Abstract
Type 1 diabetes results from the autoimmune destruction of insulin-producing beta cells by T cells specific for beta cell antigens, including insulin. In humans, the non-MHC locus conferring the strongest disease susceptibility is the insulin gene, and alleles yielding lower thymic insulin expression are predisposing. We sought to incorporate this characteristic into an HLA-transgenic model of the disease and to determine the influence of reduced thymic insulin expression on CD8+ T cell responses to preproinsulin. We examined NOD.Ins2−/− mice, which do not express insulin in the thymus and show accelerated disease, to determine whether they exhibit quantitative or qualitative differences in CD8+ T cell responses to preproinsulin. We also generated NOD.Ins2−/− mice expressing type 1 diabetes-associated HLA-A*0201 (designated NOD.β2m−/−.HHD.Ins2−/−) in an effort to obtain an improved humanized disease model. We found that CD8+ T cell reactivity to certain insulin peptides was more readily detected in NOD.Ins2−/− mice than in NOD mice. Furthermore, the proportion of insulin-reactive CD8+ T cells infiltrating the islets of NOD.Ins2−/− mice was increased. NOD.β2m−/−.HHD.Ins2−/− mice exhibited rapid onset of disease and had an increased proportion of HLA-A*0201-restricted insulin-reactive T cells, including those targeting the clinically relevant epitope Ins B10-18. Our results suggest that insulin alleles that predispose to type 1 diabetes in humans do so, at least in part, by facilitating CD8+ T cell responses to the protein. We propose the NOD.β2m−/−.HHD.Ins2−/− strain as an improved humanized disease model, in particular for studies seeking to develop therapeutic strategies targeting insulin-specific T cells.
Keywords: T cells, Autoimmunity, Diabetes
INTRODUCTION
Type 1 diabetes is an autoimmune disease that results from the T cell-mediated destruction of pancreatic beta cells, leading to permanent insulin deficiency. Insulin is a major antigen targeted during the development of type 1 diabetes both in humans and in the well-studied model of the disease, the NOD mouse (1). The human insulin gene itself, on chromosome 11p15, maps to the type 1 diabetes susceptibility locus IDDM2 (2). This association has been further mapped to the variable number of tandem repeats (VNTR)3 minisatellite 5' of the insulin gene (3). There are three VNTR allelic classes. The majority of type 1 diabetes patients carry two class I VNTR alleles (4, 5), which lead to decreased expression of insulin in the thymus (6, 7) and show a strong association with disease at homozygosity.
While in humans there is one gene encoding insulin, mice express two insulin genes, Ins1 and Ins2. The proteins they encode are highly homologous at the sequence level, varying only at two amino acids in the mature insulin protein. Although both genes are expressed in the islets, only Ins2 expression can be detected in the thymus (8, 9). Compensatory mechanisms permit normal pancreatic insulin production in Ins2−/− mice; however, thymic insulin levels are markedly reduced (10). Studies of non-autoimmune-prone mouse strains have shown that Ins2−/− mice exhibit altered T cell tolerance to insulin (10–12). For example, CD4+ T cells from Ins2−/− 129 mice respond to immunization with insulin, while T cells from wildtype mice do not (11, 12). Radioresistant thymic cells are responsible for this phenotype (12). Indeed, in Ins1−/− 129:B6 mice, deletion of Ins2 specifically in the medullary thymic epithelial cells induces autoimmune diabetes (13). In diabetes-prone NOD mice, Ins2 ablation accelerates disease onset, and altered T cell tolerance to insulin is observed (14, 15). For example, NOD.Ins2−/− mice respond to immunization with Ins1/2 A4–21, whereas wildtype NOD mice do not (15). Collectively, this body of work suggests the NOD.Ins2−/− mouse as an appropriate model of the aforementioned genetic association between reduced expression of insulin in the thymus and type 1 diabetes development in humans.
CD8+ T cells are required for diabetes development in NOD mice (16–21), and a considerable fraction of islet-infiltrating CD8+ T cells recognizes an epitope derived from insulin, Ins1/2 B15-23 (22, 23). CD8+ T cells specific for insulin peptides are also found in the peripheral blood of type 1 diabetes patients (24–30). However, the impact of reduced thymic insulin expression on CD8+ T cell reactivity to insulin has not been explored previously. Here we addressed this issue, first by using NOD.Ins2−/− mice. We studied the polyclonal population of T cells found infiltrating the islets, where the highest proportion of disease-relevant T cells is found (24). We found that the overall number of T cells targeting insulin epitopes is higher in the absence of Ins2 expression. This work also revealed two previously undescribed insulin-derived CD8+ T cell epitopes. In order to more directly translate these findings to type 1 diabetes patients, we generated Ins2-deficient NOD.β2m−/−.HHD mice. We have previously used islet-infiltrating T cells from NOD.β2m−/−.HHD mice, which express the type 1 diabetes-associated human class I MHC molecule HLA-A*0201 (31–33) and no murine class I MHC molecules, to identify beta cell peptides targeted by T cells in the context of HLA-A*0201 (34, 35). Importantly, several of these peptides have also been shown to be recognized by T cells from type 1 diabetes patients (25, 36, 37). Here we show that NOD.β2m−/−.HHD.Ins2−/− mice exhibit rapid onset of disease and have an increased proportion of HLA-A*0201-restricted insulin-reactive T cells, including those targeting the clinically relevant epitope Ins B10–18 (25–27, 29). Taken together, our findings suggest that insulin alleles that predispose to type 1 diabetes in humans do so, at least in part, by augmenting CD8+ T cell responses to the protein. We propose the NOD.β2m−/−.HHD.Ins2−/− strain as an improved humanized disease model, in particular for epitope mapping experiments and for studies seeking to develop therapeutic strategies targeting insulin-specific T cells.
MATERIALS AND METHODS
Animals
NOD and NOD.Ins2−/− mice (14) were originally obtained from the Jackson Laboratory (Bar Harbor, ME). NOD and NOD.Ins2−/− mice are maintained by brother-sister mating at the Albert Einstein College of Medicine. For breeding of NOD.Ins2−/− mice, the females were always heterozygous for Ins2. NOD.β2m−/− (18) and NOD.β2m−/−.HHD mice (35) have been previously described and are maintained by brother-sister mating at Albert Einstein College of Medicine. NOD.β2m−/−.HHD mice express a monochain chimeric HLA-A*0201 molecule consisting of human β2-microglobulin covalently linked to the α1 and α2 domains of HLA-A*0201, followed by the α3, transmembrane, and cytoplasmic portions of H-2Db. All animal experiments were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine.
Type 1 diabetes assessment
Type 1 diabetes was assessed by the presence of glucosuria as measured with Diastix reagent strips (Bayer, Elkhart, IN). Mice were considered diabetic after two consecutive positive measurements.
