Abstract
L-selectin is an adhesion molecule expressed by neutrophils that broadly directs their infiltration in to sites of inflammation, and is also present at relatively high levels in the serum of normal individuals. It is well established that L-selectin is efficiently shed from the surface of neutrophils upon their activation, a process that regulates its density and binding activity. Neutrophil programmed cell death is critical for the resolution of inflammation, and L-selectin down-regulation is induced during this process as well. The mechanisms underpinning this latter process are much less understood, and were investigated here. Using ADAM17 radiation chimeric mice, we demonstrate for the first time that during early events of death receptor-mediated neutrophil apoptosis, L-selectin down-regulation occurs primarily by ADAM17-mediated shedding. This was observed as well upon using shRNA to knock-down ADAM17 expression in Jurkat cells, a well-studied cell line in terms of the molecular processes involved in the induction of apoptosis. These findings directly reveal that ADAM17 activity occurs during programmed cell death. Hence, the cleavage of particular ADAM17 substrates may be an additional component of the anti-inflammatory program initiated by apoptotic neutrophils. Of interest was that during later stages of induced leukocyte apoptosis, soluble L-selectin production occurred independent of ADAM17, as well as membrane events such as blebbing and microparticle production. This process may provide an explanation for the lack of diminished serum L-selectin levels in ADAM17-null mice, and suggests a mechanism for the homeostatic maintenance of soluble L-selectin levels in the blood of healthy individuals.
INTRODUCTION
Ectodomain shedding is a regulated proteolytic process that directs the cleavage of cell surface proteins, typically at a juxta-membrane site, resulting in the release of a soluble extracellular domain fragment (1–3). The functional implications of ectodomain shedding are diverse, as it can promote an immediate and prolonged formation of soluble agonists and antagonists, as well as regulate the density of receptors and adhesion molecules. The majority of a growing list of shed proteins are cleaved by ADAM173, a disintegrin and metalloprotease (4). Leukocytes express ADAM17 and a number of their products undergo ectodomain shedding (5). By directly examining leukocytes deficient in functional ADAM17, it has been reported that this sheddase cleaves various inflammation regulating factors, including the pleiotropic cytokine TNFα, its two receptors TNFRI (CD120a) and TNFRII (CD120b), and L-selectin (CD62L) (6–11).
ADAM17’s enzymatic activity is inducible and its function has been primarily studied in the context of cell activation (12–14). The leukocyte-expressed adhesion molecule L-selectin is perhaps one of the best characterized shed molecules in terms of structure/function analyses and its targeting by ADAM17 (8, 14–18), and thus is an excellent model protein to study ADAM17 activity in leukocytes. L-selectin is constitutively expressed at high levels by resting neutrophils, and essentially all molecules are cleaved within minutes of neutrophil activation by a variety of physiological stimuli (19).
In addition to their pro-inflammatory role during activation, neutrophils mediate an anti-inflammatory program upon programmed cell death (20, 21), which includes an efficient down-regulation of cell surface L-selectin expression (22, 23). In our study, we directly exam ADAM17’s role of in this process by using leukocytes that lack expression of the sheddase. Our findings show that during early events of induced neutrophil apoptosis, L-selectin down-regulation occurs primarily by ADAM17-mediated ectodomain shedding, indicating ADAM17 activity during the anti-inflammatory program. We also report that at later time points of induced neutrophil apoptosis, soluble L-selectin production can occur in a manner independent of ADAM17. This process may be important for regulating the high levels of soluble L-selectin in the blood of healthy individuals, a potential anti-adhesive mechanism.
MATERIALS AND METHODS
Antibodies and other reagents
The anti-human L-selectin mAb DREG-200 has been previously described (24). The anti-L-selectin mAb LAM1-116 (detects both murine and human L-selectin) conjugated to PE, biotin, or allophycocyanin (APC), and recombinant human Fas ligand were purchased from Ancell (Bayport, MN). PE-conjugated anti-mouse LFA-1 (CD11a/CD18) mAb, PE- and APC-conjugated anti-mouse L-selectin (Ly-22) mAb, and Alexa Fluor-488 anti-mouse Ly-6G (Gr-1) were purchased from eBioscience (San Diego, CA). The anti-human LFA-1 (CD11a/CD18) mAb R3.1 has been previously described (25). The anti-Fas IgM mAb CH-11 was purchased from Upstate (Lake Placid, NY). The anti-ADAM10 and ADAM17 mAbs (clones 163003 and 111623, respectively) and TNFα were purchased from R&D Systems (Minneapolis, MN). Biotin, FITC-, PE-, and APC-conjugated F(ab')2 goat anti-mouse IgG secondary antibodies as well as APC-conjugated streptavidin were purchased from Jackson Immunoresearch (West Grove, PA). DMEM, RPMI, PBS, HBSS, Hepes, and molecular grade water were purchased from Mediatech (Hevdon, VA). FBS was purchased from Atlas Biologicals (Fort Collins, CO). Penicillin-Streptomycin solution was purchased from Cellgro (Manassas, VA). PMA was purchased from Sigma-Aldrich (St. Louis, MO). TAPI-1 was purchased from Peptides International (Louisville, KY). Leupeptin, 6-aminocaproic acid, APMSF-HCl, aprotinin, pepstatin A, and chymostatin were all purchased from Sigma. For cell apoptosis detection, a FLICA Poly Caspase kit (FAM-VAD-FMK) was purchased from ImmunoChemistry Technologies (Bloomington, MN) and FITC and PE-conjugated annexin-V-was purchased from BD Pharmingen (San Diego, CA).
Mice
All experimental procedures were performed in accordance with protocols approved by the Animal Care and Use Committee of the University of Minnesota. ADAM17 deficiency in mice is lethal between embryonic day 17.5 or soon after birth (7), and therefore radiation chimeric mice were generated by reconstitution with embryonic day 15–16, liver hematopoietic cells that either lacked or expressed functional ADAM17, as previously described (8, 9). These mice are referred to as either ADAM17 chimeric or wild-type chimeric mice, respectively. ADAM8 knock-out mice were generously provided by Dr. Andy J.P. Docherty (Celltech R&D, Slough, United Kingdom) via Dr. Carl P. Blobel (Hospital for Special Surgery, Weill Medical College of Cornell University) (26). Age-matched mice were used for all experiments.
Cell culture, transduction, and induction of cellular apoptosis
All reagents used for cell isolation and incubations were sterile and tested for endotoxin. Cell viabilities were assessed by exclusion of the vital dye trypan blue. Mouse bone marrow neutrophils were isolated as previously described (8, 9). For neutrophil treatments, cells were suspended in PBS at the indicated concentrations and initially equilibrated to culture conditions at 37°C in 5% CO2. Some cell samples were incubated with the broad-spectrum zinc metalloprotease inhibitor TAPI (50 µg/ml) for 30 min, and then were treated with TNFα (50 ng/ml) with or without cycloheximide (35 µM) for the indicated time points. Jurkat cells (an acute T cell leukemia cell line) were maintained in DMEM/F12 supplemented with 8% heat-inactivated FBS and antibiotics at 37°C in 5% CO2. For Jurkat cell treatments, cells were suspended in RPMI at the indicated concentrations and initially equilibrated to culture conditions at 37°C in 5% CO2. Some cell samples were incubated with TAPI (50 µg/ml) for 30 min, and then treated with PMA (25 ng/ml), recombinant human FasL, or anti-Fas antibody CH-11 (both of these reagents were used at a final concentration of 0.5 µg/ml) for the indicated time points. In additional experiments, Jurkat cells were incubated with TAPI and/or various broad-spectrum inhibitors of serine, cysteine, or aspartate proteases (leupeptin, 100 µM; 6-aminocaproic acid, 10mM; APMSF-HCl, 50 µM; aprotinin, 20 µg/ml; pepstatin A, 3 µM; chymostatin, 20 µM) for 30 min, and then treated with anti-Fas antibody CH-11 for the indicated time points. The working concentrations of these inhibitors were based on those frequently used in the literature (http://sciencegateway.org/resources/protease.htm).
