Abstract
Purpose of review
Systemic sclerosis is commonly complicated by pulmonary arterial hypertension (PAH-SSc) and is a leading cause of death in this population. We will review existing challenges and recent advances in the treatment of this disease.
Recent findings
Traditionally employed outcome measures in pulmonary arterial hypertension research may not be applicable in PAH-SSc. Importantly, new therapies that target abnormal cellular proliferation in the pulmonary vasculature are currently under investigation and may be particularly relevant to PAH-SSc.
Summary
Pulmonary arterial hypertension complicating systemic sclerosis occurs commonly and portends a poor prognosis. However, recent advances in our understanding of the disease in the context of systemic sclerosis may lead to novel diagnostic and therapeutic strategies that will ultimately improve quality of life and survival in this population.
Keywords: Pulmonary hypertension, systemic sclerosis, diagnosis, therapy
Introduction
Pulmonary arterial hypertension (PAH), defined as a mean pulmonary artery pressure greater than 25 mmHg, pulmonary capillary wedge pressure less than 15 mmHg, and pulmonary vascular resistance greater than 3 Wood units, is a progressive disease of the pulmonary vasculature that leads to right heart failure and death.1** PAH commonly complicates systemic sclerosis (SSc) with a prevalence estimated at 8–12%.2;3 Importantly, PAH in SSc (PAH-SSc) significantly impacts survival, and is a leading cause of mortality in SSc.4*
Recent advances in our understanding of the pathogenesis of PAH in general have led to development of specific therapies targeting the pulmonary vasculature. While these therapies have shown, to varying degrees, improvements in symptoms, functional capacity, and quality of life; few have demonstrated a survival benefit. Further, patients with PAH-SSc have a strikingly divergent response to therapy and overall worse outcome than patients with idiopathic PAH (IPAH).5–7 While the reasons for these clinical differences remain unclear, there may be several explanations ranging from inadequacy of currently employed outcome measures for SSc-related disease to distinct structural differences in the pulmonary vasculature and its interaction with the right ventricle. Thus, there remains significant room for improvement in the assessment and treatment of PAH-SSc.
Assessment of Pulmonary Arterial Hypertension in Systemic Sclerosis
There is marked geographic variation in the occurrence of SSc with the prevalence in Europe and Japan between 30–70 cases per million compared to 240 cases per million in the United States.8–10 With a conservative estimate of PAH prevalence of 10% in SSc, the prevalence of PAH-SSc in the United States may be as high as 24 cases per million, nearly 4-fold higher than the prevalence of IPAH.11 Despite this, recent data from a US-based registry study suggests that IPAH is at least 2-fold more common than PAH-SSc, perhaps reflecting under-recognition of PAH in the SSc population.12 A recently published consensus statement from the American College of Cardiology, American College of Chest Physicians, American Thoracic Society, and Pulmonary Hypertension Association recommends yearly echocardiography for patients with systemic sclerosis to screen for pulmonary hypertension.1 While this practice has been employed at many specialized centers, variations in its implementation exist, both between community-based practices and referral centers and between specialties (i.e. rheumatologists and pulmonologists).13;14 In addition, pulmonary function test abnormalities, such as decline in diffusing capacity for carbon monoxide (DLCO), alone or in combination with elevation in serum N-terminal pro-brain natriuretic peptide, or a forced vital capacity (FVC)-to-DLCO ratio less than 1.6, may identify SSc patients with PAH, but may not be routinely performed by clinicians. 15;16**
