Abstract
The pathogenesis of hypertension and its mode of progression are complex, multifactoral and incompletely understood. However, there is accumulating evidence from humans and animal models of hypertension indicating that excessive central sympathetic nerve activity (SNA) plays a pathogenic role in triggering and sustaining the essential hypertensive state (the so-called “neuroadrenergic hypothesis”). Importantly, augmented central sympathetic outflow has also been implicated in the initiation and progression of a plethora of pathophysiological processes independent of any increase in blood pressure, such as left ventricular hypertrophy and cardiac arrhythmias. Thus, the sympathetic nervous system constitutes an important putative drug target in hypertension. However, traditional pharmacological approaches for the management of essential hypertension appear ineffective in reducing central sympathetic outflow. Recently, several new and promising therapeutic strategies targeting neurogenic hypertension have been developed. The present report will provide a brief update of this topic with a particular emphasis on human studies examining the efficacy of novel pharmacological approaches (central sympatholytics, statins), lifestyle modification (aerobic exercise training, weight loss, stress reduction) and surgical intervention (renal denervation, chronic carotid baroreflex stimulation, deep brain stimulation) in reducing excessive central sympathetic activation in hypertension.
Keywords: Sympathetic nervous system, blood pressure, cardiovascular
Introduction
Over the past 50 years there have been remarkable improvements in the therapeutic management of hypertension (Chobanian, 2009), but despite such advances it is estimated that worldwide 1 billion people are hypertensive with the global prevalence projected to exceed 1.5 billion by 2025 (Kearney et al., 2005). While these alarming statistics likely result from a multitude of factors, including an absence of treatment (i.e., under diagnosed) or treatment non-compliance, it is suggested that up to 30% of patients with hypertension remain hypertensive despite multiple drug regimens (so called resistant hypertension) (Calhoun et al., 2008). Given this lack of efficacy of current drug therapies, and considering that hypertension is a powerful independent predisposing risk factor for development of cerebrovascular disease, heart failure, coronary heart disease and peripheral artery disease (Kannel & Wilson, 2003), the development of novel and effective therapeutic strategies is clearly warranted.
The pathogenesis of hypertension and its mode of progression are complex, multifactoral and incompletely understood. However, as elegantly reviewed by Grassi et al (Grassi et al., 2010) in this issue of Experimental Physiology, there is accumulating evidence from humans and animal models of hypertension indicating that excessive central sympathetic nerve activity (SNA) plays a pathogenic role in triggering and sustaining the essential hypertensive state (the “neuroadrenergic hypothesis”). The mechanisms underlying this neural dysfunction appear multifocal and may arise from alterations in circulating factors (e.g. hormones), neural afferent pathways (e.g. arterial baroreflex, chemoreflex), central neural integration (e.g. cardiovascular brainstem centres such as the nucleus tractus solitarius) and/or efferent pathways (e.g. sympathetic nerve firing, adrenergic receptor polymorphisms). Importantly, aside from a clear role in the development of hypertension, excessive central sympathetic activation has also been implicated in the initiation and progression of a plethora of pathophysiological processes independent of any increase in blood pressure, such as left ventricular hypertrophy, cardiac arrhythmias, atherosclerosis and metabolic dysfunction (Fisher et al., 2009; Grassi et al., 2010). As such, the sympathetic nervous system constitutes an important putative drug target not only for arresting hypertension but also the progression of common cardiovascular comorbidities (Grassi, 2004). The present report will begin by briefly discussing the efficacy of traditional anti-hypertensive drugs in reducing central SNA, then focus on the promise of several novel therapeutic strategies for targeting excessive central SNA in human hypertension.
A common clinical measure of whole-body SNA can be obtained by the determination of venous plasma or urinary noradrenaline concentrations, the primary neurotransmitter of the sympathetic nervous system. However, such ‘global’ measures are limited by an inability to account for local reuptake mechanisms and regional variations in SNA. A more sophisticated and specific approach is the isotope dilution methodology whereby noradrenaline spillover resulting from active sympathetic terminals of individual organs (e.g. kidneys, brain or heart) can be determined (Esler et al., 2003). Although a very robust SNA measurement technique, it is relatively invasive limiting its use in certain settings. A more easily obtainable index of SNA may be provided by spectral analysis of spontaneous oscillations in heart rate or blood pressure (Pagani et al., 1997), however, the physiological validity of this approach has been questioned (Parati et al., 2006). Given that the focus of the present review is on central sympathetic outflow, an emphasis will be placed on human studies employing direct intraneural recordings of efferent post-ganglionic SNA to the skeletal muscle vasculature or skin, using the microneurography technique. Of note, due to the potential for profound regional differentiation in the pattern of SNA in health and disease, an important caveat to the results of studies relying on a measure of SNA to a single vascular bed or target organ is that the efficacy of a therapeutic intervention in altering SNA to other regions may be overlooked (Esler et al., 2003).