For expression of shRNA in Jurkat cells, the human ADAM17 sequence 5’-GCTTGATTCTTTGCTCTCA-3’, which was determined to knock-down ADAM17 expression, or the ADAM17 sequence 5’-CAGCAGCTGGAGTCCTGTG-3’, which did not knock-down ADAM17 expression and was used as a control to assess off-target effects, were followed by a 9 nucleotide non-complementary spacer (TTCAAGAGA). With their respective reverse complement sequence, the double-stranded DNA constructs were inserted into the pLL3.7 lentiviral vector (Addgene Inc., Cambridge, MA), per the manufacture’s instructions, after vector digestion with XhoI and HpaI (New England Biolabs, Ipswich, MA). Lentivirus generation and transduction were performed as previously described (14, 17).
Flow cytometry and ELISA
For cell staining, Fc receptor and nonspecific antibody binding sites were blocked and cells were stained with particular mAbs, as previously described (8, 27). Briefly, mouse bone marrow leukocytes were triple-stained with anti-Gr-1, anti-mouse L-selectin or anti-LFA-1, and annexin-V. For cell staining that involved annexin-V (for labeling of externalized phosphatidylinositol) or FLICA (labeling of active caspases), non-fixed cells were used, and following their staining with antibodies, the cells were then treated with either reagent (per the respective manufacture’s instructions) and immediately examined by flow cytometry, as previously described (23). ADAM17 and ADAM10 are typically expressed at low levels and so their staining on Jurkat cells was amplified by treating cells with an anti-ADAM17 or anti-ADAM10 mAb, biotin-conjugated F(ab’)2 goat anti-mouse IgG, and then APC-conjugated streptavidin. Isotype-matched negative control mAbs were used to evaluate levels of nonspecific staining. All stained cells were analyzed on a FACSCanto instrument (Becton Dickinson, San Jose, CA).
Soluble murine or human L-selectin in tissue culture media supernatants were quantified by ELISA, as previously described (8, 17). In some cases, media supernatants were filtered (0.22 µm pore size) then centrifuged at 100,000 × g prior to their analysis by ELISA.
RESULTS
ADAM17 cleaves L-selectin upon the induction of apoptosis
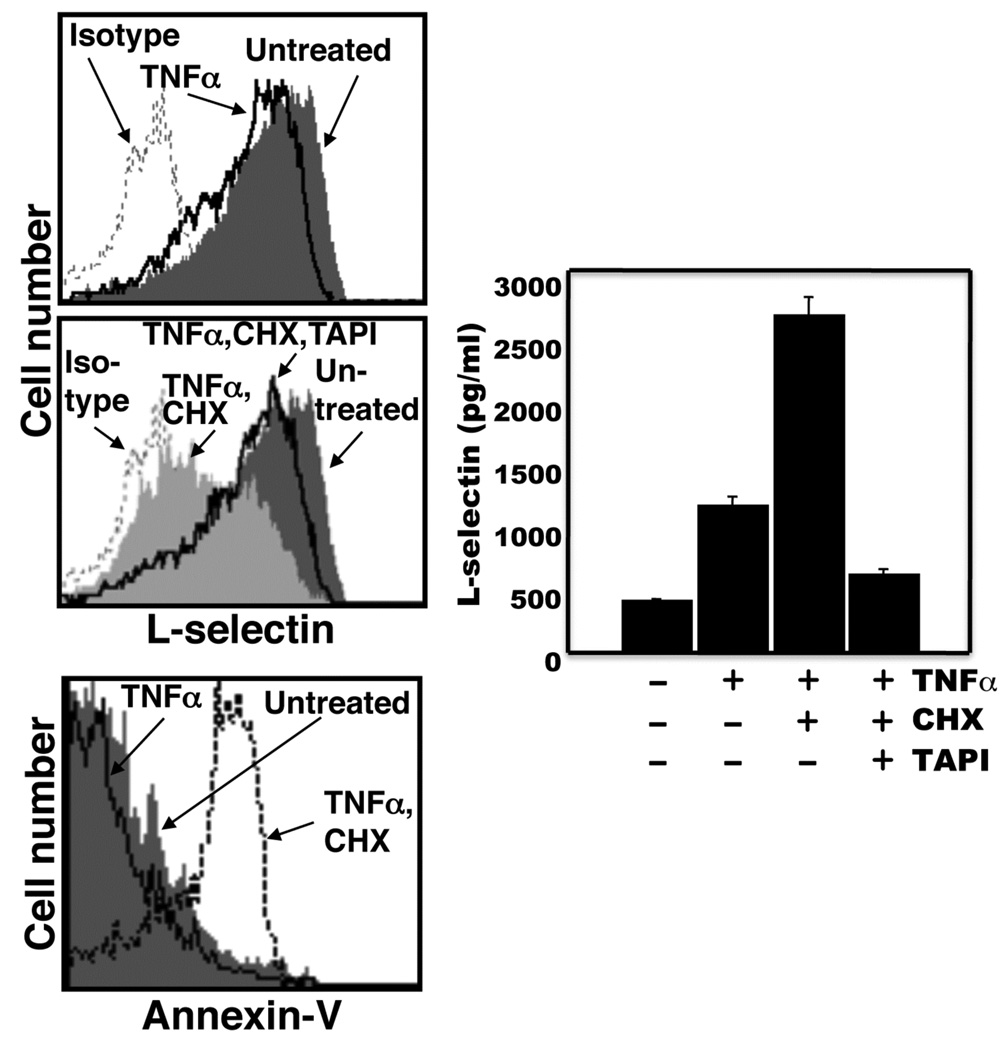
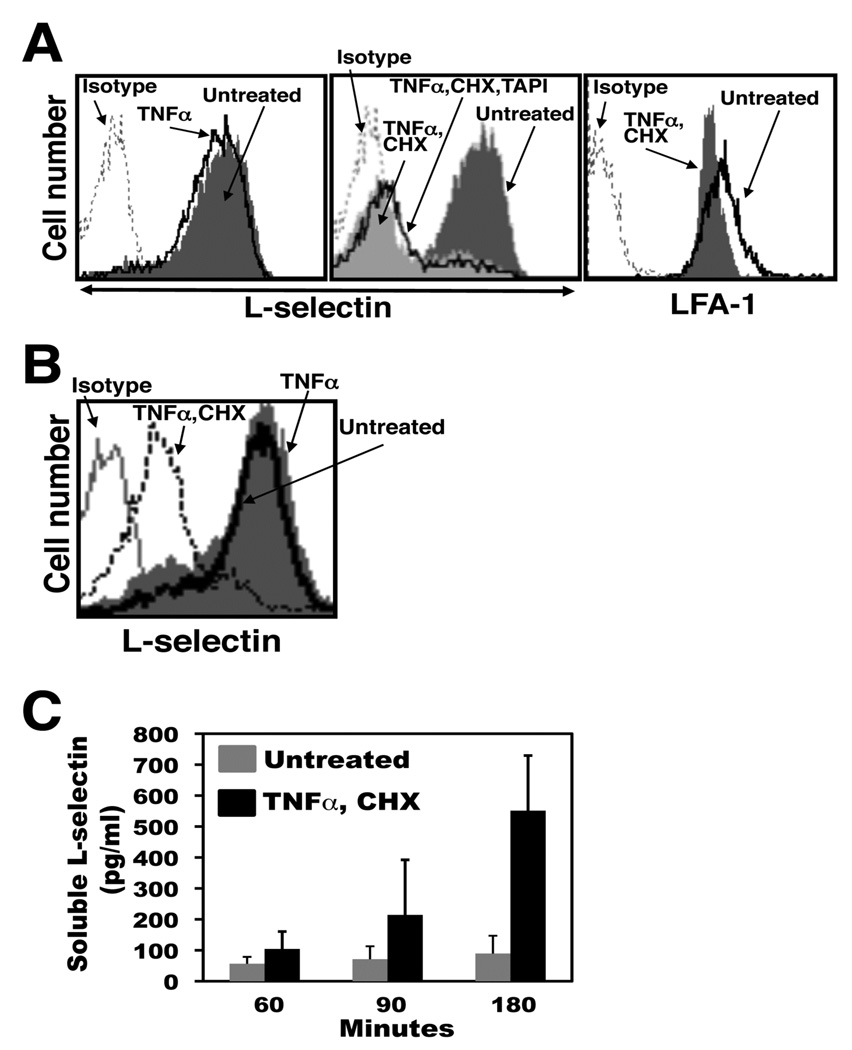
Human neutrophils upon the induction of apoptosis down-regulate their surface L-selectin in a manner involving metalloproteases (23). L-selectin is shed by ADAM17 following neutrophil activation (8), yet recent studies have revealed that other sheddases can also cleave L-selectin (28–30). To directly establish the role of ADAM17 in L-selectin shedding during death receptor-induced apoptosis, we examined murine neutrophils lacking functional ADAM17. Because important differences can occur between human and murine neutrophils (31), we initially established the effects of induced apoptosis by murine neutrophils on their expression of L-selectin. Our primary means of inducing apoptosis by isolated human neutrophils has been through the Fas receptor (23), due to its well established signaling cascades and its direct manner of caspase activation (32). However, our Fas-binding reagents for human leukocytes (see below) demonstrated little activity with murine neutrophils (data not shown). Instead, TNFα in the presence of cycloheximide was used, which is a reproducible method for inducing neutrophil apoptosis (33–36). Murine TNFα was initially tittered down to a concentration that induced nominal L-selectin shedding via neutrophil activation during the timeframe of our assays. We observed that our source of TNFα at 50 ng/ml had little effect on phosphatidylserine exposure and L-selectin shedding by murine neutrophils, whereas when combined with cycloheximide, both events were markedly enhanced (Fig. 1). L-selectin surface expression was down-regulated in a relatively rapid manner and typically occurred before appreciable reactivity by annexin-V with the neutrophils, as determined by flow cytometry. In Figures 1 and 2, the two events were evaluated at different time points. The down-regulation in L-selectin surface expression and production of its soluble extracellular fragment were consistently observed in our assays by 90 min after the induction of apoptosis (Fig. 1), and at this time point, soluble L-selectin production was essentially abolished by broad-spectrum zinc metalloprotease inhibitors, such as TAPI (Fig. 1). Such inhibitors, however, did not prevent the cells from undergoing apoptosis, as previously described for human neutrophils (23).
