Abstract
Background
There has been an increase in the utilization of single-fraction stereotactic body radiation therapy (SBRT) to treat thoracic structures, but there have been few reports describing toxicity outcomes with this treatment.
Methods
We evaluated 119 sites (114 patients) with no prior history of thoracic radiation were treated from 10/1/2003 to 10/27/2008 with single-fraction SBRT to thoracic structures. The median dose to the gross tumor volume was 2400 cGy (range 1800–2400 cGy), as was the median dose to the planning target volume (range 1600–2400 cGy). A detailed review of thoracic toxicities was performed to include pneumonitis or Grade 2 or higher esophageal and bronchial toxicity. In addition, we retrospectively contoured the esophagus and bronchus of 48 patients treated in 2004–2005, prior to the establishment of dose constraints to determine the range of doses that these structures received.
Results
Of the contoured patients, the median dose to the hottest 1 cc (D1cc) of the esophagus was 1250 cGy (range 158–2572 cGy). The median bronchial D1cc was 1101 cGy (range 260–2211 cGy). At a median follow-up of 11.6 months, there were seven Grade 2 or higher esophageal toxicities, including one Grade 3 and one Grade 4 toxicities. There were two bronchial toxicities, one Grade 2 and one Grade 3. There were no cases of pneumonitis.
Conclusions
High-dose single-fraction SBRT is well tolerated to the thoracic region, with most patients tolerating high doses to central structures without significant toxicity.
Keywords: Stereotactic body radiation therapy, Esophagus, Bronchus, Hypofractionation
Stereotactic body radiation therapy (SBRT) is being increasingly utilized for the treatment of malignancies in which precise localization is required to limit the dose to the surrounding normal structures. When used effectively, a high therapeutic ratio can be delivered without the need for standard fractionation. In the extreme case, a single fraction can be a sufficient treatment when delivering up to 2000–2400 cGy [1], thereby achieving a high biologically effective dose (BED) to the disease while sparing normal tissues.
Multiple studies have been published examining the efficacy of SBRT and have demonstrated promising results [2–6]. However, the toxicity when treating centrally located lung disease has not been fully established. In fact, prior reports with hypofractionated regimens have indicated that some caution should be exercised when treating central lesions [7].
The aim of this study was thus to examine the effect of single-fraction SBRT (SF-SBRT) to central thoracic structures by assessing the incidence of pneumonitis and Grade 2 or higher esophageal or bronchial toxicity. In addition, we retrospectively contoured the esophagus and bronchus in 48 patients treated from 2004 to 2005, when dose constraints had not yet been fully established at our institution, to determine the range that these structures received and to put the toxicity data in the context of dose. While we acknowledge that the findings for single-fraction treatment cannot be directly extrapolated to those of multiple fraction regimens for curative treatment, particularly with respect to DVH and BED comparisons, in terms of assessing thoracic structure tolerance to high fractions of radiation therapy, as has not yet been published to the high doses cited in our study, the conclusions that can be drawn are analogous.
Methods and materials
Patient and disease characteristics
This study was approved by the Institutional Review Board at Memorial Sloan-Kettering Cancer Center and consisted of a retrospective review of 119 paraspinal sites (114 patients) treated at our institution for metastasis between October 2003 and October 2008 with SF-SBRT and intensity-modulated radiation therapy. Table 1 depicts the patient and disease characteristics. To avoid confounding factors, the patients were excluded if they had a prior history of thoracic radiation therapy, or if they were receiving concurrent fractionated radiation to the thorax. The patients receiving concurrent radiation outside of the thorax, or concurrent single-fraction radiation to the thorax, were included. In the latter scenario, both sites were included for analysis.
Table 1.
