Abstract
Objectives The primary aims of the study were to: (a) describe the trajectories of adherence to daily inhaled corticosteroid (ICS) medication for a year in economically disadvantaged, African-American youth with asthma based on growth curve modeling; and (b) test the relationship of treatment adherence to symptom control, quick-relief medication, and healthcare utilization. Methods This prospective study measured adherence to daily ICS treatment using electronic monitoring in 92 children and adolescents with moderate to severe asthma for 9–12 months and assessed clinical outcomes, including asthma-related symptoms, quick-relief medication, and healthcare utilization. Results Youth showed a decrement in treatment adherence to less than half of prescribed corticosteroid treatment over the course of the study, which related to increased healthcare utilization (p < .04), but not to asthma symptoms or albuterol use. Conclusion Economically disadvantaged youth with asthma demonstrate high rates of chronic nonadherence that warrant identification and intervention to reduce asthma-related healthcare utilization.
Keywords: health care utilization, pediatric asthma, treatment adherence
Pediatric asthma is an important chronic health condition from a public health perspective, based on its high prevalence, substantial morbidity, impact on children and families, and medical care costs (Bender & Rand, 2004; Bloom & Cohen, 2007; Vinicor, 1998). Moderate to severe persistent asthma can be controlled by medical treatment, especially daily inhaled corticosteroid (ICS) treatment to prevent airway inflammation, which is a primary and effective treatment (EPR-3, 2007; Rachelefsky, 2009). However, rates of morbidity in pediatric asthma (e.g., symptoms, activity limitations, and healthcare utilization) continue to rise, especially in minority populations (Lieu et al., 2002; McQuaid & Walders, 2003). One factor that has been implicated in the high rates of asthma-related morbidity is nonadherence to prescribed medical treatment. Nonadherence to asthma treatment is highly prevalent and is recognized as a potential cause of treatment failure (Milgrom et al., 1996) that has serious clinical consequences, including increased asthma-related symptoms, functional impairment, and healthcare utilization (Bauman et al., 2002, Bender & Rand, 2004; Bender, Ikle, DuHamel, & Tinkelman, 1997, Bukstein et al., 2007).
The potential clinical significance of treatment nonadherence challenges researchers to understand the long-term trajectories of nonadherence to asthma treatment, including the relationship of adherence patterns to relevant clinical outcomes, especially in high risk, minority populations. Previous research has suggested that children and adolescents with asthma from ethnic minority families have high rates of nonadherence and also demonstrate greater disease severity, more symptoms, more school days missed, increased emergency room visits and hospitalizations, and fatalities from asthma than nonminority children and adolescents (Getahun, Demissie, & Rhoads, 2005; Grant, Lyttle, & Weiss, 2000; Kruse, Deshpande, & Venzina, 2007; Lieu et al., 2002). Moreover, a significant proportion of asthma-related morbidity among minority children and adolescents may be attributable to low levels of treatment adherence owing to substantial family and medical system barriers, which need to be addressed in proactive clinical management (Mansour, Lanphear, & DeWitt, 2000; Seid, 2008; Smith, et al., 2008).
What is known about the prevalence of long-term nonadherence in pediatric asthma and its relationship to clinical outcomes? Previous studies have documented high rates of nonadherence to inhaled corticosteroid treatment over periods of 3–4 months based on electronic monitoring (50–70%). However, inconsistent relationships have been found between treatment nonadherence and asthma control, use of rescue medication, activity limitations, and healthcare utilization, including emergency room visits and hospitalizations. Bender and Zhang (2008) found that high rates of nonadherence over a 3-month period (53–65%) were associated with increased use of short courses of oral corticosteroids but not symptom control, emergency room visits, or school absences. Milgrom et al. (1996) noted that nonadherence rates to treatment for asthma over a 3-month period were much higher among children whose asthma exacerbations required a burst of oral corticosteroids (73%) compared to those who did not (32%). McQuaid, Kopel, Klein, and Fritz (2003) described nonadherence rates of 53% for 1 month that correlated with functional impairment, including activity limitations. Finally, Walders, Kopel, Koinis-Mitchell, and McQuaid's (2005) study of short-term (1 month) adherence to pediatric asthma treatment identified nonadherence rates of 54%, which predicted frequency of emergency room visits and school absences.
Taken together, the above findings underscore the potential clinical consequences of nonadherence in pediatric asthma monitored over periods of 1–4 months. However, the absence of data concerning objective measurement of longer term treatment adherence in pediatric asthma, especially in higher risk minority populations, is a limitation of previous research. Bauman et al. (2002) measured nonadherence to asthma treatment for a year in a largely minority population and found a relationship to symptoms and healthcare utilization. However, this study used a parent self-report measure of adherence, which is potentially biased and hence less valid, than objective methods such as electronic monitoring (Bender et al., 2007). To our knowledge, only two studies (Fiese & Wamboldt, 2003; Fiese, Wamboldt, & Anbar, 2005) have measured treatment adherence for pediatric asthma based on electronic monitoring for longer than 6 months. However, these studies included a majority of Caucasian children and adolescents with asthma and did not assess the relationship of treatment adherence to asthma-related clinical outcomes (Fiese & Wamboldt, 2003; Fiese et al., 2005). Chronic nonadherence lasting for 6 months or more is of potentially clinical significance because longer periods of nonadherence that are captured in the trajectories of individual children might be expected to have greater effects on long-term health outcomes than brief periods of nonadherence. Moreover, children and adolescents who demonstrate patterns of chronic nonadherence that also predict negative health outcomes would be especially important for targeting adherence promotion interventions.
