Abstract
Background
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) recently published an equation for estimated glomerular filtration rate (eGFR) using the same variables (serum creatinine, age, gender and race) as the Modification of Diet in Renal Disease Study (MDRD) equation. Although the CKD-EPI equation estimates GFR more precisely as compared with the MDRD equation, whether this equation improves risk prediction is unknown.
Study Design
Prospective cohort study, the Atherosclerosis Risk in Communities (ARIC) Study.
Setting & Participants
13,905 middle-aged participants without a history of cardiovascular disease with median follow-up of 16.9 years.
Predictor
eGFR
Outcomes & Measurements
We compared the association of eGFR in categories (≥120, 90–119, 60–89, 30–59, <30 ml/min/1.73m2) by the CKD-EPI and MDRD equations with risk of incident end-stage renal disease (ESRD), all-cause mortality, coronary heart disease (CHD), and stroke.
Results
Median of eGFRCKD-EPI was higher than that of eGFRMDRD (97.6 vs. 88.8 ml/min/1.73m2, P<0.001). The CKD-EPI equation reclassified 44.9% (n=3,079) and 43.5% (n=151) of participants with eGFRMDRD 60–89 and 30–59, respectively, upward to a higher eGFR category but no one with eGFRMDRD 90–119 or <30, lowering the prevalence of CKD stage 3–5 from 2.7% to 1.6%. Participants with eGFRMDRD 30–59 who were reclassified upward had lower risk as compared to those who were not reclassified (ESRD incidence rate ratio, 0.10 [95% CI, 0.03–0.33], all-cause mortality, 0.30 [0.19–0.48], CHD, 0.36 [0.21–0.61], stroke, 0.50 [0.24–1.01]). Similar results were observed for participants with eGFRMDRD 60–89. More frequent reclassification of younger, female, and white participants explained some of these trends. Net reclassification improvement among participants with eGFR <120 was positive for all outcomes (P<0.001).
Limitations
Limited number of cases with eGFR <60 and no measurement of albuminuria.
Conclusions
The CKD-EPI equation more appropriately categorized individuals with respect to long-term clinical risk as compared to the MDRD equation, suggesting improved clinical usefulness in this middle-aged population.
Glomerular filtration rate (GFR) is the best overall measure of kidney function.1 However, direct measurement of GFR using radioactive agents is burdensome and expensive.1 Thus, equations for estimated GFR (eGFR) utilizing endogenous filtration markers such as serum creatinine have been developed and used for chronic kidney disease (CKD) diagnosis and staging.2 The Modification of Diet in Renal Disease (MDRD) Study equation, which incorporates information on age, gender, race, and serum creatinine concentration, is most commonly used in clinical practice and epidemiological studies to estimate kidney function.3–6
Despite its widespread use, eGFR by the MDRD equation (eGFRMDRD) has several limitations. The MDRD equation was developed in a population of individuals with CKD and reduced GFR7, 8 and systematically underestimates GFR in individuals with measured GFR ≥60 ml/min/1.73m2.8–10 Previous studies have suggested that the use of the MDRD equation may result in “over-diagnosis” of CKD.8, 9, 11
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) recently published a new equation to improve the estimation of GFR particularly among individuals with GFR ≥60 ml/min/1.73m2.12 The CKD-EPI equation was developed using data from 8,254 individuals from 10 studies including the MDRD study and validated in additional 16 studies containing 3,896 individuals.12 eGFR by the CKD-EPI equation (eGFRCKD-EPI) was more accurate at estimating measured GFR overall and among individuals with normal or mildly reduced kidney function as compared with eGFRMDRD. Importantly, the CKD prevalence was reduced from 13.1% based on the MDRD equation to 11.5% by the CKD-EPI equation in the adult US population represented by the National Health and Nutrition Examination Survey 1999–2006.12
However, performance of the CKD-EPI equation for classification of long-term clinical risk has not been evaluated. The objective of this study was to evaluate the implications of eGFR categories based on the CKD-EPI equation as compared to those based on the conventional MDRD equation for classifying individuals at risk for end-stage renal disease (ESRD), all-cause mortality, coronary heart disease (CHD), and stroke.