Assessment of insulitis by histopathology
As previously described (38), pancreata were fixed in Bouin’s solution and sectioned. Tissue was stained with aldehyde fuchsin for visualization of islets and with an H&E counterstain for identification of leukocytes. Islets (at least 20/mouse in most cases) were individually scored as follows: 0, no lesions; 1, peri-insular leukocytic aggregates, usually periductal infiltrates but no islet destruction; 2, 25% islet destruction; 3, >25–75% islet destruction; and 4, >75% islet destruction. An insulitis index was calculated by the formula: insulitis index = (total score for all islets)/(4 × number of islets examined). Diabetic mice or animals that died after becoming diabetic were assigned an insulitis index of 1, because these mice usually had <20 remaining islets and all examined islets were score 4.
Peptides
A peptide library containing all of the 8mer, 9mer, 10mer, and 11mer peptides that can be derived from murine preproinsulin 1 was synthesized by Mimotopes (Raleigh, NC) using their proprietary Truncated PepSet technology. Each mixture in the libraries contained four peptides with a common C-terminus, but having a length of 8, 9, 10, or 11 residues. The four peptides in each mixture were present in approximately equimolar amounts. Concentrated peptide stocks (2.75 mM) were prepared in 50% acetonitrile/H2O, and 40 mM (i.e., about 10 mM for each peptide in the mixture) working stocks were obtained by serial dilution in PBS (pH 6.5). Individual peptides, having a purity of ≥ 90%, were obtained from Mimotopes. Concentrated stocks (10 mM) were prepared in DMSO, and 10 mM working stocks were obtained by dilution in PBS.
Islet isolation
Islet isolation by collagenase perfusion of the common bile duct was performed as previously described (39). Briefly, the common bile duct was cannulated and the pancreas perfused with 1.5 ml of 0.625 mg/ml cold collagenase P (Roche, Indianapolis, IN). The inflated pancreas was removed, placed in a 50 ml conical tube with 0.5 ml collagenase P, and incubated for 14 min at 37°C to digest exocrine tissue. Islets were then washed and handpicked using a micromanipulator under a dissecting microscope. Islets were handpicked again and counted, and placed in culture as described below.
Culture of islet-infiltrating T cells
Culture medium for islet-infiltrating cells consisted of RPMI 1640 medium supplemented with 10% FBS (HyClone, Logan, UT), 1 mM sodium pyruvate, non-essential amino acids, 28 µM β-mercaptoethanol (designated RPMI-10), and 50 U/ml recombinant human IL-2 (PeproTech, Rocky Hill, NJ). Approximately 50 islets/well were cultured in 24-well tissue culture plates at 37°C, 5% CO2 for 7 days. As previously reported (39), in most cultures, the majority of the cells were CD8+ after this time period (e.g., for NOD mice at 7–8 weeks of age, % CD8+ T cells = 65.5 ± 17.3; range, 39.2–85.4).
IFN-γ ELISPOT assay
ELISPOT plates (MAHA S45 10; Millipore, Billerica, MA) were precoated with anti-mouse IFN-γ mAb (BD Biosciences, San Jose, CA) and blocked with 1% BSA (Fraction V; Sigma-Aldrich, St. Louis, MO). Mitomycin C-treated antigen-presenting cells (RMA-S/Kd for NOD and NOD.Ins2−/− mice, and T2 for NOD.β2m−/−.HHD and NOD.β2m−/−.HHD.Ins2−/− mice) were added at 2 × 104 cells/well and pulsed with 1 µM peptide. Cultured islet-infiltrating T cells were added at 2 × 104 cells/well, and plates were incubated at 37°C for 40 h. IFN-γ secretion was detected with a second, biotinylated anti-mouse IFN-γ mAb (BD Biosciences) and spots were developed using streptavidin-alkaline phosphatase (Zymed Laboratories, Carlsbad, CA) and 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium substrate (Sigma-Aldrich). Spots with a minimum size of 0.01 mm2 were counted using an automated ELISPOT reader system (Autoimmun Diagnostika, Strassberg, Germany). Spot counts shown are background (PBS)-subtracted.
MHC stabilization assay
Peptide binding to Db or Kd was determined as described (40). Briefly, following overnight incubation at 26°C, RMA-S/Kd cells were incubated with peptide or PBS and human β2-microglobulin (Sigma-Aldrich, St. Louis, MO) for 20 h at 37°C. Cells were stained with anti-Db (clone 34-2–12) or anti-Kd mAb (clone SF11.1, both from BD Biosciences Pharmingen, San Jose, CA), and analyzed by flow cytometry. The positive control peptide for Db was MimA2 (YAIENYLEL) and for Kd, NRP-V7 (KYNKANVFL). The fluorescence index was calculated as the ratio of the average mean fluorescence intensity (MFI) in the presence of peptide divided by the average MFI in the presence of solvent alone.
Statistical analysis
The Mann-Whitney test was used throughout the study to determine the statistical significance of the data, except for analysis of survival curves, for which the log-rank (Mantel-Cox) test was used.
RESULTS
Accelerated insulitis and diabetes in NOD.Ins2−/− mice
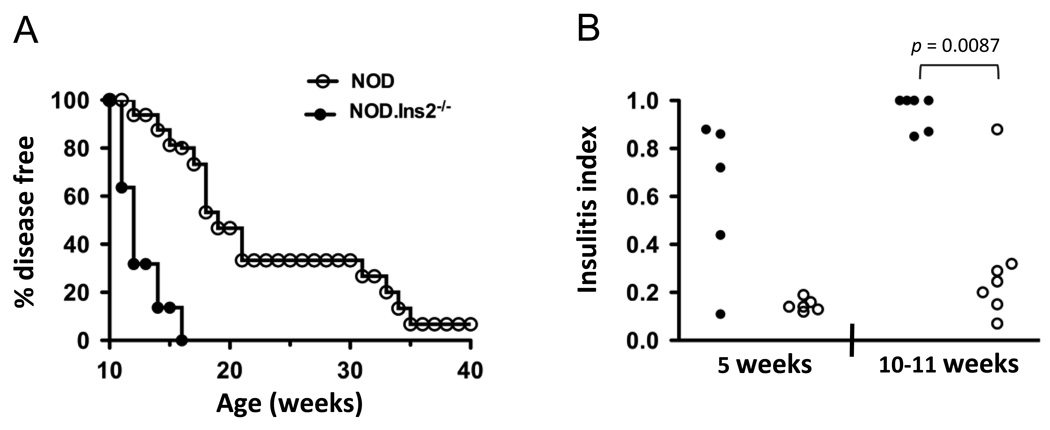
Disease development in our female NOD.Ins2−/− and NOD mice agree with the incidence of diabetes previously reported by others (14, 15). As shown in Fig. 1A, one hundred percent of NOD.Ins2−/− mice and 20% of NOD mice became diabetic by 16 weeks of age. In addition, we found that islets of young (4–5 weeks of age) and older (10–11 weeks of age) female NOD.Ins2−/− mice were more heavily infiltrated than those from female NOD mice of similar age (Fig. 1B).
FIGURE 1. Incidence of disease in female NOD.Ins2−/− mice and wildtype littermates.