An important methodological issue that has limited the scientific impact of previous research is that the primary unit of analysis of treatment adherence has been limited to a summary score, often a mean percentage of drug taken during the observation period. A mean adherence score does not encompass the many points in time that adherence is assessed and the trajectories of treatment adherence over time that are demonstrated by individual children and adolescents. An average summary score does not consider potentially important individual differences in trajectories such as acceleration or deterioration in adherence over time. Such different trajectories may have very different effects on clinically relevant health outcomes. Consequently, a mean-based summary of treatment adherence may be less sensitive in detecting asthma-related clinical outcomes than approaches such as growth curve modeling that are comprised of data based on individual patients’ trajectories (DeLucia & Pitts, 2006; Singer & Willett, 2003).
To our knowledge, no previous study has used growth curve modeling to evaluate asthma treatment adherence over an extended time period using electronic monitoring in a sample of minority children and adolescents. Studies that provide a detailed prospective description of treatment adherence in minority children and adolescents with asthma and document the relationship to clinical outcomes are an important first step toward identifying nonadherent patients who are at highest risk and hence in need of targeted adherence promotion.
To address these unmet needs, this study had a dual purpose: (a) to describe the patterns of adherence to daily inhaled corticosteroids over 9–12 months in economically disadvantaged African American children and adolescents with asthma based on growth curve modeling (DeLucia & Pitts, 2006); and (b) to test the relationship of treatment adherence to symptom control, quick relief or as needed (PRN) medication use, and healthcare utilization. Previous data suggested that the combination of minority status and economic disadvantage may confer special risk for treatment nonadherence in pediatric asthma (e.g., cultural barriers, discrimination, and access to care) (Mansour et al., 2000; Rand et al., 1994). Based on previous research (Bauman et al., 2002; Bender & Zhang, 2008; McQuaid et al., 2003), we hypothesized that nonadherence to asthma treatment would predict increased asthma-related symptoms, greater use of quick relief medication, and increased healthcare utilization.
Methods
Study Design
Data from this study were originally collected to examine the efficacy of a tailored problem-solving intervention compared with family-based education in improving adherence to treatment regimens for children diagnosed as having asthma and their caregivers (Drotar, 2006). The intervention did not result in any significant group differences in the trajectories of treatment adherence to daily inhaled corticosteroid treatment, quick relief medication, or relevant clinical outcomes, including asthma-related symptoms and healthcare utilization (Drotar, 2006). Consequently, data from the entire sample was pooled for the purpose of this analysis. Previous analyses of the baseline data from this study have described quality of life (Greenley, Josie & Drotar, 2008; Josie, Greenley & Drotar, 2007) and the relationship between risk factors and asthma severity (Josie, Greenley & Drotar, 2008).
Procedure: Patient Recruitment
This study was approved by the Institutional Review Board of University Hospitals Health Systems. Recruitment strategies included contacting physicians who provided care for children and adolescents with asthma in primary and subspecialty care, inpatient, and emergency room settings at one large pediatric tertiary care center. Parents of patients who met eligibility criteria were first contacted by their physician and then by study staff who explained procedures, obtained parental consent and youth assent, and scheduled appointments. Procedures for electronic monitoring of asthma medication were explained and verified in a home visit, and electronic monitors were downloaded at quarterly intervals.
Participants
Eligibility Criteria
Children and adolescents were eligible for participation if they were African-American, between 5 and 17 years of age, diagnosed as having persistent moderate to severe asthma that warranted the use of daily controller medications as determined by an evaluation from a study physician (NIH, 1997), and were from families with incomes below the federal poverty level (HHS, 2001). Exclusion criteria included the presence of a serious comorbid condition, serious developmental disability, or income exceeding poverty level.
Demographic Characteristics
One hundred and forty-one participants completed baseline, however, 49 participants did not complete the study. Reasons for failure to complete the study included: unresponsiveness to contact attempts/failure to show up for appointments (n = 29); family no longer interested in participating (n = 7); moved out of area (n = 4); less than 270 days of electronic monitoring data available (n = 4); and other (n = 5).
Participants were all African-American (N = 92), ranged from 5 to 17 years (M = 9.99 years; SD = 2.84 years), and were predominantly male (64 [70%]). The majority of participating caregivers were African-American (91 [99%]), the youth's biological mother (78 [85%]), and single (58 [63%]). Caregivers ranged from 24 to 71 years (M = 36.46 years; SD = 9.79 years). Among primary caregivers, 40 (44%) had completed high school and 44 (48%) were employed at least part-time. The median annual income range of the sample was $10,000-$14,999. Eighty-seven participants (94.6%) indicated some type of health insurance coverage at baseline: 84 (96.6%) had public health insurance (e.g., Medicaid, Medicaid HMO), one participant (1.1%) reported state insurance for children with a chronic condition, and two participants (2.3%) reported private/other insurance coverage. Furthermore, 87 participants (94.6%) reported that insurance covered the cost of prescriptions, four participants reported copay payment for prescriptions (4.3%), and one participant (1.1%) reported no assistance with prescription payment.
Analysis of Sample Attrition
A comparison of participating families (N = 92) with those who failed to complete the study (N = 49) indicated no differences with respect to youth age (p = .26; d = .20), youth sex (p = .24; d = .13), youth or primary caregiver (p = .46; d = .10) ethnicity, or primary caregiver relationship to youth (i.e., biological mother, biological father, or other) (p = .18; d = .14). However, the families who dropped out of the study had more children under the age of 18 in the home (M = 3.70; SD = 1.79) as compared with those who remained in the study (M = 2.75; SD = 1.51), t(129) = −3.36, p < .01, d = .59 and were associated with a younger age of the primary caregiver [dropouts, M = 32.94, SD = 8.14; participants, M = 36.51, SD = 9.83; t(139) = 2.18, p < .04, d = .39]. Fluticasone adherence information was available for 29 participants who dropped out of the study who were monitored for an average of 96.31 days (SD = 63.96). Adherence to daily corticosteroid (average number of daily puffs of ICS medication) was lower in dropouts (M = 0.89; SD = 0.52) versus completers (M = 1.61; SD = 0.78), t(119) = 4.64, p < .001, d = .99.
