Dear Editor:
Several oral medications have been shown to inhibit the intestinal absorption of levothyroxine sodium when taken simultaneously with thyroid hormone; these include bile acid-binding resins (1), iron salts (2), calcium carbonate (3), sucralfate (4), aluminum-containing antacids (5), raloxifene (6), chromium picolinate (7), and sevelamer hydrochloride (HCl) (7). In most of these cases, it is believed that the malabsorption of thyroxine is due to the binding of the hormone to the other medications, forming an insoluble or nonabsorbable complex (1,2,5,8,9). Based on their physicochemical properties or similarities to previously studied agents, we proposed that two other medications, colesevelam HCl and lanthanum carbonate, may also decrease thyroxine absorption when co-administered with levothyroxine.
Colesevelam HCl, the newest bile acid sequestrant, is a nonabsorbed polymer that binds to bile acids in the small intestine, thereby preventing their reabsorption. The other bile acid sequestrants, cholestyramine and colestipol, inhibit absorption of levothyroxine through the binding of thyroxine to the basic anion copolymer resins in the gastrointestinal tract. When compared to other bile acid sequestrants, colesevelam HCl has a unique chemical structure that results in a high affinity of binding sites for bile acids, with less affinity for other molecules. Although it has been proposed that the binding of colesevelam HCl to other drugs should be less problematic than that seen with the older bile acid sequestrants (10,11), it is probable that it too binds levothyroxine and decreases its absorption.
Lanthanum carbonate is a new phosphate binder used in the treatment of hyperphosphatemia in end-stage renal disease. Lanthanum carbonate dissociates in the acidic milieu of the upper gastrointestinal tract, resulting in the release of lanthanum ions which form insoluble complexes with dietary phosphate. Other phosphate binders used in end-stage renal disease patients, including calcium carbonate, aluminum-containing antacids, and sevelamer HCl, have already been shown to interfere with the absorption of levothyroxine (3,5,7). Lanthanum carbonate may interfere with the absorption of levothyroxine in the same manner. To evaluate these potential interactions with thyroid hormone, we conducted single-dose absorption studies of levothyroxine in normal volunteers, with and without simultaneous administration of colesevelam HCl or lanthanum carbonate. Six euthyroid, healthy, normal volunteers (four men, two women; ages 27–40 years) participated in this study. None of the subjects had a history of thyroid, renal, hepatic, intestinal, or cardiac disease. In addition, none was taking medications known to alter the absorption or metabolism of levothyroxine (e.g., calcium supplements, iron preparations, aluminum-containing antacids, sucralfate, phenytoin, carbamazepine, or oral contraceptives). Baseline serum thyroxine (T4) and thyrotropin (TSH) were normal in all subjects. The study was approved by the Institutional Review Board of Stony Brook University, and informed consent was obtained from all subjects prior to participation in the study. The subjects were paid for their participation.
We utilized a 6-hour pharmacokinetic study of levothyroxine absorption as described in several previous reports (3,4,6,7). Each subject was studied on three separate occasions; at least 3 weeks elapsed between study sessions. On each occasion the subjects fasted overnight. Following insertion of an indwelling catheter into an arm vein, blood samples were collected at 15 and 0 minutes before, and at 30, 60, 120, 240 and 360 minutes after the administration of the study medication. For the first study visit, each subject was given 1 mg (five 0.2-mg tablets) of levothyroxine sodium (Synthroid®, Abbott Laboratories, Abbott Park, IL) orally along with 240 mL of water. At each of the two subsequent study visits, the same protocol was followed, and one of the study drugs (colesevelam HCl [Welchol®, Daiichi Sankyo, Inc., Parsippany, NJ] 3.75 g or lanthanum carbonate [Fosrenol®, Shire US, Inc., Wayne, PA] 500 mg) was co-administered with the 1 mg dose of levothyroxine and 240 mL water. All subjects received colesevelam HCl on the second visit and lanthanum carbonate on the final visit. On each occasion, a light lunch was served after the 240-minute specimen was collected.
Blood samples were allowed to clot at room temperature, and serum was stored at −20°C until assayed. Total serum T4 and serum TSH were measured in the Stony Brook University Hospital clinical laboratory using Siemens Advia Centaur immunochemiluminescent assays (Siemens Healthcare Diagnostics, Deerfield, IL). All serum samples from each subject were run in a single assay for each hormone. Serum T4 was measured at each time point, while serum TSH was assayed in the specimens collected at −15, 0, +120, +240, and +360 minutes.
