Abstract
High-alcohol-drinking rats, given access to 10% ethanol, expressed an alcohol deprivation effect (ADE) only after multiple deprivations. In alcohol-preferring (P) rats, concurrent access to multiple ethanol concentrations combined with repeated cycles of EtOH access and deprivation produced excessive ethanol drinking. The current study was undertaken to examine the effects of repeated alcohol deprivations with concurrent access to multiple concentrations of ethanol on ethanol intake of HAD replicate lines of rats. HAD-1 and HAD-2 rats received access to 10, 20 and 30% (v/v) ethanol for 6 weeks. Rats from each replicate line were assigned to: (1) a non-deprived group; (2) a group initially deprived of ethanol for 2 weeks; or (3) a group initially deprived for 8 weeks. Following the restoration of the ethanol solutions, cycle of 2 weeks of ethanol exposure and 2 weeks of alcohol deprivation was repeated three times for a total of four deprivations. Following the initial ethanol deprivation period, deprived groups significantly increased ethanol intakes during the initial 24-hour re-exposure period. Multiple deprivations increased ethanol intakes, shifted preference to higher ethanol concentrations and prolonged the duration of the elevated ethanol intakes for up to 5 days. In addition, repeated deprivations increased ethanol intake in the first 2-hour re-exposure period as high as 5–7 g/kg (which are equivalent to amounts consumed in 24 hours by HAD rats), and produced blood ethanol levels in excess of 150 mg%. The results indicate that HAD rats exhibit ‘loss-of-control’ of alcohol drinking with repeated deprivations when multiple ethanol concentrations are available.
Keywords: Alcohol deprivation effect, alcohol relapse, high-alcohol-drinking rats, loss of control, multiple deprivations, sensitization
INTRODUCTION
The alcohol deprivation effect (ADE) is defined as a temporary increase in the voluntary intake of ethanol solutions and the ratio of ethanol/total fluid intake over baseline drinking conditions, when ethanol is returned following a period of alcohol deprivation (Sinclair & Senter 1967, 1968). The ADE has been hypothesized to be an animal model of alcohol craving (Sinclair & Li 1989; Heyser, Schulteis & Koob 1997), and has been utilized to examine the efficacy of pharmacological agents to prevent relapse drinking (Kornet, Goosen & Van Ree 1991; Spanagel & Zieglgansberger 1997; Heyser et al. 1998).
Research has shown that the drinking patterns of human alcoholics are segmented by multiple periods of abstinence and intake (Burish et al. 1981; Hilbrom 1990; McMillen 1997), and that multiple detoxifications are associated with less responsive treatment and greater alcohol drinking (Malcolm et al. 2000). Repeated experience of alcohol withdrawal has been postulated to increase the vulnerability and susceptibility of future withdrawal episodes (Becker, Diaz-Granados & Weathersby 1997). The successive potentiation of withdrawal symptoms after repeated cycles of ethanol exposure and deprivation has been postulated to be a form of neurobiological kindling (Ballenger & Post 1978). Therefore, it is possible that the neurobiological adaptations associated with repeated periods of alcohol exposure and abstinence may increase the hyperexcitability of the central nervous system (CNS), and enhance both the withdrawal and stimulating effects of ethanol (McCown & Breese 1990; Becker et al. 1997).
Studies from our laboratory examined the effects of multiple deprivations on expression of an ADE in alcohol-preferring (P) and high-alcohol-drinking (HAD) rats under 24-hour free-choice alcohol drinking conditions (Rodd-Henricks et al. 2000a,b), using a single ethanol concentration. In P rats given 24-hour free choice between 10% (v/v) ethanol and water, a robust ADE was observed after a single deprivation; repeated deprivations prolonged the expression of the ADE but did not alter its magnitude (Rodd-Henricks et al. 2000a). In a subsequent operant study, the effects of repeated deprivations resulted in higher breakpoint ratios compared with values for non-deprived P rats (Rodd et al. 2003), suggesting that repeated deprivations increased the reinforcing properties of ethanol. In support of this interpretation, evidence from intracranial self-administration experiments indicated that repeated deprivations increased the reinforcing effects of ethanol in the posterior ventral tegmental area (VTA) of P rats (Rodd et al. 2005).
The replicate lines of HAD rats did not show an ADE following a single deprivation under either 24-hour free-choice (Rodd-Henricks et al. 2000b) or operant (Oster et al. 2006) conditions with a single concentration of ethanol available. However, under 24-hour free-choice access, an ADE could be observed after a second deprivation, which was prolonged with repeated deprivations for the HAD-2 replicate line (Rodd-Henricks et al. 2000b); under operant conditions, both replicate lines demonstrated an ADE following a second deprivation (Oster et al. 2006). As was the case for P rats, repeated deprivations increased the breakpoint values in both replicate lines of HAD rats (Oster et al. 2006), suggesting that repeated cycles of ethanol access and abstinence increased the reinforcing effects of ethanol.
In addition, three low alcohol-consuming lines demonstrated a modest (alcohol non-preferring (NP), low alcohol drinking (LAD)-2) to significant (LAD-1) ADE after repeated cycles of ethanol drinking and deprivation, suggesting that selective breeding for low alcohol consumption is not associated with the inability to display an ADE (Bell et al. 2004). However, the ethanol intakes of these lines were low and any increases were small relative to the intakes of the P and HAD lines.
Wolffgramm & Heyne (1995) examined ethanol intakes of non-selected rats given concurrent access to 5, 10 and 20% ethanol over an extended period of time, which was segmented by periods of abstinence. These investigators reported that animals gradually progress from a controlled level to a high level of ethanol intake that is thought to reflect loss of control. The concurrent access to multiple concentrations of ethanol (10, 20 and 30%) under 24-hour free-choice conditions by P rats resulted in a robust ADE after a single deprivation, which increased in magnitude and duration with subsequent deprivations (Rodd-Henricks et al. 2001), suggesting a ‘loss of control’ under these ethanol-drinking conditions. Within the context of this experimental design, an interpretation of ‘loss of control’ is operationally defined as ethanol intakes in a 2-hour period approaching ethanol consumption levels usually attained during a 24-hour period. The markedly higher ethanol intakes observed under these relapse conditions appeared to be due to a shift in preference toward the higher ethanol concentrations (Rodd-Henricks et al. 2001). The preference for the higher concentrations of ethanol subsequently reverted back to the lower concentration across relapse days, the duration of which was related to the deprivation cycle.
In Sardinian alcohol-preferring (sP) rats, exposure to repeated cycles of alcohol deprivation and concurrent access to multiple concentrations of ethanol, increased ethanol consumption twofold during the initial 2-hour period of re-exposure, and irreversible shifted preference to the higher ethanol concentrations (Serra et al. 2003). However, ethanol intakes of sP rats during the initial re-exposure period and during the first 24-hour period following the first deprivation (Serra et al. 2003) were significantly less than observed for the P rats (Rodd-Henricks et al. 2001). Moreover, unlike findings for the P rat (Rodd-Henricks et al. 2001), repeated deprivations had little effect on altering the magnitude or duration of the ADE observed after the first deprivation (Serra et al. 2003).