Prescribed Medical Treatment
In accord with their diagnosis of asthma, eligible patients in the study were evaluated by an asthma specialist. Two daily doses of two puffs of preventative inhaled corticosteroid (fluticasone) (total of four daily doses) and quick relief medication (albuterol) for as needed (PRN) symptom control was prescribed. Patients were either prescribed medications by the study physician (C.K., S.R.) who was an asthma specialist or by their current physician. All participants had comparable prescriptions, e.g., both medications were delivered by a metered dose inhaler (MDI). Medications were not provided by the study; families were responsible for obtaining them. Sixty-six percent of eligible patients were also prescribed a leukotriene modifier. However, data on adherence to this medication are not presented in this article.
Predictor Variable: Adherence to Inhaled Corticosteroid Medication
An electronic monitoring device, the MDILog (West Med Technologies, Englewood, CO) was used over a period of 9–12 months, to continuously record and store the timing of fluticasone doses. For example, when the inhaler is actuated to administer medication, this medication use is time stamped with the date and time the medication was used. The electronic monitor can hold up to 1,320 medication events prior to downloading the data. Validation of the MDILog has been established (Bender, Milgrom, Rand, & Ackerson, 1998; Milgrom et al., 1996). Adherence was defined as the correspondence between medication doses taken each day and the prescribed dose (N = 4). Data were downloaded at quarterly intervals. Home visits were also used for purposes of quality control, that is, to download meters if families missed a clinic visit, to replace lost meters, etc. Recommended quality control procedures for electronic monitoring were followed (Riekert & Rand, 2002). Data quality was assessed by Johns Hopkins Adherence Research Center and included identification of records with missing bookmarks, broken devices, and device malfunctions.
Outcome Variables
Frequency of Asthma Symptoms
Parents were asked to rate their children's asthma symptoms (e.g., shortness of breath; tightness in the chest; wheezing without a cold; cough; a cold that won’t go away; and wheezing with a cold) every three months for a 4-week period on a 5-point Likert scale where a rating of 1 = experiencing symptoms “all of the time” and a rating of 5 = experiencing symptoms “none of the time” based on the Children's Health Survey for Asthma (Asmussen, Alon, Grant, Fagan, & Weiss, 1999). Parent-reported symptom ratings were obtained at baseline and then at quarterly intervals from 3 to 12 months.
Healthcare Utilization
Healthcare utilization was defined as the total number of hospitalizations, emergency room, and clinic visits due to problems with asthma. These data were gathered via self-report and verified by independent chart review at 3-month intervals, thus healthcare utilization information was available at baseline and at quarterly intervals from 3 to 12 months.
Use of Quick Relief Medication
The Doser-CT (Medalogic Corporation), which attached to a MDI and recorded the date and frequency of medication use, was used to measure use of quick relief medication (albuterol). Albuterol use was monitored from baseline until 9 to 12 months; and data were downloaded at quarterly intervals.
Results
Data Analytic Strategy
Individual growth curve models measured change over time at both the individual and the population level and fitted a regression equation and line for each participant. The linear individual change model summarized growth for the population and for each individual using two terms: fitted intercept and fitted slope (Singer & Willett, 2003). Time was centered at the first occasion, so that 0 represented the time of the first repeated measure, thus the fitted slopes accurately represented the annual estimated rate of change in the outcomes (DeLucia & Pitts, 2006; Singer & Willett, 2003). Time-varying predictors can be centered or uncentered; however, the overall interpretations might change when using centered versus uncentered time-varying predictors (e.g., centering variables and outcomes, might cause changes to occur in any intercept related parameter: mean, variances, and covariances) (Singer & Willett, 2003). There also are caveats to centered time-varying predictors, including, difficulties with interpretation and reciprocal causation (Singer & Willett, 2003). Growth curve models are defined by two levels: level 1 or unconditional growth curve model, in which the only predictor was time; and a conditional growth curve model or level 2 model, included static and/or dynamic predictors (Singer & Willett, 2003). The level 1 model is a random coefficients model because a core bivariate distribution of intercepts and slopes are used to define individual growth parameters, and “each individual draws [their] coefficients from an unknown random distribution of parameters …” (Singer & Willett, 2003, p. 54). Thus, parameters can vary between individuals (DeLucia & Pitts, 2006). The conditional growth curve model describes the between person change model, and accounts for general patterns like between person differences in intercepts and slopes, but also inter-individual heterogeneity within persons (Singer & Willett, 2003). The conditional growth curve model examined whether there was significant variation in initial status and/or rate of change in adherence to inhaled corticosteroids, parent reported symptoms, healthcare utilization, and/or quick relief medication use that could be explained beyond an unconditional growth curve model. The conditional growth curve model also described the between person change model (how individual changes differed across participants) and tested the significance of the relationship of corticosteroid medication adherence and rate of change in frequency of symptoms, use of quick relief medication, and healthcare utilization. The age moderation effect was tested by entering age as a predictor of the time and intercept term.
Unconditional and conditional growth curve modeling were performed using SAS Proc Mixed (SAS Institute, 1990). Restricted maximum likelihood estimations were used to avoid biased estimates of the variance components. Unstructured covariance matrices were used to allow variances and covariances to vary across time points rather than to conform to a priori constraints. The covariance and variance values are provided in Tables I and II (see variance components) (Singer & Willett, 2003). Growth curve analysis accounts for missing data in that the analysis makes use of whatever data are available for an individual subject (Singer & Willett, 2003). It is not necessary to eliminate subjects from the analysis if they have missing data.