METHODS
Study Population
We analyzed data from participants in the Atherosclerosis Risk in Communities (ARIC) Study, a population-based cohort study of middle-aged individuals from four US communities: Forsyth County, North Carolina; suburban Minneapolis, Minnesota; Washington County, Maryland; and Jackson, Mississippi. Details of the ARIC Study are described elsewhere.13 In brief, 15,792 men and women aged 45 to 64 years were enrolled from 1987 through 1989. In the present study, we excluded participants self-reporting race other than white or black (n=48) or missing serum creatinine values at baseline (n=150). We also excluded participants with a history of cardiovascular disease at baseline based on self-report or clinical examination or missing data on cardiovascular history (n=1,726), for a final study population size of 13,905 participants. As might be expected, participants who were excluded due to a history of cardiovascular disease (n=1,039) had poorer risk factor profile as compared to the final study population (mean age: 57.0 vs. 54.0 years, systolic blood pressure: 124.1 vs. 121.1 mmHg, and low-density lipoprotein cholesterol [LDL-C]: 147.4 vs. 136.7 mg/dL). Participants who were excluded due to missing information (n = 801) were similar to the final study population (mean age: 54.2 years, systolic blood pressure: 122.6 mmHg, and LDL-C: 142.7 mg/dL).
Data Collection
ARIC study participants provided information on baseline demographic and behavioral variables and medical history to a trained interviewer. Completed years of education and smoking status (current or former/never) were determined by self-report. Blood samples were collected according to standardized procedures.14 Certified technicians measured systolic and diastolic blood pressures with participants in the sitting position after 5 minutes of rest using a random-zero sphygmomanometer. The average of the second and third readings was recorded. We defined diabetes mellitus as a fasting glucose of ≥ 126 mg/dL, non-fasting glucose of ≥200 mg/dL, self-reported physician diagnosis of diabetes, or use of oral hypoglycemic medication or insulin. Plasma cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C) were determined using enzymatic methods, and LDL-C was calculated using the Friedewald equation.14 Left ventricular hypertrophy by electrocardiogram was defined by the Cornell voltage.15 Evidence of atherosclerosis of the common carotid arteries (shadowing/plaque on either side) was determined by ultrasound examination.13, 16
Estimation of GFR
Serum creatinine concentration was measured using a modified kinetic Jaffe method14, 17 and corrected for interlaboratory differences, calibrated to the Cleveland Clinic by subtraction of 0.24 mg/dL,16, 18 and standardized to the Roche enzymatic method (Roche-Hitachi PModule instrument with Roche Creatininase Plus assay, Hoffman-La Roche, Ltd., www.roche.com) by multiplication of 0.95.19 We calculated eGFR using the IDMS-traceable 4-variable MDRD Study equation[nd1]: eGFRMDRD = 175 × (standardized serum creatinine [mg/dL])−1.154 × age−0.203 × (0.742 if female) × (1.212 if black)7, 20 and also using the CKD-EPI equation: eGFRCKD-EPI = 141 × (minimum of standardized serum creatinine [mg/dL]/κ or 1)α × (maximum of standardized serum creatinine [mg/dL]/κ or 1)−1.209 × 0.993age × (1.018 if female) × (1.159 if black), where κ is 0.7 for female and 0.9 for male and α is −0.329 for female and −0.411 for male.12 The unique properties of the CKD-EPI equation compared to the MDRD Study equation include a steeper gradient for age, a less steep slope for serum creatinine less than 0.7 mg/dL in females and 0.9 mg/dL in males and a similarly steep slope at a range higher than these levels, a smaller black-to-white ratio, and a slightly higher female-to-male ratio particularly when a creatinine concentration is less than 0.9 mg/dL.12 These properties resulted in higher eGFRCKD-EPI as compared to eGFRMDRD particularly in younger population, female, and whites.12
Outcome Assessment
ARIC investigators conduct continuous, comprehensive surveillance for all cardiovascular disease-related hospitalizations and deaths in the four communities. All potential cardiovascular events are adjudicated using published criteria.21–23 We defined incident CHD as a definite or probable myocardial infarction, definite coronary death, or coronary revascularization procedure. Stroke included definite or probable cases defined as sudden or rapid onset of neurologic symptoms that lasted for 24 h or led to death in the absence of another cause.22, 23
ESRD cases included all participants with a history of hospitalization with an International Classification of Disease (ICD) code specified for kidney transplant, dialysis or a procedure indicating dialysis and for individuals with an earlier diagnosis of CKD an underlying cause of death of acute renal failure. Individuals with a code of traumatic anuria or those with a transplant or dialysis code on the same date as another code for acute renal failure (ICD-9 586, 584, 788.9) but without previous CKD were not included.