Therapeutic Strategies
Traditional Pharmacological Therapies
At present, angiotensin-converting–enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARB), beta-receptor blockers, calcium-channel blockers and diuretics, represent the primary pharmacological treatment options in patients with hypertension (Chobanian, 2009). However, the extent to which these commonly used anti-hypertensive agents, prescribed as either a monotherapy or in combination, have the potential to modulate central sympathetic outflow remains incompletely understood. Adding to the complexity of this issue is that studies of a particular anti-hypertensive agent often report conflicting results, which may partly be due to differences in the pharmacokinetic properties of the drug, the duration of treatment, or whether the patients examined have uncomplicated or complicated hypertension. However, in general minimal alterations in muscle SNA have been reported in studies examining the effects of long-term administration of traditional anti-hypertensive agents, such as angiotensin II converting enzyme (ACE) inhibitiors (Grassi et al., 1998) or angiotensin II type 1 (AT1) receptor antagonists (Krum et al., 2006), in patients with uncomplicated essential hypertension. Similarly, β-adrenergic blockade also appears to have a neutral effect on central sympathetic outflow, particularly when reductions in heart rate are accounted for (Wallin et al., 1984). Interestingly, chronic administration of some traditionally used anti-hypertensive compounds, particularly diuretics and dihydropyridine calcium channel blockers can actually stimulate central sympathetic outflow (Grassi, 2004). In this regard, recent work has indicated that chlorthalidone, a first-line drug therapy for hypertension, caused persistent activation of the sympathetic nervous system in hypertensive patients, an effect that was avoided by spironolactone even though similar reductions in blood pressure were achieved (Menon et al., 2009). Clearly, sympathetic effects of traditional blood pressure lowering drugs need to be carefully considered. Indeed, Fu et al. (Fu et al., 2005) demonstrated that successful blood pressure lowering with combined AT1 receptor antagonist and diuretic treatment, in a group of newly diagnosed patients with moderate essential hypertension, was associated with a chronic exacerbation of muscle SNA, possibly due to baroreflex unloading. Overall, these findings highlight the inadequacies of traditional hypertensive drug treatments in targeting excessive central sympathetic outflow in hypertension.
Alternative Therapeutic Strategies
Central Sympatholytics
A direct pharmacological approach to reduce excessive central sympathetic outflow in hypertension is to stimulate α2 or imidazoline receptors within the central nervous system. Indeed, the administration of either clonidine (α2 and imidazoline receptor agonist) or moxonidine (selective imidazoline receptor agonist) has been shown to effectively reduce SNA and blood pressure in essential hypertension (Esler et al., 1997; Wenzel et al., 1998). However, unpleasant side effects have been reported with clonidine and moxonidine (e.g. drowsiness, dizziness, orthostatic intolerance). While these side-effects may to some extent be circumvented with effective dosing, more promising may be a second generation of imidazoline binding agents, such as rilmenidine (Esler et al., 2004). Along with beneficial sympatholytic and blood pressure lowering effects, a particular advantage of rilmenidine is that there is a persistence of the normal blood pressure and sympathetic responses to orthostatic stress, suggesting that protection from postural hypotension is maintained (Esler et al., 2004). Furthermore, rilmenidine appears to be generally well tolerated and is effective in reducing left ventricular hypertrophy in patients with essential hypertension (Koldas et al., 2003). In light of such benefits, rilmenidine has been suggested as a “first-line antihypertensive option for all groups of hypertensive patient” (Reid, 2000).
Central Nitric Oxide
Emerging basic research suggests that nitric oxide (NO) may represent a novel therapeutic target to arrest excessive central sympathetic activation in hypertension. NO is synthesised from the conversion of the amino acid L-arginine to L-citrulline by the enzyme NO synthase (NOS). There are three NOS isoforms including endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) NOS with both eNOS and nNOS constitutively expressed in mammalian cells. While the contribution of reduced NO bioavailability to the hypertensive state has been well documented in terms of impaired endothelial-dependent vasodilation, less appreciated is the potential role of NO in central regulation of sympathetic outflow. Indeed, all NOS isoforms are expressed in the central nervous system and accumulating evidence from animal and human studies suggest that NO is a key signalling molecule involved in the tonic restraint of sympathetic outflow from the brainstem (Thomas et al., 2001). In support of this concept, systemic infusion of a competitive NOS inhibitor in healthy humans causes sympathetic activation and marked elevations in blood pressure (Lepori et al., 1998; Sander et al., 1999; Young et al., 2009) (Fig 1). The clinical relevance of such findings is that it raises the possibility that physiological accumulation of an endogenous NOS inhibitor may also raise SNA. One candidate is asymmetric dimethlarginine (ADMA), an endogenous NOS inhibitor that has received recent attention as plasma concentrations are increased in essential hypertension, and markedly elevated in secondary hypertension associated with renal failure, where excretion of ADMA by the kidney is greatly impaired (Thomas et al., 2001; Boger, 2003). Although plasma ADMA has been shown to be strongly associated with plasma concentrations of noradrenaline (Mallamaci et al., 2004), the contribution of excessive ADMA to increases of central SNA in essential hypertension remains to be fully elucidated.