Table I.
Level 1: Unconditional Growth Curve Models
| Fluticasone |
Albuterol use |
Symptom ratings |
Healthcare utilization |
|||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | |
| Fixed effects | ||||||||
| Intercept | 1.67* (0.086) | 1.50 to 1.83 | 0.623* (0.083) | 0.460 to 0.789 | 4.30* (0.087) | 4.13 to 4.47 | 0.322* (0.106) | 0.111 to 0.533 |
| Rate of change | −0.012* (0.002) | −0.015 to −0.008 | −0.002 (0.002) | −0.006 to 0.002 | −0.001 (0.001) | −0.005 to 0.003 | 0.005* (0.003) | 0.0003 to 0.011 |
| Variance components | ||||||||
| Within-person | 1.057* (0.019) | 1.021 to 1.095 | 1.099* (0.025) | 1.052 to 1.151 | 0.156* (0.003) | 0.151 to 0.162 | 0.433* (0.008) | 0.417 to 0.449 |
| In intercept | 0.615* (0.010) | 0.458 to 0.869 | 0.507* (0.093) | 0.365 to 0.752 | 0.671* (0.103) | 0.507 to 0.929 | 0.982* (0.153) | 0.740 to 1.369 |
| In rate of change | 0.0003* (0.00004) | 0.0002 to 0.0004 | 0.0002* (0.0001) | 0.0001 to 0.0003 | 0.0003* (0.0005) | 0.0002 to 0.0004 | 0.001* (0.0001) | 0.0004 to 0.0008 |
| Covariance | −0.007* (0.002) | −0.010 to −0.004 | −0.007* (0.002) | −0.010 to −0.003 | −0.011* (0.002) | −0.015 to −0.007 | −0.016* (0.003) | −0.023 to −0.010 |
*Significant p < .05
Table II.
Level 2: Conditional Growth Curve Models (Adherence is the Predictor)
| Albuterol use |
Symptom ratings |
Healthcare utilization |
||||
|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | Coefficient (SE) | 95% CI | |
| Fixed effects | ||||||
| Average intercept (time 0) | 0.635 (0.083) | 0.470 to 0.801 | 4.30* (0.086) | 4.13 to 4.47 | 0.320* (0.106) | 0.101 to 0.532 |
| Effect of Fluticasone on intercept | −0.017 (0.029) | −0.073 to 0.039 | −0.020* (0.010) | −0.040 to −0.001 | 0.032* (0.016) | 0.0003 to 0.064 |
| Average rate of change | −0.002 (0.002) | −0.006 to 0.001 | −0.001 (0.002) | −0.005 to 0.003 | 0.005* (0.003) | 0.0002 to 0.010 |
| Effect of Fluticasone on rate of change | 0.001 (0.001) | −0.0005 to 0.003 | 0.0002 (0.0003) | −0.0003 to 0.0007 | −0.001* (0.0004) | −0.002 to −0.0002 |
| Variance components | ||||||
| Within-person | 1.10* (0.025) | 1.060 to 1.160 | 0.1571* (0.0030) | 0.151 to 0.163 | 0.438* (0.008) | 0.422 to 0.455 |
| In intercept | 0.501* (0.092) | 0.363 to 0.747 | 0.6537* (0.1012) | 0.493 to 0.910 | 0.979* (0.154) | 0.740 to 1.370 |
| In rate of change | 0.0002* (0.0001) | 0.0001 to 0.0004 | 0.0003* (0.00005) | 0.0002 to 0.0004 | 0.001* (0.0001) | 0.0004 to 0.0008 |
| Covariance | −0.006* (0.002) | −0.010 to −0.003 | −0.011* (0.002) | −0.014 to −0.007 | −0.016* (0.003) | −0.023 to −0.010 |
*Significant p < .05
Primary Outcomes: Adherence to Corticosteroid Medication
The predictor variable was adherence to fluticasone medication over a period of 9 to 12 months. Adherence to fluticasone medication was defined as the number of prescribed medication doses taken each day and was based on a prescribed treatment regimen of four puffs daily. Daily fluticasone use data were averaged over 5-day intervals. Thus, a participant with 1 year of monitoring data had 73 time points, where each time point was the average daily fluticasone use for that 5-day period. If the daily number of inhaler actuations exceeded four, the number of daily puffs was truncated to four, to control for “dumping” of the inhaler and inhaler overuse. This procedure is similar to those used in previous research (Fennie, Bova, & Williams, 2006; McQuaid, et al., 2003; Walders, et al., 2005). Fluticasone use was monitored for 1 year in 67 (72.8%) participants and 9–12 months in 25 (37.2%) participants.
Changes in Adherence and Clinical Outcomes
The results of the unconditional growth curve models for adherence to inhaled corticosteroids (fluticasone) as well as the outcome variables of albuterol use, symptom ratings, and healthcare utilization are presented in Table I. Unconditional growth curve models were constructed with the predictor variables (i.e., time) centered at the first occasion; however outcome variables were uncentered to assist with interpretations.