In addition to the challenges in diagnosing PAH in patients with SSc, the outcome measures currently utilized in the assessment of PAH may not be adequate or appropriate in PAH-SSc. For instance, while right atrial pressure and cardiac index have been demonstrated to predict survival in patients with various forms of PAH, we have found in a large cohort of PAH-SSc patients from our center that neither is a significant predictor of outcome in multivariable analysis.17 Similarly, the most commonly used outcome measure in clinical trials, the six minute walk test (6MWT), has recently been challenged as an appropriate outcome measure in PAH-SSc. While this test has been validated as a measure of cardiopulmonary exercise capacity in patients with IPAH, the 6MWT has not been validated in this manner in PAH-SSc.18 Further, the minimally important difference or MID for the 6MWT, defined as the smallest difference in an outcome measure that identifies a clinically meaningful change in outcome as opposed to a solely statistically significant change in outcome, has yet to be defined in PAH-SSc. However, the MID has been recently described in a large cohort of patients with PAH from the SUPER Study that included 62 patients with PAH related to connective tissue diseases.19*;20 Unfortunately, less than half of the PAH patients with connective tissue diseases had SSc, so a sub-group analysis of this cohort would be of limited value and would be unlikely to establish a MID for PAH-SSc. Further limitations to the utility of the 6MWT in PAH-SSc include the impact of musculoskeletal disease and subclinical interstitial lung disease upon distance achieved. 21 Additionally, demographic and anthropometric characteristics such as age, height, weight, and gender, are known to influence distance achieved on 6MWT. Our experience shows that patients with SSc who develop PAH are older than patients with IPAH, thus, the distance achieved on 6MWT may not be comparable based upon differences in age.6 This has important implications regarding prognosis and assessment of response to therapy since prior studies have identified clinically important 6MWT distance thresholds both at baseline and after therapy.18;22;23 Thus, adjusting the 6MWT for demographic and anthropometric characteristics to express the distance achieved as a percentage of predicted value might provide a more valid assessment.24
Other non-invasive measures have recently been studied as potential outcome measures for PAH and PAH-SSc in particular. Serum markers, such as uric acid and sodium, may be useful for prognosis in IPAH, but neither the relevance in PAH-SSc nor the responsiveness to PAH-specific therapy have been described.25;26 Serum N-terminal pro-brain natriuretic peptide (NT-proBNP) has been demonstrated to correlate with disease severity and predict survival in IPAH.27 Recent studies in PAH-SSc have suggested the utility of NT-proBNP to predict the development of PAH in SSc and to predict survival in PAH-SSc.16;28 Further, our experience suggests that NT-proBNP levels significantly differ between IPAH and PAH-SSc despite similar hemodynamic characteristics and that NT-proBNP predicts survival only in PAH-SSc, emphasizing the potential role of NT-proBNP in the assessment of PAH in SSc patients. (Mathai SC et al. manuscript under review) Echocardiographic measures, such as the tricuspid annular plane systolic excursion (TAPSE), may offer an easily obtained, reproducible measure of PAH severity and RV function that may be useful in both IPAH and PAH-SSc.29 Cardiac MRI is being utilized with increasing frequency in the assessment of PAH in general and PAH-SSc in particular. Besides parameters of right ventricular size and function, we and others are focusing on assessment of the interaction between the pulmonary vasculature and the right ventricle in PAH-SSc compared to IPAH in hopes of elucidating novel markers of disease.
Treatment of Pulmonary Arterial Hypertension
With improved understanding of the pathogenesis of PAH, novel therapies targeting select pathways have been developed, with a focus on the chronically impaired endothelial function, affecting vascular tone and remodeling.30–32 More recently, the observation of aberrant proliferation of endothelial and smooth muscle cells in PAH, with increased expression of secreted growth factors, has led some investigators to liken PAH to a neoplastic process.33 As such, anti-neoplastic agents have recently been employed in therapy of PAH.