Excessive alcohol drinking is an important topic to study and understand. In addition to examining the P rat, it would also be important to determine if other lines of rats selectively bred for high alcohol drinking also exhibit excessive alcohol intakes under certain conditions. Therefore, the objective of this study was to determine the effects of repeated deprivations with concurrent access to multiple concentrations of ethanol on expression of an ADE by the replicate lines of HAD rats. The hypothesis to be tested is that repeated deprivations with concurrent access to multiple concentrations of ethanol will result in ‘loss-of-control’ of alcohol drinking by HAD rats.
METHODS
Animals
Adult male HAD-1 and HAD-2 rats from the 35th to the 37th generations weighing 250–325 g at the start of the experiment were used. The replicate lines of HAD rats were derived from the N/Nih heterogeneous stock rats (Li, Lumeng & Doolittle 1993). Rats were maintained on a 12-hour reversed light–dark cycle (lights off at 0900 hours). Food and water were available ad libitum throughout the experiment. Subjects were single-housed in metal cages for 48 hours to acclimate them to the testing conditions. During this time, a single bottle filled with water was placed on the front wall of each home cage. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the Institutional Animal Care and Use Committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (National Research Council, Commission on Life Sciences, National Research Council 2003).
Free-choice alcohol-drinking procedure using multiple ethanol concentrations
Following the acclimation period, rats received continuous, concurrent free-choice access in the home cage to 10, 20 and 30% (v/v) ethanol and water for 6 weeks. The water bottle was always presented on the left front side of the cage. Because of the size of the water bottle and the cage configuration, it was not possible to switch the position of the water bottle with the ethanol drinking tubes. Ethanol solutions were maintained in 25-ml serological tubes that were cut at both ends and sealed with a polyurethane (no. 13) stopper at one end and a rubber stopper fitted with a metal sipper at the other end. The positions of the three ethanol tubes on the right front side of the cage were randomly changed each day, and fluid intakes were recorded to the nearest 0.1 g by weighing the water and ethanol tubes before and after each 24-hour period. Fluid intake measures were converted into g ethanol/kg body weight/day (g/kg/day) and the ratio of intake for each solution (g solution/g total fluid intake/day). Body weights of the rats were recorded twice weekly and fluid intake was measured daily. At the end of the 6-week free-choice access period, ethanol was removed for a 2- or 8-week period (HAD-1 n = 8/group; HAD-2 n = 9/group). These two initial deprivation periods were selected because they encompassed the shortest and longest initial deprivation periods previously used for P rats (Rodd-Henricks et al. 2001). An additional group, never deprived of ethanol, served as age-matched controls (both HAD-1 and HAD-2; n = 6/line). During all deprivation periods, the position of the single water bottle remained on the left front side of the cage. After the first deprivation period, continuous free-choice access to ethanol was given for an additional 2-week period. Ethanol was returned at the onset of the dark cycle (0900 hours). Following the assigned initial deprivation period, rats were allowed a second access to the multiple concentrations of ethanol for a total of 2 weeks. After 2 weeks of ethanol re-exposure, subjects in both deprivation groups were deprived of ethanol for two additional weeks. This cycle of 2 weeks exposure followed by 2 weeks deprivation was continued until all deprived rats had undergone a total of four deprivation and re-exposure periods. Additionally, during the 24-hour period immediately prior to deprivation and during the initial 24-hour period following re-exposure, intakes of the three ethanol solutions were recorded after 2 hours into the dark cycle (0900 hours).
Statistical analyses
Baseline measurements were the average of the 3 days immediately prior to any deprivation period. Initial baseline measurements were analyzed by a between group (two deprivation groups and non-deprived group) analysis of variance (ANOVA). Subsequent baseline measurement analysis consisted of a deprivation cycle × group (initial deprivation length) mixed ANOVA with repeated measures on ‘deprivation’. The non-deprived group was excluded from the baseline data analysis because of the distinct timing of baseline sampling of each deprived group. Experimental data analysis consisted of a deprivation cycle × group (initial deprivation length) × day (baseline versus re-exposure day 1–7) mixed ANOVA with repeated measures on ‘deprivation’ and ‘day’ for ethanol intake. Analysis of the ratio of total fluid intake consisted of a deprivation cycle × group (initial deprivation length) × day (baseline versus re-exposure days 1–7) × fluid (ethanol concentration) mixed ANOVA with repeated measures on ‘deprivation’ and ‘day’.
Analysis of the amount of ethanol consumed at distinct time periods throughout the first ethanol re-exposure day consisted of a deprivation cycle × group (all three groups) × time period mixed ANOVA with repeated measures on ‘deprivation’ and ‘time’. Values for the non-deprived group were the average of the amount of fluid intake recorded during the ethanol re-exposure period for the 2- and 8-week deprived groups. Analysis of the amount of each ethanol solution consumed at distinct time periods throughout the first ethanol re-exposure day consisted of a deprivation cycle (baseline and four deprivations) × group (deprived only) × solution × time period mixed ANOVA with repeated measures on ‘deprivation’ and ‘time’. To determine if consumption of total ethanol, or preference for a particular ethanol solution, was changed following re-exposure, individual one-way ANOVAs were performed separately on each of the initial deprivation groups. All post hoc comparisons performed utilized the Tukey’s b procedure.
Determination of blood ethanol concentrations (BECs)
In a separate experiment, HAD-2 male rats, initially deprived for 2 weeks, were cycled through four deprivation/re-exposure periods; a non-deprived group was taken through at the same time. The deprived group was allowed 2 hours of ethanol re-exposure, fluid intakes were then recorded, and tail blood samples were obtained. Rats were then transported to another room to have trunk blood samples obtained. Tail and trunk blood samples were obtained at the same time for non-deprived rats. Samples were analyzed with an Analox Analyzer (model GL5, Analox Instruments USA, Lunenburg, MA, USA).
RESULTS
Over the initial 6-week period of access to the three concentrations of ethanol, the intakes of the HAD-1 and HAD-2 rats were similar and approximately 7 g/kg/day (data not shown). Ethanol intakes for the non-deprived group did not change significantly during the remainder of the experiment (F17,85 < 1.4; P < 0.14). At the end of the first 6-week period, HAD-1 and HAD-2 rats were consuming slightly more 10% than 20% and 30% (data not shown). The preferences for the different solutions for the non-deprived rats did not change significantly throughout the experimental period (P > 0.82). Water intake (data not shown) remained relatively constant throughout the experimental period.