Description of Change in Adherence to Corticosteroid Medication
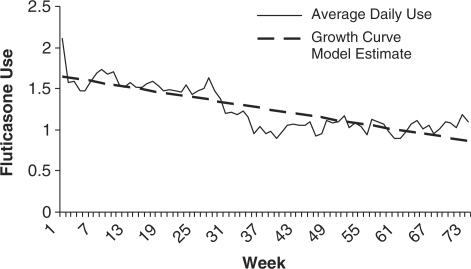
Figure 1 shows the average fluticasone use over time as well as the model estimate for the unconditional growth curve trajectory. Linear and curvilinear models (quadratic, cubic, and spline) were constructed and exploratory analysis revealed that a linear model best fit the data. This was determined by evaluating the Akaike Information Criterion (AIC) value. It should be noted that model comparisons are easier to evaluate when large differences in AIC are present because in practice the model with the lowest AIC is chosen; but when small differences exist between AIC values, the models are much harder to evaluate, thus AIC alone should not predict which model best fits the data because theoretical knowledge could enhance the final model (Singer & Willett, 2003). The average overall adherence rate (daily mean percent prescribed puffs) for all participants over the course of the study was 31.5% (SD = 32.3). In the unconditional growth model for fluticasone adherence, the intercept of 1.67 puffs per day at the onset of the study suggests that participants took 42% of prescribed puffs at the start of the study. Average daily adherence decreased by .012 puffs per week. After 1 year, participants took an average of only 0.806 puffs per day (20.2% of prescribed puffs). However, fluticasone adherence was not moderated by age, p = .72.
Figure 1.
Individual growth curve for fluticasone use.
Description of Change in: Symptoms, Albuterol Use, and Healthcare Utilization
In the unconditional growth curve model for symptom ratings, the average initial symptom rating of 4.30 (out of 5) did not significantly change over the course of the study. Furthermore, parent reported symptom ratings were not moderated by age, p = .94. On average, participants used quick relief medication (albuterol) on 14.83% (SD = 17.23) of days monitored with an average of .53 (SD = 1.21) puffs per 5-day week. The unconditional growth model for albuterol use suggested that participants took an average of .63 puffs per day of albuterol at baseline; however, this medication use did not significantly change over time; nor was medication use moderated by age, p = .31. Average healthcare utilization was .32 visits per 3-month interval and utilization increased at a rate of .01 per week, resulting in an average of 1.05 visits at the conclusion of the study (p < .04). However, healthcare utilization was not moderated by age, p = .75.
Prediction of Clinical Outcomes Based on Adherence
Given the presence of significant variability in the individual estimates (intercept or slope) for albuterol use, symptom ratings, and healthcare utilization, conditional growth curve models predicting these outcomes from fluticasone adherence were developed. The conditional models included fluticasone adherence as a time-varying predictor. Fluticasone adherence was centered around the grand mean respectively; however outcome measures were not centered given centering the outcomes makes results difficult to interpret. In other words, adherence was centered by subtracting the weekly adherence average from the grand adherence mean. The conditional growth curve results are presented in Table II. Furthermore, the t-tests presented here indicate the solutions for the fixed effects components of the model. They relate to Table II, such that, these are the t-values associated with the fixed effects (Singer & Willett, 2003).
Predicting Symptom Ratings from Fluticasone Adherence
As shown in Table II, results of the conditional model indicated a significant main effect of fluticasone adherence, t(5548) = −2.03, p < .04, d = .21. On the other hand, the main effect of time was not significant, indicating that asthma-related symptoms did not change over the course of the study. The rate of change in symptoms did not differ as a function of medication adherence as noted by the absence of a significant interaction between fluticasone adherence and time.
Predicting Albuterol Use from Fluticasone Adherence
As shown in Table II, results of the conditional model indicated that fluticasone adherence and albuterol use were not related, nor did the rate of albuterol use change over the course of the study.
Predicting Healthcare Utilization from Fluticasone Adherence
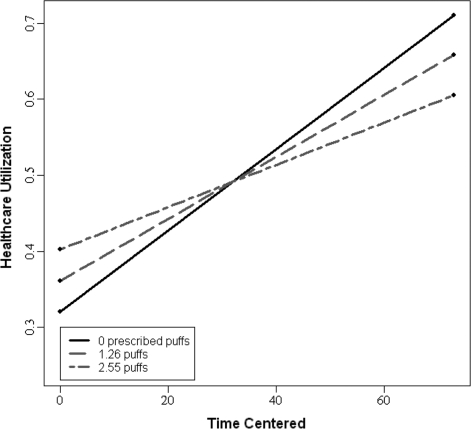
Fluticasone adherence predicted healthcare utilization, t(5572) = 1.98, p <.05, d = .21. The significant main effect of time (t(91.3) = 2.06, p <.05, d = .21), indicated a change in healthcare utilization over the course of the study. Finally, there was a significant interaction between fluticasone adherence and time, t(5581) = −2.36, p <.02, d = .23, indicating that the rate of change in healthcare utilization differed significantly by medication adherence; however, this rate of change in healthcare utilization was not moderated by age, p = .12. To illustrate the interaction between fluticasone adherence and time, widely recommended graphing practices were used (Curran, Bauer, & Willoughby, 2006). The daily average adherence rate over the course of the study was 1.26 (SD = 1.29), thus three different adherence groups were modeled: low adherence group (1SD below the mean), average adherence group, and high adherence group (1SD above the mean). As shown in Fig. 2, individuals in the low adherence group had greater increases in healthcare utilization over time, such that, utilization increased at a rate of .0053 visits over 1 year. Thus, individuals had .76 healthcare related visits at 1 year. Those in the average adherence group also had an increase in healthcare utilization visits over time; utilization increased at a rate of .0041 visits, which corresponds to .70 visits at the conclusion of the study. Those in the high adherence group had less healthcare utilization visits over time, utilization increased at a rate of .0028 visits, such that, at the conclusion of the study, the high adherence group had an average of .65 healthcare-related visits.
Figure 2.
Conditional growth curve for utilization and adherence.