Figure 1. Pharmacological inhibition of nitric oxide synthase (NOS) increases sympathetic nerve activity (SNA) in healthy humans.
Panel A: Original records illustrating skin SNA at baseline and 180 minutes after a 60 minute intravenous infusion of the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 4mg/kg). Panel B: Individual skin SNA responses from all subjects at the end of the L-NAME infusion (i.e. 60 minutes) and at 180 minutes. These findings clearly indicate that experimental inhibition of NOS increases SNA and supports a role of nitric oxide in central sympathetic control in humans. (Reproduced with permission from Young et al., 2009)
Reactive Oxygen Species
Aside from alterations in NOS activity, NO bioavailability in the peripheral vasculature and central nervous system are crucially dependent upon the balance between reactive oxygen species (ROS) and antioxidant defence systems. ROS levels are reported to be elevated in patients with hypertension, and vascular oxidative stress has been implicated in the pathogenesis of essential hypertension (Rodrigo et al., 2007). Critically, aside from scavenging NO, increases in ROS within the central nervous system may also directly activate or sensitise sympathetic neurones (Peterson et al., 2006). As such, the therapeutic targeting of ROS represents an attractive strategy to reduce vasomotor tone and blood pressure. Importantly, reducing ROS may not only provide a decrease in central sympathetic drive but would also cause an improvement of endothelial function by restoring NO dependent dilation in the periphery. In this regard, there has been considerable recent interest in the use of antioxidants vitamins in the treatment of hypertension (e.g. vitamin C and E). However, studies on the antihypertensive effects of these compounds have thus far been largely equivocal and their therapeutic effectiveness remains controversial (Thomas et al., 2001; Rodrigo et al., 2007). Alternative therapies targeting the NO pathway which may also have beneficial effects by reducing ROS include the use of HMG-CoA reductase inhibitors (statins) and exercise training.
HMG-CoA Reductase Inhibitors- Statins
Prescribed for their potent cholesterol lowering properties, statins are also known to have a number of “pleiotropic effects” including stimulation of NO production, antioxidant and anti-inflammatory effects. Of note, in an animal model of pacing-induced heart failure the ability of statin therapy to cause a marked reduction in renal SNA and improvements in heart rate variability indices of cardiac autonomic balance, have been well documented (Pliquett et al., 2003a; Pliquett et al., 2003b). This decrease in central SNA was associated with concomitant up-regulation of neuronally derived NOS and antioxidants in the brainstem as well as central suppression of pro-oxidants (Zucker & Gao, 2006). Although limited work in hypertension has been performed examining the potential for statins to cause reductions in central SNA, preliminary work is encouraging. Indeed, Kishi et al. (Kishi et al., 2003) recently demonstrated that 30 days of atorvastatin treatment (50 mg/kg per day) in stroke prone spontaneously hypertensive rats, caused up-regulation of brainstem endothelial NOS and significant reductions in SNA (assessed using urinary noradrenaline excretion) and blood pressure. While in humans, Siński et al. (Sinski et al., 2009) reported that 8 weeks of atorvastatin (20 mg daily) reduced muscle SNA from 36±7 to 29±5 bursts per minute (mean±SE) and improved baroreflex sensitivity by ~44% in patients with essential hypertension and untreated hypercholesterolaemia. However, as statins significantly reduced the baseline elevation in cholesterol observed in these patients the extent to which the sympatho-inhibitory actions are independent of lipid-lowering is unclear. Nonetheless, prospective, placebo-controlled, randomised trials in primary hypertensive patients are clearly warranted in order to validate these initial findings.
Aerobic Exercise Training
Although aerobic exercise training is a well known non-pharmacological approach to reduce blood pressure in patients with hypertension, the mechanisms underlying such a hypotensive effect remain incompletely understood. Alterations in both neuro-humoral and neural cardiovascular control are likely involved. Notably, an influence of aerobic exercise training on central SNA has not been firmly established. However, Laterza et al. (Laterza et al., 2007) recently demonstrated that 4 months of moderate intensity aerobic exercise training in never-treated hypertensive patients significantly reduced muscle SNA and increased arterial baroreflex regulation of SNA. Quite remarkably, the magnitude of the reduction in sympathetic outflow was such that following training, the excessive central sympathetic activation initially observed, had been reduced to levels of age-matched control subjects. Although the mechanisms underlying these observations remain unclear, alterations in central NOS activity and antioxidant status may have contributed. Indeed, exercise training has been shown to elicit increases in both peripheral and central NOS activity (Mueller, 2007), and is associated with a up-regulation of central antioxidants and down-regulation of central pro-oxidants (Zucker et al., 2004). Of note, an important caveat in the use of exercise training as a treatment for hypertension, or any disease, is that in order to optimise the effectiveness of a training regimen, consideration of patient motivation and provision of appropriate support is essential.
