Abstract
Background
Selenium (Se) is an essential trace element needed for the biosynthesis of selenoproteins. Selenocysteine incorporation sequence binding protein 2 (SBP2) represents a key trans-acting factor for the co-translational insertion of selenocysteine into selenoproteins. We recently described children with mutations in the SBP2 gene who displayed abnormal thyroid function tests and reduced selenoprotein concentrations. We have tried to improve selenoprotein biosynthesis and thyroid hormone metabolism in SBP2 deficient subjects by supplementing an organic and an inorganic Se form.
Methods
Three affected and two unaffected siblings received daily doses of 100, 200, or 400 μg selenomethionine-rich yeast and 400 μg sodium selenite for one month each. Serum was drawn at baseline and after supplementations. Thyroid function tests, extracellular glutathione peroxidase activity, Se, and selenoprotein P concentrations were determined.
Results
Selenomethionine-rich yeast increased serum Se concentrations in all subjects irrespective of genotype. Sodium selenite was effective in increasing the selenoprotein P concentration in normal and to a lesser degree in affected subjects. Both forms failed to increase the glutathione peroxidase activity or to correct the thyroid function abnormalities in the SBP2 deficient individuals indicating that impaired deiodinase expression was not positively affected. No adverse side effects were observed.
Conclusions
Total serum Se concentrations in SBP2 deficient subjects respond to selenomethionine supplementation but this effect is not indicative for improved selenoprotein synthesis. Se is obviously not a limiting factor in the SBP2 deficient individuals when regular daily Se intake is provided. These findings might help to identify and diagnose more individuals with selenoprotein biosynthesis defects who might present at young age irrespective of their Se supply with characteristic thyroid function test abnormalities, growth retardation, and reduced Se and selenoprotein concentrations.
Introduction
Selenium (Se) is an essential trace element for humans. It is required for the biosynthesis of a small number of selenocysteine-containing proteins (1). A complex and specific machinery of cis- and trans-acting factors has evolved to re-code the UGA stop codon for selenocysteine insertion (2) producing in humans a unique class of 25 selenoprotein genes giving rise to 30–50 selenoproteins that include five isoenzymes of the glutathione peroxidase (GPx) family, all three iodothyronine deiodinases (DIO), and all three thioredoxin reductases (3). GPxs contribute to antioxidative defense whereas DIO isoenzymes control thyroid hormone metabolism. Additionally, there are a number of specialized selenoproteins implicated in Se storage, metabolism, and transport; methionine sulfoxide reduction; and endoplasmic reticulum function or with still yet to be defined biological roles (1). Some of these proteins have been proven essential for life as demonstrated by targeted genetic inactivation in mice (4). Others appear to be of less vital importance and become only poorly synthesized when the Se supply is limiting (5). The circulating extracellular GPx3 and the ubiquitous cytosolic GPx1 belong to these unprivileged selenoproteins (6). Their expression declines markedly when Se is limiting, and therefore they can be used to assess the Se status of individuals. The transport and storage protein selenoprotein P (SePP) accounts for the majority of circulating Se and appears to be a better biomarker of Se status in humans (7). SePP fulfils an essential role for regular Se metabolism (8) and becomes strongly deregulated in certain pathologies, such as severe illness (9).
Recently, four children from two unrelated families were found to carry mutations in the selenocysteine incorporation sequence binding protein 2 (SBP2) which is essential for normal selenoprotein biosynthesis (10). These children displayed abnormal thyroid hormone metabolism, delayed growth rates, and strongly decreased serum Se and selenoprotein concentrations. These findings are in agreement with the established importance of regular Se supply and selenoprotein expression for the thyroid and growth hormone axes (11). DIO2 and GPx enzymatic activities were reduced in cultured fibroblasts from the subjects. Data on the biosynthesis of house-keeping selenoproteins, which reside high in the selenoprotein hierarchy (e.g., GPx4 or the thioredoxin reductases), are missing but these enzymes are likely expressed since their absence has proven embryonically lethal in respective mouse models (4). Moreover, the less privileged selenoenzymes such as GPx1, GPx3, DIO2, or SePP were still expressed to some extent in the affected subjects, which indicates that the SBP2 mutations render the mutant proteins not completely inactive but rather constitute hypomorphic alleles. This notion has been strengthened by respective analyses of RNA-binding and trans-acting activities with recombinant SBP2 proteins in vitro (12).
