Abstract
Background
(R /S)-Salsolinol (SAL), a condensation product of dopamine (DA) with acetaldehyde, has been speculated to have a role in the etiology of alcoholism. Earlier studies have shown the presence of SAL in biological fluids and postmortem brains from both alcoholics and non-alcoholics. However, the involvement of SAL in alcoholism has been controversial over several decades, since the reported SAL levels and their changes after ethanol exposure were not consistent, possibly due to inadequate analytical procedures and confounding factors such as diet and genetic predisposition. Using a newly developed mass spectrometric method to analyze SAL stereoisomers, we evaluated the contribution of ethanol, diet, and genetic background to SAL levels as well as its enantiomeric distribution.
Methods
Simultaneous measurement of SAL enantiomers and DA were achieved by high performance liquid chromatography-tandem mass spectrometry (HPLC / MS / MS). Plasma samples were collected from human subjects before and after banana (a food rich in SAL) intake, and during ethanol infusion. Rat plasma and brain samples were collected at various time points after the administration of SAL or banana by gavage. The brain parts including nucleus accumbens (NAC) and striatum (STR) were obtained from alcohol-non-preferring (NP) or alcohol-preferring (P) rats as well as P-rats which had a free access to ethanol (P-EtOH).
Results
Plasma SAL levels were increased significantly after banana intake in humans. Consistently, administration of banana to rats also resulted in a drastic increase of plasma SAL levels, whereas brain SAL levels remained unaltered. Acute ethanol infusion did not change SAL levels or R / S ratio in plasma from healthy humans. The levels of both SAL isomers and DA were significantly lower in the NAC of P rats in comparison to NP rats. The SAL levels in NAC of P rats remained unchanged after chronic free-choice ethanol drinking. There were decreasing trends of SAL in STR and DA in both brain regions. No changes in enantiomeric ratio were observed after acute or chronic ethanol exposure.
Conclusions
SAL from dietary sources is the major contributor to plasma SAL levels. No significant changes of SAL plasma levels or enantiomeric distribution after acute or chronic ethanol exposure suggest that SAL may not be a biomarker for ethanol drinking. Significantly lower SAL and DA levels observed in NAC of P rats may be associated with innate alcohol preference.
Keywords: Salsolinol, Diet, Ethanol, Dopamine, Alcohol-Preferring Rats, Alcohol-Non-Preferring Rats, Nucleus Accumbens, Striatum, HPLC / MS / MS
The involvement of morphine-like alkaloids in alcoholism has been extensively studied ever since the formation of dopamine-derived alkaloids, SAL (1-methyl-1,2,3,4-tetrahydro-6,7-dihydroxy-isoquinolines) and tetrahydropapaveroline (THP), was observed in vitro after rat brain homogenate was treated with ethanol or acetaldehyde (Davis and Walsh, 1970; Yamanaka et al., 1970). Subsequent identification of increased in vivo production of SAL in parkinsonian patients on L-DOPA treatment after ethanol ingestion (Sandler et al., 1973) further prompted studies of SAL as a potential biomarker for alcoholism. SAL is formed from dopamine (DA) either by nonenzymatic Pictet-Spengler condensation with acetaldehyde, a metabolite of ethanol, yielding racemic (R/S)-SAL, or with pyruvic acid followed by enzymatic decarboxylation and reduction, producing (R)-SAL. It has been also reported that enantio-selective (R)-SAL can be synthesized from DA and acetaldehyde by (R)-SAL synthase in human brain (Naoi et al., 1996). (R/S)-SAL is present in urine, plasma, cerebrospinal fluid and postmortem brains of both alcoholics and nonalcoholics (Haber et al., 1996; Sjöquist et al., 1982). The SAL is also contained in alcoholic beverages and variety of foods such as cheese, banana, beef, and milk (Duncan et al., 1984; Riggin et al., 1976).
Attempts to correlate the SAL levels in biological fluids and brain tissues to ethanol intake or to alcohol addiction behavior have been reported even though the results are controversial. Acute ethanol ingestion by nonalcoholics showed unchanged, decreased, or increased SAL levels in human biological fluids (Adachi et al., 1986; Haber et al., 1996; Sjöquist et al., 1985). A role of SAL in alcohol addiction has been suggested, as SAL infusion into rat brain ventricles promoted alcohol consumption or self-administration (Duncan and Deitrich, 1980; Melchior and Myers, 1977; Rodd et al., 2003). The SAL concentrations in plasma and urine were shown to be elevated in chronic alcoholics in comparison to nonalcoholics (Collins et al., 1979; Faraj et al., 1989; Sjöquist et al., 1981), supporting SAL as a potential clinical marker for alcohol addiction. However, others reported that SAL levels in urine (Feest et al., 1991; Musshoff et al., 1997) or in the postmortem brains (Musshoff et al., 2005; Sjöquist et al., 1983) were not different between alcoholics and controls. These controversial results prompted further studies to evaluate possible stereoselective contributions of SAL to alcoholism. Advances in analytical methods for the enantiomeric determination of SAL isomers have proved changes in R/S ratio in the brain regions of alcohol-preferring (P) rats after ethanol consumption (Haber et al., 1999; Rojkovicova et al., 2008). However, enantiomeric distribution of SAL isomers was neither changed in human urine and plasma by acute alcohol ingestion (Haber et al., 1996) nor different in postmortem brains between alcoholics and controls (Musshoff et al., 2005). The variability in the reported levels of SAL in healthy subjects and conflicting results on the influence of ethanol on SAL levels and enantiomeric distribution might be partly due to analytical problems and experimental variables including dietary conditions and genetic factors. In this study, we examined the effect of SAL-containing foods, ethanol or genetic predisposition to high alcohol drinking on the levels of SAL enantiomers and DA in humans and rats using a newly developed method (Lee et al., 2007).