Patient characteristics.
| Characteristics | Number of patients (%) |
|---|---|
| Age | |
| <45 | 39 (34) |
| 45–55 | 30 (26) |
| >55 | 45 (40) |
| Gender | |
| Male | 75 (66) |
| Female | 39 (34) |
| Primary site | |
| Renal | 20 (17) |
| Sarcoma | 16 (14) |
| Melanoma | 12 (11) |
| Lung | 11 (10) |
| Colorectal | 10 (9) |
| Prostate | 9 (8) |
| Breast | 7 (6) |
| Thyroid | 7 (6) |
| Biliary tree | 6 (5) |
| Other (all ≤3) | 16 (14) |
Treatment planning
All patients underwent CT simulation using a custom cradle specifically designed for extra-cranial IGRT treatment [8]. Approximately a 3–5 mm margin was placed from the gross tumor volume (GTV) to the clinical target volume (CTV), and an additional 1–3 mm from the CTV to the planning target volume (PTV), for a total margin of 4–8 mm from the GTV to the PTV unless otherwise limited by proximity to the spinal cord. IMRT treatment was designed using our in-house treatment planning system and an optimization algorithm developed by Spirou and Chui [9] and delivered using a sliding window technique [10]. The patients were treated using multi-field beam arrangements with a typical paraspinal treatment consisting of 5–9 posteriorly directed co-planar beams. This arrangement is depicted in Fig. 1, which demonstrates the isodose curves from a patient who experienced a significant toxicity (described in the Results section below). The median dose to the gross tumor volume (GTV) was 2400 cGy (range 1800–2400 cGy), and the median dose to the planning target volume (PTV) was 2400 cGy (range 1600–2400 cGy). Plans were normalized such that the spinal cord received a maximum point dose of 12–14 Gy and the prescription dose was delivered to the resulting 100% isodose surface.
Fig. 1.
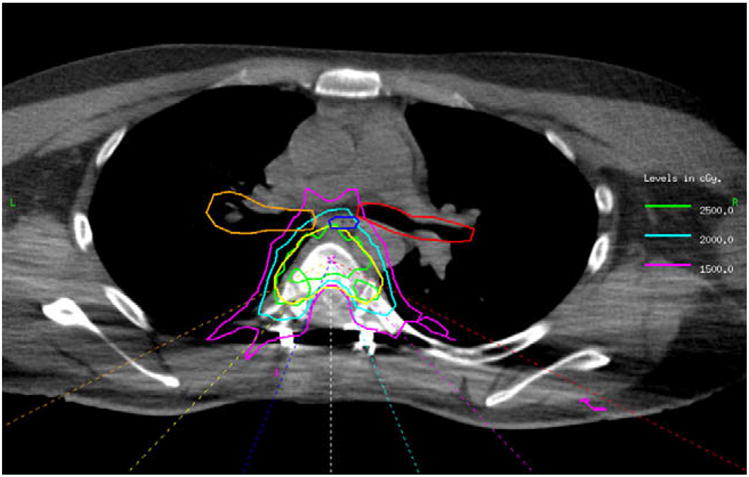
Dose distribution of the patient with Grade 4 toxicity (esophageal fistula). Dark blue, esophagus; red, left bronchus; orange, right bronchus; yellow, planning target volume (PTV); 1500 cGy isodose line, pink; 2000 cGy isodose line, turquoise; 2500 cGy, green. It is evident that a small portion of the esophagus, which lies directly outside of the PTV, received 2500 cGy.
The archived studies of the first three patients treated with this technique could not be retrieved. In the subsequent 48 patients treated, the esophagus and bronchus were retrospectively contoured on the planning CT images by two physicians (WO and DG) over the length of the PTV plus an additional one centimeter superiorly and/or inferiorly. The esophagus was contoured to include the lumen and the esophageal wall. Both the mainstem and distal bronchus were contoured to include the lumen and the bronchial wall. No modifications were made to the PTV. Forward dose calculations were then performed on the PTV and revised normal tissue contours using the IMRT plan and fields used for treatment.
Table 2 depicts the dosimetric information for the PTV, esophagus, and mainstem bronchi. The median dose to 95% of the PTV (D95) was 95.4% with a range of 58–103.7%. Twenty-one percent of the patients had a D95 <90%. The median PTV receiving at least 100% of the prescribed dose was 91.1% (range 73.6–98.3%). The data were recorded for all patients in whom at least some part of the esophagus or bronchus lay within the contouring range, i.e. the treatment volume ± 1 cm. The D1cc represents the dose to the hottest 1 cc of the esophagus, as indicated by the dose–volume histogram. The highest dose of the left and right mainstem bronchus were recorded, since the laterality of bronchial complications cannot often be determined (e.g. in the case of bronchitis).