Based on these considerations, we attempted to improve selenoprotein biosynthesis and thyroid hormone status in SBP2 deficient subjects by controlled daily supplementation of their regular diets with two different forms of Se.
Subjects and Methods
Five siblings, three affected (homozygous for SBP2 R540Q) and two phenotypically normal (heterozygous), participated in this study approved by the Institutional Review Board with signed parental agreement. They were affected subjects III-2, III-6, and III-7, ages 18, 11, and 9, respectively, and normal subjects II-3 and III-5, ages 17 and 14, respectively (see Dumitrescu et al. [10]).
Blood was drawn at baseline and after each incremental dose of Se supplementation, and serum samples were shipped and stored frozen until analysis. Three incremental doses of Se-rich yeast (100, 200, and 400 μg) were ingested daily for one month. After a wash-out period of 3 months, 400 μg sodium selenite was ingested daily for one month. Se-rich yeast tablets were purchased from CVS Corporation (Woonsocket, RI), and sodium selenite drinking ampoules were a gift from biosyn Arzneimittel GmbH (Fellbach, Germany).
Total thyroxine (T4), total triiodothyronine (T3), and thyrotropin (TSH) were measured by chemiluminescence using Elecsys 2010 technology (Roche-Boehringer Mannheim GmbH, Mannheim, Germany, and Hitachi Ltd., Indianapolis, IN). Reverse T3 (rT3) and thyroglobulin (Tg) were measured by radioimmunoassays (RIAs). Free T4 levels were estimated from the total T4 and the resin T4 uptake ratio.
Total serum Se concentrations were determined by the analytical method involving piazselenol formation and fluorimetric quantification as previously described (10,13). SePP concentrations were measured by the immunoluminometric sandwich assay established recently (9). GPx3 activity was determined by the coupled enzymatic assay with t-butyl-hydroperoxide as substrate (13).
Aliquots of serum (50 μL/sample) were analyzed by a multiplex analyzer system, Luminex 200 (Luminex Corp., Austin, TX) in combination with LINCOplex cytokine immunoassays for human interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β (Linco Research, St. Charles, MO).
Grouped data are expressed as mean ± SD. Effects of genotype, treatment type, and dose were assessed by analysis of variance, and the significance analyzed by Fisher's PLSD using StatView, version 5.0 (Abacus Concepts, Berkeley, CA) for the Macintosh computer (Apple Inc., Cupertino, CA).
Results
Baseline serum Se concentrations were lower in the three affected subjects (p < 0.0001). They increased significantly, reaching levels exceeding 100 μg/L and similar to the levels of the normal siblings while on 200 μg/d of Se-rich yeast (Fig. 1A). In both affected and normal subjects, serum Se was significantly higher than baseline when given 200 μg/d (p < 0.01) and 400 μg/d (p < 0.0001) Se-rich yeast. These results indicate an effective supplementation and compliance with the study design. The slight increases of serum Se concentrations observed during selenite administration in both affected and unaffected relatives were not statistically significant (Fig. 1A) and are in line with the impaired Se metabolism in the subjects and the sufficiently high Se status in the controls, respectively.
FIG. 1.
Effects of selenium (Se) supplementation in the form of Se-rich yeast (primarily selenomethionine) and selenite to sequence binding protein 2 (SBP2) deficient individuals and their normal siblings. (A) serum Se concentration; (B) glutathione peroxidase (GPx)3 enzymatic activity in serum; (C) serum selenoprotein P (SePP) concentration. Affected and normal individuals are identified in the legend and described in detail in the original publication (10).
Activities of circulating GPx3 in serum of affected subjects were on average less than 50% compared to their normal siblings at baseline (p < 0.0001) (Fig. 1B). The difference was not resolved by any of the two Se compounds or dosages used. This lack of effectiveness became even more obvious when the Se transport and storage protein SePP was analyzed. Affected subjects displayed on average only one third of circulating SePP concentrations compared to normal healthy Europeans (9) and concentrations were significantly lower (p < 0.001) at baseline and at all levels of Se-rich yeast and selenite supplementation (Fig. 1C). Selenite, but not Se-rich yeast, significantly increased the serum SePP levels in both affected (0.93 ± 0.23 vs. 1.33 ± 0.32; p < 0.05) and normal (3.50 ± 0.14 vs. 5.05 ± 0.35; p < 0.005) subjects. The effect was relatively small in the affected individuals. These data indicate that hepatic metabolism of Se is grossly impaired in the SBP2 deficient subjects and their low serum Se concentrations are secondary to reduced hepatic SePP and diminished renal GPx3 biosynthesis and secretion.