METHODS
Ethanol Infusion and Diet Study in Humans
Subjects were 21- to 45-year-old nonsmoking male social drinkers in good health, as determined by medical history, physical exam, ECG, and lab tests. Subjects provided informed consent for the protocol approved by the Combined Neuroscience Institutional Review Board of the NIH. Subjects arrived at the NIH Clinical Center at 8:00 am on the morning of testing, following an overnight fast, and after abstaining from alcohol for 48 hours, and were free of all medications for at least 2 weeks prior to the study. A breathalyzer test was performed to ensure a zero breath alcohol concentration, and a negative urine drug screen result was obtained before proceeding. Two indwelling intravenous catheters were inserted, one each into the ante-cubital vein of the arm using sterile technique. One catheter was used for the ethanol infusion and the other was used for blood sampling. The first group of subjects (n = 5) received 45-minute infusions of 6% v/v ethanol and saline in separate sessions at 1-week interval. The second group of subjects (n = 6) received a 45-minute infusion of 6% v/v ethanol 1 hour following a standard banana-containing breakfast. In the first group, blood samples were collected at baseline (just prior to the start of the infusion), and at 15 and 45 minutes after the start of the infusion. In the second group, a baseline blood sample (time = −60 minutes) was collected, following which participants received a standard breakfast (~300 cal) consisting of cereal, skim milk, a banana, and orange juice. At 60 minutes after the breakfast (time = 0 minutes), another blood sample was collected, following which the ethanol infusion was started. Blood samples were obtained at 15, 30, and 45 minutes after the start of the infusion. Blood samples were collected into EDTA-containing tubes and centrifuged at 1000 × g at 4°C for 10 minutes, and the plasma was obtained for SAL and DA analyses. In both groups, the ethanol infusion was administered according to an infusion-rate profile that is based on a physiologically based pharmacokinetic model for alcohol (Ramchandani and O’Connor, 2006; Ramchandani et al., 1999). The profile consisted of an exponentially increasing infusion rate from the start of the infusion until the target BrAC of 80 mg% is reached at 15 minutes, followed by an exponentially decreasing infusion rate which tapered to a constant steady-state value, to maintain the BrAC at the target value for a predetermined interval of 30 minutes. This infusion-rate profile was computed using individualized estimates of the model parameters, based on the participant’s height, weight, age, and gender. This method has been used successfully in several studies of the pharmacokinetics and pharmacological effects of alcohol in humans. Serial breathalyzer measurements were obtained, using the Alcotest 7410 handheld breathalyzer (Drager Safety Diagnostics Inc., Irving, TX), to ensure that the BrACs were within 5 mg% of the target, and to enable minor adjustments to the infusion rates to overcome errors in parameter estimation and experimental variability.
Animal Study
Male Sprague–Dawley rats were administrated by gavage with 1 ml SAL solution (10 μg/ml; n = 3 for each time point) or 3 g banana (n = 2 for each time point) homogenized in saline (~1 g/ml). Rats were sacrificed by decapitation and blood and brain samples were collected. To study the effect of ethanol on SAL levels, adult male alcohol-preferring (P) rats and alcohol-non-preferring (NP) rats were used. Rats were double-housed upon arrival maintained on a 12-hour light–dark cycle for a week (with lights on at 07:00 hours). Afterwards, rats were single-housed in hanging banks and the P-EtOH rats (n = 7) were assigned to the drinking group and received free concurrent access to 10 and 30% EtOH (v /v) in addition to tap water for 8 weeks. The P-naïve (n = 7) rat group and all NP rats (n = 7) received water as their only liquid. All rats had rat chow ad libitum. Animals were habituated to handling and to the guillotine daily between 09:00 and 10:00 hours for 3 days prior to sacrifice. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the Institutional Animal Care and Use Committee and are in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, NIH, and the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996. Animals were sacrificed by decapitation between 17:00 and 18:00 hours. The head was immediately placed in liquid nitrogen for 3 seconds then the brain was quickly removed and placed on a glass plate for dissection on ice. The NAC and STR were dissected. Trunk blood was collected from each rat, centrifuged to collect serum. Tissue samples were placed into microcentrifuge tubes and weighed and immediately frozen on dry ice and stored at −80°C prior to extraction for analysis.
Quantitation of SAL / DA
Enantiomeric (R/S)-SAL and DA were determined by a highly specific and reliable quantitative method using HPLC/electrospray ionization-tandem mass spectrometry (ESI-MS/MS) according to the assay as described previously with a minor modification (Lee et al., 2007). In brief, plasma spiked with (S)-SAL-d4, (R)-SAL-d4, and DA-d4 as internal standards were acidified with 1 M HClO4 containing 0.01% EGTA, 0.02% semicarbazide, and 0.02% sodium metabisulfite. Then, the sample was centrifuged at 2000 × g for 15 minutes at 4°C. The supernatant was heated for 30 minutes at 80°C to hydrolyze any conjugated SAL. Brain tissue was homogenized with PBS buffer, transferred into centrifuge tubes containing phenylhydrazine hydrochloride (1 M, 20 μl) to ensure trapping any residual acetaldehyde, and acidified with 1 M HClO4 solution. The brain homogenates were centrifuged at 2000 × g for 20 minutes at 4°C. The supernatant was not subjected to acid hydrolysis since conjugated SAL and DA levels in brain tissue were found to be negligible. The sample buffered at pH 8.5 was loaded on the conditioned PBA cartridge. After the cartridge was conditioned with water and methanol, SAL was eluted with 0.1 M HCl:MeOH (1:1). The elution aliquot was adjusted to pH 8.2, and 10% PFBBr and 10% DIPEA in acetonitrile were added. Then, the sample solution was kept 70°C for 2.5 hours. The derivatized SAL /DA was extracted with hexane and then washed with water. The hexane layer was removed, evaporated to dryness, and re-dissolved in methanol for the analysis. Derivatized samples were analyzed by a Finnigan TSQ Quantum mass spectrometer in a positive ion electrospray ionization mode. The chiral separation was achieved using an Agilent 1100 HPLC with a Chiralpak AD-H (2.1 × 150 mm) column. The mobile phase consisted of methanol (A) and isopropanol (B), and the separation was achieved using an isocratic A:B (3:2) with flow rate of 0.12 ml/min or a gradient from A:B (8:2) to A:B (3:7) in 5 minutes, and then held for 15 minutes with flow rate of 0.12 ml/min. Quantitation was performed by multiple reaction monitoring (MRM) as follows: m/z 720 → 210 for SAL, m/z 724 → 210 for SAL-d4, m / z 874 → 497 for DA, and m/z 878 → 501 for DA-d4.
Statistical Analysis
All data were expressed as mean ± SD. Statistical analysis was performed using Student’s t-tests (unpaired two-tailed). *p < 0.05; **p <0.01.