Figure 1. L-selectin shedding occurs by murine neutrophils upon the induction of apoptosis.
A, Mouse bone marrow-derived neutrophils (2×106/ml) were either untreated or treated with TNFα, TNFα and cycloheximide (CHX), or TNFα, CHX, and TAPI. The cells were cultured at 37°C for 90 min prior to L-selectin detection and for 180 min prior to phosphatidylserine detection. Relative L-selectin surface expression levels and annexin-V reactivity were determined by flow cytometry, as described in the Materials and Methods. Data shown are representative of at least three independent experiments using neutrophils isolated from separate animals. Negative control antibody staining of untreated cells is indicated (Isotype). The x axis = Log 10 fluorescence. To assess soluble L-selectin levels, neutrophils (4×106/ml) were treated as described above for 90 min at 37°C, and media supernatants from the respective samples were subjected to ELISA, as described in the Material and Methods. The ELISA data are the mean (± SD) of three independent experiments performed in duplicate.
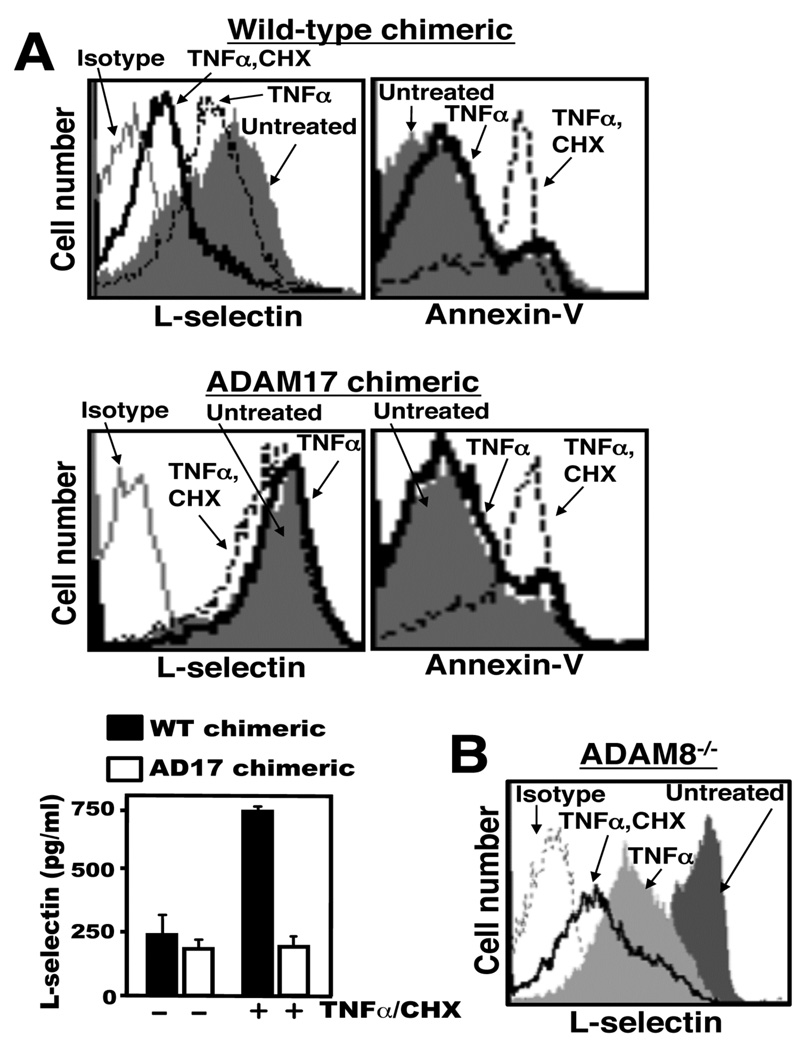
Figure 2. ADAM17, but not ADAM8, mediates L-selectin shedding at early time points of induced apoptosis.
A, Mouse bone marrow-derived neutrophils (2×106/ml) from wild-type chimeras or ADAM17 chimeras were either untreated or treated with TNFα or TNFα and CHX for 90 min (panels marked L-selectin) or for 180 min (panels marked annexin-V) at 37°C. B, Mouse bone marrow-derived neutrophils (2×106/ml) from ADAM8 knock-out mice were treated as described above. Relative L-selectin surface expression levels and annexin-V reactivity were determined by flow cytometry, and data shown are representative of at least three independent experiments using neutrophils isolated from separate animals. Negative control antibody staining of untreated cells is indicated (Isotype). The x axis = Log 10 fluorescence. Soluble L-selectin levels in the media supernatants were determined by ELISA, and data shown are the mean (± SD) of 3 independent experiments performed in duplicate.
Targeting the Adam17 gene in all cells of mice is lethal and most die soon after birth (7). However, radiation chimeric mice reconstituted with hematopoietic cells lacking functional ADAM17 (ADAM17 chimeras) are viable (8, 9). An assessment of neutrophils from wild-type chimeras (reconstituted with wild-type hematopoietic cells) revealed that they also shed their L-selectin upon the induction of apoptosis by TNFα/cycloheximide, as determined by flow cytometry and ELISA (Fig. 2A). In contrast to these cells, the shedding of L-selectin by neutrophils from ADAM17 chimeras was quite minimal at early time points of induced apoptosis (Fig. 2A). Neutrophils from both chimeras demonstrated similar levels of phophatidylserine exposure after their treatment with TNFα/cycloheximide (Fig. 2A), indicating that a lack of functional ADAM17 did not prevent the apoptotic process.
ADAM8 has also been implicated in L-selectin shedding by activated neutrophils (29), and thus we examined its contribution in the shedding of L-selectin by apoptotic neutrophils as well. This was also done in a direct manner by using neutrophils obtained from ADAM8 knock-out mice. In contrast to ADAM17-deficient neutrophils, however, we observed that ADAM8-deficient neutrophils efficiently down-regulated their surface L-selectin upon their treatment with TNFα/cycloheximide (Fig. 2B).