Effects of the length of the initial deprivation period on daily ethanol intakes across re-exposure days
For HAD-1 and HAD-2 rats, baseline ethanol consumption did not differ (P = 0.46 and P = 0.53, respectively) between the non-deprived and alcohol-deprived groups (Figs 1 & 2). Additionally, the proportion of the three solutions of ethanol consumed under baseline conditions was similar (group P = 0.96 and P = 0.73; group × concentration: P = 0.91 and P = 0.69, respectively) for the non-deprived and alcohol-deprived groups (Figs 3 & 4). Under these baseline conditions, all groups displayed a preference for the 10% concentration (P < 0.001).
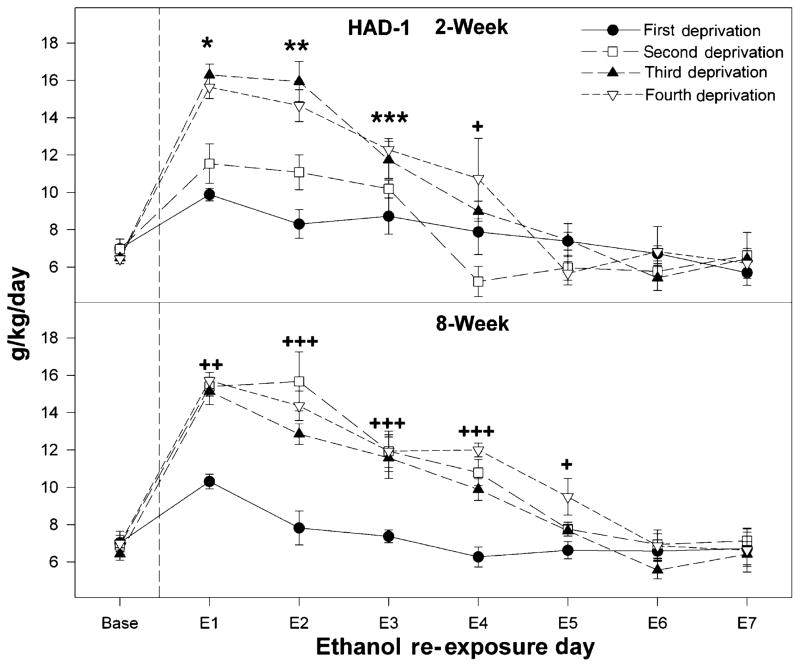
Figure 1.
Ethanol intakes of high-alcohol-drinking-1 (HAD-1) rats initially deprived of alcohol for 2 or 8 weeks and then taken through cycles of 2 weeks ethanol access and 2 weeks ethanol deprivation for a total of four cycles. Baseline values were determined from the average of the last 3 days just before deprivation. A single asterisk (*) indicates that ethanol intake (a) is increased over baseline levels following all deprivation periods; (b) following the second deprivation is greater than following the first deprivation; and (c) following the third and fourth deprivation period is greater than following the first and second deprivation. Double asterisks (**) indicate that ethanol intake is increased over baseline levels following the second, third and fourth deprivation periods, and that intake following the third and fourth deprivation is significantly more than during the second deprivation period. Triple asterisks (***) indicate that ethanol intake is significantly higher than baseline levels following the third and fourth deprivation cycles. A single plus (+) indicates that ethanol consumption following the fourth deprivation period is significantly greater than baseline intake values. Double pluses (++) indicate that ethanol intake is increased, following all deprivation periods over baseline level of intake, and that ethanol intake following the second, third and fourth deprivation period is greater than that observed, following the first deprivation period. Triple pluses (+++) indicate the ethanol intake following the second, third and fourth deprivation period is elevated compared to baseline levels, and following a single deprivation
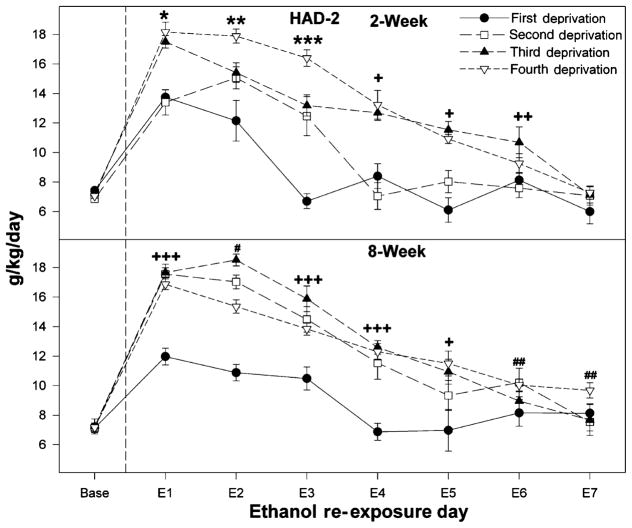
Figure 2.
Ethanol intakes of high-alcohol-drinking-2 (HAD-2) rats initially deprived of alcohol for 2 or 8 weeks and then taken through cycles of 2 weeks ethanol access and 2 weeks ethanol deprivation for a total of four cycles. Baseline values were determined from the average of the last 3 days just before deprivation. A single asterisk (*) indicates that ethanol intake is increased over baseline levels following all deprivation periods and that intake following the third and fourth deprivation period is greater than following the first and second deprivation. Double asterisks (**) indicate that ethanol intake (a) is increased over baseline levels following all deprivation periods; (b) following the second and third deprivation is greater than following the first deprivation; and (c) following the fourth deprivation period is greater than following all other deprivation periods. Triple asterisks (***) indicate that ethanol intake (a) is increased over baseline levels following the second, third and fourth deprivation periods; (b) following the second and third deprivation is greater than following the first deprivation; and (c) following the fourth deprivation period is greater than following all other deprivation periods. A single plus (+) indicates that ethanol consumption following the third and fourth deprivation period is significantly greater than baseline intake values. Double pluses (++) indicate that ethanol intake is increased following the fourth deprivation period. Triple pluses (+++) indicate that ethanol intake is increased over baseline levels following all deprivation periods, and that ethanol intake following the second, third and fourth deprivation period is greater than following the first deprivation. A single pound (#) indicates that ethanol consumption is increased, following all deprivations cycles compared to baseline intake, and all deprivations cycles are different from each other. A double pound (##) symbol indicates that ethanol is significantly elevated, following the fourth deprivation period compared to baseline intake values
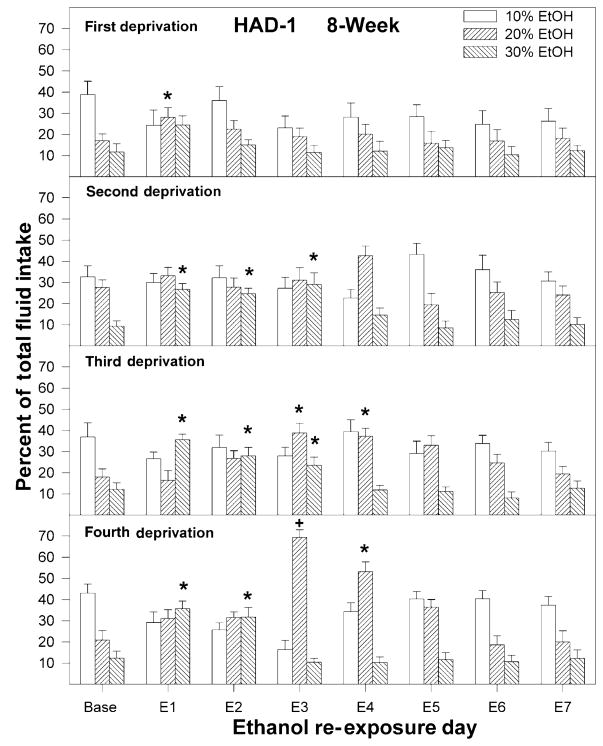
Figure 3.