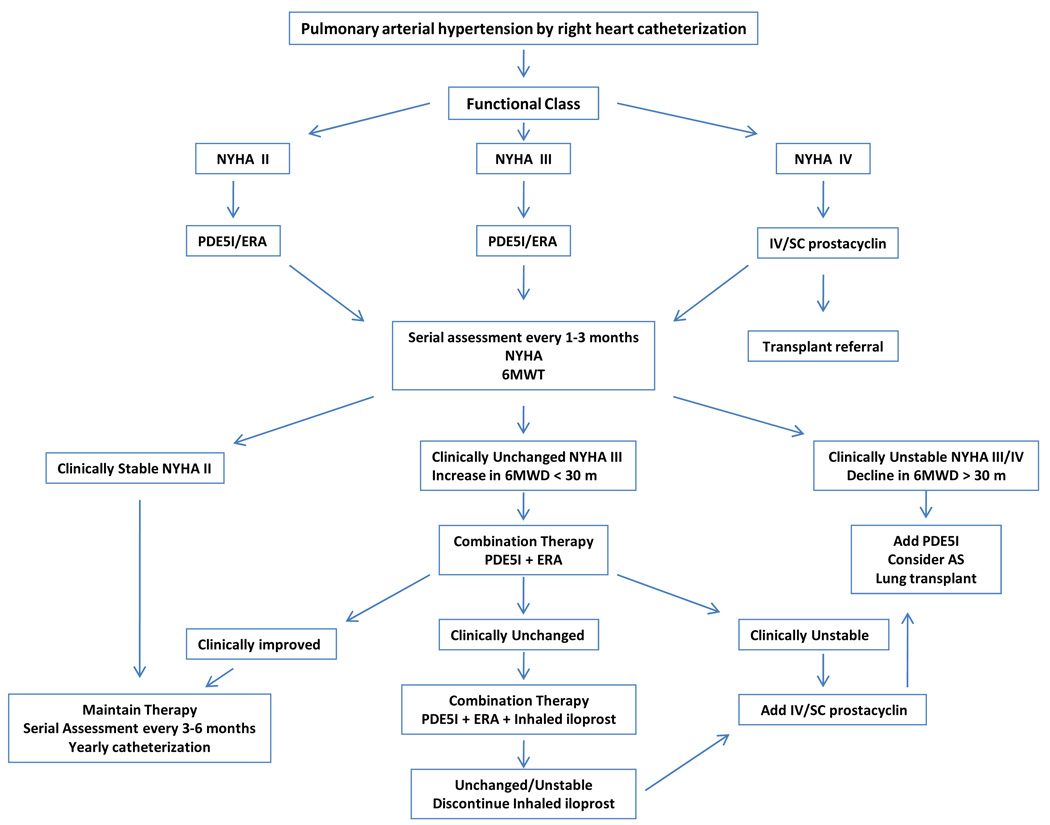
While similarities in certain aspects of the putative pathogenesis of PAH exist in PAH-SSc, clinical differences in response to therapy and outcomes suggest that distinct pathobiologic mechanisms may be involved in PAH-SSc. We will first review more traditional therapies for PAH and their relevance in PAH-SSc, then discuss novel therapies for PAH-SSc. Figure 1 depicts our treatment algorithm for PAH-SSc.
Figure 1. Proposed Treatment Algorithm for PAH-SSc.
Treatment algorithm utilized by the Johns Hopkins Pulmonary Hypertension Program. This algorithm is based on a combination of available evidence from clinical trials and clinical experience.
Clinical treatment algorithm for PAH-SSc employed by Johns Hopkins Pulmonary Hypertension Program. Note that this algorithm reflects our current practice patterns and includes aspects that have not been studied in clinical trials.
General Measures
Although limited or, in most cases, no specific data exists for either IPAH or PAH-SSc, consensus guidelines recommend the use of 1) supplemental oxygen in patients who are hypoxic at rest or with exercise (oxygen saturation < 90%), 2) diuretics for the management of volume overload and in overt right heart failure, and 3) digoxin for management of refractory right heart failure complicated by atrial arrhythmias.
Calcium Channel Blockers
Vasodilator therapy with high dose calcium channel blockers is an effective long-term therapy only in minority of patients with IPAH (about 12%) who demonstrate acute vasodilation during hemodynamic testing; in half of these initial responders, vasoreactivity wanes over time.34 Importantly, the prevalence of PAH-SSc patients who demonstrate acute vasodilation during hemodynamic testing is only about 1%, suggesting limited utility for calcium channel blockers in this disease state.34 Thus, high-dose calcium channel blocker therapy is rarely indicated in PAH-SSc though many patients may receive these drugs at low doses for Raynaud’s syndrome.
Anticoagulation
While pulmonary thromboembolic arterial disease and thrombosis in situ has been found in pathologic studies of patients with IPAH, there are few reports in the literature of similar findings in PAH-SSc.35 Similarly, there are no data on the role of anticoagulation in the treatment of PAH-SSc. Several retrospective studies and one non-randomized prospective study have suggested improved outcomes in IPAH, while two retrospective studies suggested no clinical benefit.36 Still, despite a lack of randomized, controlled trials, consensus guidelines recommend anticoagulation for all IPAH patients and for patients with PAH related to connective tissue diseases who have “advanced” disease.1 While we encourage all of our PAH-SSc patients to begin oral anticoagulation, our experience in over 100 PAH-SSc patients suggests that less than 50% remain on long-term anticoagulation therapy. Most often, patients develop occult lower gastrointestinal bleeding that necessitates cessation of anticoagulation.