We also directly examined the role of ADAM17 in L-selectin shedding by human leukocytes. This was done by using shRNA specific for ADAM17 to knock-down its expression. Targeting ADAM17 in human neutrophils is problematic considering that they are short-lived and that their manipulation by transfection or transduction consistently induced some level of activation, which may confound our results. Instead, Jurkat cells, a human T cell line, were used. The advantages of these cells are that they express high levels of endogenous L-selectin, unlike most human myeloid cell lines, and that Jurkat cells are one of the best studied cell types with regard to Fas-induced apoptosis (37). We found that the engagement of their Fas receptor led to efficient L-selectin shedding, which was performed by using either soluble recombinant human FasL (rhFasL) or the anti-Fas IgM mAb CH-11 (supplemental Fig. 1A–C). As with neutrophils, the induction of L-selectin shedding by Jurkat cells occurred as an early apoptotic event prior to phosphatidylserine exposure (supplemental Fig. 1A). The treatment of Jurkat cells with rhFasL consistently induced L-selectin shedding and phosphatidylserine exposure more rapidly than CH-11 treatment (data not shown), which may have to do with different manners of Fas engagement.
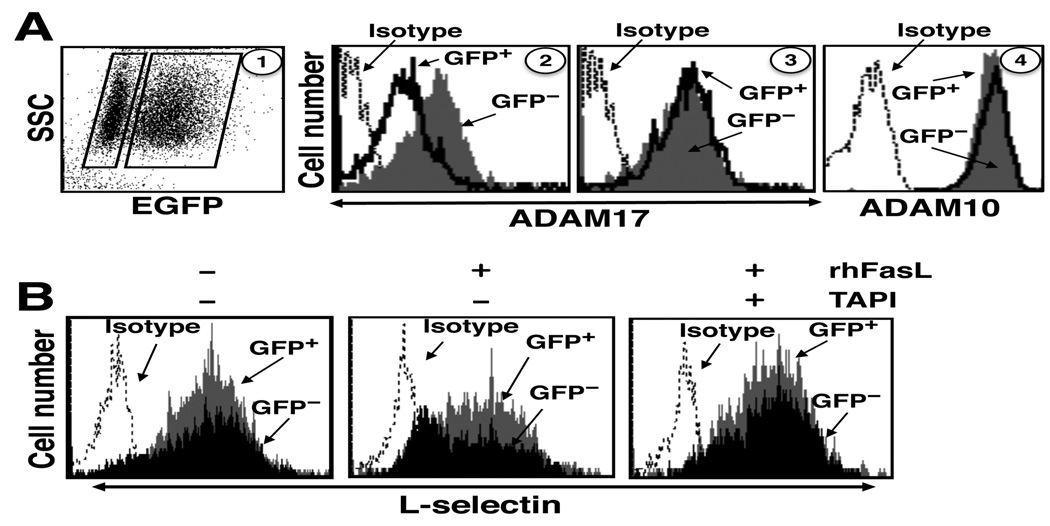
Jurkat cells were transduced with a bicistronic lentiviral vector that expressed GFP and ADAM17 shRNA in a proportional manner. Greater than 50% of the transduced Jurkat cells expressed GFP, and the GFP-positive cells expressed ≈50% less cell surface ADAM17 than the GFP-negative cells (Fig. 3A), whereas surface ADAM10 expression, the ADAM family member most similar to ADAM17 (38), was equivalent for both populations (Fig. 3A). Control ADAM17 shRNA was observed to have no effect on the surface levels of ADAM17 expression (Fig. 3A). Following the treatment of Jurkat cells transduced with active ADAM17 shRNA with rhFasL, L-selectin surface expression down-regulated to a greater extent by GFP-negative cells than by GFP-positive cells (Fig. 3B). When the transduced cells were treated with TAPI prior to the induction of apoptosis, L-selectin shedding by the GFP-negative cells was blocked, demonstrating that their down-regulation of L-selectin occurred by ectodomain shedding (Fig. 3B). Taken together, the above findings reveal ADAM17 activity upon the induction of leukocyte apoptosis, and that it is the primary sheddase of L-selectin during this process.
Figure 3. shRNA specific for ADAM17 diminishes L-selectin shedding upon Fas engagement.
A, Jurkat cells were transduced with a bicistronic lentiviral vector expressing GFP and shRNA directed against human ADAM17 or control shRNA, as described in the Materials and Methods. Transduction efficiency was >50%, as indicated by GFP expression. A representative dot plot indicating the proportion of GFP expressing cells is shown in panel 1. GFP-negative and -positive cells (left and right boxes, respectively, panel 1) were electronically gated and each population was analyzed for their relative expression levels of ADAM17 and ADAM10 by flow cytometry. Transduced Jurkats cells expressing ADAM17 shRNA were stained for cell surface ADAM17 (panel 2) or ADAM10 (panel 4). Transduced Jurkats cells expressing control shRNA were stained for ADAM17 expression (panel 3). B, The transduced Jurkats cells were treated with rhFasL in the presence or absence of TAPI, as indicated, at 37°C for 30 min. Relative L-selectin surface expression levels were determined by flow cytometry. Negative control antibody staining of all cells is indicated (Isotype). The x axis = Log 10 fluorescence. SSC = laser light side scatter. The data are representative of three independent experiments.
Soluble L-selectin is produced at later stages of induced leukocyte apoptosis independent of ADAM17
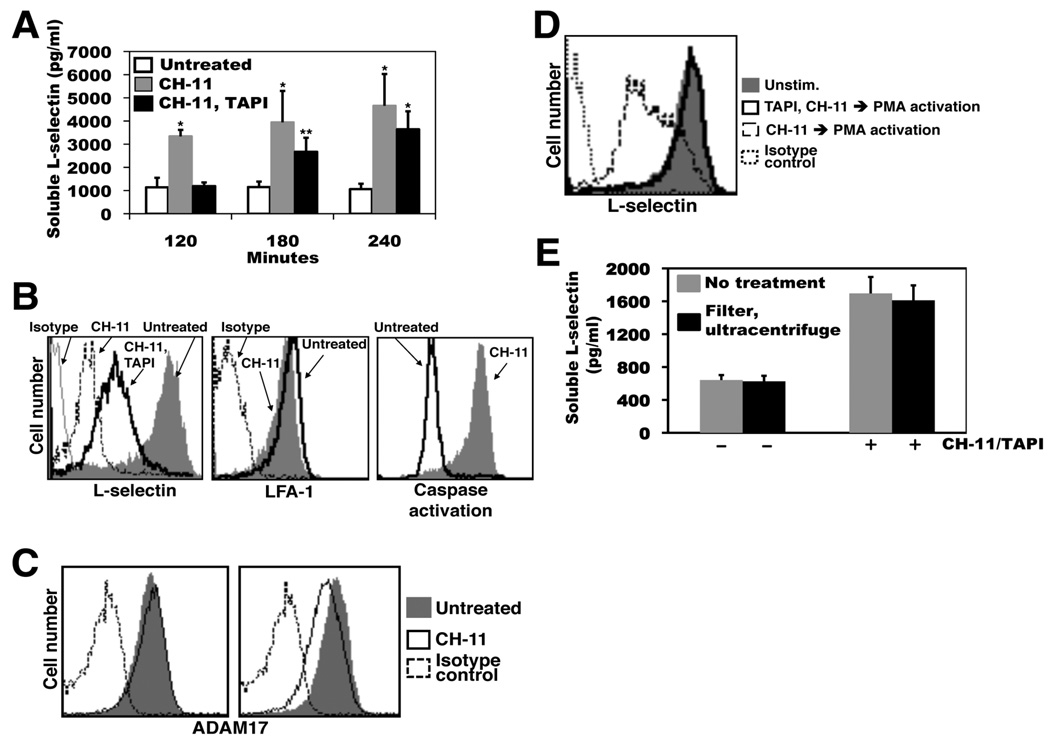
When monitoring L-selectin shedding by Jurkat cells undergoing induced apoptosis in the presence of TAPI over an extended time period, we noted that though initially blocked, considerable production of soluble L-selectin did occur at later time points. As shown in Figure 4A, soluble L-selectin production was assayed for up to 4 hr by Jurkat cells that were either untreated, treated with CH-11, or treated with CH-11 and TAPI. Two hours after the induction of apoptosis, high levels of soluble L-selectin were detected, which was essentially abolished by the presence of TAPI. At the 3 and 4 hr time points, however, apoptotic Jurkat cells in the presence of TAPI produced significantly higher levels of soluble L-selectin than time-matched, untreated cells (Fig. 4A). At these later time points of apoptosis, cell surface L-selectin also underwent a corresponding down-regulation in expression in the presence of TAPI (Fig. 4B). This late apoptotic process did not result in a general down-regulation in the expression of cell surface molecules, as the surface levels of LFA-1 on the same cells were only modestly decreased (Fig. 4B). We also monitored ADAM17 surface expression on Jurkat cells at various time points of apoptosis. Jurkat cells treated with CH-11 for 2 hours demonstrated surface ADAM17 expression levels equivalent to time-matched, untreated cells, whereas cells treated for with CH-11 for 4 hours demonstrated a marginal decrease in ADAM17 staining when compared to time-matched, untreated cells. Hence, soluble L-selectin production occurred at later stages of apoptosis in the presence of a broad-spectrum metalloprotease inhibitor and during the down-regulation of ADAM17 surface expression.