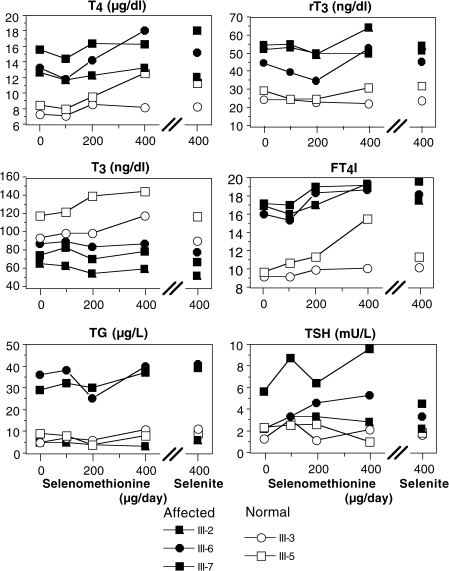
Parameters of thyroid function were determined in the same serum samples. They confirmed the previously reported (10) significant (p < 0.0001) increase in total T4, total rT3 and free thyroxine index (FT4I), and decrease in total T3. TSH and Tg concentrations were significantly (p < 0.01) higher in the affected individuals. These differences persisted with both Se supplementation forms and doses, without significant change from baseline values (Fig. 2).
FIG. 2.
Effect of Se supplementation on thyroid function tests of SBP2 deficient individuals and their normal siblings. Note that these treatments did not alter any of the measured parameters. Affected and normal individuals are identified in the legend and described in detail in the original publication (10).
Se deficiency impairs the immune system (14) and reduced expression of selenoprotein S has been linked to increased circulating levels of IL-6, IL-1β, and TNF-α (15). To test whether SBP2 deficient subjects have increased proinflammatory cytokine levels and whether the Se supplementations successfully corrected them, we analyzed serum samples for these biomarkers before and after supplementations. Concentrations of either cytokine were close to or below the detection limit of 3.2 pg/mL and did not differ between affected and unaffected siblings.
Discussion
All subjects, irrespective of genotype, responded to treatment by increase in total serum Se concentrations after Se-rich yeast supplementation. Selenomethionine is the main compound in Se-rich yeast which can be incorporated nonspecifically into all circulating serum proteins (16). Therefore, not only the normal siblings but also the SBP2 deficient children, metabolized this Se form successfully. This positive supplementation effect was in contrast to selenite which is metabolized and inserted as selenocysteine into the growing peptide chain of selenoproteins (2). Here, the affected subjects failed to show similarly strong supplementation success as the normal siblings, and displayed only slightly increased SePP concentrations without a positive effect on circulating GPx3 activity. We do not know whether biosynthesis of housekeeping selenoproteins was improved in parallel, but these enzymes did not seem to be limiting in the affected subjects, otherwise it would have produced a more severe phenotype.
Yet it is known from clinical studies that a high Se status confers reduced health risk and protects against some chronic diseases (17). Therefore, improving the Se status might still be of benefit to the affected individuals. The results show that Se-rich yeast might be the compound of choice, because it readily increased the Se concentrations in serum markedly, which might serve as an important Se reservoir during times of need, such as in severe illness. Nevertheless, the subjects were obviously not residing in a Se-deplete area since the unaffected siblings displayed normal circulating Se levels.
Thyroid function test abnormalities with elevated TSH, Tg, T4, and rT3 concentrations persisted in the SBP2 deficient individuals. As a matter of fact, with both Se preparations and at all doses, no significant changes from baseline were observed in subjects with both genotypes. These findings indicate that the pituitary thyroid feedback axis is functioning (e.g. inverse correlation of T3 and TSH), but that T3 formation by DIO1 and DIO2 selenoprotein activities is impaired in the affected siblings leading to high circulating T4 and rT3 concentrations. We conclude that the pituitary DIO activities remained reduced in the affected siblings explaining their high TSH despite the increased T4 serum concentrations.