Prostaglandins
Prostacyclin (epoprostenol) has proven effective in the management of PAH, demonstrating improvements in exercise capacity, cardiopulmonary hemodynamics, functional classification (New York Heart Association classification) and symptoms in patients with IPAH.37;38 In fact, epoprostenol remains the only PAH-specific therapy to demonstrate a survival benefit in a randomized clinical trial.37 In PAH-SSc, continuous intravenous epoprostenol improved exercise capacity and hemodynamics compared to conventional therapy, but failed to improve survival.39 Similar, albeit modest, responses to the prostacyclin analogue treprostinil, delivered through continuous subcutaneous infusion, were noted between IPAH and PAH related to connective tissue diseases.40;41 However, while functional capacity, exercise capacity, and hemodynamics improved in both IPAH and PAH-CTD, only half of the PAH-CTD group had SSc. Further, the efficacy of treprostinil appears to be dose-related and the subcutaneous route of administration can be limited by infusion site pain. Recently, the intravenous form of treprostinil has been approved by regulatory agencies for the treatment of PAH, including PAH-SSc. Several investigators have reported the safety and efficacy of this formulation of treprostinil.42;43 Although required maintenance doses are usually twice as high as compared to epoprostenol (affecting the cost of administration), the safety profile and drug stability offers potentially significant advantages in PAH-SSc.44 For example, the lack of requirement for ice packing may be particularly important for SSc patients who suffer from Raynaud’s phenomenon. Still, despite the potential efficacy of prostacyclin agents, the need for continuous infusion, meticulous catheter care, and daily preparation of the medication can be challenging in patients whose manual dexterity may be impaired by significant Raynaud’s phenomenon, sclerodactyly, and digital ulcerations. At our center, many PAH-SSc patients are unable to receive parenteral therapy as a result of these physical limitations.
Although inhaled prostacyclin analogues have been developed for the treatment of PAH, no studies or specific sub-group analyses on efficacy of inhaled iloprost in PAH-SSc have been reported. However, in our practice, we often will use inhaled iloprost in combination with oral therapies, especially in patients in whom parenteral or subcutaneous infusions are not practical due to physical limitations. The utility of inhaled iloprost is limited by the frequency with which the medication must be dosed; many patients are unable or unwilling to take the medication 6–9 times a day as prescribed.
Endothelin Receptor Antagonists
Bosentan, the first oral therapy approved for therapy of PAH, has been shown to improve NYHA functional class, 6MWD, time-to-clinical worsening, and hemodynamics in a short-term trial of patients with PAH.45 In a sub-group analysis of patients with PAH-SSc included in this initial study, there was a non-significant trend towards improvement in 6MWD in patients treated with bosentan compared to placebo. A recent 48-week, open-label study of patients with various forms of CTD, the majority of whom had SSc, demonstrated functional improvements in over 25% of these patients while the one-year survival was 92%.46 However, no 6MWT were reported and quality of life, as assessed by the Short Form-36 and Health Assessment Questionnaire modified for SSc, did not improve. Our experience suggests that long-term outcome of PAH-SSc patients receiving bosentan as initial therapy is inferior compared to IPAH patients, with no improvement in functional class and poorer survival.47 Still, recent guidelines from the European League against Rheumatism (EULAR) recommend bosentan as initial therapy for PAH-SSc based upon the quantity and quality of available data.48**
Selective endothelin receptor antagonists, such as sitaxsentan and ambrisentan, have recently been approved for treatment of PAH, although sitaxsentan is only approved in Europe. These agents differ from bosentan in their selectivity for the endothelin-A receptor that allows for preservation of the vasodilatory action of the endothelin-B receptor while antagonizing the vasoconstrictive effect of the endothelin-A receptor. Sitaxsentan has been shown to improve exercise capacity, as assessed by change in peak VO2 over 12 weeks, in PAH subjects who received 300mg daily.49 However, ten-percent of these subjects experienced elevation in liver enzymes greater than 3-fold above the upper limit of normal. While 6MWT, functional class, cardiac index, and pulmonary vascular resistance improved significantly in the 100mg daily group, subjects on this dose did not achieve a significant increase in peak VO2. Additionally, there were no improvements in quality of life overall. However, there were significant improvements in the sub-group analysis of 42 patients with CTD, including 19 SSc patients, in 6MWD, functional class, hemodynamics, and in the physical functioning as assessed by the SF-36.50 In a sub-group analysis of the one year follow up of patients included in a prospective, open-label study that included 84 PAH patients on bosentan therapy, suggested a possible differential response to sitaxsentan compared to bosentan in post-hoc analysis.51 PAH-CTD patients were more than twice as likely to fail monotherapy and experience clinical worsening with bosentan compared to sitaxsentan. Further, there was a non-significant trend towards poorer survival in the bosentan monotherapy group, with 1-year survival at 88% compared to 96% in the sitaxsentan group. While the proportion of CTD patients included in the study who had SSc was not reported, these data suggest that sitaxsentan may be an appropriate initial therapy in PAH-SSc.