Table 2.
Dosing characteristics of structures.
| Structure | Median value | Range | 1st quartile | 3rd quartile |
|---|---|---|---|---|
| PTV (cc) | 51.25 | 7.6–204.82 | 29.3 | 84.29 |
| Prescribed dose to PTV (cGy) | 1800 | 1600–2400 | 1800 | 2400 |
| Esophagus D1cc (cGy) | 1249 | 158–2572 | 920 | 1876 |
| Bronchus D1cc (cGy) (higher of left vs. right mainstem bronchus) | 1101 | 260–2211 | 534 | 1395 |
Treatment characteristics
All patients received SF-SBRT on a Varian Clinac 2100 EX equipped with an On-Board Imaging (OBI) device (Varian, Palo Alto, CA). After setup in the custom cradle used during simulation, pretreatment image acquisition and registration were performed using either orthogonal kV radiographs matched to DRR reference images or cone beam CT (CBCT) registered to the planning scan. Setup errors of 2 mm or more in any direction were corrected prior to the start of treatment.
Follow-up
The patients were followed at 2–3 month intervals for two years, then every six months for the next three years, and yearly thereafter. The last follow-up date was recorded as either the most recent clinic appointment or date of death. Toxicities were scored by the clinicians on follow-up and confirmed retrospectively, according to the Common Terminology Criteria for Adverse Events [11].
Results
Disease outcomes
The median follow-up was 10.7 months (range 1–55 months). Fifty percent of patients (n = 65) died of disease. The 12- and 24- month rates of overall survival were 63% and 45%, respectively.
Esophageal toxicity
Seven patients experienced Grade 2 or higher esophageal toxicity, and in two patients the toxicity was Grade 3 or higher. The first patient (Grade 3) began to experience significant pain and difficulty with swallowing 2 weeks after treatment. The symptoms persisted and the patient was referred for a swallowing evaluation two months after the treatment, the results of which are unavailable. At the last clinic follow-up, approximately 3 months after treatment, the swallowing difficulties persisted. The D1cc in this patient was 2288 cGy. The patient was not receiving chemotherapy.
The second patient experienced odynophagia in the month following radiation therapy. Four months after treatment, the patient underwent an upper gastrointestinal endoscopy that demonstrated an esophageal ulcer and necrosis. The patient required a gastric pull-up procedure for a fistula that was presumed to be secondary to radiation therapy. The patient’s symptoms then improved. However, five months after the gastric pull-up procedure (10 months after treatment) he experienced an episode of massive fatal hemoptysis. The etiology of this episode was uncertain, as the patient also had lung metastases at the time of death, but radiation could not be ruled out as a contributing factor. In addition, he had previously received doxorubicin/ifosfamide and in the time preceding his death he received sorafenib. Because radiation was certainly responsible for the gastric pull-up, but the role of radiation in the episode of massive hemoptysis was unclear, this patient was scored as having Grade 4 toxicity. The esophagus D1cc was 2431 cGy. Fig. 1 demonstrates the isodose distributions from this patient.
Bronchial toxicity
Two patients experienced Grade 2 or higher bronchial toxicity. The first patient also had a history of lung metastases and had a mild cough prior to treatment. However, the cough persisted, and approximately 6 weeks after the treatment became productive prior to resolution over the following month (Grade 2). The bronchus D1cc was 1101 cGy. This patient (who also had Grade 2 esophageal toxicity) was receiving erlotinib chemotherapy.
The second patient initially presented with dysphagia. However, a bronchoscopy was performed that demonstrated both extrinsic compression by the tumor, but also 80% stenosis of the left mainstem bronchus that was thought to be at least partially due to radiation therapy. The patient underwent balloon dilatation (Grade 3 toxicity) and one month later died from progression of the malignancy. The bronchus D1cc was 2211 cGy. The patient was not receiving chemotherapy.
Pneumonitis
There were no cases of pneumonitis in this study.