Percent of total fluid intake consumed by high-alcohol-drinking-1 (HAD-1) rats of 10, 20 and 30% ethanol following each deprivation for the rats initially deprived of alcohol for 8 weeks. Baseline values were determined from the average of the last 3 days just before deprivation. An asterisk symbol (*) indicates that preference for that solution was increased compared to baseline levels. A pound (#) symbol indicates preference for that solution decreased compared to baseline values. A plus symbol (+) indicates that the preference for that solution was increased compared to baseline levels, and compared to preference following all other deprivation cycles on that re-exposure day
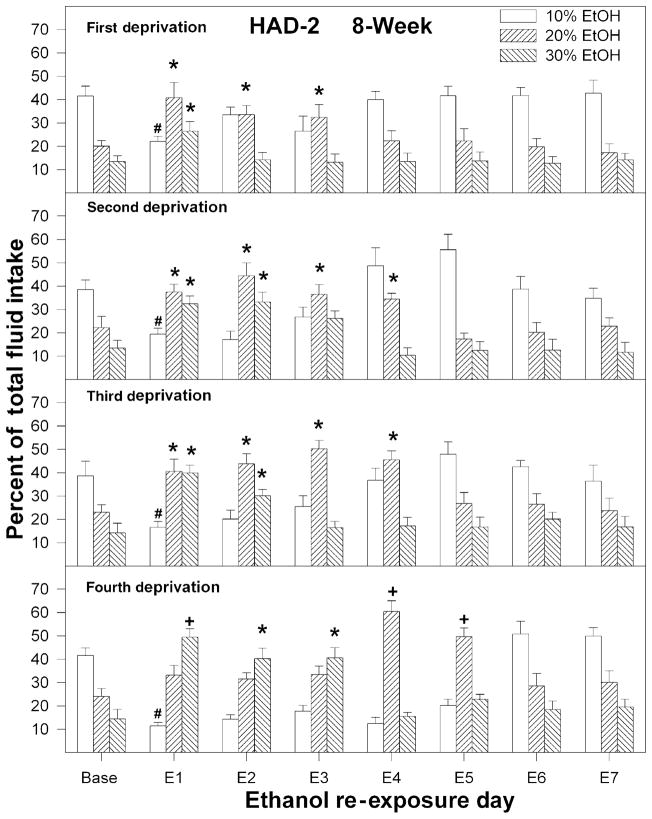
Figure 4.
Percent of total fluid intake consumed by high-alcohol-drinking-2 (HAD-2) rats of 10, 20 and 30% ethanol following each deprivation for the rats initially deprived of alcohol for 8 weeks. Baseline values were determined from the average of the last 3 days just before deprivation. An asterisks symbol (*) indicates that preference for that solution was increased compared to baseline levels. A plus symbol (+) indicates that the preference for that solution was increased compared to baseline levels and compared to preference following all other deprivation cycles
For HAD-1 rats, both the 2- and 8-week deprivation groups displayed significant increases (Day; F4,56 = 10.1; P < 0.001) in the amount of ethanol consumed following the first ethanol re-exposure (Fig. 2). There was no effect of the initial length of deprivation, i.e. 2 versus 8 weeks, on the amount of ethanol consumed (F1,14 = 1.2; P = 0.29), nor was the interaction between day and group significant (F4,56 = 0.51; P = 0.55). In both deprived groups, ethanol intake was elevated from 7 to 10 g/kg/day on the first re-exposure day, and returned to near baseline levels on the second re-exposure day.
For HAD-2 rats (Fig. 3), there was a significant increase in ethanol intake in both deprived groups (Day; F4,64 = 43.6; P < 0.001); there was also an effect of the initial deprivation length on ethanol intake (Day × Group interaction; F4,56 = 5.9; P = 0.006). The significant interaction term was decomposed by holding the within-subject factor ‘Day’ constant. ANOVAs performed on each individual re-exposure day indicated that on the first re-exposure day, HAD-2 rats initially deprived for 2 weeks consumed more ethanol than rats initially deprived for 8 weeks (F1,16 = 5.5; P = 0.032; Fig. 3; 12 versus 14 g/kg/day). On the other hand, on the third re-exposure day, the 8-week group still had elevated intakes and consumed more ethanol than the 2-week group (F1,16 = 16.7; P < 0.0001).
Effects of length of the initial deprivation period on relative consumption of the 10, 20 and 30% ethanol solutions
For HAD-1 and HAD-2 rats, consumption of the different ethanol solutions upon re-exposure to ethanol for the 8-week deprivation groups is depicted in Figs 4 and 5, respectively (data for 2-week group are not shown and are similar to the data for the 8-week group). For both HAD-1 and HAD-2 rats, following the first deprivation, there was increased preference for the higher concentrations of ethanol. For both rat lines, there was an effect of Day and Concentration during the first re-exposure period (P < 0.001) with no significant effect of group (P > 0.33) and no Day × Group interaction (P > 0.27).
Figure 5.