A, Glucosuria was measured weekly in NOD.Ins2−/− (filled symbols, n=22) and NOD mice (open symbols, n=15) until 40 weeks of age (p < 0.0001). One hundred percent of NOD.Ins2−/− and 20% of NOD females developed diabetes by 16 weeks of age. B, Histological analysis of the islet infiltration of NOD.Ins2−/− (filled symbols) and NOD mice (open symbols) at 5 and 10–11 weeks of age is reported as the insulitis index, calculated as described in Research Design and Methods. Each symbol represents one mouse. The difference in islet infiltration between NOD.Ins2−/− and NOD mice was below statistical significance at 5 weeks of age (p = 0.15), but highly significant at 10–11 weeks of age (p = 0.0087).
Novel CD8+ T cell epitopes targeted in NOD.Ins2−/− mice
We studied islet-infiltrating CD8+ T cells from NOD and NOD.Ins2−/− mice, and tested them by IFN-γ ELISPOT assay, screening an exhaustive peptide library that spans the preproinsulin 1 molecule. As shown in Fig. 2A, the only epitope for which we were able to reliably demonstrate reactivity in NOD mice was the previously described NOD epitope Ins1/2 B15–23 (23). Not surprisingly, reactivity to Ins1/2 B15–23 was also observed in NOD.Ins2−/− mice. However, in NOD.Ins2−/− mice, we also found reactivity to a novel epitope cluster shared between preproinsulin 1 and 2, designated “99–101 cluster” (mixes 99, 100, and 101) (Fig. 2A and D). As shown in Fig. 2B, we found that the 99–101 cluster contained two novel epitopes from the prepro- and mature Ins1 and Ins2 molecules, Ins1/2 A11–19 (the 9mer peptide in mix 99) and Ins1/2 A13–21 (the 9mer peptide in mix 101), defining a novel epitope “hot-spot” in the insulin molecule.
FIGURE 2. Novel CD8+ T cell specificities identified in the islet infiltrates of NOD.Ins2−/− mice.
A, A representative experiment is shown in which a Truncated Pepset library spanning preproinsulin 1 was screened by IFN-γ ELISPOT with the cultured islet-infiltrating cells from 7-week-old female NOD (left panel) and NOD.Ins2−/− mice (right panel). The known Ins1/2 B15–23 epitope cluster and the novel cluster designated 99–101 are indicated with arrows. The data shown are representative of multiple library screens performed with islet-infiltrating cells from NOD and NOD.Ins2−/− mice of different ages. B, The minimal epitopes for the two specificities present in the 99–101 cluster were determined by testing the individual peptides with the islet-infiltrating cells (85% CD8+ T cells) of a 9-week-old mouse. The two novel epitopes, Ins1/2 A11–19 and Ins1/2 A13–21, are indicated with arrows and are both 9mers. The peptide sequences are underlined in (D), where the other peptides in the mixes corresponding to cluster 99–101 are shown. Data in (A) and (B) are background-subtracted. C, In a cell-based MHC stabilization assay, variants of Ins1/2 A11–19 and A13–21 mutated at position 9 show binding to the Db molecule. The positive control peptide for Db was MimA2 (YAIENYLEL) and for Kd, NRP-V7 (KYNKANVFL). The fluorescence index was calculated as the ratio of the average MFI in the presence of peptide divided by the average MFI in the presence of solvent alone.
We next sought to determine the MHC restriction of the two novel epitopes by performing IFN-γ ELISPOT with antigen-presenting cells that express either Db or Kd, and which were washed of excess peptide to prevent T cells from presenting peptide to each other. However, we only observed a response to the peptides when excess peptide was present during the assay (data not shown). This suggests that the peptides have low affinity for MHC, and/or that the cysteines in the peptides allow them to dimerize or become otherwise modified in ways that reduce their binding to MHC. We therefore tested variants of Ins1/2 A11–19 and Ins1/2 A13–21 where the anchor position 9 was mutated to a preferred residue (methionine and isoleucine for Db, and isoleucine for Kd) (41), or where the cysteines were mutated to serines. As shown in Fig. 2C, using a cell-based MHC stabilization assay, we confirmed that the natural versions of the epitopes have very low binding affinity, and importantly, we demonstrated that both epitopes are presented in the context of Db when the anchor position 9 is mutated to a preferred residue. Of note, the I9 variant (an anchor residue for both Kd and Db) of Ins1/2 A11–19, the weakest-binding of the two novel epitopes, shows no detectable affinity for Kd (Fig. 2C). In this assay, peptide variants containing serines instead of cysteines did not show improved binding to the MHC.
Higher frequency of insulin-reactive CD8+ T cells in NOD.Ins2−/− mice
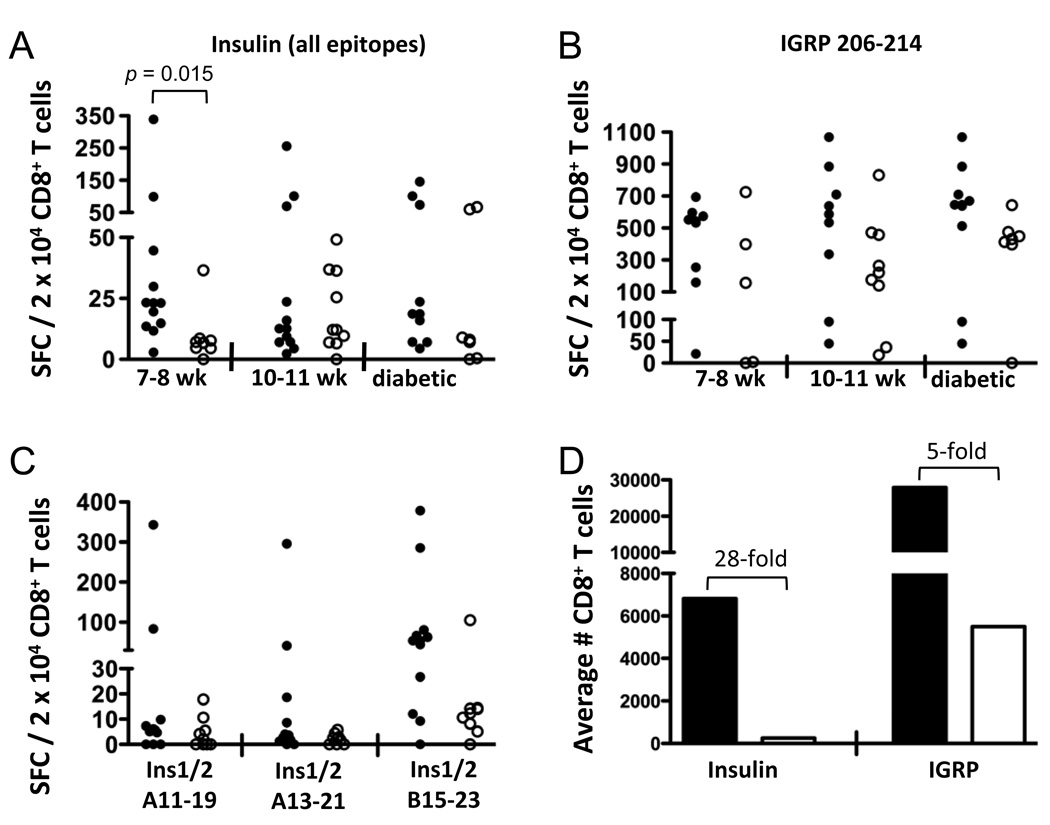
After finding that they target novel epitopes, we investigated whether CD8+ T cells recognizing insulin peptides were also quantitatively increased in NOD.Ins2−/− compared to NOD mice. The average reactivity to Ins1/2 B15–23 and the novel A11–19 and A13–21 insulin epitopes was six times higher in young NOD.Ins2−/− mice (7–8 weeks of age) compared to wildtype animals (Fig. 3A). In addition, in this age group, we obtained 4.7-fold more CD8+ T cells after culturing islet infiltrates from NOD.Ins2−/− mice as compared with wildtype littermates (data not shown). Therefore, the average number of insulin-reactive CD8+ T cells was 28 times higher in NOD.Ins2−/− than in standard NOD female mice (Fig. 3D). This marked difference in insulin reactivity was not observed in older or diabetic mice (Fig. 3A), suggesting that it likely reflects a difference in the precursor frequency of insulin-specific CD8+ T cells, and not a result of a more advanced disease stage in NOD.Ins2−/− versus NOD mice. This is further supported by our finding that reactivity to islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) 206–214, a major CD8+ T cell epitope in NOD mice (22, 42), did not differ significantly between NOD.Ins2−/− and wildtype females at 7–8 weeks of age (Fig. 3B).