Figure 4. Soluble L-selectin is generated by Jurkat cells in the presence of TAPI at later time points of induced apoptosis.
A, Jurkat cells (2×106/ml) were incubated with the anti-Fas antibody CH-11 in the presence or absence of TAPI for the indicated time points at 37°C. The presence of soluble L-selectin in the cell supernatants was determined by ELISA. B, Jurkat cells (2×106/ml) were incubated with the anti-Fas antibody CH-11 in the presence or absence of TAPI, or were left untreated, as indicated, for 4 hr at 37°C. Relative L-selectin or LFA-1 surface expression levels, and caspase activity (FLICA reactivity) were determined by flow cytometry. C, Jurkat cells (2×106/ml) were incubated with the anti-Fas antibody CH-11 or were left untreated, as indicated, for 2 hr (left panel) or 4 hr (right panel) at 37°C. Relative ADAM17 surface expression levels were determined by flow cytometry. D, Jurkat cells (2×106/ml) were incubated with the anti-Fas antibody CH-11 in the presence or absence of TAPI, as indicated (TAPI, CH-11 or CH-11, respectively) for 4 hr at 37°C. Tissue culture media supernatants from the two cell treatments were removed of cells by centrifugation, filtered (0.22µm), and then used to suspend previously untreated Jurkat cells (2×106/ml). These cells were then incubated with PMA to induce their overt activation. Relative L-selectin surface expression levels were determined by flow cytometry. Negative control antibody (Isotype) staining of untreated cells is indicated (B–D). The x axis = Log 10 fluorescence. E, Jurkat cells were incubated in the absence or presence of the anti-Fas antibody CH-11 plus TAPI, as indicated, for 4 hr at 37°C. The tissue culture media supernatant from both cell treatments was divided into two equal aliquots, and one of the aliquots was subjected to filtration (0.22µm) then ultracentrifugation (100,000 × g) for 1 hr. The levels of soluble L-selectin in the two aliquots for both cell treatments were determined by ELISA. For panels B-D, data are representative of at least three independent experiments. Data in panels A and E are the mean (± SD) of 3 independent experiments performed in duplicate. *, p < 0.001 vs. untreated; **, p < 0.05 vs. untreated. Statistical significance was determined by an unpaired student t test.
An obvious consideration for assays involving TAPI is its stability during extended cell incubation periods. We examined this in a cell-based assay by collecting media supernatants from Jurkat cells treated with CH-11 for 4 hr in the presence or absence of TAPI, and using the media supernatants to suspend untreated Jurkat cells, which were then overtly activated with PMA to induce L-selectin shedding. We found that activated Jurkat cells incubated in media supernatant from the CH-11/TAPI-treated cells did not down-regulate their expression of surface L-selectin, whereas Jurkat cells incubated in media supernatant from the CH-11-treated cells did (Fig. 4C), indicating activity by the metalloprotease inhibitor. We have also added TAPI to Jurkat cells in culture prior to and at regular time points after the induction of apoptosis, and again noted a significant down-regulation in L-selectin expression at the later time points of apoptosis (data not shown).
In addition to a broad-spectrum metalloprotease inhibitor, we examined the effects of other protease inhibitors on the late apoptosis release of soluble L-selectin, in particular inhibitors of serine proteases, which can also cleave L-selectin and have been implicated in ectodomain shedding (39–41). As shown in supplemental Figure 2, Jurkat cells treated with TAPI to block the initial shedding of L-selectin during the induction of apoptosis along with various other broad-spectrum protease inhibitors produced similar levels of soluble L-selectin as cells treated with TAPI alone.
Membrane blebbing and microparticle production are additional mechanisms by which certain surface proteins can be down-regulated during leukocyte apoptosis. Interestingly, CD43 and CD16, which undergo ectodomain shedding upon neutrophil activation (42), can be down-regulated in expression during neutrophil apoptosis by these processes (43, 44). To determine whether L-selectin released from the surface of Jurkat cells treated with CH-11 and TAPI was associated with membrane particles, these were removed from the media supernatant by filtration and ultracentrifugation. For instance, media supernatant from Jurkat cells treated with CH-11 and TAPI for 4 hr was divided into two equal volumes and one of the aliquots was filtered (0.22 µm pore size) then centrifuged at 100,000 × g for 1 hr. Soluble L-selectin levels in the two aliquots were then compared by ELISA. If soluble L-selectin produced by apoptotic Jurkat cells in the presence of TAPI was primarily associated with membrane particles, we would expect that its levels would be significantly decreased after the described treatments; however, this was not the case (Fig. 4D).
Soluble L-selectin occurs at high levels in the blood of healthy humans and mice (45, 46). Interestingly, the levels of soluble L-selectin in the blood of ADAM17 and wild-type chimeric mice are not significantly different (8), suggesting another mechanism for its production. As with Jurkat cells, we observed that murine neutrophils also down-regulated their surface L-selectin at later time points of induced apoptosis in the presence of TAPI. For instance, by 3 hr of induced apoptosis, neutrophils from unmanipulated mice treated with TNFα/cycloheximide in the presence of TAPI demonstrated an appreciable down-regulation in L-selectin expression, as determined by flow cytometry (Fig. 5A). Again, surface levels of LFA-1 on the same cells decreased only marginally at this time point (Fig. 5A). In contrast to early time points following the induction of apoptosis, neutrophils isolated from ADAM17 chimeras also demonstrated a down-regulation in surface L-selectin expression as apoptosis progressed (Fig. 5B), resulting in a corresponding increase in soluble L-selectin levels (Fig. 5C). Altogether, the above findings reveal that soluble L-selectin production can occur by distinct mechanisms invoked at different stages of induced apoptosis.
Figure 5. ADAM17-deficient neutrophils release L-selectin at later time points of induced apoptosis.
A, Mouse bone marrow-derived neutrophils (2×106/ml) were either untreated or treated with TNFα or TNFα/CHX in the presence or absence of TAPI for 180 min at 37°C. B, Mouse bone marrow-derived neutrophils (2×106/ml) from ADAM17 chimeras were either untreated or treated with TNFα or TNFα/CHX for 180 min at 37°C. For panels A and B, relative L-selectin or LFA-1 surface expression levels and annexin-V reactivity were determined by flow cytometry. Negative control antibody staining of untreated cells is indicated (Isotype). The x axis = Log 10 fluorescence. Data are representative of at least three independent experiments using neutrophils isolated from separate animals. C, Mouse bone marrow-derived neutrophils (2×106/ml) from ADAM17 chimeras were either untreated or treated with TNFα/CHX at 37°C for the indicated time points. The presence of soluble L-selectin in the cell supernatants was determined by ELISA. Data are represented as the mean (± SD) of 3 independent experiments performed in duplicate.