Statistical Analyses
We categorized eGFR using the following clinically relevant cut-points established by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI): ≥120, 90–119, 60–89, 30–59, <30 ml/min/1.73m2.3 Participants with eGFR <15 ml/min/1.73m2 (CKD stage 5) were not considered separately in the present study, as there were few participants in this category (n=16 for eGFRCKD-EPI and n=15 for eGFRMDRD). Individuals with eGFR ≥120 ml/min/1.73m2 were considered separately from those with eGFR 90–119 ml/min/1.73m2 under the assumption that high eGFR may result from very low creatinine due to muscle loss related to ill health and may not necessarily be associated with better long-term outcomes. 16, 24 We compared baseline characteristics of the population across these eGFR categories.
We evaluated the continuous association between eGFR by both equations and the incidence rates of clinical outcomes using a Poisson regression model incorporating linear spline terms for eGFR (knots at 45, 60, 75, 90, and 105 ml/min/1.73 m2) with and without adjustment for age, gender, and race. We also evaluated the risk of clinical outcomes according to categories of eGFR, using eGFR of 90–119 ml/min/1.73m2 as the reference group and after adjusting for multiple covariates. Model discrimination was assessed using Harrell’s C-statistic.25 We defined follow-up time as the period to the first outcome or loss to follow-up. Individuals who were free of the above outcomes by January 1, 2006 (January 1, 2005 for ESRD) were subject to administrative censoring.
To assess reclassification, we created a 5 × 5 cross-tabulation of the eGFRMDRD and the eGFRCKD-EPI categories, calculated the proportion of participants reclassified by eGFRCKD-EPI in each category of eGFRMDRD, and assessed whether risk of clinical outcomes differed between participants reclassified and those not reclassified. To further evaluate overall improvement in reclassification, we calculated net reclassification improvement[nd2],26 calculated as the sum of the proportion of participants reclassified downward to a lower eGFR category among individuals with an outcome and the proportion of participants reclassified upward to a higher eGFR category among individuals without an outcome, less the sum of the proportion of participants reclassified upward among individuals with an outcome and the proportion of participants reclassified downward among individuals without an outcome. This calculation represents the sum of the two terms corresponding to “clinically correct” reclassification, less the two terms reflecting “clinically incorrect” reclassification. In sensitivity analyses, we also assessed net reclassification improvement using 10-year risk categories (<5%, 5-<10%, 10-<20%, and ≥20%) of each outcome predicted from Cox proportional hazards models.27 All analyses were conducted using Stata 10.1 software (Stata Corp, www.stata.com) and a P-value of less than 0.05 was considered statistically significant.
RESULTS
Characteristics of Study Participants
Participants with CKD stage 3 (eGFRCKD-EPI of 30–59 ml/min/1.73m2) or stage 4/5 (eGFRCKD-EPI <30 ml/min/1.73m2) were more likely to be older, female, and black and have more comorbidities including diabetes as compared to individuals with eGFRCKD-EPI of 90–119 ml/min/1.73m2 (Table 1). The category of eGFRCKD-EPI ≥120 ml/min/1.73m2 mainly consisted of black females who also tended to have higher prevalence of diabetes and left ventricular hypertrophy, more often reported taking anti-hypertensive medications, and have higher body mass index compared to those with eGFRCKD-EPI of 90–119 ml/min/1.73m2. The mean age in this group, however, was lower compared to the other categories. Similar results were observed across categories of eGFRMDRD (Table S1; available as online supplementary material associated with this article at www.ajkd.org).