RESULTS
Sensitive and Reliable Quantitation of SAL Enantiomers and DA in Biological Samples
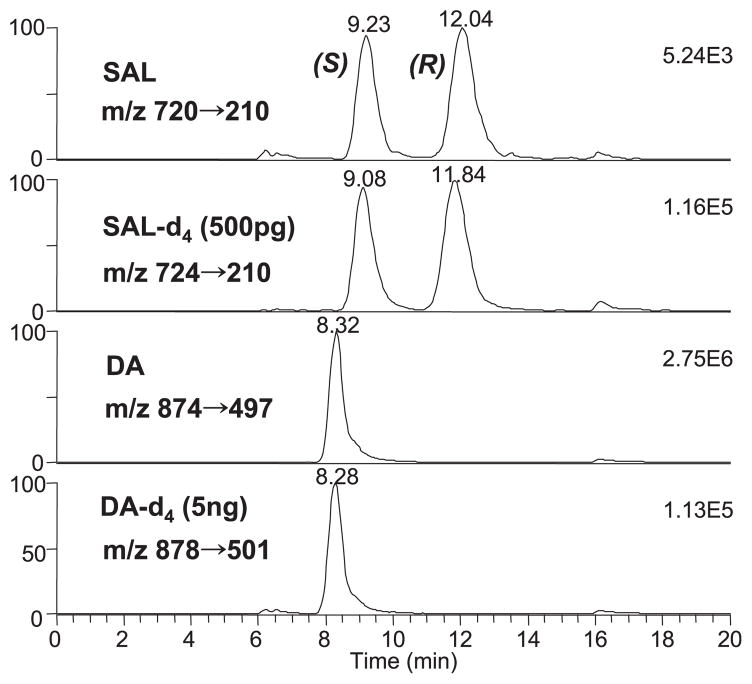
The chemical derivatization coupled chiral phase HPLC/ MS/MS method described earlier (Lee et al., 2007) allowed reliable and sensitive quantitation of both SAL enantiomers and DA in biological fluids. The excellent reproducibility and accuracy as evidenced by less than 10% for both coefficient of variance and error were achieved using inherently selective MRM detection and deuterium labeled internal standards. The limits of quantitation were further improved to be below 2 and 20 pg for each SAL isomer and DA, respectively, allowing us to analyze brain tissues reliably in the 5 to 10 mg range. Representative ESI-MS/MS ion chromatograms (Fig. 1) clearly demonstrate that the assay allows reliable determination of SAL isomers and DA in biological samples.
Fig. 1.
Representative MRM chromatogram of SAL enantiomers and DA found in rat brain NAC (17 mg) spiked with (S)-, (R)-SAL-d4, and DA-d4 as internal standards. (S)- and (R)-SAL were found to be 0.9 and 1.0 pg / mg, respectively, and DA level was 4.5 ng / mg. The separation was achieved using a mobile phase changing from MeOH:IPA 8:2 to 3:7 in 5 minutes with the flow rate of 0.12 ml / min.
Effect of Ethanol Infusion and Intake of Biogenic Amine Containing Food on Plasma SAL and DA Levels in Humans
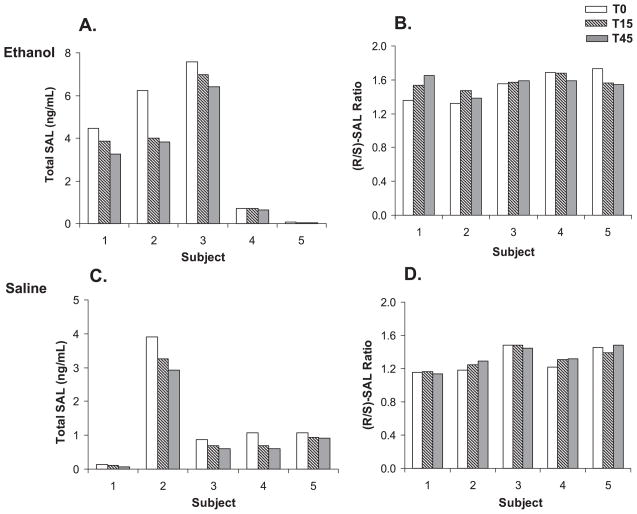
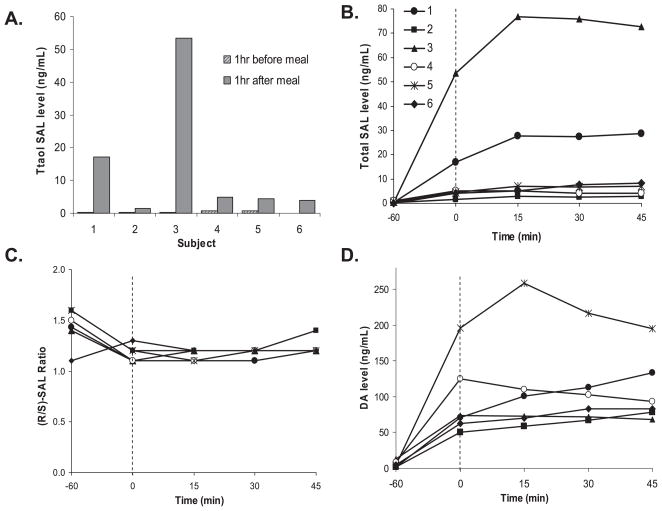
As shown in Fig. 2A, ethanol infusion appeared to time-dependently decrease plasma SAL levels in all five subjects in the first group. For comparison, plasma SAL and DA levels were examined during saline infusion administered to the same subjects to evaluate if this decline was ethanol-induced. Saline infusion also showed similar time-dependent decrease in SAL levels (Fig. 2C), suggesting that ethanol may not be the cause for SAL decrease observed during ethanol infusion. No significant change in enantiomeric composition (R/S ratio) was observed in plasma samples obtained during ethanol or saline infusion (Fig. 2B,D). Plasma DA levels showed the similar trend and mean levels at T0, T15, and T45 during saline infusion were 10.6 ± 5.0, 9.7 ± 4.0, and 9.0 ± 4.1 ng/ml, respectively. Basal SAL levels in the plasma of individual subjects were found to be widely different. Moreover, high intra-individual variations were observed in basal SAL levels determined at 1 week interval. To test the effect of consuming SAL-rich foods on plasma SAL levels, plasma concentrations of SAL and DA were determined in the second group of healthy subjects (n = 6) before and after consuming banana which is known to contain considerable amounts of biogenic amines. The total free and conjugated SAL and DA levels in banana at different ripening stages were found to be in the range of 7 to 1905 ng/g for (R)-SAL, 6 to 1878 ng/g for (S)-SAL, and 19 to 112 μg/g for DA, which is similar to those reported by Riggin and colleagues (1976). The degrees of conjugation of DA and SAL were about 60% and 8%, respectively. Ingestion of banana caused substantial increases in plasma SAL and DA levels, as shown in Fig. 3. A meal including biogenic amines resulted in greatly increased levels of SAL from 0.16 ± 0.12 to 5.8 ± 7.6 ng/ml (range: 0.8 to 28 ng/ml) for (S)-SAL and from 0.23 ± 0.16 to 6.6 ± 8.7 ng/ml (range: 0.7 to 25 ng/ml) for (R)-isomer (Fig. 3A). Within 1 to 1.5 hours after banana