Ambrisentan has recently been approved by the Food and Drug Administration for use in PAH, based upon the results of a large, placebo-controlled, randomized clinical trial.52 In this study, statistically significant improvements in 6MWD were noted, but the effect of therapy differed between IPAH and PAH-CTD; the range of placebo-corrected improvement in 6MWD was 50–60 meters versus 15–23 meters respectively. No other outcome measures (time to clinical worsening, change in functional class, quality of life, change in brain natriuretic peptide level, or safety) were reported by PAH-type. Further, the proportion of CTD patients with SSc was not reported. While ambrisentan is generally well tolerated, peripheral edema is common and congestive heart failure has been reported.
Phosphodiesterase Inhibitors
Sildenafil, a phosphodiesterase type V inhibitor that reduces the catabolism of cGMP, leading to enhanced effects of nitric oxide, has been widely employed in the treatment of PAH. A large clinical trial demonstrated improvement in 6MWD in subjects with various forms of PAH, including CTD-related disease.20 A post-hoc analysis of the PAH-CTD subjects in the larger study found improvements in 6MWD, functional class, and hemodynamics after 12 weeks of therapy with 20 mg TID. No further improvements were noted in subjects who received either 40 mg TID or 80 mg TID doses. Importantly, less than 50% of the CTD patients in this study had SSc, thereby limiting the generalizability to PAH-SSc. Still, given the favorable safety profile, sildenafil is an attractive agent for initial therapy in PAH-SSc.
The results of a large, randomized study of tadalafil, a once-daily phosphodiesterase inhibitor, have been recently reported.53 The treatment effect upon 6MWD, time to clinical worsening, and quality of life was significant in subjects who received 40 mg daily. Statistically significant improvements in 6MWD were noted in the PAH-CTD group, though the proportion of patients with SSc was not reported. Importantly, over half of the participants were on therapy with bosentan 125 mg BID at enrollment, which may have impacted the magnitude of response to additional therapy with tadalafil. Thus, tadalafil may be a useful alternative to sildenafil in the treatment of PAH-SSc given its safety profile and ease of administration.
Regardless of the oral therapy selected as initial therapy, the effect upon exercise capacity may not be significant. As demonstrated in a recent systematic review of all randomized controlled trials evaluating the efficacy of bosentan, sitaxsentan, and sildenafil, the effect size, defined as the ratio of the treatment effect (mean differences in 6MWD between treatment and placebo groups) to the pooled standard deviation of the differences, was small to moderate at best and not statistically significant for any drug studied.54* While this study highlights the limited response in 6MWD to oral therapies in PAH-SSc, it also emphasizes the need for appropriate outcome measures in PAH-SSc as discussed previously.
Combination Therapy
Given the possible synergistic effects of the available PAH therapies that target separate pathways involved in the pathogenesis of the disease, combination therapy has become common practice in pulmonary hypertension centers. Several multi-center trials are now exploring the efficacy of various combinations of oral drugs, oral and inhaled drugs, and oral and intravenous drugs. The recently completed PACES trial demonstrated that adding sildenafil at 80 mg TID to intravenous epoprostenol improved exercise capacity, time to clinical worsening, quality of life, and hemodynamics in patients with PAH.55 However, while no sub-group analyses were reported, the improvement noted was mainly in IPAH subjects and in those whose exercise capacity was better at baseline. Further, only 11% of the cohort had PAH-SSc. Therefore, it remains to be seen if addition of sildenafil to epoprostenol is efficacious in PAH-SSc.