Taken together, our study shows that the SBP2 deficient individuals do not profit from surplus Se supplementation as judged by those parameters that are readily measurable and directly related to the impairment. Our analysis also highlights that total serum Se concentrations do not always mirror circulating selenoproteins. The phenotype does not depend on the daily Se intake and therefore would likely have a similar presentation in the different geographical areas of the world. These findings might help to identify and diagnose more individuals with selenoprotein biosynthesis defects who might present at young age with growth retardation, characteristic thyroid function test abnormalities, and reduced Se and selenoprotein concentrations as described (10).
Acknowledgments
We thank the families for their participation in the study. We gratefully acknowledge the technical assistance of Silke Kappler, Jennifer Merz, and Katja Schreiber and the staff of the Clinical Endocrinology Laboratory at the University of Chicago. We thank our colleagues Ulrich Schweizer, Kostja Renko, and Eva Wirth for constructive discussions and technical help. This work was supported in part by grants (DK 15070 and RR 04999) from the National Institutes of Health (USA), and by the Deutsche Krebshilfe (10-1792-SchoII) and Deutsche Forschungsgemeinschaft (Scho849/2-1 and GraKo1208).
Disclosure Statement
No competing financial interests exist.
References
- 1.Papp LV. Lu J. Holmgren A. Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 2.Xu XM. Carlson BA. Mix H. Zhang Y. Saira K. Glass RS. Berry MJ. Gladyshev VN. Hatfield DL. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2007;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kryukov GV. Castellano S. Novoselov SV. Lobanov AV. Zehtab O. Guigo R. Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 4.Schweizer U. Schomburg L. New Insights into the Physiological Actions of Selenoproteins from Genetically Modified Mice. IUBMB Life. 2005;57:1–8. doi: 10.1080/15216540500364255. [DOI] [PubMed] [Google Scholar]
- 5.Bermano G. Nicol F. Dyer JA. Sunde RA. Beckett GJ. Arthur JR. Hesketh JE. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J. 1995;311(Pt 2):425–430. doi: 10.1042/bj3110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller C. Wingler K. Brigelius-Flohé R. 3′UTRs of glutathione peroxidases differentially affect selenium-dependent mRNA stability and selenocysteine incorporation efficiency. Biol Chem. 2003;384:11–18. doi: 10.1515/BC.2003.002. [DOI] [PubMed] [Google Scholar]
- 7.Xia Y. Hill KE. Byrne DW. Xu J. Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 8.Burk RF. Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–235. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 9.Hollenbach B. Morgenthaler NG. Struck J. Alonso C. Bergmann A. Kohrle J. Schomburg L. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J Trace Elem Med Biol. 2008;22:24–32. doi: 10.1016/j.jtemb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Dumitrescu AM. Liao XH. Abdullah MS. Lado-Abeal J. Majed FA. Moeller LC. Boran G. Schomburg L. Weiss RE. Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 11.Köhrle J. Jakob F. Contempre B. Dumont JE. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- 12.Bubenik JL. Driscoll DM. Altered RNA binding activity underlies abnormal thyroid hormone metabolism linked to a mutation in selenocysteine insertion sequence-binding protein 2. J Biol Chem. 2007;282:34653–34662. doi: 10.1074/jbc.M707059200. [DOI] [PubMed] [Google Scholar]
- 13.Schomburg L. Schweizer U. Holtmann B. Flohé L. Sendtner M. Köhrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J. 2003;370:397–402. doi: 10.1042/BJ20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthur JR. McKenzie RC. Beckett GJ. Selenium in the immune system. J Nutr. 2003;133:1457S–1459S. doi: 10.1093/jn/133.5.1457S. [DOI] [PubMed] [Google Scholar]
- 15.Curran JE. Jowett JB. Elliott KS. Gao Y. Gluschenko K. Wang J. Abel Azim DM. Cai G. Mahaney MC. Comuzzie AG. Dyer TD. Walder KR. Zimmet P. MacCluer JW. Collier GR. Kissebah AH. Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 16.Burk RF. Norsworthy BK. Hill KE. Motley AK. Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15:804–810. doi: 10.1158/1055-9965.EPI-05-0950. [DOI] [PubMed] [Google Scholar]
- 17.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]