Discussion
Our primary descriptive finding was a significant decrement in the trajectory of treatment adherence to inhaled corticosteroid medication over the course of a year. On average, children and adolescents with asthma took less than half of their prescribed medication at the beginning of the monitoring period, and this declined to 20% after 1 year of study participation. At least one other study has documented low rates of treatment adherence over the course of a year in a largely minority sample of children with asthma (Bauman et al., 2002). However, to our knowledge this is the first study to describe treatment adherence over a year's duration based on growth curve analysis in an economically disadvantaged African-American sample of children and adolescents with asthma.
The factors that accounted for these high rates of treatment nonadherence were not evaluated in this study. However, a wide range of potential barriers to treatment adherence including parental mental health status (Weil et al, 1999); family health beliefs (Mansour et al., 2000); over reliance on emergency medical treatment, problematic family allocation of responsibilities for asthma treatment (Walders, Drotar, & Kercsmar, 2000); and access to care (Rand et al., 1994; Seid, 2008) have been described in minority and low income samples of children and adolescents with asthma and may be influential here.
With respect to the primary hypotheses concerning the relationship of adherence to inhaled corticosteroid treatment and asthma-related clinical outcomes, only the predicted relationship between adherence to treatment and healthcare utilization was supported. Overall rates of treatment adherence (<50%) that also decelerated over a year were associated with increased healthcare utilization (emergency room visits, hospitalizations, and clinic visits due to problems with asthma). The finding indicated that a trajectory of low initial adherence to daily ICS treatment coupled with deceleration in adherence over time may place children at risk for increased healthcare utilization.
While others have noted similar findings (Bauman et al., 2002; Walders et al., 2005), this study documented a relationship between adherence and healthcare utilization based on growth curve analyses of objective electronic monitoring of treatment adherence over a sustained period of time in a minority population. Our findings also suggest that families who were nonadherent to prescribed corticosteroid treatment may have had difficulty independently managing asthma symptoms and instead relied on visits to health care providers for symptom management in clinic or emergency room visits or hospitalizations. The fact that quick-relief medication use did not increase over time but healthcare utilization did is consistent with this explanation.
Contrary to hypotheses, the overall group trajectory of adherence to pediatric asthma treatment did not relate to frequency of symptoms and frequency of quick relief medications. Moreover, the lack of overall change in asthma-related symptoms and use of beta-agonist medications despite low rates of daily inhaled corticosteroid medication adherence was unexpected. Some of these findings may reflect individual differences in how children with asthma responded to corticosteroid treatment due to genetic and factors other than medication use (Drazen, Silverman, & Lee, 2000). Furthermore, it is also possible that nonadherence may be associated with improvement in symptoms over time. For example, as children and adolescents experience fewer symptoms they may begin to believe that daily medication is not necessary. It is also possible that changes in families’ access to medication owing to changes in insurance status may have accounted for declines in adherence. Finally, seasonal effects on symptoms or other outcomes were not measured.
The present findings should be interpreted in light of several methodological limitations. In the absence of an experimental design, it is impossible to isolate the variance in asthma-related clinical outcomes that can be attributed solely to treatment adherence. The frequency of sample attrition was high. Moreover, families with more risk factors and more problematic adherence were more likely to drop out of the study, which has been found in other studies (Zebracki et al., 2003). Additionally, adherence to daily inhaled corticosteroid medications was lower in dropouts compared to completers, thus the overall rates of adherence to inhaled corticosteroid treatment may have been even lower and the relationship with outcome variables greater had the entire sample been available for study. Furthermore, because this sample only included economically disadvantaged African American children and adolescents, these findings might not generalize to other, more representative samples.
The present findings have a number of implications for future research. Studies should assess the relationship of treatment nonadherence to clinical outcomes in children with asthma with larger samples as measured by electronic monitoring. Moreover, the methods of statistical analyses used in this research (DeLucia & Putts, 2006) are well suited for use in studies of long-term trajectories of treatment adherence in pediatric asthma, as well as, other chronic conditions but can also be extended (Jones, Nagin, & Roeder, 2001). For example, with a larger sample size, it would be possible to identify subgroups of children and adolescents who demonstrate different trajectories of nonadherence and evaluate the relationship of these trajectories to clinical outcomes (Nagin, 2005). Other methods of analysis of adherence to asthma treatment may be sensitive to differences in clinical outcomes. For example, gaps in medication treatment adherence defined as number of consecutive days with no prescribed medication use have been found to relate to clinical outcomes in adults with asthma (Williams et al., 2004) and should be studied in pediatric asthma.
Finally, the present findings have potential clinical implications. For example, the findings suggest that economically disadvantaged, African American children and adolescents who demonstrate chronically low levels of adherence over the course of time are also at risk for increased healthcare utilization related to asthma. Several process variables that might be affecting adherence to daily ICS treatment in economically disadvantaged and African-American families similar to the sample studies here may involve increased risk for parental distress or depression, which limits parental energy and attention to the child's asthma treatment (Weil et al., 1999); giving the child independence or responsibility for asthma management, which the child is not ready to assume (Walders et al., 2000); beliefs about asthma medication (e.g., that it is not effective and causes side effects) (Bender & Bender, 2005; Orrell-Valente et al., 2007); or problematic family management strategies (e.g., anxiety and/or absence of coordinated response to asthma symptoms) (Fiese & Wamboldt, 2003); each of these processes and barriers are potentially modifiable and can be targeted in problem-solving interventions. For example, adherence promotion interventions might be focused specifically on enhancing family management of acute symptoms at home by making more appropriate use of quick relief medication in order to reduce healthcare utilization (Guendelman, Meade, Benson, Chen, & Samuels, 2002).