The hormone concentrations in the sera obtained at −15 and 0 minutes were averaged for a baseline measurement of T4 and TSH. Mean, standard deviation (SD), and standard error (SE) of serum T4 and TSH concentrations were calculated for all subjects at each time point. The response area under the T4 concentration curve (above baseline) was calculated using trapezoidal integration. Paired Student t tests were used to determine the significance of the differences observed in the T4 response areas; Bonferroni correction was applied, and a p value of <0.025 was required for statistical significance.
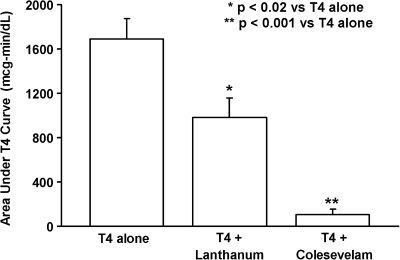
As expected, serum T4 levels increased in all subjects following the administration of 1 mg of levothyroxine alone. However, the rise in serum T4 was blunted by the co-administration of either colesevelam HCl or lanthanum carbonate (Fig. 1). The mean baseline-subtracted response area for levothyroxine given alone was 1692 ± 183.5 (SE) μg-min/dL, 107.5 ± 45.8 μg-min/dL for levothyroxine plus colesevelam HCl (3.8% of the area of levothyroxine alone) and 982.5 ±172.3 μg-min/dL for levothyroxine plus lanthanum carbonate (59.1% of the area of levothyroxine alone). The T4 area under the curve response to levothyroxine plus colesevelam HCl was significantly lower than the levothyroxine-alone response (p < 0.001), as was the T4 area under the curve response to levothyroxine plus lanthanum carbonate (p < 0.02) (Fig. 2).
FIG. 1.
Mean ± SE serum concentrations of thyroxine (T4) over a 6-hour period following administration of 1 mg of levothyroxine with and without study medications in six normal subjects.
FIG. 2.
Mean ± SE area (above baseline) under the serum T4 concentration curve in six normal subjects receiving levothyroxine alone or simultaneously with study medications. Co-administration of colesevelam HCl or lanthanum carbonate significantly decreased the absorption area compared to T4 given alone.
Serum TSH levels fell slightly during each 6-hour collection period, but returned to baseline by the time of the next testing (at least 3 weeks later). Mean (±SE) baseline serum TSH levels obtained on each test day were 2.28 ± 0.7 μU/mL for levothyroxine alone, 2.92 ± 0.9 μU/mL for levothyroxine plus colesevelam, and 2.42 ± 0.8 μU/mL for levothyroxine plus lanthanum carbonate. None of the subjects experienced symptoms of thyrotoxicosis during the study.
It is relatively well known that levothyroxine should be taken separately from food, since food adsorbs thyroid hormone thereby decreasing its availability for intestinal absorption (12,13). Moreover, several medications are known to interfere with the intestinal absorption of levothyroxine when taken simultaneously (1–9). We proposed that both colesevelam HCl and lanthanum carbonate would have a negative impact on the absorption of levothyroxine due to their pharmacological properties. This study demonstrates that both drugs result in a blunted rise in the serum T4 concentration when given concurrently with levothyroxine. This effect is more pronounced with colesevelam HCl but is significant for both drugs studied.
After the present study was initiated, the manufacturer of colesevelam HCl amended its drug-prescribing information to indicate that co-administration of 3.75 g of colesevelam with 0.6 mg of levothyroxine reduced the absorption of the levothyroxine by 22% (14). Our results, using the same dose of colesevelam HCl but a larger (1 mg) dose of levothyroxine, suggest that the reduction in levothyroxine absorption may be substantially larger—about 96% in the current study. The manufacturer's prescribing information further indicates that ingestion of colesevelam HCl either 1 or 4 hours after administration of levothyroxine does not alter the absorption of the thyroid hormone (14).