intake, both SAL and DA appeared to reach the highest concentration in plasma of most subjects, which appeared to be sustained or show a small duration for the remainder of the ethanol infusion period (Fig. 3B). This suggests that the ethanol infusion did not appear to significantly alter the plasma SAL level and its enantiomeric distribution. The mean R/S ratios changed from 1.4 ± 0.2 (T = −60 minutes) to 1.2 ± 0.1 (T = 0 minutes) indicating the contribution from racemic SAL in banana (Fig. 3C). DA levels also increased substantially from 5.8 ± 3.8 to 92.5 ± 44.5 ng/ml after the same meal (Fig. 3D). It should be noted that the plasma from 2 subjects showed peak SAL concentrations more than 5- (subject 1) and 13-fold (subject 3) higher than the mean SAL level observed in the remaining participants. In addition, DA levels showed substantial increases in 2 different subjects 4 and 5, but their SAL levels were low, suggesting individual differences in adsorption or metabolism of DA or SAL.
Fig. 2.
Effect of ethanol infusion on plasma SAL levels and enantiomeric distribution in nonalcoholic human subjects. Total SAL level (A) and R / S ratio (B) in plasma collected from volunteers during ethanol infusion (T = 0, 15, 45 minutes) are shown. Also shown are total SAL level (C) and R / S ratios (D) in plasma during saline infusion in the same subjects that participated in the ethanol infusion.
Fig. 3.
Influence of banana intake and ethanol infusion on plasma SAL and DA in nonalcohol human subjects. Total SAL levels before and after banana intake (A) are shown with time profiles of total SAL levels (B), R / S ratios (C), and DA levels (D). Plasma samples were collected from healthy subjects at various time points; T = −60 (1 hour before banana intake), T = 0 (beginning of ethanol infusion marked with a dashed line; 1 hour after banana intake), and T = 15, 30, 45 minutes (duration of ethanol infusion).
Influence of SAL or Banana Intake on Plasma and Brain SAL Levels in Rats
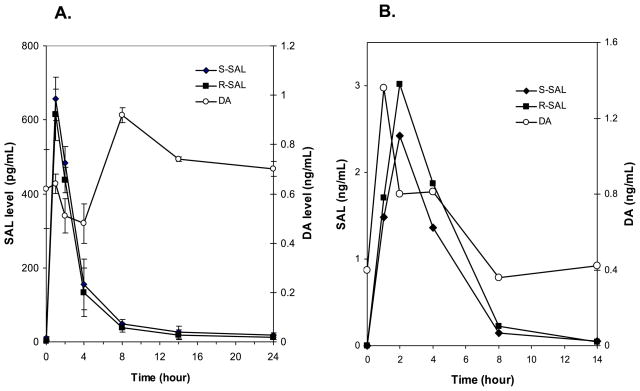
To examine the time course of SAL or DA clearance, rats were given SAL or banana in solution by gavage. A single administration of 10 μg SAL in saline resulted in a significant elevation of rat plasma SAL levels and sharply declined to near basal levels by 14 hours, as shown in Fig. 4A. The mean plasma concentrations of (S)- and (R)-SAL at 1 hour after SAL administration were 650 ± 46 and 614 ± 42 pg/ml, respectively. The mean basal (S)- and (R)-SAL levels were 11 ± 4 and 10 ± 1 pg/ml, respectively. The total (R/S)-SAL and DA levels in chow were 6 ng/g (R/S ratio ~1.0) and 0.27 μg/g, respectively. As expected, the DA concentration was not altered (basal: 0.6 ± 0.1; (24 hours: 0.7 ± 0.1 ng/ml). A single dose of 3 g banana (corresponding to 75 μg SAL) also increased the plasma SAL concentration markedly. In comparison to SAL ingestion, banana ingestion resulted in a slower increase of SAL (peak concentrations occurred at 2 hours) but showed the similar time course of clearance, reaching near basal level at 14 hours (Fig. 4B). The DA levels also followed the similar time course after feeding banana. Despite the increases seen in plasma SAL or DA levels after banana ingestion, those levels in the brain were not changed either in NAC or STR at any time points examined (Table 1).
Fig. 4.
Time course of rat plasma SAL and DA levels following the i.g. administration of 10 μg SAL (A) and 3 g banana (B).
Table 1.