Another potential clinical implication of our findings is that routine measurement of adherence to treatment based on objective methods could facilitate clinical management of pediatric asthma by identifying problematic patterns of nonadherence that lead to under treatment and increased healthcare utilization. Reliable identification of patterns of nonadherence is necessary to provide feedback and to implement targeted behavioral interventions to parents and children to reduce the high levels of treatment nonadherence encountered in high risk populations of children and adolescents with asthma (Onyirimba et al., 2003).
Funding
National Institutes of Health (R01HL695471 to D.D.).
Conflicts of interest: None declared.
Acknowledgments
The assistance of Jill Goodman, Leigh Josie, Tara Moore, Kristin Barret, and Terri Casey in conducting this study and Meggie Bonner in typing this article is gratefully acknowledged.
References
- Asmussen L, Olson LM, Grant EN, Fagan J, Weiss K. Reliability and validity of the Children's Health Survey for Asthma. Pediatrics. 1999;104:e71–e77. doi: 10.1542/peds.104.6.e71. [DOI] [PubMed] [Google Scholar]
- Bauman LJ, Wright E, Leickly FE, Crain E, Kruszon-Moran D, Wade SL, et al. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002;110:6–12. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- Bender BG, Bartlett SJ, Rand CS, Turner C, Wamboldt FS, Zhang L. Impact of interview mode on accuracy of child and parent report of adherence with asthma-controller medication. Pediatrics. 2007;120:471–477. doi: 10.1542/peds.2006-3457. [DOI] [PubMed] [Google Scholar]
- Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: Responses to interviews, focus groups, and questionnaires. Immunology and Allergy Clinics of North America. 2005;25:107–130. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Bender BG, Ikle DN, DuHamel T, Tinkelman DG. Retention of asthmatic patients in a longitudinal clinical trial. Journal of Allergy and Clinical Immunology. 1997;99:197–203. doi: 10.1016/s0091-6749(97)70096-4. [DOI] [PubMed] [Google Scholar]
- Bender B, Milgrom H, Rand C, Ackerson L. Psychological factors associated with medication nonadherence in asthmatic children. Journal of Asthma. 1998;35:347–353. doi: 10.3109/02770909809075667. [DOI] [PubMed] [Google Scholar]
- Bender BG, Rand C. Medication non-adherence and asthma treatment costs. Current Opinions in Allergy and Clinical Immunology. 2004;4:191–195. doi: 10.1097/00130832-200406000-00009. [DOI] [PubMed] [Google Scholar]
- Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. Journal of Allergy and Clinical Immunology. 2008;122:490–495. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Bloom B, Cohen RA. Summary Health Statistics for U.S. Children: National Health Interview Survey, 2006. National Center for Health Statistics. Vital Health Statistics. 2007;10(234) [PubMed] [Google Scholar]
- Bukstein DA, Murphy KR, Katz LM, Ramachandran S, Doyle JJ, Stern LS. Outcomes among a young population of pediatric asthma patients using controller therapies: Results from a retrospective database analysis. Pediatric Asthma, Allergy and Immunology. 2007;20(4):211–222. [Google Scholar]
- Curran PJ, Bauer DJ, Willoughby MT. Testing and probing interactions in hierarchical linear growth models. In: Bergeman CS, Boker SM, editors. The notre dame series on quantitative methodology, Volume 1: Methodological issues in aging research. Mahwah, NJ: Lawrence Erlbaum Associates; 2006. pp. 99–129. [Google Scholar]
- DeLucia C, Pitts S. Applications of individual growth curve modeling for pediatric psychology research. Journal of Pediatric Psychology. 2006;31(10):1002–1023. doi: 10.1093/jpepsy/jsj074. [DOI] [PubMed] [Google Scholar]
- Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. British Medical Bulletin. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- Drotar D. Overcoming barriers to treatment adherence in minorities and persons living in poverty. 2006. Presentation at National Institutes of Health of Investigators funded under RFA, Bethesda, MD. [Google Scholar]
- EPR-3. Bethesda, MD: U.S. Department of Health and Human Services; National Institute of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program; Expert panel report 3: guidelines for the diagnosis and management of asthma (EPR-3, 2007). NIH Publication No. 97-4051. 1997. [Google Scholar]
- Fennie KP, Bova CA, Williams AB. Adjusting and censoring electronic monitoring device data: implications for study outcomes. Journal of Acquired Immune Deficiency Syndrome. 2006;43(S1):S88–S95. doi: 10.1097/01.qai.0000248336.97814.2f. [DOI] [PubMed] [Google Scholar]
- Fiese BH, Wamboldt FS. Tales of pediatric asthma management: Family-based strategies related to medical adherence and health care utilization. Journal of Pediatrics. 2003;143:457–467. doi: 10.1067/S0022-3476(03)00448-7. [DOI] [PubMed] [Google Scholar]
- Fiese BH, Wamboldt FS, Anbar RD. Family asthma management routines: Connections to medical adherence and quality of life. Journal of Pediatrics. 2005;146:171–176. doi: 10.1016/j.jpeds.2004.08.083. [DOI] [PubMed] [Google Scholar]
- Getahun D, Demissie K, Rhoads GG. Recent trends in asthma hospitalization and mortality in the United States. Journal of Asthma. 2005;42:373–378. doi: 10.1081/JAS-62995. [DOI] [PubMed] [Google Scholar]
- Grant EN, Lyttle CS, Weiss KB. The relation of socioeconomic factors and racial/ethnic differences in US asthma mortality. American Journal of Public Health. 2000;90:1923–1925. doi: 10.2105/ajph.90.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self-management behaviors of inner-city children: A randomized trial of the health buddy interactive device and an asthma diary. Archives in Pediatric and Adolescent Medicine. 2002;156:114–120. doi: 10.1001/archpedi.156.2.114. [DOI] [PubMed] [Google Scholar]