DISCUSSION
Emerging evidence indicates that ectodomain shedding by leukocytes is a key post-translational mechanism for regulating inflammation. In part, this process modulates the activity of various cytokines, cytokine receptors, and adhesion molecules (5). ADAM17 induction occurs upon cell activation, and its biological functions have been mainly examined in this context. The fate of neutrophils following their infiltration into sites of inflammation is programmed cell death, which is an effector activity that plays a vital role in resolving acute inflammation (20, 21). In this study, we provide the first direct evidence that ADAM17 cleaves L-selectin upon the induction of neutrophil apoptosis, which appears to be an early apoptotic event. We also report that as the apoptotic process progresses, L-selectin can be released from the surface of leukocytes by a mechanism distinct from ADAM17-mediated shedding and the membrane events of blebbing and microparticle production.
We have previously reported that L-selectin undergoes efficient shedding upon the induction of human neutrophil apoptosis by a mechanism that involves metalloproteases (23). L-selectin shedding has been reported to occur in an ADAM17-dependent and independent manner (8, 28–30), and thus we directly examined its role in L-selectin shedding upon death receptor-induced leukocyte apoptosis using two distinct genetic approaches. Fas signaling of apoptosis in Jurkat cells has been extensively studied (37), and these cells, which express high levels of surface L-selectin, were employed to knock-down ADAM17 expression using shRNA. This approach decreased the expression of cell surface ADAM17, but not the expression of its most similar family member ADAM10, and also reduced L-selectin shedding upon Fas engagement when compared with control cells. We also examined the shedding of L-selectin by neutrophils lacking functional ADAM17 following the induction of apoptosis. In contrast to wild-type neutrophils, ADAM17-deficient neutrophils demonstrated a near complete abrogation of L-selectin shedding at early time points of induced apoptosis. ADAM8 has been implicated in L-selectin shedding by neutrophils as well (29), and we also directly examined its role by using neutrophils from ADAM8 knock-out mice. We observed that these cells efficiently down-regulated their surface L-selectin expression soon after the induction of apoptosis. Hence, our findings indicate that in addition to neutrophil activation, ADAM17 is a primary sheddase of L-selectin upon death receptor-induced apoptosis.
Activation-induced L-selectin shedding regulates the receptor’s cell surface density and neutrophil adhesiveness (18, 47). The purpose of L-selectin shedding during neutrophil apoptosis is less clear at this time, though receptor down-regulation by apoptotic neutrophils likely contributes to a general diminution in their responsiveness to inflammatory cues (22). Neutrophils that have recently infiltrated sites of inflammation already express low levels of L-selectin due to their activated state (8, 18, 48), and thus it would seem that nominal levels of cell surface L-selectin would be available for further ADAM17-mediated cleavage upon the induction of neutrophil apoptosis. However, activated neutrophils express L-selectin mRNA, actively synthesize the adhesion molecule, and they can even re-express appreciable levels of surface L-selectin following their activation, but prior to undergoing apoptosis (23) (49, 50). Consequently, ADAM17-mediated shedding during neutrophil apoptosis at sites of inflammation may produce physiologically relevant levels of localized soluble L-selectin, among additional substrates. Other settings in which ADAM17-dependent shedding may occur upon leukocyte apoptosis is during the clearance of senescent neutrophils from the blood and the turnover of lymphocytes during negative selection, which may be a mechanism for the down-regulation of various receptors and/or the production of assorted soluble agonists and antagonists.
ADAM17’s enzymatic activity is robustly induced upon cell activation with various stimuli, which occurs in part through an intrinsic process (51, 52). We speculate that ADAM17’s enzymatic activity is enhanced upon the induction of leukocyte apoptosis as well, though at this time we cannot rule out that changes in L-selectin’s conformation and/or cell surface distribution may also contribute to its shedding during this cellular process. We have recently reported that redox modifications of cysteinyl sulfhydryl groups in the ectodomain of mature ADAM17 up-regulates its enzymatic activity (14). It will be interesting to determine whether this inducer mechanism may regulate ADAM17’s activity upon death receptor-induced neutrophil apoptosis as well. In addition, increased conversion of ADAM17’s pro-form to mature-form has also been reported during leukocyte apoptosis (53).
Our data also reveals that during the late stages of neutrophil apoptosis, L-selectin expression can be down-regulated by a mechanism distinct from ADAM17, which resulted in the production of soluble L-selectin. CD16 and CD43 can undergo ectodomain shedding as well upon neutrophil activation, and have been reported to be released from the surface of neutrophils by membrane blebbing and microparticle production during apoptosis (43, 44). An approach used to discern soluble adhesion molecules that are cleaved from those that are associated with membrane particles is by sedimentation of the latter using ultracentrifugation (44, 54). We found that a combination of filtration (≥ 0.22 µm particle size) as well as ultracentrifugation (100,000 × g) did not significantly reduce the levels of soluble L-selectin in the tissue culture media supernatant from cells undergoing prolonged apoptosis in the presence of the broad spectrum zinc metalloprotease inhibitor TAPI, suggesting that it may have been produced by a proteolytic process.
ADAM10 has been reported to cleave L-selectin in the absence of its primary sheddase ADAM17 (30). However, it would seem unlikely that ADAM10 is playing a major role in soluble L-selectin production by apoptotic leukocytes considering that it has been shown to down-regulate in expression during this process (23), and that soluble L-selectin production occurred in the presence of TAPI, which is known to block ADAM10 activity (55–57). Serine proteases have been implicated as sheddases as well and can also cleave L-selectin (39–41). However, various broad-spectrum serine protease inhibitors, as well as inhibitors of aspartic and cysteine proteases, also did not block soluble L-selectin production by late apoptotic leukocytes. Thus, the precise proteolytic process by which soluble L-selectin production occurs during later stages of leukocyte apoptosis remains to be determined. In addition, it will be interesting to determine whether this putative protease targets other cell surface determinants. For instance, surface expression of PSGL-1, a ligand of L-selectin (58), can also be down-regulated during neutrophil apoptosis, which appears to involve a non-ADAM17 proteolytic process as well (54).
The current study is the first to establish mechanisms of soluble L-selectin production upon death receptor-induced, neutrophil apoptosis. We have previously reported that ADAM17 deficient neutrophils can generate soluble L-selectin upon spontaneous apoptosis, which appears to involve other metalloproteases (8). The biological significance of ADAM17-independent processes that mediate the release of surface L-selectin from apoptotic neutrophils is unclear at this time. Soluble L-selectin is maintained at high levels in the blood of healthy individuals and mice (45, 46). We have reported that the levels of soluble L-selectin in the blood of ADAM17 and wild-type chimeric mice are not significantly different (8), suggesting that ADAM17 may not be the principle means of maintaining blood levels of soluble L-selectin. However, Venturi et al. have reported that soluble L-selectin in the blood is primarily derived by proteolytic cleavage, as gene-targeted mice expressing non-cleavable L-selectin have considerably lower levels of serum L-selectin (18). In conjunction with our findings, it is tempting to speculate that soluble L-selectin levels in the blood of normal individuals may be maintained by a proteolytic mechanism other than ADAM17, perhaps occurring during leukocyte apoptosis.
In conclusion, ADAM17 appears to be active during the various phases of a neutrophil’s short life span; such as during their time in circulation [e.g. maintains L-selectin at an appropriate cell surface density (8)], upon their activation (8), and during death receptor-mediated apoptosis. The induction of ectodomain shedding during leukocyte apoptosis likely has a much broader purpose than just cleaving L-selectin. Indeed, various leukocyte determinants that undergo ectodomain shedding have an important role in regulating the resolution of inflammation, including TNFα, TNFRI, TNFRII, FasL, TRAIL, and IL-6R (53, 59–63), and thus this process may be another component of the anti-inflammatory program initiated by neutrophils (20) (21).