FIGURE 3. CD8+ T cell reactivity to insulin epitopes in the islet infiltrates of NOD and NOD.Ins2−/− mice.
A, Female NOD.Ins2−/− (filled circles) and NOD mice (open circles) of different ages (7–8 and 10–11 weeks of age) or diabetic were studied for reactivity to insulin epitopes by IFN-γ ELISPOT. The numbers of spot-forming cells in response to three insulin epitopes (Ins1/2 B15–23, A11–19, and A13–21) in each culture of islet-infiltrating cells were subtracted for PBS, normalized to the percent of CD8+ T cells (determined by flow cytometric analysis of an aliquot of each culture), and averaged. The data are expressed as the number of spot-forming cells per 2 × 104 CD8+ T cells. Each data point represents one mouse or the pooled islets of multiple mice. B, Reactivity to IGRP 206–214 is shown. In (A) and (B), the group of NOD.Ins2−/− mice at 10–11 weeks of age includes mice that were diabetic. C, Reactivity of mice at 7–8 weeks of age for the individual insulin epitopes is shown. These data are normalized as in (A) to the percent of CD8+ T cells in each culture. D, The total number of islet-infiltrating CD8+ T cells reactive to insulin (the added reactivity to the three insulin epitopes) or to IGRP 206–214 is shown for NOD.Ins2−/− (black bars) and NOD mice (white bars) at 7–8 weeks of age.
We investigated whether the difference in insulin-reactive CD8+ T cells in young mice was due to the difference in reactivity to any particular insulin epitope. Even though there was higher reactivity to each of the three insulin epitopes in NOD.Ins2−/− than in NOD mice, these differences were not on their own statistically significant (Fig. 3C). (There was, however, a trend for Ins1/2 B15–23; p = 0.083.) Therefore, the quantitative difference observed in overall insulin reactivity is due to CD8+ T cells specific for all three epitopes studied.
Disease acceleration in NOD.β2m−/−.HHD.Ins2−/− mice
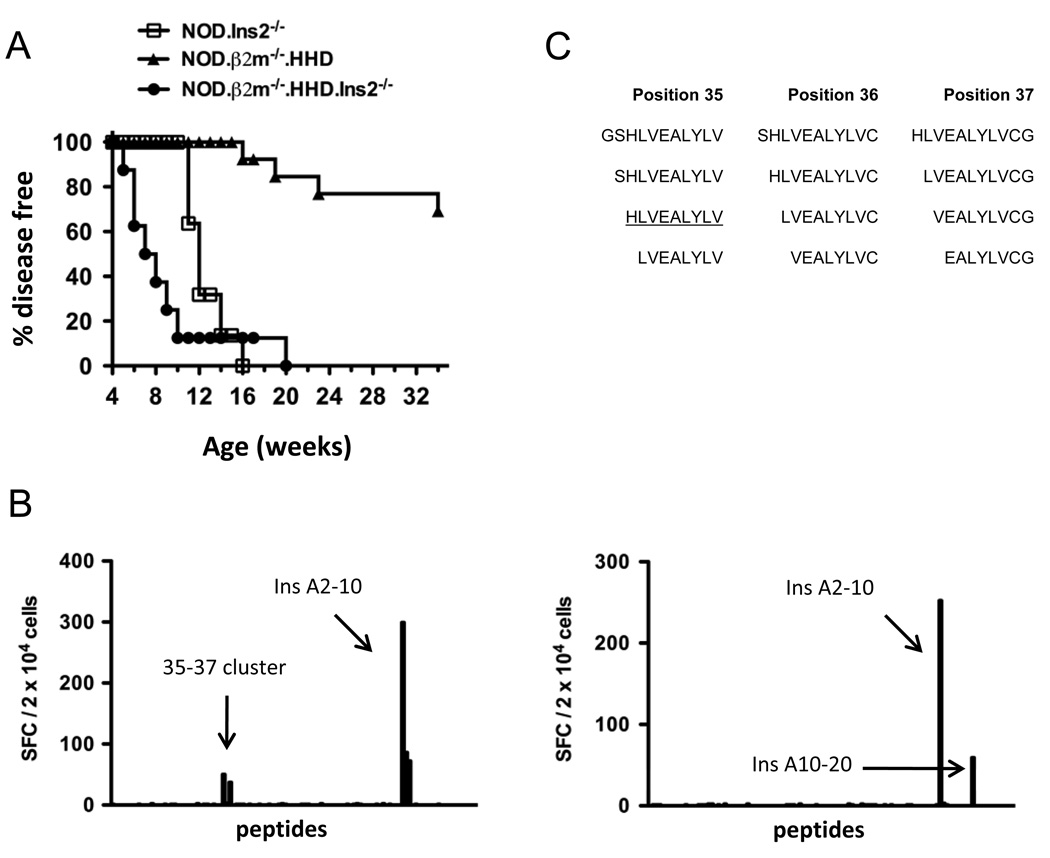
To translate our findings of the CD8+ T cell population in NOD.Ins2−/− mice more directly to human patients, we generated NOD.β2m−/−.HHD.Ins2−/− mice expressing the human class I MHC molecule HLA-A*0201, but no murine class I MHC molecules, and also lacking Ins2 expression. Introduction of the Ins2 deficiency markedly accelerated diabetes development in NOD.β2m−/−.HHD mice (Fig. 4A; p < 0.001). While NOD.β2m−/−.HHD mice in our colony began developing diabetes at 16 weeks of age, disease onset was as early as 5 weeks of age in the NOD.β2m−/−.HHD.Ins2−/− stock. Further, ninety percent of NOD.β2m−/−.HHD.Ins2−/− mice developed diabetes by 10 weeks of age. Interestingly, the onset of diabetes in NOD.β2m−/−.HHD.Ins2−/− mice was also accelerated with respect to NOD.Ins2−/− mice (Fig. 4A; p < 0.001). This suggested that the HLA-A*0201-restricted T cell response is affected to a greater degree than the Db and Kd response by the lack of thymic insulin expression, and establishing the NOD.β2m−/−.HHD.Ins2−/− mouse as an improved model of type 1 diabetes.