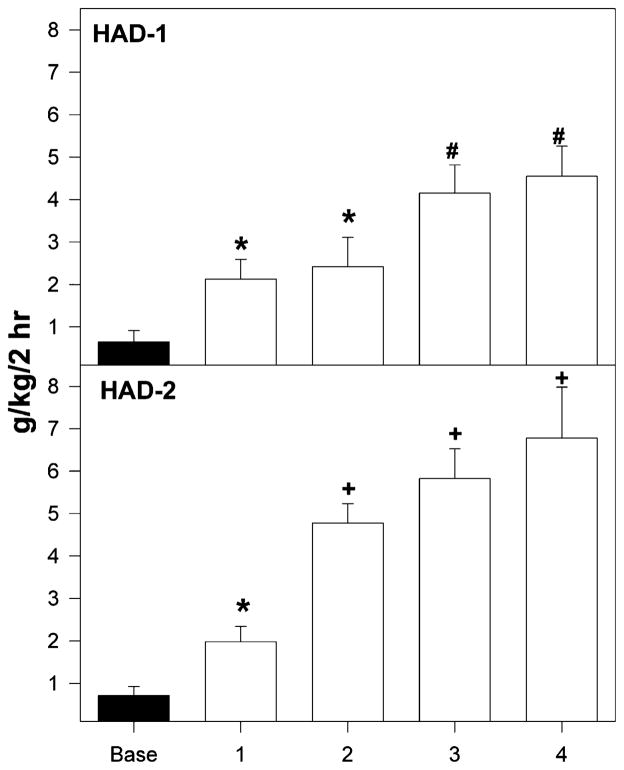
Ethanol intakes during the first two hours of access following a deprivation period by high-alcohol-drinking-1 (HAD-1) and HAD-2 rats, initially deprived for 8 weeks and then exposed to repeated cycles of ethanol access and deprivation. An asterisks symbol (*) indicates that ethanol consumption increased compared to baseline levels. A pound (#) symbol indicates that ethanol intake increased compared to baseline levels and intake following the first deprivation period. A plus symbol (+) indicates that ethanol consumption increased compared to (a) baseline levels, (b) intake following the first deprivation, and (c) intakes observed for HAD-1 rats in that deprivation cycle
For HAD-1 rats (both 2- and 8-week groups), consumption of 20% ethanol was increased for only 1 day (P < 0.03). For HAD-2 rats, consumption of 20 and 30% ethanol was increased during the first re-exposure day for both the 2- and 8-week deprived groups (P < 0.02). Consumption of 20% ethanol was also increased over baseline for both deprived groups (P < 0.02) during the second re-exposure day and for HAD-2 rats deprived for 8 weeks during the third re-exposure day (P < 0.03). In addition, the percentage of 10% ethanol consumed during the first re-exposure day was reduced in both HAD-2 deprived groups (P < 0.001).
Effects of repeated deprivations on daily ethanol intakes
Comparing baseline ethanol consumption (g/kg/day) on the last 3 days of the initial 6 weeks of exposure with baseline ethanol intake on the last 3 days of the 2-week ethanol re-exposure periods (Figs 1 & 2) did not reveal a significant effect of deprivation cycle, group, or group × cycle interaction for either HAD-1 or HAD-2 rats (all F < 1.46; all P > 0.27).
For HAD-1 rats, the 2- and 8-week deprivation groups displayed higher ethanol intakes (Fig. 1), following the second through the fourth deprivation compared with the first deprivation or baseline. There was a significant effect of deprivation cycle (F3,42 = 21.18; P < 0.001) and a significant interaction between deprivation cycle, initial deprivation length and day (F21,294 = 11.37; P < 0.001). Examining the effects of repeated deprivations on ethanol intake in rats initially deprived for 2 weeks (Fig. 1, top) indicated that ethanol intakes following the third and fourth deprivations were higher than the intakes following the first and second deprivation periods. One-way ANOVAs across re-exposure days indicated that, following the second and third deprivations, ethanol consumption was elevated above baseline levels for the initial 3 days of re-exposure (P < 0.04), whereas following the fourth deprivation, ethanol intake was increased over baseline levels for the initial 4 days of re-exposure (P < 0.03).
The effects of repeated deprivations on ethanol intake in HAD-1 rats initially deprived for 8 weeks (Fig. 1, bottom) indicated that the second through the fourth deprivation increased ethanol intakes to the same level during the initial 24-hour period of re-exposure compared with levels after the initial deprivation period (P < 0.001). In addition, the elevated ethanol intakes extended for 4 days following the second and third deprivation periods (P < 0.002), and for 5 days following the fourth deprivation (P values < 0.03).
For HAD-2 rats in the 2- and 8-week deprivation groups (Fig. 2), there was a significant effect of deprivation cycle (F3,48 = 75.14; P < 0.001) and a significant interaction between deprivation cycle, initial deprivation length and day (F21,336 = 4.7; P < 0.001) on ethanol intake.
Examining the effects of repeated deprivations on ethanol intake in rats initially deprived for 2 weeks (Fig. 2, top), there was a significant effect of deprivation cycle on ethanol consumption during the initial 24-hour period of re-exposure (P < 0.001); ethanol intakes following the third and fourth deprivations were higher than intakes following the first and second deprivations, which were similar to each other. One-way ANOVAs performed across days indicated that ethanol intakes were elevated above baseline levels for the initial 3 days of re-exposure following the second deprivation (P < 0.001), and for the initial 6 days of re-exposure following the third and fourth deprivations (P < 0.044).
The effects of repeated deprivations for rats initially deprived for 8 weeks (Fig. 2, bottom) indicated that the second through the fourth deprivation increased ethanol intake about the same amount during the initial 24-hour period of re-exposure, and intakes were all higher than intakes following the first deprivation (P < 0.001). In addition, ethanol intakes were elevated for 4 consecutive days following the second deprivation period (P < 0.02), and for 5 and 7 days following the third and fourth deprivation period, respectively (P < 0.05).
Contrasting the effects of exposure to repeated cycles of ethanol access and deprivation for both HAD-1 and HAD-2 rats indicated a significant cycle × day × group × line interaction (F42,840 = 1.93; P < 0.001). In general, HAD-2 rats had higher ethanol intakes that persisted across days compared with HAD-1 rats.
Effects of repeated deprivations on consumption of water and the 10, 20 and 30% ethanol solutions
Baseline water intakes prior to deprivation averaged 7.4 ± 2 and 7.9 ± 2 ml/day for HAD-1 and HAD-2 rats, respectively. For HAD-1 and HAD-2 rats, during the initial 24-hour ethanol re-exposure period following the first deprivation, water consumption for both the 2-week and 8-week groups were not significantly different from water intake for the non-deprived group or from baseline values. With repeated alcohol deprivations, water intakes were not significantly different from water intakes for the non-deprived HAD rats (data not shown).
The relative intakes of the three different ethanol solutions for the HAD-1 and HAD-2 rats in the 8-week deprived group, as a function of re-exposure day and number of deprivations, are depicted in Figs 3 and 4. To minimize the number of complex graphs, only data for the 8-week group are shown. Comparable patterns of preferences for the three different ethanol solutions following a single deprivation or repeated deprivations were observed for the 2-week groups. The relative preferences for the three solutions of ethanol under baseline conditions (last 3 days prior to deprivation) did not change significantly during the course of the experiment (main effects of deprivation and group, and group × deprivation × concentration interaction were not significant; P > 0.28). For HAD-1 and HAD-2 rats, both the 2- and 8-week deprivation groups displayed a shift in ethanol solution preference following repeated deprivation cycles (P < 0.001). This shift in preference occurred primarily on days when increased ethanol intake was also observed.
