patients with bipolar I disorder. J Clin Psychiatry. 2004;65:1483–1490. doi: 10.4088/jcp.v65n1107. [DOI] [PubMed] [Google Scholar]
- 15.Kaye NS, Graham J, Roberts J, Thompson T, Nanry K. Effect of open-label lamotrigine as monotherapy and adjunctive therapy on the self-assessed cognitive function scores of patients with bipolar I disorder. J Clin Psychopharmacol. 2007;27:387–391. doi: 10.1097/jcp.0b013e3180a76dd2. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 17.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 19.Reitan RM. Trail Making Test: Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1986. [Google Scholar]
- 20.Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 21.Sharma T, Bilder R. Standardisation And Cross-Validation Study of Cogtest, an Automated Neurocognitive Battery for Use in Clinical Trials. Newark, DE: Cogtest; 2002. [Google Scholar]
- 22.Weschler D. Weschler Adult Intelligence Scale-Revised; Manual. New York: The Psychological Corporation; 1987. [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale-3rd Edition (WMS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 24.Delis DC, Kramer JH, Kaplan E, Ober A. California Verbal Learning Test-Children's Version (CVLT-C) manual. San Antonio, TX: The Psychological Corporation; 1994. [Google Scholar]
- 25.Poznanski E, Grossman J, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Adolesc Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986;27:490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang SJ, Huang CC, Hsu KS, Tsai JJ, Gean PW. Presynaptic inhibition of excitatory neurotransmission by lamotrigine in the rat amygdalar neurons. Synapse. 1996;24:248–255. doi: 10.1002/(SICI)1098-2396(199611)24:3<248::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Lancaster B, Peakman T, Garthwaite J. Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type IIA Na+ channels and with native Na+ channels in rat hippocampal neurones. Pflugers Arch. 1995;430:437–446. doi: 10.1007/BF00373920. [DOI] [PubMed] [Google Scholar]
- 29.Haldane M, Jogia J, Cobb A, Kozuch E, Kumari V, Frangou S. Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with Lamotrigine monotherapy. Eur Neuropsychopharmacol. 2008;18:48–54. doi: 10.1016/j.euroneuro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Pavuluri M, West A, Hill K, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: Three-year follow-up shows cognitive development lagging behind healthy youth. J Am Acad Child Adolesc Psychiatry. 2009;48:299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle AE, Wilens TE, Kwon A, et al. Neuropsychological functioning in youth with bipolar disorder. Biol Psychiatry. 2005;58:540–548. doi: 10.1016/j.biopsych.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Henin A, Mick E, Biederman J, et al. Can bipolar disorder-specific neuropsychological impairments in children be identified? J Consult Clin Psychol. 2007;75:210–220. doi: 10.1037/0022-006X.75.2.210. [DOI] [PubMed] [Google Scholar]
- 33.Dickstein DP, Treland JE, Snow J, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 34.Dickstein DP, Nelson EE, McClure EB, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- 35.McClure EB, Treland JE, Snow J, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 36.Ferrier IN, Stanton BB, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry. 1999;175:246–251. doi: 10.1192/bjp.175.3.246. [DOI] [PubMed] [Google Scholar]
- 37.Ferrier IN, Thompson JM. Cognitive impairment in bipolar affective disorder: implications for the bipolar diathesis. Br J Psychiatry. 2002;180:293–295. doi: 10.1192/bjp.180.4.293. [DOI] [PubMed] [Google Scholar]
- 38.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychological impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 39.Pavuluri M, O';Connor MM, Harral E, Sweeney JA. Neurocognitive outcome of lamotrigine in pediatric bipolar disorder. Poster presented at the 45th Annual Meeting of the American College of Psychopharmacology; December 3-7, 2006; Hollywood, FL.. [Google Scholar]