We have found poorer response to a combination of oral therapies in PAH-SSc compared to IPAH.56 While the addition of sildenafil to bosentan monotherapy improved 6MWD and functional class in IPAH subjects, PAH-SSc subjects did not experience significant improvement. However, clinical deterioration may have been slowed in these patients. Importantly, there were significantly more side-effects in PAH-SSc subjects compared to IPAH, including hepatotoxicity. Clinically important interactions between sildenafil and bosentan, including decreasing serum concentrations of sildenafil and increasing serum levels of bosentan, can occur when co-administered.57 Whether these interactions are the same in PAH-SSc remains unknown, but underlying gastrointestinal disorders such as esophageal dysmotility, gastroparesis, small bowel malabsorption, and pancreatic insufficiency may interfere with drug absorption and metabolism.
Novel Therapies
Recent discoveries that highlight the aberrant proliferation of endothelial and smooth muscle cells in PAH have prompted the study of anti-neoplastic drugs initially in experimental models, and now in clinical trials.58 Two strategies are currently under investigation in randomized controlled trials: disruption of the platelet-derived growth factor (PDGF) pathway and the vascular-endothelial growth factor (VEGF) pathway. STI-571/imatinib (Gleevec®/Glivec®), which was originally developed to inhibit the Bcr-Abl kinase in the treatment of chronic myelogenous leukemia, is a dual PDGF and VEGF inhibitor that is currently under investigation for the treatment of PAH. Several case reports have suggested its utility, including one in a patient with PAH-SSc and in a patient with pulmonary veno-occlusive disease (PVOD).59–63 Since PVOD is thought to be common in PAH-SSc and a potential reason for poorer response to PAH-specific therapy, imatinib offers a potentially attractive alternative therapy.64 A phase II study to evaluate the safety, tolerability, and efficacy of imatinib in PAH has been completed, but the results are pending. Interestingly, based upon its mechanism of action, this agent is also being studied for the treatment of SSc-related interstitial lung disease and SSc-related skin disease as dysregulated proliferation and increased VEGF have been demonstrated in SSc and may be involved in the pathogenesis of SSc.65
Lung Transplantation
Despite the advances in medical therapy for PAH, lung transplantation remains the only cure. Although CTD is not an absolute contraindication to lung transplantation, SSc patients tend to have multi-organ disease that increases peri- and post-operative risk. In particular, esophageal dysmotility, common in SSc, may increase the risk of aspiration and post-transplant dysfunction. Clinical and subclinical renal disease may also increase the likelihood of complications related to the prolonged use of potentially nephrotoxic immunosuppressive agents. For these reasons, PAH-SSc patients are often denied lung transplantation. However, our experience shows that with proper screening, patients with SSc have similar rates of survival after lung transplantation compared to patients with pulmonary fibrosis or IPAH.66*
Conclusion
PAH commonly complicates systemic sclerosis and is likely under-recognized. Unfortunately, despite advances in therapy for PAH in general, patients with PAH-related to SSc have a poorer response to therapy and poorer survival. Similarly, currently employed markers of disease severity and outcome measures are inadequate. Therefore, there is an urgent need to identify biomarkers of disease severity and outcome measures relevant to PAH-SSc along. Further, increased understanding of the pathogenesis of PAH in SSc is imperative to develop therapies that target pathways specific to this disease.
Acknowledgments
Supported by P50 HL084946
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. ** This is the most recent consensus statement regarding the epidemiology, classification, diagnosis, and treatment of pulmonary arterial hypertension.
- 2.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–3800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 4. Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. * A longitudinal study of patients with systemic sclerosis that offers insight into the natural history of the disease and its complications, including pulmonary arterial hypertension.