For HAD-1 rats, there was no significant effect of group (P = 0.26) or group × day × deprivation cycle × concentration interaction (F42,546 = 0.79; P = 0.82), but there was a significant effect of deprivation cycle × day × concentration (F42,546 = 3.1; P < 0.001). For brevity, only the analysis of the 8-week group will be detailed (Fig. 3), but similar results were observed for the 2-week group. One-way ANOVAs revealed that, following the second and third deprivations, the amount of 30% ethanol consumed was elevated compared with baseline levels for the initial 3 re-exposure days (Day; F7,56 > 3.76; P < 0.002; post hoc P < 0.05); following the fourth deprivation cycle, preference for the 30% ethanol solution was increased only for the first 2 days. There was not much change in the preference for 20% ethanol during the first 2 re-exposure days with repeated deprivations. However, preference for 20% ethanol increased during the third and fourth re-exposure days following the third and fourth deprivations compared with preference observed following the first deprivation and baseline values (ANOVA P < 0.001, post hoc Tukey’s b test).
For HAD-2 rats, there was a significant group × day × deprivation cycle × concentration interaction (F42,630 = 3.6; P < 0.0001) and deprivation cycle × day × concentration (F42,630 = 3.8; P < 0.001). For brevity, only the analysis of the 8-week group will be detailed (Fig. 4), but similar results were observed with the 2-week group data. One-way ANOVAs revealed that, following the second and third deprivation periods, preference for 20% ethanol was increased for 4 consecutive days, and preference for 30% ethanol was increased for 2 consecutive days. Following the fourth deprivation, preference for 30% ethanol was increased for 3 consecutive days, and preference for 20% ethanol was increased during re-exposure days 4 and 5 (Fig. 4). The cycle × day × concentration interaction effect was decomposed to reveal that the preference for 20% and 30% ethanol increased over deprivation cycles. On the other hand, preference for the 10% ethanol solution decreased on the first re-exposure day following each deprivation.
Ethanol intake in the first 2 hours of the first re-exposure day
Repeated deprivations enhanced the amount of ethanol consumed within the first 2 hours of ethanol re-exposure in HAD-1 and HAD-2 rats initially deprived for either 2 or 8 weeks when compared with the level of intake for non-deprived controls (Fig. 5 shows data for the 8-week group; similar results were obtained with the 2-week group). For HAD-1 rats, there was a significant effect of cycle (F3,56 = 19.2; P < 0.001) and group (F2,19 = 21.2; P < 0.001), but no cycle × group interaction (F3,56 = 0.7; P = 0.63). For HAD-2 rats, there was a significant effect of cycle, group and a cycle × group interaction (P < 0.001). Contrasting HAD-1 and HAD-2 rats, the analysis revealed a significant effect of line and a line × cycle × group interaction (P < 0.001). HAD-1 rats had comparable intakes of 2–2.5 g/kg following the first and second deprivation; following the third and fourth deprivations, intakes increased to 4–4.5 g/kg in the first 2 hours of access. The intakes of HAD-2 rats following the first deprivation were around 2 g/kg; however, with subsequent deprivations, ethanol intakes for the HAD-2 rats progressively increased from 5 to almost 7 g/kg (Fig. 5). Ethanol intakes of the HAD-2 rats were higher than intakes for HAD-1 rats following the second through the fourth deprivation.
BECs
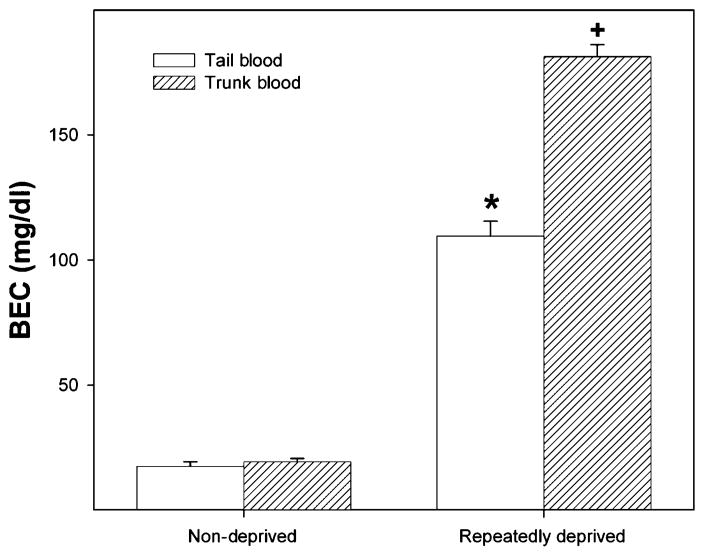
In a separate experiment, BECs were determined in HAD-2 rats after the first 2 hours of access following the fourth deprivation (Fig. 6). Ethanol intakes in the initial 2-hour time period were 1.1 ± 0.3 g/kg for the non-deprived group, and 5.8 ± 1.1 g/kg for the deprived rats. There was a significant effect of group (F1,13 = 665.56; P < 0.001), sample region (F1,13 = 61.59; P < 0.001) and a sample region × group interaction (F1,13 = 55.87; P < 0.001). The data indicated little difference in BECs between tail and trunk sampling in non-deprived rats (17 versus 19 mg%, respectively). In contrast, BECs were approximately 70% higher in the trunk blood samples than tail blood samples in the deprived group, and were in excess of 150 mg%.
Figure 6.
Tail and trunk blood ethanol concentrations (BECs) following the first 2 hour of ethanol access by high-alcohol-drinking-2 (HAD-2) rats after the fourth deprivation. An asterisks symbol (*) indicates that BECs were higher than baseline levels. A plus symbol (+) indicates that BECs were higher compared to baseline levels and tail blood values
DISCUSSION
The most significant findings of this study are that HAD-1 and HAD-2 rats, when given concurrent access to multiple high concentrations of ethanol, (1) display a robust ADE following a single deprivation (Figs 2 & 3); (2) show a more pronounced ADE in both magnitude and duration with repeated deprivations (Figs 2 & 3); and (3) exhibit ‘loss-of-control’ of alcohol drinking with repeated deprivations (Fig. 6).
In the present study, HAD-1 rats (Fig. 2) demonstrated a 1.5-fold increase in ethanol intake over baseline, and HAD-2 rats (Fig. 3) had a nearly twofold higher increase in ethanol intake over baseline after a single deprivation. A previous study (Rodd-Henricks et al. 2000b) reported that an ADE was not observed after a single deprivation for either HAD-1 or HAD-2 rats under similar drinking conditions, when only a single concentration (10%) of ethanol was available. The expression of an ADE after a single deprivation is not a result of higher baseline ethanol intakes or duration of ethanol intake because baseline intakes (~7 g/kg/day) and duration of drinking (6 weeks) were similar between the two studies. The marked difference in ethanol intake following a single deprivation between the two studies is a result of HAD rats, in the present study, shifting their preference from 10% ethanol to 20 and 30% ethanol in the first re-exposure day (Figs 4 & 5). The shift in preference was a function of the prolonged deprivation because preference for the 10% concentration of ethanol returned to baseline levels within the 2-week ethanol access period (Figs 4 & 5), and preferences for the three concentrations of ethanol for the non-deprived group did not change over time with continuous access to ethanol (Fig. 1). In the present study, the reasons for the shift in preference for the higher concentrations of ethanol following the 2 weeks of alcohol abstinence are not known but may be due to taste factors and/or an attempt to more rapidly attain the previous ‘alcohol state’. Excessive alcohol drinking that is observed following a history of dependence might be a result of the development of an abnormal homeostatic state (Roberts et al. 2000).