Time Course of Total (R / S)-SAL and DA Levels (mean ± SD) in Rat Brain STR and NAC After Administration of 3 g Banana
| Time (hours) |
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 14 | |
| STR | |||||
| (R / S)-SAL (pg / mg) | 2.0 ± 0.2 | 2.7 ± 0.4 | 2.1 ± 0.5 | 2.8 ± 0.0 | 1.9 ± 0.6 |
| DA (ng / mg) | 10.8 ± 0.5 | 12.7 ± 0.2 | 12.1 ± 0.7 | 11.3 ± 0.8 | 12.1 ± 0.1 |
| NAC | |||||
| (R / S)-SAL (pg / mg) | 1.0 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.5 | 1.5 ± 0.1 | 1.0 ± 0.1 |
| DA (ng / mg) | 4.2 ± 1.9 | 5.0 ± 0.5 | 4.8 ± 2.0 | 6.6 ± 1.7 | 6.1 ± 0.9 |
Brain SAL and DA Levels in Alcohol-Preferring Rats and the Effect of Ethanol Consumption
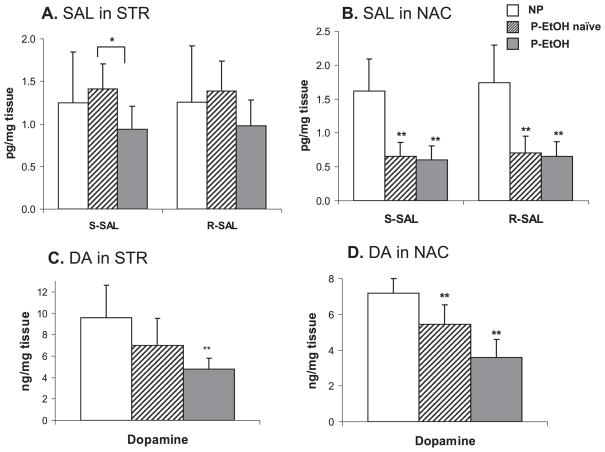
To examine whether SAL is involved in ethanol drinking behavior, we analyzed SAL and DA levels in two brain regions and plasma of alcohol-preferring (P) rats with (P-EtOH) or without (P-naïve) free access to ethanol in comparison to alcohol-non-preferring (NP) rats. As shown in Fig. 5, the basal SAL levels were markedly lower in the NAC of P than NP rats (NP: 1.6 ± 0.5; P: 0.7 ± 0.5 pg/mg for each isomer; p < 0.005). The mean DA levels in both regions were approximately 25% lower in P rats although statistical significance was reached only for the NAC (NP: 7.2 ± 2.5; P: 5.4 ± 1.1 ng/mg; p < 0.008). In the STR, no differences in SAL levels were observed between P-naïve and NP rats (NP: 1.3 ± 0.6; vs. P: 1.4 ± 0.3 pg/mg for each isomer). The SAL levels in the NAC of P rats were not changed after 8 weeks free-choice alcohol drinking (P-EtOH) [0.6 ± 0.2 and 0.7 ± 0.2 pg/mg for (S)- and (R)-SAL, respectively]. Ethanol consumption decreased SAL levels in the STR region of P rats although only the S form showed statistically significant difference (P-naïve: 1.4 ± 0.3; P-EtOH: 0.9 ± 0.3 pg/mg for S isomer; p < 0.02). The stereoisomer distribution of SAL in these brain regions was not different in both NP and P rats (Table 2). Chronic ethanol drinking did not result in changes of enantiomeric distribution, either. No statistical differences in plasma SAL and DA concentrations were found among the NP, P-naïve, and P-EtOH groups. The plasma levels of (R/S)-SAL and DA were not different between NP and P groups as well as P-naïve and P-EtOH rats (Table 3).
Fig. 5.
The levels of SAL and DA in the STR (A, C) and NAC (B, D) of NP, P-naïve, and P-EtOH rats. Values represent mean ± SD (n = 7 animals / group). Significantly lower SAL level was observed in NAC of P-rats in comparison to NP-rats. DA levels were also lower in both brain regions of P-rats. Free-choice ethanol drinking in P-rats did not increase SAL levels. Statistical significance was tested against NP rats. *p < 0.05; **p < 0.01.
Table 2.
Enantiomeric Distribution of SAL (R / S Ratio) in NAC and STR of NP, P-Naïve, and P-EtOH Rats
| (R / S)-SAL ratio |
|||
|---|---|---|---|
| NP | P-naïve | P-EtOH | |
| SIR | 1.01 ± 0.07 | 0.98 ± 0.09 | 1.02 ± 0.08 |
| NAC | 1.06 ± 0.06 | 1.05 ± 0.07 | 1.08 ± 0.07 |
Table 3.
Plasma SAL and DA Levels (Mean ± SD) of NP, P-Naïve, and P-EtOH Rats
| NP | P-naïve | P-EtOH | |
|---|---|---|---|
| (S)-SAL (pg / ml) | 8 ± 5 | 8 ± 2 | 11 ± 6 |
| (R)-SAL (pg / ml) | 8 ± 5 | 6 ± 2 | 9 ± 6 |
| DA (ng / ml) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
DISCUSSION
Despite the extensive studies, the influence of acute or chronic ethanol on SAL levels in humans as well as in experimental animals has been controversial (Adachi et al., 1986; Collins et al., 1979, 1990; Faraj et al., 1989; Feest et al., 1991; Musshoff et al., 1997, 2005; Rommelspacher et al., 1995; Sjöquist et al., 1982, 1985). The present study demonstrated that ethanol infusion did not increase plasma SAL levels in healthy subjects (Fig. 2A,B), although the small number of subjects used in this study may require careful interpretation. It is possible that the duration of ethanol infusion used in this study may not have been long enough to produce the changes in SAL concentrations in the circulating system. Nevertheless, our results are consistent with the previous findings that acute ethanol exposure did not influence SAL levels in humans (Haber et al., 1996; Sjöquist et al., 1985) or in rats (Baum et al., 1999; Myers et al., 1985b). The slight decreases in plasma SAL levels observed during ethanol infusion does not appear to be associated with ethanol since a similar trend was observed also with saline infusion (Fig. 2C,D). The intra-individual variation along with time-dependent decreases in plasma SAL levels observed with both saline and ethanol infusion in healthy subjects suggested significant influence of SAL-containing food intake on plasma SAL levels, although the contribution of endogenous SAL formation from ethanol-derived acetaldehyde may not be excluded. Indeed, intake of banana caused significant increases of not only DA but also SAL levels in both human and rat plasma (Figs. 3 and 4). It has been reported that plasma DA sulfate is dramatically increased after ingestion of biogenic amine containing foods (Davidson et al., 1981; Eisenhofer et al., 1999), even though negligible influence of diet on urinary SAL concentrations has been reported (Benedetti et al., 1989). Unlike human subjects, rats receiving the same chow diet did not show individual variations of SAL or DA levels, further supporting dietary influence. The enantiomeric distribution of the R/S SAL ratio was lowered with banana intake but not altered after ethanol infusion (Fig. 3C). High inter-individual variations of SAL and DA levels observed in human plasma collected after banana intake may reflect individual differences in SAL formation, absorption, clearance rates or metabolism of dietary SAL. Amounts of foods rich in biogenic amines consumed as the dietary habits of the volunteers might have also contributed to some of high inter-individual variations of basal SAL and DA levels observed in human plasma. To clarify the influence of diet on SAL levels, consumption of meals containing foods with low or high amounts of SAL should be systematically analyzed in relation to the plasma SAL levels using a larger number of subjects. In addition to diet, genetic background could have influenced SAL level as well, as was observed for the low SAL levels in the NAC of P rats (Fig. 5B).