Table 1.
Characteristics of Participants according to Clinical Categories of eGFRCKD-EPI
| Categories of eGFRCKD-EPI (ml/min/1.73m2) |
|||||
|---|---|---|---|---|---|
| >=120 | 90–119 | 60–89 | 30–59 | <30 | |
| Characteristic | (n = 716) | (n = 9,035) | (n = 3,931) | (n = 196) | (n = 27) |
| Age (y) | 49.4 ± 3.9 | 53.5 ± 5.5 | 55.5 ± 6.0 | 58.7 ± 5.3 | 56.4 ± 6.7 |
| Men | 188 (26) | 3,791 (42) | 1,956 (50) | 79 (40) | 7 (26) |
| Black race | 679 (95) | 2,267 (25) | 733 (19) | 63 (32) | 25 (93) |
| Educational level completed (n = 13,883) | |||||
| <12 yr | 253 (35) | 2,003 (22) | 798 (20) | 68 (35) | 16 (59) |
| 12 to 16 yr | 224 (31) | 3,771 (42) | 1,620 (41) | 69 (35) | 9 (33) |
| >16 yr | 238 (33) | 3,246 (36) | 1,507 (38) | 59 (30) | 2 (7) |
| Current smokers (n = 13,893) | 233 (33) | 2,545 (28) | 761 (19) | 45 (23) | 5 (19) |
| BMI (kg/m2) (n = 13,895) | 29.9 ± 6.9 | 27.4 ± 5.4 | 27.7 ± 4.8 | 28.5 ± 5.2 | 29.3 ± 5.5 |
| Diabetes mellitus (n = 13,882) | 145 (20) | 911 (10) | 383 (10) | 50 (26) | 15 (56) |
| Antihypertensive medication (n = 13,897) | 257 (36) | 2,220 (25) | 1,230 (31) | 124 (63) | 18 (67) |
| Systolic BP (mm Hg) (n = 13,900) | 126.6 ± 20.1 | 120.3 ± 18.4 | 121.3 ± 18.4 | 127.6 ± 22.3 | 153.5 ± 34.0 |
| Diastolic BP (mm Hg) (n = 13,900) | 79.2 ± 12.4 | 73.2 ± 11.1 | 73.9 ± 10.9 | 74.9 ± 12.2 | 80.2 ± 13.3 |
| LDL-C (mg/dL) (n = 13,625) | 127.4 ± 40.7 | 135.6 ± 38.5 | 140.4 ± 39.4 | 147.0 ± 45.4 | 153.3 ± 67.1 |
| HDL-C (mg/dL) (n = 13,814) | 57.2 ± 19.1 | 52.8 ± 17.1 | 50.3 ± 16.6 | 48.1 ± 16.6 | 48.4 ± 19.5 |
| Triglycerides (mg/dL) (n = 13,815) | 111.1 ± 81.8 | 127.1 ± 87.1 | 135.2 ± 91.6 | 165.1 ± 110.1 | 174.9 ± 145.1 |
| Left ventricular hypertrophy (n = 13,587) | 25 (4) | 157 (2) | 80 (2) | 9 (5) | 6 (22) |
| Carotid atherosclerosis (n = 13,473) | 46 (7) | 603 (7) | 338 (9) | 26 (14) | 7 (30) |
| Serum creatinine (mg/dL) | 0.61 ± 0.10 | 0.75 ± 0.13 | 0.96 ± 0.14 | 1.33 ± 0.24 | 7.00 ± 5.02 |
| eGFRCKD-EPI (ml/min/1.73m2) | 125.5 ± 5.3 | 102.1 ± 7.3 | 79.8 ± 7.4 | 53.0 ± 6.3 | 13.9 ± 10.1 |
| eGFRMDRD (ml/min/1.73m2) | 139.0 ± 19.4 | 99.2 ± 13.4 | 73.5 ± 6.8 | 50.5 ± 5.8 | 14.2 ± 9.9 |
Note: Values expressed as mean ± SD or number (percent). Conversion factors for units: LDL-C and HDL-C in mg/dL to mmol/L, ×0.02586; triglycerides in mg/DL to mmol/L, ×0.01129; creatinine in mg/dL to µmol/L, ×88.4; eGFR in ml/min/1.73m2 to mL/s/1.73 m2, ×0.01667.