FIGURE 4. NOD.β2m−/−.HHD.Ins2−/− mice show accelerated disease.
A, Female NOD.β2m−/−.HHD (triangles, n=13) and NOD.β2m−/−.HHD.Ins2−/− mice (circles, n=8) were followed for disease development. The incidence of diabetes in NOD.Ins2−/− mice from Fig. 1A is included for comparison. B, Representative IFN-γ ELISPOT results are shown for the screening of the preproinsulin 1 peptide library with islet-infiltrating T cells from two female NOD.β2m−/−.HHD.Ins2−/− mice (top and bottom panels). Arrows labeled “35–37 cluster” and Ins A10–20 point to the IFN-γ responses observed in these mice that had not been previously observed in NOD.β2m−/−.HHD mice. C, The peptide sequences contained within the 35–37 cluster are shown, and the sequence of Ins B10–18 is underlined.
Identification of insulin epitopes targeted in NOD.β2m−/−.HHD.Ins2−/− mice
To identify the insulin epitopes targeted in NOD.β2m−/−.HHD.Ins2−/− mice, we tested islet-infiltrating cells for reactivity to our exhaustive library of preproinsulin 1 peptides by IFN-γ ELISPOT. CD8+ T cells from NOD.β2m−/−.HHD.Ins2−/− mice recognized insulin epitopes previously described by us to be targeted in the context of HLA-A*0201 in NOD.β2m−/−.HHD mice (34) (Fig. 4B and Fig 5B). However, we also identified responses to two other epitopes that were not previously demonstrated to be targets of islet-infiltrating CD8+ T cells from NOD.β2m−/−.HHD mice (Fig. 4B). These reactivities were named the “35–37 cluster” and Ins A10–20. The 35–37 cluster contained Ins1/2 B10–18 (Fig. 4C), a peptide completely conserved between mice and humans, and reported to be targeted by HLA-A*0201-restricted T cells from type 1 diabetes patients (25–27, 29). Reactivity within this cluster was subsequently attributed to Ins1/2 B10–18-reactive T cells (Fig. 5B). We were unable to determine the minimal epitope required for a T cell response from the Ins A10–20 mixture because of the unavailability of sufficient numbers of cells to perform these experiments. As with peptides Ins1/2 A11–19 and Ins1/2 A13–21, binding of all peptides from Ins A10–20 (mix 100, Fig. 2D) to HLA-A*0201 in a cell-based assay was very weak (data not shown). However, this reactivity can most likely be attributed to Ins A12–20, a peptide known to be recognized by HLA-A*0201-restricted T cells from type 1 diabetes patients (25).
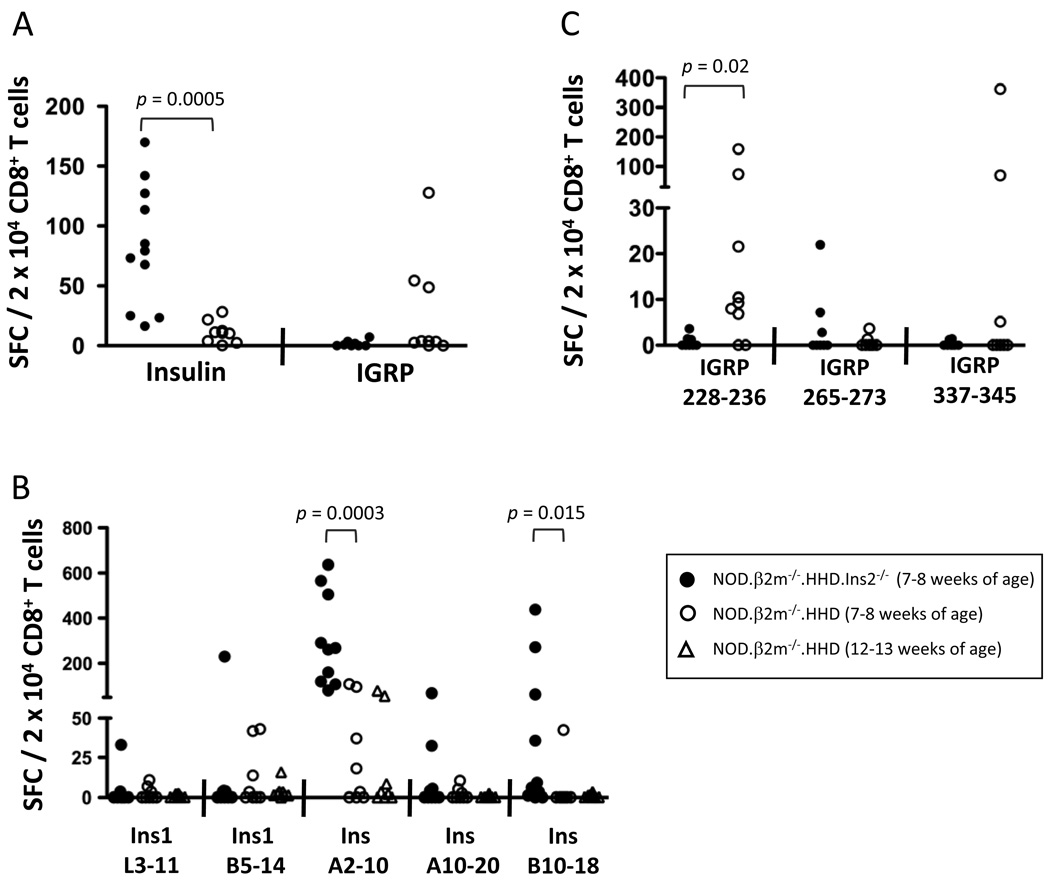
FIGURE 5. Altered frequency of islet-infiltrating cells specific for HLA-A*0201-restricted insulin and IGRP epitopes in NOD.β2m−/−.HHD.Ins2−/− compared to NOD.β2m−/−.HHD mice.
Cultured islet-infiltrating cells from female NOD.β2m−/−.HHD (open symbols) and NOD.β2m−/−.HHD.Ins2−/− mice (filled symbols) at 7–8 weeks of age were tested by IFN-γ ELISPOT for reactivity to insulin and IGRP CD8+ T cell epitopes. A, The reactivity to all insulin (Ins A10–20, Ins B10–18, Ins1 L3–11, Ins1 B5–14, and Ins1/2 A2–10) or IGRP epitopes (IGRP 228–336, IGRP 265–273, and IGRP 337–345) studied was averaged for each mouse after background subtraction. Reactivity to the individual epitopes is shown for insulin (B) and IGRP (C). The data were normalized to the percentage of CD8+ T cells (determined by flow cytometric analysis of an aliquot of each culture). Each symbol represents an individual mouse.I(B), NOD.β2m−/−.HHD mice at 12–13 weeks of age were also examined.