The ethanol intakes of the HAD-2 rats following the first deprivation appear to be higher than that for HAD-1 rats (approximately 12–13 g/kg/day for HAD-2 and 10 g/kg/day for HAD-1 rats; Figs 2 & 3). The elevated ethanol intakes of the HAD-2 rats are comparable with intakes observed for P rats under similar deprivation and drinking conditions (Rodd-Henricks et al. 2001). In addition, the duration of the ADE following the single deprivation was similar for the HAD-2 (Fig. 3) and P rats. With a single ethanol concentration following repeated deprivations, the ADE is usually observed only on the first re-exposure day (Rodd-Henricks et al. 2000b), whereas with concurrent access to multiple ethanol concentration, HAD-2 rats exhibit elevated ethanol intakes for up to 3 days (Fig. 3). These latter results are also similar to findings for the P rat under the same experimental conditions (Rodd-Henricks et al. 2001). Under 24-hour drinking conditions, with multiple ethanol concentrations available, sP rats show a modest increase in ethanol intake (6 g/kg/day at baseline to 7.5 g/kg/day on first re-exposure day) following a single deprivation (Serra et al. 2003). A similar increase in ethanol intake was not observed in sP rats following a single deprivation when a single concentration of ethanol was available (Agabio et al. 2000). Although the magnitude of the ADE was different among the four lines of rats selectively bred for alcohol drinking (P, HAD-1, HAD-2 and sP rats), the overall results indicate that concurrent availability of multiple high concentrations of ethanol has a significant impact on expression of an ADE, suggesting that there may be environmental factors interacting with one or more genes to influence relapse drinking.
Repeated deprivations increased the magnitude and duration of the ADE in both lines of HAD rats (Figs 2 & 3). Moreover, the initial 8-week deprivation period had a greater effect than the 2-week deprivation period on the magnitude and duration of ethanol drinking following the second deprivation. For the HAD-1 rats, ethanol intakes were 11–12 g/kg/day over 3 days for the 2-week group following the second deprivation, whereas for the 8-week group, ethanol intakes were nearly 16 g/kg/day within this 3-day period. Moreover, for the 8-week group, intakes were still significantly higher than control levels on re-exposure day 5. A similar difference was observed for the 2- and 8-week groups of HAD-2 rats following the second deprivation, although intakes were higher for the HAD-2 than HAD-1 rats (Figs 2 & 3). The finding that differences were observed between the 2- and 8-week groups following the second but not the first deprivation suggests that additional abstinence is needed for certain neuronal alterations to occur to influence expression of an ADE. This effect may be limited to HAD rats because similar effects following the second deprivation were not observed between the 2- and 8-week groups of P rats under the same alcohol drinking conditions (Rodd-Henricks et al. 2001).
The availability of multiple ethanol concentrations exacerbates the effects of repeated deprivations on ethanol drinking by HAD rats (Figs 2 & 3). Following the third and fourth deprivations, ethanol intakes were as high as 16–18 g/kg/day for HAD rats. Intakes of at least 12 g/kg/day were observed over a 4-day period. These intakes are twice the amount normally consumed under baseline and would qualify as a ‘bender’. These intakes would be the equivalent of a normal size man consuming over two-fifths of whiskey a day, and would be considered very dangerous drinking. The high ethanol intakes and the duration of these effects with repeated deprivations were also observed for P rats (Rodd-Henricks et al. 2001). However, these findings do not generalize to all selectively bred rat lines because repeated deprivations with concurrent access to multiple concentrations of ethanol did not significantly increase ethanol intakes of sP rats (Serra et al. 2003).
The peak increases of 16–18 g/kg/day observed in the present study following repeated deprivations (Figs 2 & 3) are significantly higher than peak ethanol intakes of 10–11 g/kg/day observed for HAD and P rats given access to a single (10%) solution of ethanol (Rodd-Henricks et al. 2000a,b) These results suggest that certain subjects with a genetic vulnerability to HAD will drink even more excessively under relapse conditions when strong alcoholic drinks are available. This would be the equivalent of a man switching from beer or wine to shots of whiskey, i.e. more alcohol taken in per unit drink. In fact, the HAD-2 rats exhibit excessive alcohol drinking after the second deprivation, whereas the HAD-1 rats show excessive alcohol consumption after the third deprivation. This excessive alcohol consumption could be categorized as ‘loss-of-control’, as indicated by HAD rats consuming nearly as much alcohol in 2 hours (Fig. 6) as they would normally consume in a 24-hour period (Fig. 1). BECs were in excess of 150 mg% in HAD-2 rats after the fourth deprivation. P rats also demonstrated ‘loss-of-control’ upon re-exposure following a prolonged deprivation period (Rodd-Henricks et al. 2001). In this latter study, however, P rats showed this effect after the first deprivation, and ethanol intakes during the first 2 hours of re-exposure increased with subsequent deprivations (Rodd-Henricks et al. 2001). In contrast, sP rats did not exhibit this level of ethanol intake in the first hour of re-exposure following repeated deprivations with multiple ethanol concentrations available (Serra et al. 2003). Overall, the findings with the different selectively bred rat lines suggest that different combination of genes may influence ethanol intake under maintenance conditions versus relapse conditions. Moreover, the differences observed between the replicate HAD lines under relapse conditions suggest that there may be differences in their genetic makeup. Analogous differences between the HAD-1 and HAD-2 lines were observed under operant conditions (Oster et al. 2006).
The very high ethanol intakes observed with repeated deprivations suggest that tolerance to the aversive effects of ethanol may have developed; the immediate relapse of drinking suggests that sensitivity to the reinforcing effects of ethanol may have persisted during prolonged abstinence. A previous study demonstrated that chronic alcohol drinking alone increased the threshold to the aversive effects of ethanol (Stewart et al. 1991), suggesting the development of tolerance. It is possible that repeated deprivations might exacerbate the effects observed with chronic drinking alone. An intracranial self-administration study indicated that chronic alcohol drinking by P rats increased the sensitivity of the posterior VTA to the reinforcing effects of ethanol and also appeared to produce tolerance to a non-reinforcing concentration of ethanol (Rodd et al. 2005). Moreover, this study (Rodd et al. 2005) showed that repeated deprivations exacerbated the effects of chronic drinking on both sensitivity to low ethanol doses and the ‘apparent’ tolerance to a high ethanol concentration. An operant oral self-administration study with HAD-1 and HAD-2 rats (Oster et al. 2006) indicated that higher breakpoint ratios were attained by HAD rats that had been repeatedly deprived compared with non-deprived HAD rats, suggesting that the reinforcing effects of ethanol increased with multiple deprivations. Therefore, the effects of repeated deprivations may increase the rewarding effects of alcohol and produce tolerance to the aversive effects of alcohol. The net result of this is that HAD rats are more likely to relapse, and when they do relapse, they are likely to drink in large excess, especially when high concentrations of ethanol solutions are available. This experimental procedure may be a suitable model for studying neurobiological mechanisms underlying relapse and excessive alcohol drinking.