Discussion
This study has shown that central thoracic structures such as the esophagus and mainstem bronchus are tolerant of high single- fraction doses of radiation therapy. Lung cancer continues to be a challenge to the treating physician, because of both the inherent aggressiveness of the disease and the fact that many patients are elderly and/or medically debilitated with baseline cardiac and pulmonary disease. Therefore, many patients are not ideal surgical candidates, and it is in this setting that SBRT has become an alternative treatment option for early stage disease. However, the technique has been limited by the potential toxicity to mediastinal organs when treating central structures. The goal of this study was to provide further toxicity data on central structures with SBRT by extrapolating from the treatment of intrathoracic metastatic sites. However, there are two primary differences between this study and those previously mentioned in the literature to date. First, all patients were treated with single-fraction regimens, and second, the number of patients analyzed was larger than most previous studies.
One of the initial reports of hypofractionated SBRT for the treatment of intrathoracic structures was by Timmerman et al. [4], who performed a phase I trial examining 37 medically inoperable patients with non-small cell lung cancer (NSCLC) in a dose escalation study, starting with 800 cGy × 3 fractions and escalating the dose by 200 cGy per fraction. Doses of up to 2000 cGy × 3 fractions were tolerated, and high-grade toxicity was limited to one case of Grade 3 pneumonitis and another patient with Grade 3 hypoxia. In a subsequent phase II study by the same institution, however, the authors found that Grades 3–5 toxicity occurred in 14/70 patients, and that the 2-year freedom from severe toxicity was 83% in peripheral tumors but only 54% with central lesions, with “central” being defined as within 2 cm of the mediastinum or major airways [7]. This difference in toxicity was sustained in the recently reported 50-month follow-up, with 10.4% and 27.3% Grades 3–5 toxicity in peripheral and central tumors, respectively [12]. For this reason, RTOG 0236, the phase 2 study examining three-dimensional conformal SBRT to lung tumors (2000 cGy × 3 fractions), has established an anatomical limit of two centimeters within the bronchial tree as an exclusion criteria.
In addition to the Timmerman study, there have been several analyses of radiation for the treatment of early stage and/or medically inoperable NSCLC with various fractionation schemes, many of which have utilized SBRT to achieve higher doses per fraction. For instance, Hayakawa et al., examined 36 patients with peripheral stage I NSCLC treated with doses of 60–81 Gy at 2 Gy/fraction, and found no Grade 3 or higher toxicity. Zimmermann et al. [13] reviewed the treatment of 30 patients with stage I NSCLC treated to 24–37.5 Gy in 7–12.5 Gy fractions and found that 14 patients experienced pneumonitis, but only one patient had Grade 3 or higher toxicity [6]. Nyman et al. assessed 45 patients with stage I NSCLC, treated to 45 Gy in 15 Gy fractions, and found that four patients experienced either bronchitis or pneumonia, including one patient with pneumonitis [5]. Finally, Onishi et al. performed a retrospective study with 257 patients, receiving 18–75 Gy in 1–22 Gy fractions, and found that 28 patients had Grade 2 or higher complications. One patient experienced Grade 3 or higher bronchitis and one patient had Grade 3 esophagitis [3].
Our study also demonstrates a very low rate of Grade 3 or higher toxicity, with only two high-grade complications of the esophagus, one of the bronchus, and no cases of pneumonitis. It should be noted that the Grade 3 or higher complications occurred by April 2006, when the technique was relatively new at our institution and the published data from other institutions were less mature. There is certainly a learning curve in terms of defining appropriate dose constraints, and Table 3 depicts our current dosing guidelines for the lung, bronchus, and esophagus in single-fraction therapy at our institution. These guidelines were established through interdepartmental meetings and a statistical analysis of dose–volume histogram (DVH) data of single fraction patients using a method known as the DVH atlas, developed by Jackson et al. [14] and being updated in an upcoming publication. It is notable that as the respective esophageal doses with Grade 3 or higher toxicity in the current study were 2288 and 2431 cGy, neither of these patients would have fit our current dose constraints. Furthermore, the patient with the highest esophageal D1cc (2572 cGy), while not experiencing a documented toxicity, died of disease after 1.2 months. Strict bronchial constraints are now under development, and the current policy is to avoid hot spots (in relation to the PTV) in the region of the mainstem or distal bronchi.
Table 3.