Abbreviations and definitions: eGFRCKD-EPI, estimated glomerular filtration rate (eGFR) calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation; eGFRMDRD, estimated glomerular filtration rate calculated using the Modification of Diet in Renal Disease (MDRD) Study equation; BP, blood pressure; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; MDRD, Modification of Diet in Renal Disease
Mean eGFRCKD-EPI was higher among persons with eGFR of 30–89 ml/min/1.73m2 but lower than eGFRMDRD among individuals with eGFR ≥120 ml/min/1.73m2 (Table 1 and Figure 1). Mean eGFRCKD-EPI and eGFRMDRD were similar for categories of eGFR 90–119 and less than 30 ml/min/1.73m2. Overall, the median (interquartile range) of eGFRCKD-EPI was higher than that of eGFRMDRD (97.6 [87.3–105.6] and 88.8 [79.8–102.1] ml/min/1.73m2, respectively, P<0.001). In particular, median eGFRCKD-EPI was higher than eGFRMDRD among female, whites, and younger individuals (+8.8 ml/min/1.73m2 in female vs. +6.9 in male, +10.0 in whites vs. +5.8 in blacks, and +9.5 in younger [45–54 y] vs. +7.3 in older [55–64 y]) as expected. The distribution of eGFRCKD-EPI and eGFRMDRD are shown in Panel A of Figure 2.
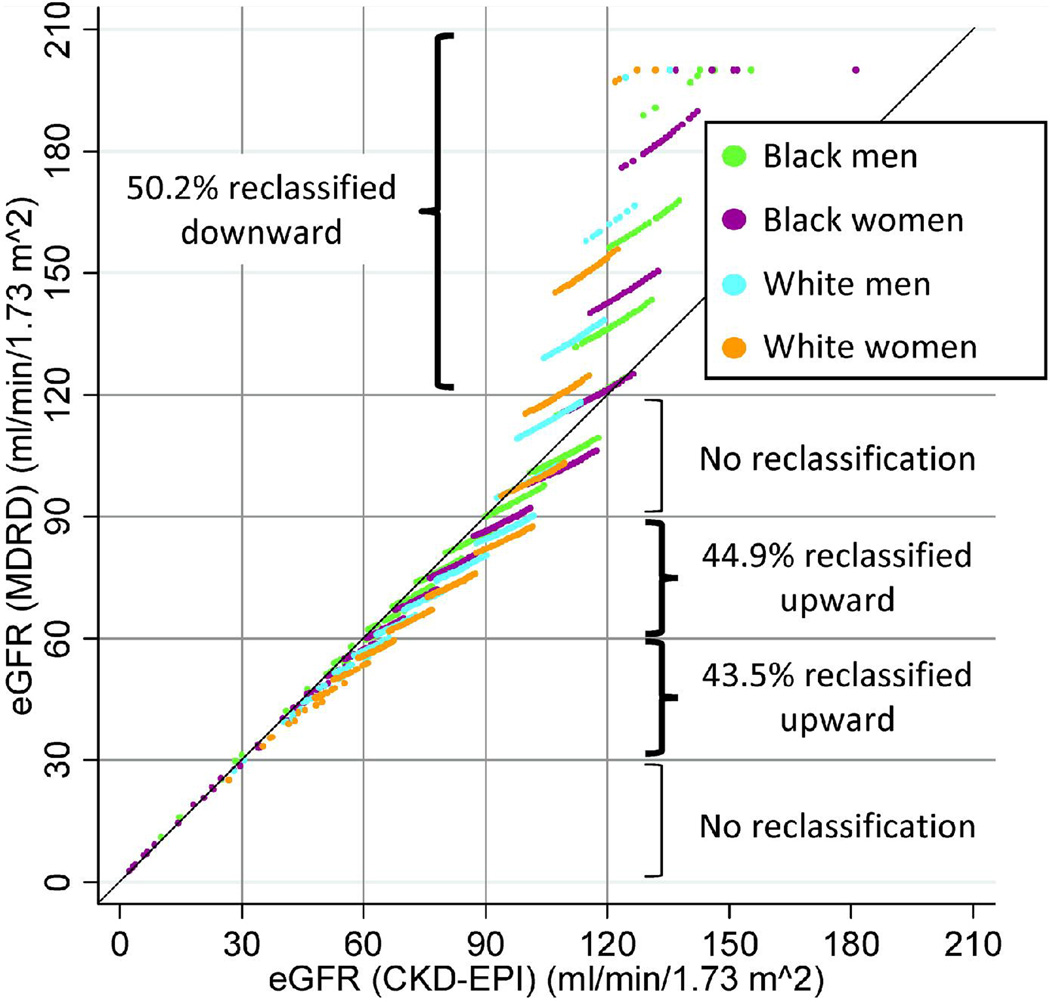
Figure 1.
Scatter plot of eGFRCKD-EPI and eGFRMDRD according to the combination of gender and race. Points in off-diagonal boxes indicate individuals who are reclassified into a higher (below the diagnonal) or lower (above the diagonal) eGFR category by eGFRCKD-EPI compared to eGFRMDRD. The graph includes 13,905 individuals but points with the same eGFR plot on top of each other. Individuals with eGFRMDRD >200 are plotted at 200.
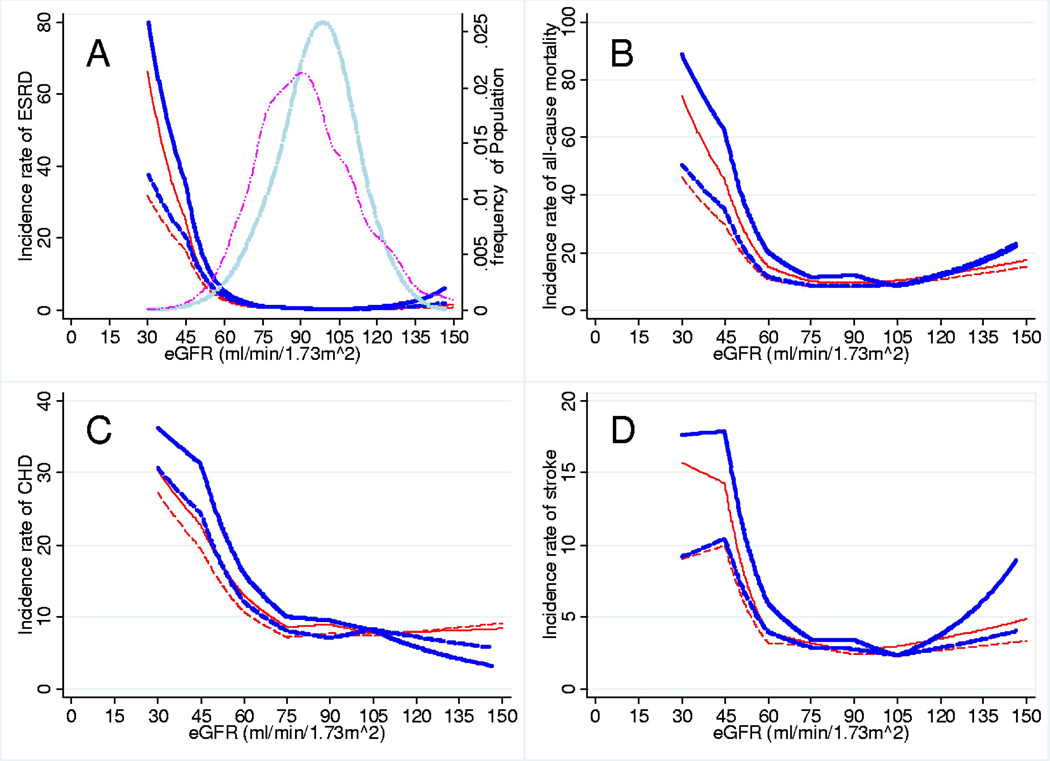
Figure 2.