In addition to the alterations in the rewarding and aversive properties of ethanol contributing to relapse behavior, it is also possible that stress may be produced with repeated deprivations (Holter et al. 1998) and this could contribute to relapse behavior. Another factor that could contribute to relapse behavior is that dependence could have developed with the alcohol drinking conditions used in the present study. Physical signs of withdrawal were not examined in the present study. In the preceding instances, relapse drinking may be initiated to reduce stress and the negative symptoms of dependence (Liu & Weiss 2002a,b).
Similar to the results with P rats (Rodd-Henricks et al. 2001), in the present study, the effects of prolonged deprivations were reversible, because ethanol intakes (Figs 2 & 3) and preferences for different ethanol solutions (Figs 4 & 5) returned to baseline values within 2 weeks of ethanol access. These results suggest that the presence of ethanol reversed the neuronal alterations that had developed during the ethanol-free period. Moreover, the alterations that did occur during deprivation appeared to persist beyond the initial 2-week deprivation, as indicated by similar expression of an ADE being observed in the 2- and 8-week groups following the first deprivation (Figs 2 & 3).
Neurobiological mechanisms underlying relapse and excessive alcohol drinking are not well understood. It is likely that changes in multiple neurotransmitter systems in multiple regions are involved in mediating relapse and excessive alcohol drinking. Local cerebral glucose utilizatio studies (Smith et al. 2001, 2002) indicated that chronic alcohol drinking reduced functional glucose utilization in several limbic regions and that some of these changes were still evident after 2 weeks of deprivation, having partially recovered or not recovered at all, whereas functional activity in other limbic regions had completely recovered. These results suggest that neuronal imbalances may exist within and between limbic regions following prolonged deprivation, which may contribute to relapse drinking.
The dose–response effects of 5-HT3 receptor antagonists in reducing ethanol drinking were shifted to the right, following a long-term deprivation (Rodd-Henricks et al. 2000c) in P rats, suggesting that alterations in the 5-HT3 receptor system have developed with alcohol drinking. Alterations in the opioid system may have also developed with repeated deprivations, as suggested by the finding that naltrexone was less effective in reducing reinstatement in rats that have undergone multiple withdrawals (Ciccocioppo et al. 2003). A microdialysis study (Thielen et al. 2004) indicated that chronic alcohol drinking by P rats increased dopamine neurotransmission in the nucleus accumbens, which could be a result in part of reduced dopamine D2 autoreceptor function. Moreover, these changes in dopamine neuronal activity and D2 autoreceptor function persisted in the absence of ethanol for at least 2 weeks (Thielen et al. 2004). In addition, alterations in excitatory and inhibitory neurotransmission may have occurred in stock Wistar rats with repeated episodes of withdrawal (Dahchour & De Witte 2003). Overall, the above findings indicated that chronic alcohol drinking and deprivations altered multiple neurotransmitter systems and affected several CNS regions, and that some of these changes persist in the absence of alcohol and might be factors contributing to relapse.
In summary, the present results indicate that HAD-1 and HAD-2 rats display a robust ADE following a single deprivation when concurrent access to multiple high concentrations is available. With repeated deprivations, HAD-1 and HAD-2 rats show a ‘loss-of-control’ over alcohol drinking that is a result of a shift in preference from 10% ethanol to 20 and 30% ethanol.
Acknowledgments
This study was supported in part by NIAAA grants AA07611, AA10721 and AA11261.
References
- Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G. Development of short-lasting alcohol deprivation effect in Sardinian alcohol-preferring rats. Alcohol. 2000;21:59–62. doi: 10.1016/s0741-8329(00)00072-0. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, Li T-K, McBride WJ. Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol—consuming rats. Alcohol Clin Exp Res. 2004;28:1867–1874. doi: 10.1097/01.alc.0000148101.20547.0a. [DOI] [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42:1013–1020. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology. 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- Hilbrom ME. Alcohol withdrawal seizures and binge versus chronic drinking. In: Port RJ, Mattson RH, Cramer JA, Diamond I, editors. Alcohol and Seizures: Basic Mechanisms and Clinical Concepts. Philadelphia, PA: FA Davis; 1990. pp. 206–215. [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Kornet M, Goosen C, Van Ree JM. Effect of naltrexone on alcohol consumption during chronic alcohol drinking and after a period of imposed abstinence in free-choice drinking rhesus monkeys. Psychopharmacology. 1991;104:367–376. doi: 10.1007/BF02246038. [DOI] [PubMed] [Google Scholar]
- Li T-K, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behav Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus non-dependent rats. J Pharmacol Exp Ther. 2002a;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotrophin-releasing factor and opioid mechanisms. J Neurosci. 2002b;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22:159–164. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol ‘kindles’ inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- McMillen BA. Toward a definition of a valid animal model of alcoholism: multiple animal models for multiple diseases. Alcohol. 1997;14:409–419. doi: 10.1016/s0741-8329(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA. Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines. Alcohol. 2006;38:155–164. doi: 10.1016/j.alcohol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li T-K, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005;315:648–657. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Edmundson VE, Dagon CL, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of 5-HT3 receptor antagonists on daily alcohol intake under acquisition, maintenance, and relapse conditions in alcohol preferring (P) rats. Alcohol. 2000c;21:73–85. doi: 10.1016/s0741-8329(00)00083-5. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li T-K. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000b;24:747–753. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li T-K. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000a;24:8–16. [PubMed] [Google Scholar]
- Serra S, Brunetti G, Vacca G, Lobina C, Carai MAM, Gessa GL, Colombo G. Stable preference for high ethanol concentrations after ethanol deprivation in Sardinian alcohol-preferring (sP) rats. Alcohol. 2003;29:101–108. doi: 10.1016/s0741-8329(03)00003-x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Li T-K. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following deprivation. Psychon Sci. 1967;8:11–12. [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li T-K, Murphy JM. Long-term effects of alcohol drinking on cerebral glucose utilization in alcohol-preferring rats. Pharmacol Biochem Behav. 2001;69:543–553. doi: 10.1016/s0091-3057(01)00553-6. [DOI] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li T-K, Murphy JM. Local cerebral glucose utilization after relapse in ethanol drinking in alcohol-preferring (P) rats. Alcohol. 2002;27:115–126. doi: 10.1016/s0741-8329(02)00216-1. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–59. [PubMed] [Google Scholar]
- Stewart RB, McBride WJ, Lumeng L, Li T-K. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology. 1991;105:530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]