Dose constraints for the lung, esophagus, and bronchus in single-fraction SBRT at Memorial Sloan-Kettering Cancer Center.
| Structure | Dose constraint |
|---|---|
| Total lung volume | V20 ≤ 10% |
| Esophagus | Level 1: ≤15 Gy/2 cc |
| Level 2: ≤20 Gy/2 cc | |
| Attempt Level 1. If not, use Level 2 | |
| Bronchus (mainstem/distal) | Maximum bronchus dose ≤ prescription dose |
Therefore, the conclusions that we can draw from this study are as follows. First, in agreement with prior studies, with a large number of patients we have shown that the esophagus, bronchus, and lung can tolerate high dose fractions, with very little significant toxicity. Indeed, when we quantitated the esophageal and bronchial doses in our initial cohort of patients with these structures inside or in proximity to the treatment field, the median D1cc values were 1249 and 1101 cGy in a single fraction, respectively. Second, our data can be extrapolated to central tumors as well, as almost all the patients included in the analysis were treated to the paraspinal region, in proximity to mediastinal structures. Finally, while patients with higher doses to the esophagus (D1cc greater than 2200 cGy) were at greater risk for toxicity, further data are needed to determine more definitive guidelines for single-fraction SBRT treatments, particularly with respect to the bronchus and lung.
In addition to the weaknesses inherent in any retrospective analysis, there are several limitations to this study. First, as alluded to above, the low rate of overall complications restricted the ability to perform reliable statistical tests on the data to better establish dose thresholds. Furthermore, the follow-up is short as a result of limited life expectancies in these patients with metastatic disease. Thus, with longer follow-up periods, as would be expected in the patients being treated with localized malignancies, more toxicity may manifest which would alter the thresholds outlined in this study. However, it should be noted that our median follow-up was almost one year, and we would anticipate a significant proportion of the expected toxicity, such as acute fistula, pneumonitis, bronchitis, and esophageal or bronchial stenosis to occur within this time period.
Third, we chose to analyze the esophagus and bronchus toxicity data using the D1cc. We realize that the parameter utilized to evaluate dose constraints varies from institution to institution (i.e. maximumdose, dose to the hottest 5% of the organ), and that because we determined this parameter by examining the dose–volume histogram (rather than as a contiguous volume), this dose could have been spread throughout the organ. However, we still feel that it remains more informative than a point dose (as in the maximum dose, Dmax), yet also holds the advantage of describing the dose received to an absolute, rather than relative, volume of tissue, an important factor in considering the contouring variations between institutions.
Indeed, the application of DVH dose constraints in the setting of SBRT has been utilized with varying efficacy. For instance, in a study by Yamashita et al., 25 patients were treated with SBRT to a dose of 48 Gy in four fractions at the University of Tokyo hospital. In all these patients, dose constraints were applied such that the mean lung dose (MLD), volume of lung receiving 20 Gy (V20), and volume of lung receiving 15 Gy (V15) were kept below 18.0 Gy, 20%, and 25%, respectively. The authors found an unexpectedly high rate of radiation pneumonitis (RP), as 7/25 patients had symptomatic Grades 2–5 toxicity, with three deaths. While MLD showed a significant correlation with the V5–V20 levels, it did not correlate with the development of RP [15]. In addition, a study by Baumann et al. examining the incidence of toxicity-related chronic obstructive pulmonary disease and cardiovascular disease in SBRT for stage I NSCLC could not find clear correlations between DVH parameters and toxicity when the size of the tumor was taken into account [16]. These studies indicate that caution should be exercised when applying strict DVH parameters to hypofractionated regimens until more definitive data have been published regarding predictive parameters in this setting.
Next, as alluded to in the Introduction, our study differs from those previously published in that all patients were treated with a single palliative dose. Therefore, this study does not allow for a direct extrapolation in the case of multiple fractions for curative intent. These studies have been cited above solely to put the current findings in the context of what has been previously published, as prior outcomes with single-fraction treatment to the thoracic region are sparse. Rather, the utility of this study lies in the finding that thoracic structures can be treated to high single doses with reasonable toxicity. Whether these high doses are delivered in the palliative setting or for definitive treatment does not affect this conclusion. Establishing specific dose constraints for single-fraction treatment on the central structures of the esophagus and bronchus, which has not yet been defined, is the subject of a current study at our institution.