Weight Loss
Large scale epidemiological studies indicate a strong association between weight gain and hypertension (Brown et al., 2000). In obesity-related hypertension elevations in muscle SNA, and renal noradrenaline spillover have been identified (Rumantir et al., 1999; Grassi et al., 2000). Although the underlying mechanisms remain to be fully elucidated impairments in arterial baroreflex function, alterations in central neural pathways, activation of the renin-angiotensin-aldosterone system, sleep disordered breathing, genetic factors and elevations in circulating concentrations of insulin and leptin have all been implicated (Grassi et al., 2000; Davy & Orr, 2009). Importantly, weight loss following caloric restriction alone or in combination with exercise training has been associated with reductions in muscle SNA (Trombetta et al., 2003; Straznicky et al., 2005) and heart rate variability derived indices of cardiac SNA (Ashida et al., 2007), in obese individuals. Given the increasing prevalence of obesity, particularly in the Western world, weight loss may represent the single most effective way to reduce SNA on a population-wide basis. More recently, the linkage of the melanocortin-4 receptor gene to obesity related phenotypes in humans has led to an increased research focus on the hypothalamic melanocortin system. The findings that the melanocortin-4 receptor can alter central sympathetic outflow suggests that central melanocortinergic signalling pathways may provide potential therapeutic targets for obesity-related sympathoexcitation (Haynes et al., 1999).
Reductions in Psychosocial Stress
Psychosocial stress has been implicated in the pathogenesis of essential hypertension (Esler et al., 2008). Indeed, elevated work-related stress is linked to raised daytime ambulatory blood pressure, particularly where the degree of job control (i.e. autonomy) was perceived to be low (Steptoe & Willemsen, 2004). More recently, Granado et al. (Granado et al., 2009) reported that the incidence of hypertension is elevated in military personnel who are exposed to multiple stressful combat situations compared to those who are not. Importantly, elevated stress has also been associated with increased risk of atherosclerosis (Rozanski et al., 1999) and acute cardiovascular events (Rosengren et al., 2004). While the mechanisms linking stress to such pathophysiological processes appear complex and multifactoral, the sympathetic nervous system may play an important role (Rosengren et al., 2004; Esler et al., 2008). Indeed, acute laboratory-based stress tasks (i.e. mental arithmetic) have been shown to evoke increases in sympathetic vasoconstrictor tone and blood pressure (Wallin et al., 1992), and a greater reactivity to such acute stressors has been suggested to be prognostic for the future development of hypertension (Chida & Steptoe, 2010; Flaa et al., 2008). Moreover, links have been identified between chronic psychosocial stress, markers of excessive sympathetic activation and elevated blood pressure (Lucini et al., 2005). As such, stress reduction methods may confer beneficial health effects as a result of lowering SNA and blood pressure (Rozanski et al., 1999), thus this is clearly an area worthy of further investigation.
Surgical Therapeutic Strategies
Due to the increasing prevalence of resistant hypertension (Calhoun et al., 2008), more aggressive approaches to reducing blood pressure are warranted. In this regard the hypotensive utility of two novel surgical interventions targeting the sympathetic nervous system has recently been described, namely therapeutic renal sympathetic denervation (Krum et al., 2009) and carotid sinus baroreceptor stimulation (Wustmann et al., 2009). Interestingly, in both these cases it seems that modern technological progress has reinvigorated approaches to hypertension management first described in basic work many years previously.
Renal Denervation
Regional surgical sympathectomy for the treatment of hypertension was used to treat resistant hypertension before the availability of antihypertensive medications; however the procedure was fraught with complications and had high rates of postoperative morbidity and mortality (Morrissey et al., 1953). More recently, Krum et al. (Krum et al., 2009) performed a proof-of-principle trial of catheter-based renal sympathetic denervation in patients with resistant hypertension defined as marked hypertension despite three or more medications. A robust reduction in blood pressure (−27 systolic/−17 diastolic mmHg [95% CI 16/11]) was reported one year post-procedure. Notably, the benefits of this procedure, in comparison with earlier approaches to surgical sympathectomy, are that it is brief (median procedure time 38 min), appears to have no deleterious effects on renal function and can be performed without long-term complications. Specifically, the procedure requires a catheter to be inserted into the femoral artery at the groin and advanced to the renal artery, where radiofrequency ablations to the afferent and efferent sympathetic nerves supplying each kidney are performed. An approximate 50% reduction in renal noradrenaline spillover was observed following the procedure verifying denervation of renal efferent sympathetic fibres. Furthermore, in an associated case study the renal ablation procedure was reported to also elicit pronounced reductions in muscle SNA, from 56 bursts per minute at baseline to 19 bursts per minute one year post-procedure. This quite remarkable reduction in sympathetic neural activity of ~66% is perhaps indicative of the beneficial consequents of disruption of renal afferent signalling to the central nervous system (Schlaich et al., 2009). Randomised controlled trials are required to further explore the therapeutic potential of these exciting initial findings.