Our data indicated no correlation between the plasma and brain SAL levels in rats (Fig. 4B, Table 1). It is well recognized that most catecholamines in plasma are not incorporated into the central nervous system (CNS) due to inability to cross the blood–brain barrier (BBB). The SAL detected in the brain is likely to be derived from in situ synthesis (Origitano et al., 1981), although sodium-independent organic cation transporter (OCT-2) has been recently recognized as a possible active transporter of SAL (Taubert et al., 2007). Our data indicated no changes in SAL concentrations in rat brains despite significant increases of plasma SAL levels after banana administration, supporting the idea that peripheral SAL may not significantly contribute to the brain SAL concentration.
The central dopaminergeric system is a well-recognized target for addiction behavior (Dar and Wooles, 1984; Heinz et al., 2005). We found lower basal DA levels in the STR or NAC of P-rats in comparison to NP-rats. The present study is in agreement with previous reports showing that the contents of DA were lower in the various brain regions of P rats as compared with the NP line (Bustmante et al., 2008; Murphy et al., 1983; Strother et al., 2005) supporting the notion that an innate deficiency of the mesolimbic DA system may be a contributing factor for the genetically predisposed alcohol preference in ethanol-preferring rats (McBride and Li, 1998; Murphy et al., 1987).
SAL, a DA metabolite, has been also shown to provoke alcohol drinking behavior (Duncan and Deitrich, 1980; Rodd et al., 2003, 2008). The present study indicated the lower basal levels of both SAL isomers in the NAC of P rats relative to that of NP rats as has been previously reported (Haber et al., 1999). Baum and colleagues (1999) also reported that (S)-SAL levels in the extracellular space in NAC were lower in alcohol-preferring strains than alcohol-avoiding rats.
Effects of ethanol on DA levels have been shown to vary depending on many parameters such as dose or duration of ethanol treatment or the brain regions examined (Dar and Wooles, 1984; Murphy et al., 1983; Myers et al., 1985a; Sjöquist et al., 1982). A decreasing trend of DA levels in both NAC and STR observed in our study is in agreement with previous findings. Previous studies indicated that alcohol self-administration stimulates DA release in the NAC (Weiss et al., 1993). Also, increased DA release in rat NAC and putamen by repeated alcohol exposure has been shown to enhance DA uptake rates (Budygin et al., 2007). In abstinent alcoholic patients, striatal DA deficit in conjunction with reduced DA2 / 3 receptor availability was found to correlate with alcohol craving (Heinz et al., 2005).
There are no coherent results concerning ethanol-induced change of SAL in rat brain. Chronic ethanol treatment has been reported to have no effect on SAL levels in rat hypothalamus or striatum (Collins et al., 1990; Haber et al., 1999; Myers et al., 1985b), whereas others reported that the SAL levels increased in the various rat brain regions (Haber et al., 1999; Matsubara et al., 1987; Myers et al., 1985a; Sjöquist et al., 1982; Starkey et al., 2006). Recently, Rojkovicova and colleagues (2008) also reported that SAL levels increased and R/S ratio decreased in both putamen and midbrain regions of P rats following alcohol consumption, suggesting an increase in the ethanol-induced nonenzymatic production of SAL. In contrast, we did not find that ethanol caused increased SAL levels in either STR or NAC regions of P rats. As shown in Table 2, the enantiomeric distribution of basal SAL was nearly racemic and was not different in both NP and P rats. Moreover, R/S ratio remained unaltered by ethanol exposure. This inconsistency of the influence of ethanol on brain SAL levels might be due to the differences in the brain regions studied. Nevertheless, our results are in line with the previous findings that brain acetaldehyde level was very low due to the presence of aldehyde dehydrogenase (ALDH) in capillary endothelium and brain cells despite the ethanol-dependent increase of circulating acetaldehyde concentration (Sippel, 1974; Westcott et al., 1980). Although acetaldehyde can be formed from ethanol in the brain, partly through a catalase-mediated reaction (Zimatkin and Buben, 2007), the amount and duration of alcohol consumption in our studies may not have increased acetaldehyde concentrations enough to increase SAL levels.
The plasma SAL levels were significantly affected after food intake in both rats and humans, indicating that dietary influences should be considered when interpreting changes in plasma SAL. The inconsistencies of the influence of ethanol on SAL levels in previous studies are likely due to inadequate control of diet. As the plasma SAL level returns to the near basal value after 14 hours, blood samples should be collected at least after overnight fasting or with a biogenic amine-restricted diet for 24 hours preceding studies. It is worthwhile to note that (R)-SAL was higher than (S)-isomer in human plasma, suggesting involvement of (R)-stereospecific enzymatic pathway in SAL formation in addition to nonenzymatic condensation. In contrast, rat plasma or brain showed racemic distribution of (R/S)-SAL, suggesting that SAL formation or metabolism may be different between rats and humans.
SAL has been discussed controversially as an alcoholism marker. For SAL to be a good biomarker for ethanol exposure or alcohol addiction, SAL should represent a specific product from ethanol consumption and provide a good correlation between its level and ethanol exposure. The presence of SAL in plasma samples of humans or animals does not necessarily provide evidence for in vivo condensation between DA and ethanol-derived acetaldehyde. It is clear that the SAL levels can be altered even in healthy subjects by the dietary intake of SAL. In addition, neither acute nor chronic ethanol exposure resulted in increases in SAL levels in plasma or brain tissues. These data may argue against the possibility of SAL as a biomarker for ethanol consumption, although the potential role of SAL in ethanol addiction remains interesting. Enantiomeric determination of SAL will be helpful in clarifying their postulated in vivo formations and metabolisms. As a genetic disturbance in SAL and/or DA biosynthesis could be a factor contributing to the development of alcohol addiction, further studies are required to clarify a possible role of SAL underlying alcoholism.
Acknowledgments
This work was supported by the intramural program of NIAAA and AA014437 and AA07611 (EAE and WJM).