- Greeenley RN, Josie KL, Drotar D. Self-reported quality of life among innner city youth with asthma, an empirical examination of the PedsQL3.0. Annals of Allergy, Asthma and Immunology. 2008;100:106–111. doi: 10.1016/S1081-1206(10)60418-8. [DOI] [PubMed] [Google Scholar]
- HHS. Annual update of the HHS Poverty Guidelines. Federal Register. 2001;66(33):10695–10697. [Google Scholar]
- Jones BL, Nagin DS, Roeder KA. A SAS Procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Josie KL, Greeenley RN, Drotar D. Health-related quality-of-life measures for children with asthma: Reliability and validity of the Children's Health Survey for Asthma and the Pediatric Quality of Life Inventory 3.0 Asthma Module. Annals of Allergy, Asthma Immunology. 2007;98:218–24. doi: 10.1016/S1081-1206(10)60710-7. [DOI] [PubMed] [Google Scholar]
- Josie KL, Greeenley RN, Drotar D. Cumulative risk and asthma outcomes in inner-city African American youth. Journal of Asthma. 2008;44:535–541. doi: 10.1080/02770900701496114. [DOI] [PubMed] [Google Scholar]
- Kruse LK, Deshpande S, Vezina M. Disparities in asthma hospitalizations among children seen in the emergency department. Journal of Asthma. 2007;44:833–837. doi: 10.1080/02770900701750163. [DOI] [PubMed] [Google Scholar]
- Lieu TA, Lozano P, Finkelstein JA, Chi FW, Jensvold NG, Capra AM, et al. Racial/ethnic variation in asthma status and management practices among children in managed medicaid. Pediatrics. 2002;109:857–865. doi: 10.1542/peds.109.5.857. [DOI] [PubMed] [Google Scholar]
- Mansour ME, Lanphear BP, DeWitt TG. Barriers to asthma care in urban children: Parents perspectives. Pediatrics. 2000;106:512–519. doi: 10.1542/peds.106.3.512. [DOI] [PubMed] [Google Scholar]
- Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. Journal of Allergy and Clinical Immunology. 1996;98:1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: Reasoning, responsibility, and behavior. Journal of Pediatric Psychology. 2003;28:323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Walders N. Pediatric asthma. In: Roberts MC, editor. Handbook of pediatric psychology. New York: The Guilford Press; 2003. pp. 209–285. [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- National Institutes of Health. National asthma education program expert panel: Clinical guidelines expert panel report 2: guidelines for the diagnosis and management of asthma. Washington, DC: U.S. Government Printing Office; 1997. 1997. DHHS publication 97-4051. [Google Scholar]
- Onyirimba F, Apter A, Reisine S, Litt M, McCusker C, Connors M, ZuWallack R. Direct clinician-to-patient feedback discussion of inhaled steroid use: Its effect on adherence. Annals in Allergy and Immunology. 2003;90:411–415. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- Orrell-Valente JK, Jarlsberg LG, Rait MA, Thyne SM, Rubash T, Cabana MD. Parents’ specific concerns about daily asthma medications for children. Journal of Asthma. 2007;44:385–390. doi: 10.1080/02770900701364221. [DOI] [PubMed] [Google Scholar]
- Rachelefsky G. Inhaled corticosteroids and asthma control in children assessing impairment and risk. Pediatrics. 2009;121:353–366. doi: 10.1542/peds.2007-3273. [DOI] [PubMed] [Google Scholar]
- Rand CS, Butz AM, Huss K, Eggleton P, Thompson L, Malveax FJ. Adherence to therapy and access to care. The relationship to excess asthma morbidity in African American children. Pediatric Asthma, Allergy, and Immunology. 1994;8:179–184. [Google Scholar]
- Riekert KA, Rand CS. Electronic monitoring of adherence: When is high-tech the best? Journal of Clinical Psychology in Medical Settings. 2002;9:25–34. [Google Scholar]
- Seid M. Barriers to care and primary care for vulnerable children with asthma. Pediatrics. 2008;122:994–1002. doi: 10.1542/peds.2007-3114. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford Press; 2003. [Google Scholar]
- Smith L, Bokhour B, Hohman K, Miroshnik I, Kleinman K, Cohn E, et al. Modifiable risk factors for suboptimal control and controller medication underuse among children with asthma. Pediatris. 2008;122:760–769. doi: 10.1542/peds.2007-2750. [DOI] [PubMed] [Google Scholar]
- Vinicor F. Diabetes mellitus and asthma: “Twin” challenges for public health and managed care systems. American Journal of Preventive Medicine. 1998;14:87–92. doi: 10.1016/s0749-3797(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Walders N, Drotar D, Kercsmar C. The allocation of family responsibility for asthma management tasks in African-American adolescents. Journal of Asthma. 2000;37:89–99. doi: 10.3109/02770900009055432. [DOI] [PubMed] [Google Scholar]
- Walders N, Kopel SL, Koinis-Mitchell D, McQuaid EL. Patterns of quick-relief and long-term controller medication use in pediatric asthma. Journal of Pediatrics. 2005;146:177–182. doi: 10.1016/j.jpeds.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, et al. Relationship between adherence to inhaled corticosteroid and poor outcomes among adults with asthma. Journal of Allergy and Clinical Immunology. 2004;114:1288–1293. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychological factors and asthma morbidity in inner city children with asthma. Pediatrics. 1999;104:1274–1280. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- Zebracki K, Drotar D, Kirchner KL, Schluchter M, Redline S, Kerscmar CM, et al. Predicting attrition in a pediatric asthma intervention study. Journal of Pediatric Psychology. 2003;28:519–528. doi: 10.1093/jpepsy/jsg042. [DOI] [PubMed] [Google Scholar]