Chronic Carotid Baroreflex Stimulation
An alternative approach to targeting the sympathetic nervous system in resistant hypertension is by way of surgical implantation of a device which elicits chronic stimulation of the carotid baroreflex. While the importance of the arterial baroreflex in the moment-to-moment regulation of blood pressure is well established, there has been a long history of debate surrounding its involvement in the long-term regulation of blood pressure (Thrasher, 2006). Early reports suggesting that dogs with denervated arterial baroreceptors do not develop hypertension, and that baroreceptor firing adapts during prolonged experimental elevations in pressure in rats, questioned the importance of the baroreceptors in long-term blood pressure regulation. This possibly limited exploration of the arterial baroreflex as a therapeutic target for hypertension (Uppuluri et al., 2009). Nevertheless, it is almost 50 years ago since the use of an implantable baroreflex stimulator in man was described (Bilgutay & Lillehei, 1965). Indeed, Brauwald and colleagues demonstrated that a carotid nerve stimulator implanted in patients for the treatment of intractable angina pectoris elicited consistent reductions in blood pressure and symptomatic relief of angina (Epstein et al., 1969; Braunwald et al., 1970). However the occurrence of perioperative complications and problems with technology at the time (e.g. battery life, current leakage) limited the widespread use of such devices (Uppuluri et al., 2009). More recent technological advances have lead to the development of smaller and more reliable devices (Illig et al., 2006). Following encouraging experimental work in hypertensive animals demonstrating marked and sustained reductions in blood pressure and SNA (Lohmeier et al., 2005), the safety and efficacy of the procedure has recently been established in patients with resistant hypertension (Illig et al., 2006). In brief, the device implantation requires surgical exposition of the carotid sinus and bilateral implantation of electrodes into the perivascular space surrounding the carotid sinus of the neck. The electrodes are then tunnelled subcutaneously and connected to a battery-powered impulse generator which is implanted in the infraclavicular space, in a similar manner to the traditional implantable cardiac pacemaker. In the largest clinical trial of such a device to date, Wustmann et al. (Wustmann et al., 2009) investigated the potential for blood pressure lowering with 3 months of electrical stimulation of the carotid baroreflex in 21 patients with resistant hypertension. Changes in time and frequency domain measures of heart rate variability were also evaluated as an indication of cardiac autonomic control. Following chronic carotid baroreflex stimulation blood pressure was markedly reduced (from 185±31 / 109±24 to 154±23 / 95±16 mmHg), while heart rate also fell (81±11 to 76±10 beats per minute). Changes in heart rate variability suggested a beneficial attenuation in cardiac sympathetic activity and an increase in parasympathetic activity. However, of note, in 6 subjects (~30%) no reductions in blood pressure were observed. While the reasons for such inter-individual differences are unclear, differences in co-existing drug treatments or underlying autonomic dysfunction may have contributed. It remains to be seen whether these encouraging observations persist in longer-term investigations in hypertensive patients, and what the specific effects are on central sympathetic outflow to different regions (e.g. muscle SNA).
Deep Brain Stimulation
A final point worthy of note is that a recent case report has identified a potential highly novel approach to reducing blood pressure in resistant hypertension using the technique of deep brain stimulation (Pereira et al., 2009). Briefly, stimulation of the periaqueductal gray matter area was shown to elicit a sustained reduction in blood pressure in a 58 year-old man treated for neuropathic facial pain. Reductions in blood pressure were accompanied by concomitant reductions in pain. However, given that the periaqueductal gray matter area has projections to cardiovascular areas of the brainstem it is tempting to speculate that obtaining a hypotensive effect independent of any changes in pain may also be possible. This seems reasonable given the number of previous studies showing the importance of this region in the control of blood pressure in humans (Thornton et al., 2001; Green & Paterson, 2008). As such, aside from providing unique treatment for chronic neuropathic pain refractory to medical therapy, this radical approach may also have clinical utility in the future management of resistant hypertension.
Summary
In summary, evidence from animal and human studies suggests that the origin of excessive central sympathetic activity in hypertension is multifocal and may arise from alterations in neural afferent pathways, central neural integration and/or efferent pathways. As such all represent viable targets for therapeutic interventions. Traditional pharmacological approaches to the management of essential hypertension appear ineffective in reducing central sympathetic outflow and in many cases blood pressure. However, several novel and promising therapeutic strategies targeting neurogenic hypertension have recently been developed as the result of an effective 'bench to bedside' approach taken by collectives of basic scientists and clinicians.