References
- Adachi J, Mizoi Y, Fukunaga T, Kogame M, Ninomiya I, Naito T. Effect of acetaldehyde on urinary salsolinol in healthy man after ethanol intake. Alcohol. 1986;3:215–220. doi: 10.1016/0741-8329(86)90047-9. [DOI] [PubMed] [Google Scholar]
- Baum SS, Hill R, Kiianmaa K, Rommelspacher H. Effect of ethanol on (R)- and (S)-salsolinol, salsoline, and THP in the nucleus accumbens of AA and ANA rats. Alcohol. 1999;18:165–169. doi: 10.1016/s0741-8329(98)00080-9. [DOI] [PubMed] [Google Scholar]
- Benedetti MS, Dostert P, Carminati P. Influence of food intake on the enantiomeric composition of urinary salsolinol in man. J Neural Transm [GenSec] 1989;78:43–51. doi: 10.1007/BF01247112. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Läck AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology. 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Bustmante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y, Herrera-Marschitz M. Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. Euro J Pharm. 2008;591:153–158. doi: 10.1016/j.ejphar.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Nijm WP, Borge GF, Teas G, Goldfarb C. Dopamine-related tetrahydroisoquinolines: significant urinary excretion by alcoholics after alcohol consumption. Science. 1979;206:1184–1186. doi: 10.1126/science.505002. [DOI] [PubMed] [Google Scholar]
- Collins MA, Ung-Chhun N, Cheng BY, Pronger D. Brain and plasma tetrahydroisoquinolines in rats: effects of chronic ethanol intake and diet. J Neurochem. 1990;55:1507–1513. doi: 10.1111/j.1471-4159.1990.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Dar MS, Wooles WR. The effect of acute ethanol on dopamine metabolism and other neurotransmitters in the hypothalamus and the corpus striatum of mice. J Neural Transm. 1984;60:283–294. doi: 10.1007/BF01249100. [DOI] [PubMed] [Google Scholar]
- Davidson L, Vandongen R, Beilin LJ. Effect of eating bananas on plasma free and sulfate-conjugated catecholamines. Life Sci. 1981;29:1773–1778. doi: 10.1016/0024-3205(81)90187-9. [DOI] [PubMed] [Google Scholar]
- Davis VE, Walsh MJ. Alcohol, amines, and alkaloids: a possible biochemical basis for alcohol addiction. Science. 1970;167:1005–1007. doi: 10.1126/science.167.3920.1005. [DOI] [PubMed] [Google Scholar]
- Duncan C, Deitrich RA. A critical evaluation of tetrahydroisoquinoline induced ethanol preference in rats. Pharmacol Biochem Behav. 1980;13:265–281. doi: 10.1016/0091-3057(80)90083-0. [DOI] [PubMed] [Google Scholar]
- Duncan MW, Smythe GA, Nicholson MV. Comparison of high-performance liquid chromatography with electrochemical detection and gas chromatography-mass fragmentography for the assay of salsolinol, dopamine and dopamine metabolites in food and beverage samples. J Chromatogr. 1984;336:199–209. doi: 10.1016/s0378-4347(00)85142-7. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Coughtrie MWH, Goldstein DS. Dopamine sulfate: an enigma resolved. Clin Exp Pharmacol Physiol. 1999;26(Suppl):S41–S53. [PubMed] [Google Scholar]
- Faraj BA, Camp VM, Davis DC, Lenton JD, Kutner M. Elevation of plasma salsolinol sulfate in chronic alcoholics as compared to nonalcoholics. Alcohol Clin Exp Res. 1989;13:155–163. doi: 10.1111/j.1530-0277.1989.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Feest U, Kemper A, Nickel B, Rabe H, Koalick F. Comparison of salsolinol excretion in alcoholics and nonalcoholic controls. Alcohol. 1991;9:49–52. doi: 10.1016/0741-8329(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Haber H, Dumaual N, Bare DJ, Melzig MF, McBride WJ, Lumeng L, Li T-K. The quantitative determination of R- and S-salsolinol in the striatum and adrenal gland of rats selectively bred for disparate alcohol drinking. Addict Biol. 1999;4:181–189. doi: 10.1080/13556219971687. [DOI] [PubMed] [Google Scholar]
- Haber H, Winkler A, Putscher I, Henklein P, Baeger I, Georgi M, Melzig MF. Plasma and urine salsolinol in humans: effect of acute ethanol intake on the enantiomeric composition of salsolinol. Alcohol Clin Exp Res. 1996;20:87–91. doi: 10.1111/j.1530-0277.1996.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Gründer G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2 / 3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Lee J, Huang BX, Yuan Z, Kim HY. Simultaneous determination of salsolinol and dopamine in human plasma and cerebrospinal fluid by chemical derivatization coupled to chiral liquid chromatography / electrospray ionization-tandem mass spectrometry. Anal Chem. 2007;79:9166–9173. doi: 10.1021/ac0715827. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Fukushima S, Fukui Y. A systematic regional study of brain salsolinol levels during and immediately following chronic ethanol ingestion in rats. Brain Res. 1987;413:336–343. doi: 10.1016/0006-8993(87)91025-0. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Myers RD. Alcohol drinking induced in the rat after chronic injections of tetrahydropapaveroline (THP), salsolinol or noreleagnine in the brain. In: Thurman RG, Williamson JR, Chance B, Drott HR, editors. Alcohol and Acetaldehyde Metabolizing Systems. III. Academic Press; New York: 1977. pp. 545–554. [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Monoamine and metabolite levels in CNS regions of the P line of alcohol-preferring rats after acute and chronic ethanol treatment. Pharmacol Biochem Behav. 1983;19:849–856. doi: 10.1016/0091-3057(83)90092-8. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Contents of monoamines in forebrain regions of alcohol-preferring (P) and –nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 1987;26:389–392. doi: 10.1016/0091-3057(87)90134-1. [DOI] [PubMed] [Google Scholar]
- Musshoff F, Daldrup T, Bonte W, Leitner A, Lesch OM. Salsolinol and norsalsolinol in human urine samples. Pharmacol Biochem Behav. 1997;58:545–550. doi: 10.1016/s0091-3057(97)00251-7. [DOI] [PubMed] [Google Scholar]