Supplementary Material
ACKNOWLEDGMENTS
We greatly appreciate the gifts of ADAM17(TACE)+/ΔZn mice from Dr. Roy Black (Amgen Inc.) and ADAM8 knock-out mice from Dr. Andy Docherty (Celltech R&D) and Dr. Carl Blobel (Weill Medical College of Cornell University). We thank Adam Nettles for his technical assistance.
Footnotes
The study described was supported by Grant Number R01HL61613 from the National Institutes of Health.
Abbreviations used in this paper: ADAM17, a disintegrin and metalloprotease; APC, allophycocyanin; rhFasL, recombinant human Fas ligand; CHX, cycloheximide
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
REFERENCES
- 1.Black RA. Tumor necrosis factor-alpha converting enzyme. Int. J. Biochem. Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 2.Arribas J, Borroto A. Protein ectodomain shedding. Chem. Rev. 2002;102:4627–4638. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- 3.Dello Sbarba P, Rovida E. Transmodulation of cell surface regulatory molecules via ectodomain shedding. Biol. Chem. 2002;383:69–83. doi: 10.1515/BC.2002.007. [DOI] [PubMed] [Google Scholar]
- 4.Tousseyn T, Jorissen E, Reiss K, Hartmann D. (Make) stick and cut loose--disintegrin metalloproteases in development and disease. Birth Defects Res. Part C, Embryo Today. 2006;78:24–46. doi: 10.1002/bdrc.20066. [DOI] [PubMed] [Google Scholar]
- 5.Garton KJ, Gough PJ, Raines EW. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J. Leukoc. Biol. 2006;79:1105–1116. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- 6.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 7.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Brazzell J, Herrera A, Walcheck B. ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood. 2006;108:2275–2279. doi: 10.1182/blood-2006-02-005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell JH, Herrera AH, Li Y, Walcheck B. Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J. Leukoc. Biol. 2007;82:173–176. doi: 10.1189/jlb.0307193. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP. Cutting Edge: TNF-α-Converting Enzyme (TACE/ADAM17) Inactivation in Mouse Myeloid Cells Prevents Lethality from Endotoxin Shock. J. Immunol. 2007;179:2686–2689. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Boyd K, Dempsey PJ, Vignali DA. Non-cell autonomous expression of TNF-alpha-converting enzyme ADAM17 is required for normal lymphocyte development. J. Immunol. 2007;178:4214–4221. doi: 10.4049/jimmunol.178.7.4214. [DOI] [PubMed] [Google Scholar]
- 12.Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends. Biochem. Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Sagi I, Milla ME. Application of structural dynamic approaches provide novel insights into the enzymatic mechanism of the tumor necrosis factor-alpha-converting enzyme. Anal. Biochem. 2008;372:1–10. doi: 10.1016/j.ab.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J. Immunol. 2009;182:2449–2457. doi: 10.4049/jimmunol.0802770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migaki GI, Kahn J, Kishimoto TK. Mutational analysis of the membrane-proximal cleavage site of L- selectin: Relaxed sequence specificity surrounding the cleavage site. J. Exp. Med. 1995;182:549–557. doi: 10.1084/jem.182.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen A, Engel P, Tedder TF. Structural requirements regulate endoproteolytic release of the L-selectin (CD62L) adhesion receptor from the cell surface of leukocytes. J. Exp. Med. 1995;182:519–530. doi: 10.1084/jem.182.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matala E, Alexander SR, Kishimoto TK, Walcheck B. The cytoplasmic domain of L-selectin participates in regulating L- selectin endoproteolysis. J. Immunol. 2001;167:1617–1623. doi: 10.4049/jimmunol.167.3.1617. [DOI] [PubMed] [Google Scholar]
- 18.Venturi GM, Tu L, Kadono T, Khan AI, Fujimoto Y, Oshel P, Bock CB, Miller AS, Albrecht RM, Kubes P, Steeber DA, Tedder TF. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19:713–724. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 20.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol. Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 22.Dransfield I, Stocks SC, Haslett C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–3273. [PubMed] [Google Scholar]
- 23.Walcheck B, Herrera AH, Hill CS, Mattila PE, Whitney AR, Deleo FR. ADAM17 activity during human neutrophil activation and apoptosis. Eur. J. Immunol. 2006;36:968–976. doi: 10.1002/eji.200535257. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: A rapidly down-regulated adhesion molecule. Proc. Natl. Acad. Sci. USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woska JR, Jr, Last-Barney K, Rothlein R, Kroe RR, Reilly PL, Jeanfavre DD, Mainolfi EA, Kelly TA, Caviness GO, Fogal SE, Panzenbeck MJ, Kishimoto TK, Giblin PA. Small molecule LFA-1 antagonists compete with an anti-LFA-1 monoclonal antibody for binding to the CD11a I domain: development of a flow-cytometry-based receptor occupancy assay. J. Immunol. Methods. 2003;277:101–115. doi: 10.1016/s0022-1759(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 26.Kelly K, Hutchinson G, Nebenius-Oosthuizen D, Smith AJ, Bartsch JW, Horiuchi K, Rittger A, Manova K, Docherty AJ, Blobel CP. Metalloprotease-disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev. Dyn. 2005;232:221–231. doi: 10.1002/dvdy.20221. [DOI] [PubMed] [Google Scholar]
- 27.Walcheck B, Leppanen A, Cummings RD, Knibbs RN, Stoolman LM, Alexander SR, Mattila PE, McEver RP. The monoclonal antibody CHO-131 binds to a core 2 O-glycan terminated with sialyl-Lewis x, which is a functional glycan ligand for P-selectin. Blood. 2002;99:4063–4069. doi: 10.1182/blood-2001-12-0265. [DOI] [PubMed] [Google Scholar]
- 28.Walcheck B, Alexander SR, St Hill CA, Matala E. ADAM-17-independent shedding of L-selectin. J. Leukoc. Biol. 2003;74:389–394. doi: 10.1189/jlb.0403141. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Gaviro M, Dominguez-Luis M, Canchado J, Calafat J, Janssen H, Lara-Pezzi E, Fourie A, Tugores A, Valenzuela-Fernandez A, Mollinedo F, Sanchez-Madrid F, Diaz-Gonzalez F. Expression and regulation of the metalloproteinase ADAM-8 during human neutrophil pathophysiological activation and its catalytic activity on L-selectin shedding. J. Immunol. 2007;178:8053–8063. doi: 10.4049/jimmunol.178.12.8053. [DOI] [PubMed] [Google Scholar]
- 30.Le Gall SM, Bobe P, Reiss K, Horiuchi K, Niu XD, Lundell D, Gibb DR, Conrad D, Saftig P, Blobel CP. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha, L-selectin, and tumor necrosis factor alpha. Mol. Biol. Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itou T, Collins LV, Thoren FB, Dahlgren C, Karlsson A. Changes in activation states of murine polymorphonuclear leukocytes (PMN) during inflammation: a comparison of bone marrow and peritoneal exudate PMN. Clin. Vaccine Immunol. 2006;13:575–583. doi: 10.1128/CVI.13.5.575-583.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akgul C, Edwards SW. Regulation of neutrophil apoptosis via death receptors. Cell Mol. Life Sci. 2003;60:2402–2408. doi: 10.1007/s00018-003-3110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stringer RE, Hart CA, Edwards SW. Sodium butyrate delays neutrophil apoptosis: role of protein biosynthesis in neutrophil survival. Br. J. Haematol. 1996;92:169–175. doi: 10.1046/j.1365-2141.1996.00307.x. [DOI] [PubMed] [Google Scholar]
- 34.Whyte MK, Savill J, Meagher LC, Lee A, Haslett C. Coupling of neutrophil apoptosis to recognition by macrophages: coordinated acceleration by protein synthesis inhibitors. J. Leukoc. Biol. 1997;62:195–202. doi: 10.1002/jlb.62.2.195. [DOI] [PubMed] [Google Scholar]