- 5.Kawut SM, Taichman DB, Archer-Chicko CL, Palevsky HI, Kimmel SE. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123:344–350. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 6.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 8.Tamaki T, Mori S, Takehara K. Epidemiological study of patients with systemic sclerosis in Tokyo. Arch Dermatol Res. 1991;283:366–371. doi: 10.1007/BF00371817. [DOI] [PubMed] [Google Scholar]
- 9.Allcock RJ, Forrest I, Corris PA, Crook PR, Griffiths ID. A study of the prevalence of systemic sclerosis in northeast England. Rheumatology (Oxford) 2004;43:596–602. doi: 10.1093/rheumatology/keh124. [DOI] [PubMed] [Google Scholar]
- 10.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–254. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 11.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 12.Badesch DB, Benza RL, Krichman AM, Raskob GE, Giles S. REVEAL Registry: Baseline characteristics of the first 1,226 enrolled patients. Chest. 2007;132:473S. (Abstract) [Google Scholar]
- 13.Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study) Arthritis Rheum. 2005;52:2125–2132. doi: 10.1002/art.21131. [DOI] [PubMed] [Google Scholar]
- 14.Mangat P, Conron M, Gabbay E, Proudman S. Scleroderma lung disease: Variation in screening, diagnosis and treatment practices between rheumatologists and respiratory physicians. Intern Med J. 2009 doi: 10.1111/j.1445-5994.2009.01990.x. epub before print. [DOI] [PubMed] [Google Scholar]
- 15.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48:516–522. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 16. Allanore Y, Borderie D, Avouac J, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum. 2008;58:284–291. doi: 10.1002/art.23187. * This study reports a novel screening algorithm for identification of PAH in patients with systemic sclerosis.
- 17.Campo A, Mathai SC, Zaiman A, et al. Survival of pulmonary arterial hypertension related to systemic sclerosis: A single center longitudinal study. Proc Am Thorac Soc. 2009:A2657. (Abstract) [Google Scholar]
- 18.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 19. Gilbert C, Brown MC, Cappelleri JC, Carlsson M, McKenna SP. Estimating a minimally important difference in pulmonary arterial hypertension following treatment with sildenafil. Chest. 2009;135:137–142. doi: 10.1378/chest.07-0275. * First study to report the minimally important difference for the six minute walk test in patients with pulmonary arterial hypertension. This study has important implications for assessing the response to PAH-specific therapy as it may establish a threshold for treatment effect.
- 20.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 21.Garin MC, Highland KB, Silver RM, Strange C. Limitations to the 6-minute walk test in interstitial lung disease and pulmonary hypertension in scleroderma. J Rheumatol. 2009;36:330–336. doi: 10.3899/jrheum.080447. [DOI] [PubMed] [Google Scholar]
- 22.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 23.Provencher S, Sitbon O, Humbert M, Cabrol S, Jais X, Simonneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006;27:589–595. doi: 10.1093/eurheartj/ehi728. [DOI] [PubMed] [Google Scholar]
- 24.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 25.Nagaya N, Uematsu M, Satoh T, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–492. doi: 10.1164/ajrccm.160.2.9812078. [DOI] [PubMed] [Google Scholar]
- 26.Forfia PR, Mathai SC, Fisher MR, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364–1369. doi: 10.1164/rccm.200712-1876OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fijalkowska A, Kurzyna M, Torbicki A, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 28.Williams MH, Handler CE, Akram R, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27:1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 29.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 30.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 31.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 32.Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 33.Adnot S. Lessons learned from cancer may help in the treatment of pulmonary hypertension. J Clin Invest. 2005;115:1461–1463. doi: 10.1172/JCI25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 35.Pietra GG, Edwards WD, Kay JM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989;80:1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 36.Johnson SR, Mehta S, Granton JT. Anticoagulation in pulmonary arterial hypertension: a qualitative systematic review. Eur Respir J. 2006;28:999–1004. doi: 10.1183/09031936.06.00015206. [DOI] [PubMed] [Google Scholar]
- 37.Barst RJ, Rubin LJ, Long WA, et al. The Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 38.Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 39.Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132:425–434. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 40.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 41.Oudiz RJ, Schilz RJ, Barst RJ, et al. Treprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue disease. Chest. 2004;126:420–427. doi: 10.1378/chest.126.2.420. [DOI] [PubMed] [Google Scholar]
- 42.Tapson VF, Gomberg-Maitland M, McLaughlin VV, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest. 2006;129:683–688. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 43.Sitbon O, Manes A, Jais X, et al. Rapid switch from intravenous epoprostenol to intravenous treprostinil in patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2007;49:1–5. doi: 10.1097/FJC.0b013e31802b3184. [DOI] [PubMed] [Google Scholar]
- 44.Oudiz RJ, Farber HW. Dosing considerations in the use of intravenous prostanoids in pulmonary arterial hypertension: an experience-based review. Am Heart J. 2009;157:625–635. doi: 10.1016/j.ahj.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 46.Denton CP, Pope JE, Peter HH, et al. Long-term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases. Ann Rheum Dis. 2008;67:1222–1228. doi: 10.1136/ard.2007.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Girgis RE, Mathai SC, Krishnan JA, Wigley FM, Hassoun PM. Long-term outcome of bosentan treatment in idiopathic pulmonary arterial hypertension and pulmonary arterial hypertension associated with the scleroderma spectrum of diseases. J Heart Lung Transplant. 2005;24:1626–1631. doi: 10.1016/j.healun.2004.12.113. [DOI] [PubMed] [Google Scholar]
- 48. Kowal-Bielecka O, Landewe R, Avouac J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Ann Rheum Dis. 2009;68:620–628. doi: 10.1136/ard.2008.096677. ** Consensus recommendations for the treatment of PAH complicating systemic sclerosis.