- Musshoff F, Lachenmeier DW, Schmidt P, Dettmeyer R, Madea B. Systematic regional study of dopamine, norsalsolinol, and (R / S)-salsolinol levels in human brain areas of alcoholics. Alcohol Clin Exp Res. 2005;29:46–52. doi: 10.1097/01.alc.0000150011.81102.c2. [DOI] [PubMed] [Google Scholar]
- Myers WD, Mackenzie L, Ng KT, Singer G, Smythe GA, Duncan MW. Salsolinol and dopamine in rat medial basal hypothalamus after chronic ethanol exposure. Life Sci. 1985a;36:309–314. doi: 10.1016/0024-3205(85)90115-8. [DOI] [PubMed] [Google Scholar]
- Myers WD, Ng KT, Singer G, Smythe GA, Duncan MW. Dopamine and salsolinol levels in rat hypothalami and striatum after schedule-induced self-injection (SISI) of ethanol and acetaldehyde. Brain Res. 1985b;358:122–128. doi: 10.1016/0006-8993(85)90955-2. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Dostert P, Kohda K, Kaiya T. A novel enzyme enantio-selectively synthesizes (R)salsolinol, a precursor of a dopaminergic neurotoxin, N-methyl(R)salsolinol. Neurosci Lett. 1996;212:183–186. doi: 10.1016/0304-3940(96)12807-x. [DOI] [PubMed] [Google Scholar]
- Origitano T, Hannigan J, Collins MA. Rat brain salsolinol and blood-brain barrier. Brain Res. 1981;224:446–451. doi: 10.1016/0006-8993(81)90876-3. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li T-K, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S. Studying alcohol elimination using the alcohol clamp method. Alcohol Res Health. 2006;29:286–290. [PMC free article] [PubMed] [Google Scholar]
- Riggin RM, McCarthy MJ, Kissinger PT. Identification of salsolinol as a major dopamine metabolite in the banana. J Agric Food Chem. 1976;24:189–191. doi: 10.1021/jf60203a027. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Goldstein A, Zaffaroni A, McBride WJ, Li TK. Salsolinol produces reinforcing effects in the nucleus accumbens shell of alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2003;27:440–449. doi: 10.1097/01.ALC.0000056612.89957.B4. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Oster SM, Ding ZM, Toalston JE, Deehan G, Bell RL, Li TK, McBride WJ. The reinforcing properties of salsolinol in the ventral tegmental area: evidence for regional heterogeneity and the involvement of serotonin and dopamine. Alcohol Clin Exp Res. 2008;32:230–239. doi: 10.1111/j.1530-0277.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Rojkovicova T, Mechref Y, Starkey JA, Wu G, Bell RL, McBride WJ, Novotny MV. Quantitative chiral analysis of salsolinol in different brain regions of rats genetically predisposed to alcoholism. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;863:206–214. doi: 10.1016/j.jchromb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Baum SS, Dufeu P, Schmidt LG. Determination of (R)- and (S)-salsolinol sulfate and dopamine sulfate levels in plasma of nonalcoholics and alcoholics. Alcohol. 1995;12:309–315. doi: 10.1016/0741-8329(95)00004-b. [DOI] [PubMed] [Google Scholar]
- Sandler M, Carter SB, Hunter KR, Stern GM. Tetrahyroisoquinoline alkaloids: in vivo metabolites of L-dopa in man. Nature. 1973;241:439–443. doi: 10.1038/241439a0. [DOI] [PubMed] [Google Scholar]
- Sippel HW. The acetaldehyde content in rat brain during ethanol metabolism. J Neurochem. 1974;23:451–452. doi: 10.1111/j.1471-4159.1974.tb04380.x. [DOI] [PubMed] [Google Scholar]
- Sjöquist B, Borg S, Kvande H. Salsolinol and methylated salsolinol in urine and cerebrospinal fluid from healthy volunteers. Subst Alcohol Actions Misuse. 1981;2:73–77. [PubMed] [Google Scholar]
- Sjöquist B, Johnson HA, Borg S. The influence of acute ethanol on the catecholamine system in man as reflected in cerebrospinal fluid and urine. A new condensation product, 1-carboxysalsolinol. Drug Alcohol Depend. 1985;16:241–249. doi: 10.1016/0376-8716(85)90048-1. [DOI] [PubMed] [Google Scholar]
- Sjöquist B, Liljequist S, Engel J. Increased salsolinol levels in rat striatum and limbic forebrain following chronic ethanol treatment. J Neurochem. 1982;39:259–262. doi: 10.1111/j.1471-4159.1982.tb04730.x. [DOI] [PubMed] [Google Scholar]
- Sjöquist B, Perdahl E, Winblad B. The effect of alcoholism on salsolinol and biogenic amines in human brain. Drug Alcohol Depend. 1983;12:15–23. doi: 10.1016/0376-8716(83)90050-9. [DOI] [PubMed] [Google Scholar]
- Starkey JA, Mechref Y, Muzikar J, McBride WJ, Novotny MV. Determination of salsolinol and related catecholamines through on-line pre-concentration and liquid chromatography / atmospheric pressure photoionization mass spectrometry. Anal Chem. 2006;78:3342–3347. doi: 10.1021/ac051863j. [DOI] [PubMed] [Google Scholar]
- Strother WN, Lumeng L, Li TK, McBride WJ. Dopamine and serotonin content in select brain regions of weanling and adult alcohol drinking rat lines. Pharmacol Biochem Behav. 2005;80:229–237. doi: 10.1016/j.pbb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Taubert D, Grimberg G, Stenzel W, Schömig E. Identification of the endogenous key substrates of the human organic cation transporter OCT2 and their implication in function of dopaminergic neurons. PLoS One. 2007;4:1–11. doi: 10.1371/journal.pone.0000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Westcott JY, Weiner H, Shultz J, Myers RD. In vivo acetaldehyde in the brain of the rat treated with ethanol. Biochem Pharmacol. 1980;29:411–417. doi: 10.1016/0006-2952(80)90521-3. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Walsh MJ, Davis VE. Salsolinol, an alkaloid derivative of dopamine formed in vitro during alcohol metabolism. Nature. 1970;227:1143–1144. doi: 10.1038/2271143a0. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Buben AL. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007;42:529–532. doi: 10.1093/alcalc/agm059. [DOI] [PubMed] [Google Scholar]