One of the potential limitations of the present study is that our subjects were euthyroid rather than hypothyroid. We do not believe the results would be substantially different in hypothyroid patients; they were not included in our study population and this possibility was not explored in the current investigation. Secondly, this study measured serum T4 for only 6 hours after drug ingestion. It seems unlikely that a longer period of observation would change the conclusions of this study; indeed, the levothyroxine absorption data reported in the manufacturer's prescribing information for colesevelam HCl measured total levothyroxine absorption, not just for the first 6 hours, and noted reduced absorption of levothyroxine when given simultaneously with the resin. Thirdly, the interval between study visits was relatively short. It is possible that a mild levothyroxine-induced hyperthyroidism persisted and influenced the results of subsequent visits; however, baseline serum TSH was similar on all three test days, making this unlikely. In addition, hyperthyroidism would be expected to enhance the absorption of thyroid hormone (12), and this is not consistent with the decrease in levothyroxine absorption seen with both colesevelam HCl and lanthanum carbonate. Finally, the possibility exists that the decreased absorption of levothyroxine seen with colesevelam HCl or lanthanum carbonate is due to a mechanism distinct from the interaction of other medications with levothyroxine, perhaps involving an effect of colesevelam HCl or lanthanum carbonate on the intestinal epithelium. If this is the case, administration of these drugs separately from levothyroxine may not be effective in improving thyroxine absorption. Rather, an increase in the dose of levothyroxine may be necessary. However, given the likelihood that these drugs decrease absorption of thyroxine due to their direct interaction with levothyroxine in the intestinal lumen, hypothyroid patients should be advised to take their thyroid hormone well separated in time from colesevelam HCl and lanthanum carbonate; the manufacturer's current prescribing information recommends an interval of 4 hours between ingestion of levothyroxine and colesevelam HCl (14). The manufacturer's prescribing information for lanthanum carbonate states that drugs known to interact with antacids (e.g., levothyroxine) should be taken at least 2 hours apart from the lanthanum carbonate (15).
We wish to thank the staff of the General Clinical Research Center at Stony Brook University Hospital for their assistance in conducting this study. This work was supported by funds from the General Clinical Research Center, Stony Brook University Hospital, NIH grant number MO1RR10710, and it was presented at the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, June 15–18, 2008.
Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Northcutt RC. Stiel JN. Hollifield JW. Stant EG., Jr The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208:1857–1861. [PubMed] [Google Scholar]
- 2.Campbell NRC. Hasinoff BB. Stalts H. Rao B. Wong NCW. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010–1013. doi: 10.7326/0003-4819-117-12-1010. [DOI] [PubMed] [Google Scholar]
- 3.Singh N. Weisler SL. Hershman JM. The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid. 2001;11:967–971. doi: 10.1089/105072501753211046. [DOI] [PubMed] [Google Scholar]
- 4.Sherman SI. Tielens ET. Ladenson PW. Sucralfate causes malabsorption of l-thyroxine. Am J Med. 1994;96:531–535. doi: 10.1016/0002-9343(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 5.Liel Y. Sperber AD. Shany S. Nonspecific intestinal adsorption of levothyroxine by aluminum hydroxide. Am J Med. 1994;97:363–365. doi: 10.1016/0002-9343(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 6.Siraj ES. Gupta MK. Reddy SSK. Raloxifene causing malabsorption of levothyroxine. Arch Intern Med. 2003;163:1367–1370. doi: 10.1001/archinte.163.11.1367. [DOI] [PubMed] [Google Scholar]
- 7.John-Kalarickal J. Pearlman G. Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763–765. doi: 10.1089/thy.2007.0060. [DOI] [PubMed] [Google Scholar]
- 8.Singh N. Singh PN. Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822–2825. doi: 10.1001/jama.283.21.2822. [DOI] [PubMed] [Google Scholar]
- 9.Havrankova J. Lahaie R. Levothyroxine binding by sucralfate. Ann Intern Med. 1992;117:445–446. doi: 10.7326/0003-4819-117-5-445_3. [DOI] [PubMed] [Google Scholar]
- 10.Steinmetz KL. Colesevelam hydrochloride. Am J Health Syst Pharm. 2002;59:932–939. doi: 10.1093/ajhp/59.10.932. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson TA. Armani A. McKenney JM. Guyton JR. Safety considerations with gastrointestinally active lipid-lowering drugs. Am Journal Cardiol. 2007;99:S47–S55. doi: 10.1016/j.amjcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Hays MT. Thyroid hormone and the gut. Endocr Res. 1988;14:203–224. doi: 10.3109/07435808809032986. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel KW. Kirschsieper HE. Aspects of the absorption of oral l-thyroxine in normal man. Metabolism: Clinical & Experimental. 1977;26:1–8. doi: 10.1016/0026-0495(77)90121-4. [DOI] [PubMed] [Google Scholar]
- 14.Daiichi Sankyo, Inc. Welchol® prescribing information. www.welchol.com/pdf/Welchol_PI.pdf. [Aug.2008 ]. www.welchol.com/pdf/Welchol_PI.pdf
- 15.Shire US, Inc. Fosrenol® prescribing information. www.fosrenol.com/PDFs/PrescribingInfo.pdf. [Nov.2008 ]. www.fosrenol.com/PDFs/PrescribingInfo.pdf