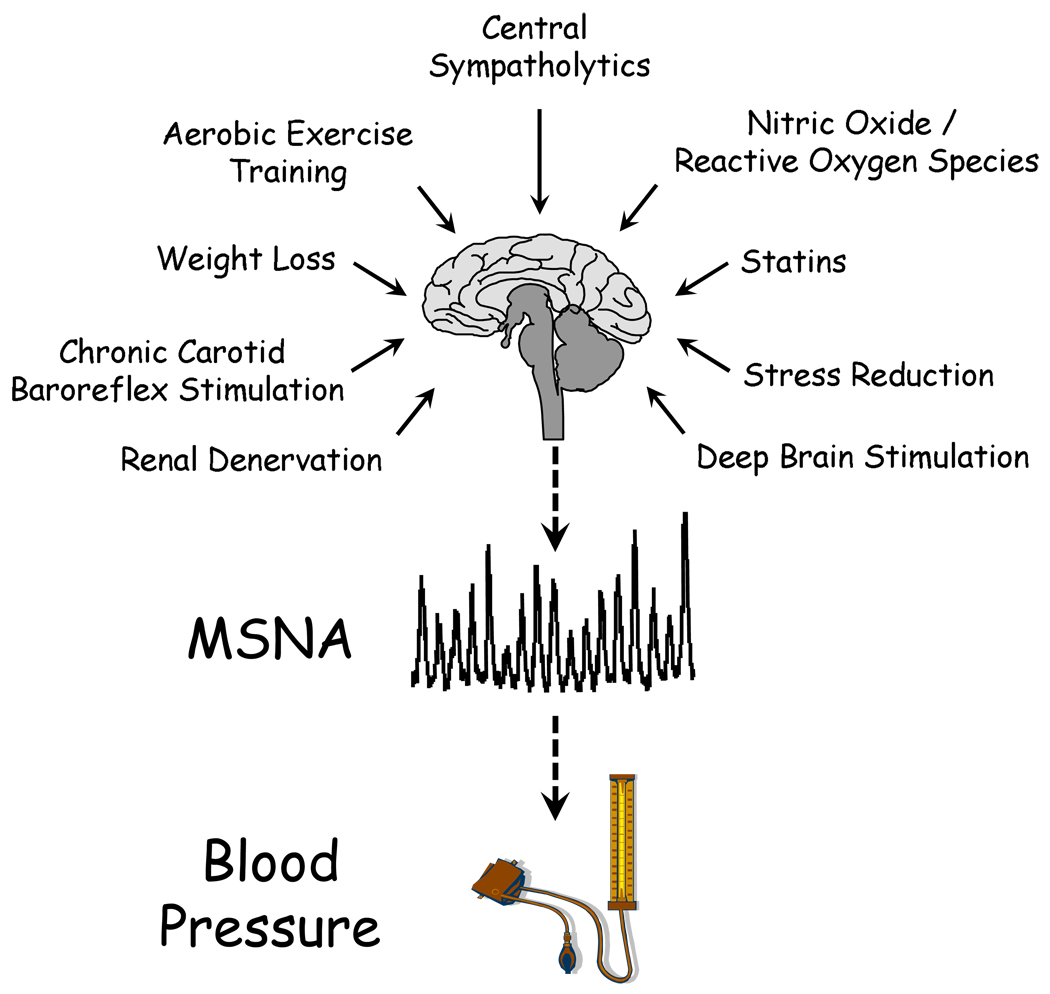
Figure 2. A schematic representation of the current therapeutic strategies and targets for reducing excessive central sympathetic nerve activity in hypertension.
The sympathetic nervous system constitutes an important putative drug target in hypertension. As noted in the text of this review, traditional pharmacological approaches for the management of essential hypertension appear ineffective in reducing central sympathetic outflow. This schematic depicts several novel and promising therapeutic strategies for targeting neurogenic hypertension in the future. SNA, sympathetic nerve activity
Acknowledgements
This review and the original research presented by the authors were, in part, supported by NIH Grant #DK-076636 to PJF.