Higher frequency of insulin-reactive CD8+ T cells in NOD.β2m−/−.HHD.Ins2−/− mice
We tested cultured islet-infiltrating cells from mice at 7–8 weeks of age against our panel of peptides, which included Ins1 L3–11, Ins1 B5–14, Ins1/2 A2–10 (previously identified in our laboratory (34)), as well as Ins1/2 B10–18 and Ins1/2 A10–20. As shown in Fig. 5A, NOD.β2m−/−.HHD.Ins2−/− mice had a much higher frequency of insulin-reactive CD8+ T cells infiltrating their islets (p = 0.0005). The average proportion of insulin-specific CD8+ T cells in the islet infiltrate of NOD.β2m−/−.HHD.Ins2−/− mice was almost 8 times higher than in the NOD.β2m−/−.HHD stock. There was a higher proportion of both Ins1/2 A2–10-specific and Ins1/2 B10–18-specific cells in NOD.β2m−/−.HHD.Ins2−/− compared to NOD.β2m−/−.HHD mice (Fig. 5B; p = 0.0003 and p = 0.015, respectively). These differences were observed even when older NOD.β2m−/−.HHD mice were examined (Fig. 5B), confirming that they were not simply due to the altered kinetics of diabetes development in the two strains (Fig. 4A). The response to IGRP epitopes tended to be greater in NOD.β2m−/−.HHD than NOD.β2m−/−.HHD.Ins2−/− mice, and analysis of the response to individual IGRP epitopes revealed a significant difference in the response to IGRP 228–236 (Fig. 5C; p = 0.02), the immunodominant IGRP epitope in NOD.β2m−/−.HHD mice (35).
DISCUSSION
Defects in central tolerance, particularly in the thymic expression of autoantigens, are known to play a crucial role in the development of autoimmunity (6, 7, 43, 44). Intrathymic injection of insulin and other autoantigens targeted by T cells in mouse models of type 1 diabetes leads to protection from autoimmune disease (45, 46). Ins2 deficiency has been previously studied to model the genetic association of alleles at the insulin promoter leading to low thymic expression of insulin and susceptibility to type 1 diabetes in humans (10–12, 14, 15). Chentoufi and Polychronakos demonstrated that Ins2−/− mice do not have decreased insulin expression in the pancreas, and that these mice have higher T cell responses to insulin as compared to Ins1−/− mice (10). Recently, Ins2 was specifically deleted in the medullary thymic epithelial cells of mice. When these animals were also rendered Ins1-deficient to eliminate residual thymic insulin expression, autoimmune diabetes was induced (13). Although these studies were not performed in the NOD background, they helped to solidify the Ins2−/− phenotype as a result of the absence of Ins2 expression in the thymus and not the islet. However, careful analysis of changes in the specificity of T cells infiltrating the pancreatic islets of NOD.Ins2−/− mice, particularly CD8+ T cells, has been lacking. We hypothesized that such insights would provide opportunities for further translation to human patients carrying class I VNTR alleles.
We chose to examine the peptide specificity of islet-infiltrating T cells, rather than those at other sites, because the largest proportion of islet-specific T cells is found within the islets themselves (24). Furthermore, studies in NOD-based models of autoimmune diabetes have revealed that similar epitope hierarchies are found in the islets, spleen, pancreatic lymph nodes, and blood (24). Our approach, examining the peptide specificity of islet-infiltrating T cells, led us to demonstrate for the first time here that Ins1/2 A11–19 and Ins1/2 A13–21 are targeted by CD8+ T cells in NOD.Ins2−/− mice in addition to the Kd-restricted Ins1/2 B15–23 epitope (23). When assessing overall reactivity to insulin epitopes, there was a significantly higher proportion of insulin-reactive CD8+ T cells in the islets of NOD.Ins2−/− compared to NOD mice. Therefore, in addition to targeting novel epitopes, the frequency of insulin-reactive CD8+ T cells at 7–8 weeks of age is six times higher in NOD.Ins2−/− than NOD mice. In fact, the absolute number of T cells recognizing the Kd and Db insulin epitopes is 28 times higher in NOD.Ins2−/− mice as compared to NOD. Despite differences in the degree to which tolerance is achieved for particular insulin epitopes, lack of insulin expression in the thymus leads to overall greater levels of insulin-reactive CD8+ T cells which correlate with exacerbated disease. The pathogenicity of insulin-specific CD8+ T cells is further indicated by our previous work in which we utilized an in vivo cytotoxicity assay to show that CD8+ T cells specific for Ins B15–23/H-2Kd, Ins A2–10/HLA-A2, and Ins B5–14/HLA-A2 can all kill peptide-bearing targets in vivo (34).
Based on previous studies, we know that CD8+ T cell epitopes identified in HLA-transgenic NOD mice can be excellent candidates for testing in patients, as two of the three IGRP epitopes identified by our laboratory using T cells from HLA-A*0201-transgenic NOD mice (35) were later shown to be targeted in type 1 diabetes patients (25, 36, 37). This led us to generate the novel NOD.β2m−/−.HHD.Ins2−/− mouse strain, in which two factors that contribute to type 1 diabetes in humans, i.e., diminished insulin expression in the thymus and the presence of the MHC class I susceptibility allele HLA-A*0201 (31–33), are represented. The strikingly accelerated onset of disease in NOD.β2m−/−.HHD.Ins2−/− mice indicates that the presence of both susceptibility factors in the NOD background may provide a better “humanized” model of type 1 diabetes. Particularly, careful study of the HLA-A*0201-restricted T cell responses in this stock may lead to critical insights into the mechanisms of type 1 diabetes development in humans. Evidence that NOD.β2m−/−.HHD.Ins2−/− mice represent an improved model of the disease comes from the finding that they are characterized by CD8+ T cells that frequently target Ins B10–18. Several independent laboratories established the importance of Ins B10–18-specific CD8+ T cells during development of type 1 diabetes in humans. This epitope is processed and presented by cells expressing HLA-A*0201 (47) and specifically targeted by T cells from peripheral blood of recent-onset and long-standing type 1 diabetes patients (25, 26, 29). Further, this epitope is targeted by HLA-A*0201-restricted CD8+ T cells from peripheral blood of patients with recurrent autoimmunity after islet transplantation (27). However, extensive analyses failed to identify Ins B10–18-specific T cells infiltrating the islets of NOD.β2m−/−.HHD mice (34). The work presented here, demonstrating that this epitope is only targeted in NOD.β2m−/−.HHD mice in the context of Ins2 deficiency, strongly supports the NOD.β2m−/−.HHD.Ins2−/− strain as an improved model of type 1 diabetes.
Islet-infiltrating CD8+ T cells from NOD.β2m−/−.HHD.Ins2−/− mice were also found to target the Ins1/2 A10–20 peptide mixture (mix 100, Fig. 2D). This reactivity can most likely be attributed to Ins A12–20, as this 9mer peptide was found to be processed and presented by preproinsulin-expressing HLA-A*0201-positive cells (47) and to be recognized by HLA-A*0201-restricted T cells from type 1 diabetes patients (25). Ins1/2 A10–20 is in the same region of the insulin protein as Ins1/2 A11–19 and Ins1/2 A13–21, the novel epitopes targeted in NOD.Ins2−/− mice in the context of Db. Interestingly, C57BL/6 mice transgenically expressing CD80 on their beta cells develop diabetes when immunized with a preproinsulin-encoding plasmid, and the diabetogenic CD8+ T cells target Ins1/2 A12–21 in the context of Kb (48). The fact that several peptides, varying in sequence by very few amino acids, are targeted by islet-infiltrating T cells in the context of three different class I MHC alleles (Db, HLA-A*0201, and Kb) establishes this region of the molecule as an epitope “hot-spot.”