- 49.Barst RJ, Langleben D, Frost A, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 50.Girgis RE, Frost AE, Hill NS, et al. Selective endothelin A receptor antagonism with sitaxsentan for pulmonary arterial hypertension associated with connective tissue disease. Ann Rheum Dis. 2007;66:1467–1472. doi: 10.1136/ard.2007.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benza RL, Barst RJ, Galie N, et al. Sitaxsentan for the treatment of pulmonary arterial hypertension: a 1-year, prospective, open-label observation of outcome and survival. Chest. 2008;134:775–782. doi: 10.1378/chest.07-0767. [DOI] [PubMed] [Google Scholar]
- 52.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 53.Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 54. Avouac J, Wipff J, Kahan A, Allanore Y. Effects of oral treatments on exercise capacity in systemic sclerosis related pulmonary arterial hypertension: a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67:808–814. doi: 10.1136/ard.2007.077149. * Systemic review of the treatment effect of PAH-therapy on six minute walk distance in patients with systemic sclerosis suggests a non-significant impact upon exercise capacity overall and emphasizes the limitations of this test as an outcome measure in PAH-SSc.
- 55.Simonneau G, Rubin LJ, Galie N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 56.Mathai SC, Girgis RE, Fisher MR, et al. Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur Respir J. 2007;29:469–475. doi: 10.1183/09031936.00081706. [DOI] [PubMed] [Google Scholar]
- 57.Paul GA, Gibbs JS, Boobis AR, Abbas A, Wilkins MR. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol. 2005;60:107–112. doi: 10.1111/j.1365-2125.2005.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 59.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 60.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med. 2006;145:152–153. doi: 10.7326/0003-4819-145-2-200607180-00020. [DOI] [PubMed] [Google Scholar]
- 61.Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax. 2006;61:736. doi: 10.1136/thx.2006.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overbeek MJ, van Nieuw Amerongen GP, Boonstra A, Smit EF, Vonk-Noordegraaf A. Possible role of imatinib in clinical pulmonary veno-occlusive disease. Eur Respir J. 2008;32:232–235. doi: 10.1183/09031936.00054407. [DOI] [PubMed] [Google Scholar]
- 63.ten Freyhaus H, Dumitrescu D, Bovenschulte H, Erdmann E, Rosenkranz S. Significant improvement of right ventricular function by imatinib mesylate in scleroderma-associated pulmonary arterial hypertension. Clin Res Cardiol. 2009;98:265–267. doi: 10.1007/s00392-009-0752-3. [DOI] [PubMed] [Google Scholar]
- 64.Dorfmuller P, Humbert M, Perros F, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 65.Grigoryev DN, Mathai SC, Fisher MR, et al. Identification of candidate genes in scleroderma-related pulmonary arterial hypertension. Transl Res. 2008;151:197–207. doi: 10.1016/j.trsl.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schachna L, Medsger TA, Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954–3961. doi: 10.1002/art.22264. * Although short-term mortality may be poorer in PAH-SSc than idiopathic pulmonary fibrosis or IPAH, longer-term survival is comparable. Thus, patients with PAH-SSc should be considered for lung transplant at centers experienced with the management of this disease.