References
- Ashida T, Ono C, Sugiyama T. Effects of short-term hypocaloric diet on sympatho-vagal interaction assessed by spectral analysis of heart rate and blood pressure variability during stress tests in obese hypertensive patients. Hypertens Res. 2007;30:1199–1203. doi: 10.1291/hypres.30.1199. [DOI] [PubMed] [Google Scholar]
- Bilgutay AM, Lillehei CW. Treatment of Hypertension with an Implantable Electronic Device. JAMA. 1965;191:649–653. doi: 10.1001/jama.1965.03080080039010. [DOI] [PubMed] [Google Scholar]
- Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- Braunwald NS, Epstein SE, Braunwald E. Carotid sinus nerve stimulation for the treatment of intractable angina pectoris: surgical technic. Ann Surg. 1970;172:870–876. doi: 10.1097/00000658-197011000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater Cardiovascular Responses to Laboratory Mental Stress Are Associated With Poor Subsequent Cardiovascular Risk Status. A Meta-Analysis of Prospective Evidence. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Chobanian AV. Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. N Engl J Med. 2009;361:878–887. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 2009;33:116–124. doi: 10.1016/j.neubiorev.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein SE, Beiser GD, Goldstein RE, Stampfer M, Wechsler AS, Glick G, Braunwald E. Circulatory effects of electrical stimulation of the carotid sinus nerves in man. Circulation. 1969;40:269–276. doi: 10.1161/01.cir.40.3.269. [DOI] [PubMed] [Google Scholar]
- Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Dawood T, Kaye D, Barton D, Pier C, Guo L, Brenchley C, Jennings G, Lambert E. Chronic mental stress is a cause of essential hypertension: presence of biological markers of stress. Clin Exp Pharmacol Physiol. 2008;35:498–502. doi: 10.1111/j.1440-1681.2008.04904.x. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–284. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Vaz M, Thompson J, Kaye D, Kalff V, Kelly M, Turner A, Jennings G. Central nervous system monoamine neurotransmitter turnover in primary and obesity-related human hypertension. Clin Exp Hypertens. 1997;19:577–590. doi: 10.3109/10641969709083171. [DOI] [PubMed] [Google Scholar]
- Esler M, Lux A, Jennings G, Hastings J, Socratous F, Lambert G. Rilmenidine sympatholytic activity preserves mental stress, orthostatic sympathetic responses and adrenaline secretion. J Hypertens. 2004;22:1529–1534. doi: 10.1097/01.hjh.0000125453.28861.b8. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci. 2009;148:5–15. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18-year follow-up study. Hypertension. 2008;52:336–341. doi: 10.1161/HYPERTENSIONAHA.108.111625. [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy: a potential mechanism for long term morbidity? Hypertension. 2005;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- Granado NS, Smith TC, Swanson GM, Harris RB, Shahar E, Smith B, Boyko EJ, Wells TS, Ryan MA. Newly reported hypertension after military combat deployment in a large population-based study. Hypertension. 2009;54:966–973. doi: 10.1161/HYPERTENSIONAHA.109.132555. [DOI] [PubMed] [Google Scholar]
- Grassi G. Sympathetic and baroreflex function in hypertension: implications for current and new drugs. Curr Pharm Des. 2004;10:3579–3589. doi: 10.2174/1381612043382756. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F. The 'neuroadrenergic hypothesis' in hypertension: current evidence. Exp Physiol. 2010 doi: 10.1113/expphysiol.2009.047381. [DOI] [PubMed] [Google Scholar]
- Grassi G, Turri C, Dell'Oro R, Stella ML, Bolla GB, Mancia G. Effect of chronic angiotensin converting enzyme inhibition on sympathetic nerve traffic and baroreflex control of the circulation in essential hypertension. J Hypertens. 1998;16:1789–1796. doi: 10.1097/00004872-199816120-00012. [DOI] [PubMed] [Google Scholar]
- Green AL, Paterson DJ. Identification of neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: implications for exercise control. Exp Physiol. 2008;93:1022–1028. doi: 10.1113/expphysiol.2007.039461. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- Illig KA, Levy M, Sanchez L, Trachiotis GD, Shanley C, Irwin E, Pertile T, Kieval R, Cody R. An implantable carotid sinus stimulator for drug-resistant hypertension: surgical technique and short-term outcome from the multicenter phase II Rheos feasibility trial. J Vasc Surg. 2006;44:1213–1218. doi: 10.1016/j.jvs.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Kannel W, Wilson PWE. Cardiovascular risk factors and hypertension. In: Izzo Jl, Black Hr., editors. Hypertension primer: the essentials of high blood pressure. Council for High Blood Pressure Research (American Heart Association); 2003. pp. 235–239. [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kishi T, Hirooka Y, Mukai Y, Shimokawa H, Takeshita A. Atorvastatin causes depressor and sympatho-inhibitory effects with upregulation of nitric oxide synthases in stroke-prone spontaneously hypertensive rats. J Hypertens. 2003;21:379–386. doi: 10.1097/00004872-200302000-00030. [DOI] [PubMed] [Google Scholar]
- Koldas L, Ayan F, Ikitimur B. Short-term effects of rilmenidine on left ventricular hypertrophy and systolic and diastolic function in patients with essential hypertension: comparison with an angiotensin converting enzyme inhibitor and a calcium antagonist. Jpn Heart J. 2003;44:693–704. doi: 10.1536/jhj.44.693. [DOI] [PubMed] [Google Scholar]
- Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1706–H1712. doi: 10.1152/ajpheart.00885.2005. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE, Rondon MU. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. 2007;49:1298–1306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- Lepori M, Sartori C, Trueb L, Owlya R, Nicod P, Scherrer U. Haemodynamic and sympathetic effects of inhibition of nitric oxide synthase by systemic infusion of N(G)-monomethyl-L-arginine into humans are dose dependent. J Hypertens. 1998;16:519–523. doi: 10.1097/00004872-199816040-00013. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Barrett AM, Irwin ED. Prolonged activation of the baroreflex: a viable approach for the treatment of hypertension? Curr Hypertens Rep. 2005;7:193–198. doi: 10.1007/s11906-005-0009-0. [DOI] [PubMed] [Google Scholar]
- Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. 2005;46:1201–1206. doi: 10.1161/01.HYP.0000185147.32385.4b. [DOI] [PubMed] [Google Scholar]
- Mallamaci F, Tripepi G, Maas R, Malatino L, Boger R, Zoccali C. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J Am Soc Nephrol. 2004;15:435–441. doi: 10.1097/01.asn.0000106717.58091.f6. [DOI] [PubMed] [Google Scholar]
- Menon DV, Arbique D, Wang Z, Adams-Huet B, Auchus RJ, Vongpatanasin W. Differential effects of chlorthalidone versus spironolactone on muscle sympathetic nerve activity in hypertensive patients. J Clin Endocrinol Metab. 2009;94:1361–1366. doi: 10.1210/jc.2008-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey DM, Brookes VS, Cooke WT. Sympathectomy in the treatment of hypertension; review of 122 cases. Lancet. 1953;1:403–408. doi: 10.1016/s0140-6736(53)91589-x. [DOI] [PubMed] [Google Scholar]
- Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:676–678. doi: 10.1152/japplphysiol.00446.2006. discussion 681–672. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Wang S, Paterson DJ, Stein JF, Aziz TZ, Green AL. Sustained reduction of hypertension by deep brain stimulation. J Clin Neurosci. 2009 doi: 10.1016/j.jocn.2009.02.041. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Pliquett RU, Cornish KG, Peuler JD, Zucker IH. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation. 2003a;107:2493–2498. doi: 10.1161/01.CIR.0000065606.63163.B9. [DOI] [PubMed] [Google Scholar]
- Pliquett RU, Cornish KG, Zucker IH. Statin therapy restores sympathovagal balance in experimental heart failure. J Appl Physiol. 2003b;95:700–704. doi: 10.1152/japplphysiol.00265.2003. [DOI] [PubMed] [Google Scholar]
- Reid JL. Rilmenidine: a clinical overview. Am J Hypertens. 2000;13:106S–111S. doi: 10.1016/s0895-7061(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol. 2007;21:111–127. doi: 10.1111/j.1472-8206.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- Sinski M, Lewandowski J, Ciarka A, Bidiuk J, Abramczyk P, Dobosiewicz A, Gaciong Z. Atorvastatin reduces sympathetic activity and increases baroreceptor reflex sensitivity in patients with hypercholesterolaemia and systemic arterial hypertension. Kardiol Pol. 2009;67:613–620. [PubMed] [Google Scholar]
- Steptoe A, Willemsen G. The influence of low job control on ambulatory blood pressure and perceived stress over the working day in men and women from the Whitehall II cohort. J Hypertens. 2004;22:915–920. doi: 10.1097/00004872-200405000-00012. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Zhang W, Victor RG. Nitric oxide deficiency as a cause of clinical hypertension: promising new drug targets for refractory hypertension. Jama. 2001;285:2055–2057. doi: 10.1001/jama.285.16.2055. [DOI] [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol. 2001;533:823–836. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher TN. Arterial baroreceptor input contributes to long-term control of blood pressure. Curr Hypertens Rep. 2006;8:249–254. doi: 10.1007/s11906-006-0058-z. [DOI] [PubMed] [Google Scholar]
- Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrao CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. 2003;285:H974–H982. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- Uppuluri SC, Storozynsky E, Bisognano JD. Baroreflex device therapy in the treatment of hypertension. Curr Hypertens Rep. 2009;11:69–75. doi: 10.1007/s11906-009-0013-x. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G, Stromgren E, Aberg H. Sympathetic outflow to muscles during treatment of hypertension with metoprolol. Hypertension. 1984;6:557–562. doi: 10.1161/01.hyp.6.4.557. [DOI] [PubMed] [Google Scholar]
- Wenzel RR, Spieker L, Qui S, Shaw S, Luscher TF, Noll G. I1-imidazoline agonist moxonidine decreases sympathetic nerve activity and blood pressure in hypertensives. Hypertension. 1998;32:1022–1027. doi: 10.1161/01.hyp.32.6.1022. [DOI] [PubMed] [Google Scholar]
- Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y, Delacretaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54:530–536. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD, Fadel PJ. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J Physiol. 2009;587:4977–4986. doi: 10.1113/jphysiol.2009.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Gao L. Statins and sympathetic nerve activity in heart failure. J Card Fail. 2006;12:759. doi: 10.1016/j.cardfail.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev. 2004;32:107–111. doi: 10.1097/00003677-200407000-00006. [DOI] [PubMed] [Google Scholar]