Finally, our study differs from that of previous analyses in that only a small subset of the patients had NSCLC. Indeed, patients with lung malignancies tend to have lower pulmonary function secondary to the tumor and cardiopulmonary co-morbidities, and in turn these patients tend to have less tolerance to high doses of radiation to the bronchus and lung. In addition, prior studies have demonstrated the difficulty of distinguishing the etiology of post-radiation adverse events in patients with a history of underlying cardiopulmonary disease. For example, in the above-noted study by Baumann et al. which assessed the incidence of COPD and CVD in patients treated with SBRT, the authors describe these challenges, stating that patients with severe underlying COPD could have an increased propensity to radiation pneumonitis, due to greater activation of interleukins in the lung parenchyma, but that the decreased amount of normal tissue could also reduce this susceptibility of the lungs to this toxicity [16]. In another study, Kopek et al. found that both the number and severity of co-morbidities can be used to predict the survival outcomes in medically inoperable early-stage NSCLC treated with SBRT [17].
We acknowledge the limitation of the differences in our population and those previously studied in the setting of hypofractionated treatment to the thorax. We accept that this study represents a select subset of patients, many of whom have favorable pulmonary characteristics. While esophageal toxicity is not affected by pulmonary function, we advocate an individualistic approach in regards to pulmonary dose constraints, particularly with respect to severely debilitated, medically inoperable patients. However, we must emphasize that although the majority of the patients included in this study have a relatively favorable pulmonary status, we believe that there still remains a great deal of validity in the results, particularly given that such high fractions were utilized in a relatively large patient population.
Acknowledgments
The authors thank the Florence Chu Foundation at Memorial Sloan-Kettering Cancer Center for their assistance in this project.
References
- 1.Wright JL, et al. Clinical outcomes after reirradiation of paraspinal tumors. Am J Clin Oncol. 2006;29:495–502. doi: 10.1097/01.coc.0000227559.32799.db. [DOI] [PubMed] [Google Scholar]
- 2.Joyner M, et al. Stereotactic body radiation therapy for centrally located lung lesions. Acta Oncol. 2006;45:802–7. doi: 10.1080/02841860600915322. [DOI] [PubMed] [Google Scholar]
- 3.Onishi H, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–55. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 5.Nyman J, Johansson KA, Hulten U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer – mature results for medically inoperable patients. Lung Cancer. 2006;51:97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann FB, et al. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer. 2005;48:107–14. doi: 10.1016/j.lungcan.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman R, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 8.Lovelock DM, et al. Accurate setup of paraspinal patients using a noninvasive patient immobilization cradle and portal imaging. Med Phys. 2005;32:2606–14. doi: 10.1118/1.1951042. [DOI] [PubMed] [Google Scholar]
- 9.Spirou SV, Chui CS. A gradient inverse planning algorithm with dose–volume constraints. Med Phys. 1998;25:321–33. doi: 10.1118/1.598202. [DOI] [PubMed] [Google Scholar]
- 10.Nicolini G, Fogliata A, Cozzi L. IMRT with the sliding window: comparison of the static and dynamic methods. Dosimetric and spectral analysis. Radiother Oncol. 2005;75:112–9. doi: 10.1016/j.radonc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Common Terminology Criteria for Adverse Events, Version 3.0. 2003 Available from: http://ctep.cancer.gov/reporting.
- 12.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa K, et al. Limited field irradiation for medically inoperable patients with peripheral stage I non-small cell lung cancer. Lung Cancer. 1999;26:137–42. doi: 10.1016/s0169-5002(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 14.Jackson A, Yorke ED, Rosenzweig KE. The atlas of complication incidence: a proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol. 2006;16:260–8. doi: 10.1016/j.semradonc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita H, et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol. 2007;2:21. doi: 10.1186/1748-717X-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann P, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer – a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol. 2008;88:359–67. doi: 10.1016/j.radonc.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Kopek N, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009 doi: 10.1016/j.radonc.2009.06.002. [DOI] [PubMed] [Google Scholar]