- 35.Ward C, Chilvers ER, Lawson MF, Pryde JG, Fujihara S, Farrow SN, Haslett C, Rossi AG. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 1999;274:4309–4318. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J. Biol. Chem. 2003;278:28443–28454. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- 37.Valavanis C, Hu Y, Yang Y, Osborne BA, Chouaib S, Greene L, Ashwell JD, Schwartz LM. Model cell lines for the study of apoptosis in vitro. Methods Cell Biol. 2001;66:417–436. doi: 10.1016/s0091-679x(01)66019-9. [DOI] [PubMed] [Google Scholar]
- 38.Maskos K, Fernandez-Catalan C, Huber R, Bourenkov GP, Bartunik H, Ellestad GA, Reddy P, Wolfson MF, Rauch CT, Castner BJ, Davis R, Clarke HR, Petersen M, Fitzner JN, Cerretti DP, March CJ, Paxton RJ, Black RA, Bode W. Crystal structure of the catalytic domain of human tumor necrosis factor-alpha-converting enzyme. Proc. Natl. Acad. Sci. U S A. 1998;95:3408–3412. doi: 10.1073/pnas.95.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jutila MA, Kishimoto TK, Finken M. Low-dose chymotrypsin treatment inhibits neutrophil migration into sites of inflammation in vivo: Effects on Mac-1 and MEL-14 adhesion protein expression and function. Cell. Immunol. 1991;132:201–214. doi: 10.1016/0008-8749(91)90019-8. [DOI] [PubMed] [Google Scholar]
- 40.Palecanda A, Kurk S, Jutila D, Jesaitis A, Jutila MA. Complete inhibition of crosslinking and activation induced shedding of L-selectin by the serine protease inhibitor diisopropylfluorophosphate (DFP) J. Immunol. 1993;150 304A-3040. [Google Scholar]
- 41.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl. Acad. Sci. U S A. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middelhoven PJ, van Buul JD, Kleijer M, Roos D, Hordijk PL. Actin polymerization induces shedding of FcgammaRIIIb (CD16) from human neutrophils. Biochem. Biophys. Res. Commun. 1999;255:568–574. doi: 10.1006/bbrc.1999.0244. [DOI] [PubMed] [Google Scholar]
- 43.Nusbaum P, Laine C, Seveau S, Lesavre P, Halbwachs-Mecarelli L. Early membrane events in polymorphonuclear cell (PMN) apoptosis: membrane blebbing and vesicle release, CD43 and CD16 down-regulation and phosphatidylserine externalization. Biochem. Soc. Trans. 2004;32:477–479. doi: 10.1042/BST0320477. [DOI] [PubMed] [Google Scholar]
- 44.Nusbaum P, Laine C, Bouaouina M, Seveau S, Cramer EM, Masse JM, Lesavre P, Halbwachs-Mecarelli L. Distinct signaling pathways are involved in leukosialin (CD43) down-regulation, membrane blebbing, and phospholipid scrambling during neutrophil apoptosis. J. Biol. Chem. 2005;280:5843–5853. doi: 10.1074/jbc.M413405200. [DOI] [PubMed] [Google Scholar]
- 45.Kishimoto TK, Kahn J, Migaki G, Mainolfi E, Shirley F, Ingraham R, Rothlein R. Regulation of L-selectin expression by membrane proximal proteolysis. Agents Actions Suppl. 1995;47:121–134. doi: 10.1007/978-3-0348-7343-7_11. [DOI] [PubMed] [Google Scholar]
- 46.Tu L, Poe JC, Kadono T, Venturi GM, Bullard DC, Tedder TF, Steeber DA. A functional role for circulating mouse L-selectin in regulating leukocyte/endothelial cell interactions in vivo. J. Immunol. 2002;169:2034–2043. doi: 10.4049/jimmunol.169.4.2034. [DOI] [PubMed] [Google Scholar]
- 47.Walcheck B, Kahn J, Fisher JM, Wang BB, Fisk RS, Payan DG, Feehan C, Betageri R, Darlak K, Spatola AF, Kishimoto TK. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature. 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 48.Jutila MA, Rott L, Berg EL, Butcher EC. Function and regulation of the neutrophil MEL-14 antigen in vivo: Comparison with LFA-1 and Mac-1. J. Immunol. 1989;143:3318–3324. [PubMed] [Google Scholar]
- 49.Ord DC, Ernst TJ, Zhou LJ, Rambaldi A, Spertini O, Griffin J, Tedder TF. Structure of the gene encoding the human leukocyte adhesion molecule-1 (TQ1, Leu-8) of lymphocytes and neutrophils. J. Biol. Chem. 1990;265:7760–7767. [PubMed] [Google Scholar]
- 50.Kahn J, Ingraham RH, Shirley F, Migaki GI, Kishimoto TK. Membrane proximal cleavage of L-selectin: Identification of the cleavage site and a 6-kD transmembrane peptide fragment of L- selectin. J. Cell Biol. 1994;125:461–470. doi: 10.1083/jcb.125.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doedens JR, Mahimkar RM, Black RA. TACE/ADAM-17 enzymatic activity is increased in response to cellular stimulation. Biochem. Biophys. Res. Commun. 2003;308:331–338. doi: 10.1016/s0006-291x(03)01381-0. [DOI] [PubMed] [Google Scholar]
- 52.Black RA, Doedens JR, Mahimkar R, Johnson R, Guo L, Wallace A, Virca D, Eisenman J, Slack J, Castner B, Sunnarborg SW, Lee DC, Cowling R, Jin G, Charrier K, Peschon JJ, Paxton R. Substrate specificity and inducibility of TACE (tumour necrosis factor alpha-converting enzyme) revisited: the Ala-Val preference, and induced intrinsic activity. Biochem. Soc. Symp. 2003;70:39–52. doi: 10.1042/bss0700039. [DOI] [PubMed] [Google Scholar]
- 53.Chalaris A, Rabe B, Paliga K, Lange H, Laskay T, Fielding CA, Jones SA, Rose-John S, Scheller J. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood. 2007;110:1748–1755. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- 54.Stampfuss JJ, Censarek P, Fischer JW, Kaber G, Rauch BH, Freidel K, Fischer U, Schulze-Osthoff K, Grosser T, Grandoch M, Schror K, Weber AA. Complete downmodulation of P-selectin glycoprotein ligand in monocytes undergoing apoptosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1375–1378. doi: 10.1161/ATVBAHA.108.166629. [DOI] [PubMed] [Google Scholar]
- 55.Vincent B, Paitel E, Saftig P, Frobert Y, Hartmann D, De Strooper B, Grassi J, Lopez-Perez E, Checler F. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J. Biol. Chem. 2001;276:37743–37746. doi: 10.1074/jbc.M105677200. [DOI] [PubMed] [Google Scholar]
- 56.Lemieux GA, Blumenkron F, Yeung N, Zhou P, Williams J, Grammer AC, Petrovich R, Lipsky PE, Moss ML, Werb Z. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J. Biol. Chem. 2007;282:14836–14844. doi: 10.1074/jbc.M608414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulte M, Reiss K, Lettau M, Maretzky T, Ludwig A, Hartmann D, de Strooper B, Janssen O, Saftig P. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007;14:1040–1049. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- 58.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J. Clin. Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray J, Barbara JA, Dunkley SA, Lopez AF, Van Ostade X, Condliffe AM, Dransfield I, Haslett C, Chilvers ER. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- 60.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat. Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 61.Mullberg J, Althoff K, Jostock T, Rose-John S. The importance of shedding of membrane proteins for cytokine biology. Eur. Cytokine Netw. 2000;11:27–38. [PubMed] [Google Scholar]
- 62.Baran J, Weglarczyk K, Mysiak M, Guzik K, Ernst M, Flad HD, Pryjma J. Fas (CD95)-Fas ligand interactions are responsible for monocyte apoptosis occurring as a result of phagocytosis and killing of Staphylococcus aureus. Infect. Immun. 2001;69:1287–1297. doi: 10.1128/IAI.69.3.1287-1297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamohara H, Matsuyama W, Shimozato O, Abe K, Galligan C, Hashimoto S, Matsushima K, Yoshimura T. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor expression in human neutrophils. Immunology. 2004;111:186–194. doi: 10.1111/j.0019-2805.2003.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.