The proportion of islet-infiltrating CD8+ T cells specific for insulin epitopes is almost eight times higher in NOD.β2m−/−.HHD.Ins2−/− than NOD.β2m−/−.HHD mice. Further, the proportion of islet-infiltrating T cells reactive to Ins1/2 A2–10 is 9 times higher in NOD.β2m−/−.HHD.Ins2−/− compared to NOD.β2m−/−.HHD mice at 7–8 weeks of age. Interestingly, this epitope was the immunodominant epitope in NOD.β2m−/−.HHD mice (34). This is perhaps an indication that the precursor frequency of T cells specific for this epitope is high, and while some escape tolerance in NOD.β2m−/−.HHD mice, many more do so and expand when insulin expression is lacking in the thymus. Ins1/2 A2–10 is thought to be an important target of the pathogenic process, as we previously reported in vivo cytotoxicity to cells displaying this peptide in NOD.β2m−/−.HHD mice (34).
Our finding that the HLA-A*0201-restricted T cell response to IGRP epitopes is repressed in NOD.β2m−/−.HHD.Ins2−/− mice may reflect the complexity of the dynamics of expansion of CD8+ T cell specificities. The inability of IGRP-reactive T cells to expand in the islets of young NOD.β2m−/−.HHD.Ins2−/− mice may be due to competition with insulin-specific CD8+ T cells for interaction with antigen-presenting cells. The markedly higher precursor frequency of CD8+ T cells specific for insulin epitopes may be at a threshold such that expansion of other specificities is limited. Because the insulin-specific CD8+ T cell response is particularly affected in Ins2-deficient mice in the context of HLA-A*0201, this may explain why we observed a decrease in the IGRP-reactive T cell response in NOD.β2m−/−.HHD.Ins2−/− mice but not in the NOD.Ins2−/− strain.
Our results indicate there is epitope dependence in terms of which insulin-reactive T cells undergo negative selection when encountering an antigenic ligand in the thymus. While tolerance to some T cell specificities is almost complete in the context of thymic insulin expression (e.g., Ins1/2 A10–20 and Ins1/2 B10–18), tolerance to others is clearly only partial (e.g., Ins 1/2 A2–10). Differences in the efficiency with which these autoreactive T cells are thymically deleted may be due to several factors, including the preferential processing and presentation of particular regions of the insulin molecule, and the precursor frequency of a specific autoreactive CD8+ T cell population.
All in all, the markedly accelerated incidence of disease in NOD.β2m−/−.HHD.Ins2−/− mice strongly supports the Ins2 deficiency as an excellent model for the association between low thymic expression of insulin and susceptibility to type 1 diabetes development in humans. Our studies of the CD8+ T cell response to insulin and IGRP in the context of the human HLA-A*0201 allele establish the necessary tools that would allow novel therapies to be tested in NOD.β2m−/−.HHD.Ins2−/− mice, as many T cell specificities relevant to disease have been identified and can be monitored. This is particularly exciting in view of recent findings suggesting that insulin therapy in combination with anti-CD3 treatment is a promising strategy to peripherally tolerize insulin-specific T cells (49). Further, it is possible that patients with class I VNTR insulin alleles will respond differently to this therapy compared to patients carrying the dominantly protective class III alleles.
ACKNOWLEDGMENTS
The authors are grateful to David V. Serreze for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
DISCLOSURES
The authors have no financial conflict of interest.
This work was supported by NIH Grants R01 DK064315, P01 DK052956, P60 DK020541 (Albert Einstein College of Medicine’s Diabetes Research and Training Center), and by grants from the Juvenile Diabetes Research Foundation and the Irma T. Hirschl/Monique Weill-Caulier Trust. The flow cytometry facility at Albert Einstein College of Medicine is supported by NIH Cancer Center Grant P30 CA013330.
Abbreviations used in this paper: IGRP, islet-specific glucose-6-phosphatase catalytic subunit-related protein; MFI, mean fluorescence intensity; SFC, spot-forming cells; VNTR, variable number of tandem repeats
REFERENCES
- 1.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3:235–249. doi: 10.1038/sj.gene.6363875. [DOI] [PubMed] [Google Scholar]
- 3.Lucassen AM, Julier C, Beressi JP, Boitard C, Froguel P, Lathrop M, Bell JI. Susceptibility to insulin dependent diabetes mellitus maps to a 4.1 kb segment of DNA spanning the insulin gene and associated VNTR. Nat. Genet. 1993;4:305–310. doi: 10.1038/ng0793-305. [DOI] [PubMed] [Google Scholar]
- 4.Bennett ST, Wilson AJ, Cucca F, Nerup J, Pociot F, McKinney PA, Barnett AH, Bain SC, Todd JA. IDDM2-VNTR-encoded susceptibility to type 1 diabetes: dominant protection and parental transmission of alleles of the insulin gene-linked minisatellite locus. J. Autoimmun. 1996;9:415–421. doi: 10.1006/jaut.1996.0057. [DOI] [PubMed] [Google Scholar]
- 5.Perez De Nanclares G, Bilbao JR, Calvo B, Vitoria JC, Vazquez F, Castano L. 5'-Insulin gene VNTR polymorphism is specific for type 1 diabetes: no association with celiac or Addison's disease. Ann. N. Y. Acad. Sci. 2003;1005:319–323. doi: 10.1196/annals.1288.050. [DOI] [PubMed] [Google Scholar]
- 6.Pugliese A, Zeller M, Fernandez A, Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 7.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 8.Deltour L, Leduque P, Blume N, Madsen O, Dubois P, Jami J, Bucchini D. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc. Natl. Acad. Sci. U. S. A. 1993;90:527–531. doi: 10.1073/pnas.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath VL, Moore NC, Parnell SM, Mason DW. Intrathymic expression of genes involved in organ specific autoimmune disease. J. Autoimmun. 1998;11:309–318. doi: 10.1006/jaut.1998.0210. [DOI] [PubMed] [Google Scholar]
- 10.Chentoufi AA, Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51:1383–1390. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- 11.Faideau B, Briand JP, Lotton C, Tardivel I, Halbout P, Jami J, Elliott JF, Krief P, Muller S, Boitard C, Carel JC. Expression of preproinsulin-2 gene shapes the immune response to preproinsulin in normal mice. J. Immunol. 2004;172:25–33. doi: 10.4049/jimmunol.172.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Faideau B, Lotton C, Lucas B, Tardivel I, Elliott JF, Boitard C, Carel JC. Tolerance to proinsulin-2 is due to radioresistant thymic cells. J. Immunol. 2006;177:53–60. doi: 10.4049/jimmunol.177.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriyama H, Abiru N, Paronen J, Sikora K, Liu E, Miao D, Devendra D, Beilke J, Gianani R, Gill RG, Eisenbarth GS. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10376–10381. doi: 10.1073/pnas.1834450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thebault-Baumont K, Dubois-Laforgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, Morin J, Laloux V, Lehuen A, Carel JC, Jami J, Muller S, Boitard C. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J. Clin. Invest. 2003;111:851–857. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor α chain gene rearrangement. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12538–12543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur. J. Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 18.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 19.Sumida T, Furukawa M, Sakamoto A, Namekawa T, Maeda T, Zijlstra M, Iwamoto I, Koike T, Yoshida S, Tomioka H, Taniguchi M. Prevention of insulitis and diabetes in β2-microglobulin-deficient non-obese diabetic mice. Int. Immunol. 1994;6:1445–1449. doi: 10.1093/intimm/6.9.1445. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur. J. Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 21.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. β2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J. Immunol. 2004;173:6727–6734. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- 23.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CA., Jr Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 24.Enee E, Martinuzzi E, Blancou P, Bach JM, Mallone R, van Endert P. Equivalent specificity of peripheral blood and islet-infiltrating CD8+ T lymphocytes in spontaneously diabetic HLA-A2 transgenic NOD mice. J. Immunol. 2008;180:5430–5438. doi: 10.4049/jimmunol.180.8.5430. [DOI] [PubMed] [Google Scholar]
- 25.Mallone R, Martinuzzi E, Blancou P, Novelli G, Afonso G, Dolz M, Bruno G, Chaillous L, Chatenoud L, Bach J-M, van Endert P. CD8+ T-cell responses identify β-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–621. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang Q, Standifer NE, Qin H, Gottlieb P, Verchere CB, Nepom GT, Tan R, Panagiotopoulos C. Recognition of HLA class I-restricted β-cell epitopes in type 1 diabetes. Diabetes. 2006;55:3068–3074. doi: 10.2337/db06-0065. [DOI] [PubMed] [Google Scholar]
- 27.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with β cell destruction in type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skowera A, Ellis RJ, Varela-Calvino R, Arif S, Huang GC, Van-Krinks C, Zaremba A, Rackham C, Allen JS, Tree TIM, Zhao M, Dayan CM, Sewell AK, Unger W, Drijfhout JW, Ossendorp F, Roep BO, Peakman M. CTLs are targeted to kill β cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toma A, Haddouk S, Briand JP, Camoin L, Gahery H, Connan F, Dubois-Laforgue D, Caillat-Zucman S, Guillet JG, Carel JC, Muller S, Choppin J, Boitard C. Recognition of a subregion of human proinsulin by class I-restricted T cells in type 1 diabetic patients. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10581–10586. doi: 10.1073/pnas.0504230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toma A, Laika T, Haddouk S, Luce S, Briand JP, Camoin L, Connan F, Lambert M, Caillat-Zucman S, Carel JC, Muller S, Choppin J, Lemonnier F, Boitard C. Recognition of human proinsulin leader sequence by class I-restricted T-cells in HLA-A*0201 transgenic mice and in human type 1 diabetes. Diabetes. 2009;58:394–402. doi: 10.2337/db08-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fennessy M, Metcalfe K, Hitman GA, Niven M, Biro PA, Tuomilehto J, Tuomilehto-Wolf E. A gene in the HLA class I region contributes to susceptibility to IDDM in the Finnish population. Childhood Diabetes in Finland (DiMe) Study Group. Diabetologia. 1994;37:937–944. doi: 10.1007/BF00400951. [DOI] [PubMed] [Google Scholar]
- 32.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robles DT, Eisenbarth GS, Wang T, Erlich HA, Bugawan TL, Babu SR, Barriga K, Norris JM, Hoffman M, Klingensmith G, Yu L, Rewers M. Identification of children with early onset and high incidence of anti-islet autoantibodies. Clin. Immunol. 2002;102:217–224. doi: 10.1006/clim.2001.5171. [DOI] [PubMed] [Google Scholar]
- 34.Jarchum I, Baker JC, Yamada T, Takaki T, Marron MP, Serreze DV, Dilorenzo TP. In vivo cytotoxicity of insulin-specific CD8+ T cells in HLA-A*0201-transgenic NOD mice. Diabetes. 56:2551–2560. doi: 10.2337/db07-0332. [DOI] [PubMed] [Google Scholar]
- 35.Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, DiLorenzo TP, Serreze DV. HLA-A*0201-restricted T cells from "humanized" NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J. Immunol. 2006;176:3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 36.Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP. Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin. Immunol. 2008;127:359–365. doi: 10.1016/j.clim.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinuzzi E, Novelli G, Scotto M, Blancou P, Bach JM, Chaillous L, Bruno G, Chatenoud L, van Endert P, Mallone R. The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes. 2008;57:1312–1320. doi: 10.2337/db07-1594. [DOI] [PubMed] [Google Scholar]
- 38.Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, Illarionov P, Ndonye R, Howell AR, Santamaria P, Besra GS, Dilorenzo TP, Porcelli SA. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J. Immunol. 2007;178:1415–1425. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 39.Jarchum I, Takaki T, DiLorenzo TP. Efficient culture of CD8+ T cells from the islets of NOD mice and their use for the study of autoreactive specificities. J. Immunol. Methods. 2008;339:66–73. doi: 10.1016/j.jim.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regner M, Claesson MH, Bregenholt S, Ropke M. An improved method for the detection of peptide-induced upregulation of HLA-A2 molecules on TAP-deficient T2 cells. Exp. Clin. Immunogenet. 1996;13:30–35. [PubMed] [Google Scholar]
- 41.Rammensee HG, Bachmann J, Stevanovic S. MHC Ligands and Peptide Motifs. Austin: Landes Bioscience; 1997. [Google Scholar]
- 42.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, Santamaria P, DiLorenzo TP. Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 44.Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat. Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 45.Cetkovic-Cvrlje M, Gerling IC, Muir A, Atkinson MA, Elliott JF, Leiter EH. Retardation or acceleration of diabetes in NOD/Lt mice mediated by intrathymic administration of candidate beta-cell antigens. Diabetes. 1997;46:1975–1982. doi: 10.2337/diab.46.12.1975. [DOI] [PubMed] [Google Scholar]
- 46.Marodon G, Fisson S, Levacher B, Fabre M, Salomon BL, Klatzmann D. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood. 2006;108:2972–2978. doi: 10.1182/blood-2006-03-010900. [DOI] [PubMed] [Google Scholar]
- 47.Hassainya Y, Garcia-Pons F, Kratzer R, Lindo V, Greer F, Lemonnier FA, Niedermann G, van Endert PM. Identification of naturally processed HLA-A2--restricted proinsulin epitopes by reverse immunology. Diabetes. 2005;54:2053–2059. doi: 10.2337/diabetes.54.7.2053. [DOI] [PubMed] [Google Scholar]
- 48.Karges W, Rajasalu T, Spyrantis A, Wieland A, Boehm B, Schirmbeck R. The diabetogenic, insulin-specific CD8 T cell response primed in the experimental autoimmune diabetes model in RIP-B7.1 mice. Eur. J. Immunol. 2007;37:2097–2103. doi: 10.1002/eji.200737222. [DOI] [PubMed] [Google Scholar]
- 49.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J. Clin. Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]