Incidence Rates (per 1,000 person-years) of Outcomes according to eGFR by the CKD-EPI and MDRD study equations. Incidence rates of (A) ESRD, (B) all-cause mortality, (C) CHD, and (D) stroke and eGFR (ml/min/1.73m2) by the CKD-EPI equation (blue and thick) and the MDRD equation (red) without (solid lines) and with (dash lines) adjustment for mean values of age, gender, and race. The long-dash two dots lines in the panel A demonstrate kernel density plots of the distributions of eGFRCKD-EPI (light blue and thick) and eGFRMDRD (magenta).
Incidence Rates According to eGFR by Each Equation
During median follow-up of 16.9 years, there were 192 cases of ESRD, 2,455 deaths, 1,850 cases of CHD, and 648 cases of stroke. The continuous relationships between eGFR by both equations and incidence rates of the clinical outcomes with and without adjusting for age, gender and race are shown in Figure 2. Although the trends for both equations were quite similar, incidence rates of the four outcomes were higher throughout the range of moderately decreased GFRCKD-EPI as compared with the same levels of eGFRMDRD. The positive slopes of eGFR with ESRD, all-cause mortality, and stroke at a range of eGFR >105 ml/min/1.73m2 were steeper for GFRCKD-EPI than eGFRMDRD. In contrast, CHD risk was lower even at eGFRs above 120 ml/min/1.73m2 for eGFRCKD-EPI but not for eGFRMDRD.
The adjusted incidence rate ratios for each outcome comparing eGFR categories of the CKD-EPI and MDRD equations are presented in Table 2. For both equations, the categories of eGFR <60 ml/min/1.73m2 were consistently associated with similarly greater risks of each of the outcomes. Individuals with eGFR 60–89 ml/min/1.73m2 by either equation had higher risks of incident ESRD and stroke as compared with the reference groups. The incidence rates of ESRD and all-cause mortality among participants with eGFR ≥120 ml/min/1.73m2 by both equations were significantly higher as compared to the reference groups for both equations. Harrell’s C-statistics were obtained after Cox proportional hazards models with covariates used in Table 2 and were nearly identical for eGFRCKD-EPI and eGFRMDRD (ESRD: 0.912 vs. 0.908, all-cause mortality: 0.746 vs. 0.747, CHD: 0.751 vs. 0.750, and stroke: 0.760 vs. 0.761, respectively).
Table 2.
Adjusted Incidence Rate Ratio (95% CI) for Outcomes according to eGFR Categories Determined by Either CKD-EPI or MDRD Study Equation
| Categories of eGFR, ml/min/1.73m2 | |||||
|---|---|---|---|---|---|
| >=120 | 90–119 | 60–89 | 30–59 | <30 | |
| No. Participants | |||||
| CKD-EPI* | 641 | 8,357 | 3,645 | 171 | 21 |
| MDRD* | 1,297 | 4,830 | 6,380 | 307 | 21 |
| Outcomes | |||||
| ESRD | |||||
| CKD-EPI | 2.07 (1.13–3.79) | (ref) | 2.89 (1.94–4.30) | 16.70 (10.13–27.53) | 54.43 (26.69–111.00) |
| MDRD | 2.04 (1.13–3.67) | (ref) | 2.85 (1.80–4.50) | 16.09 (9.33–27.75) | 59.06 (28.17–123.81) |
| All-cause mortality | |||||
| CKD-EPI | 1.34 (1.10–1.64) | (ref) | 1.01 (0.91–1.11) | 1.69 (1.33–2.15) | 5.53 (3.48–8.80) |
| MDRD | 1.27 (1.10–1.49) | (ref) | 0.99 (0.90–1.09) | 1.56 (1.25–1.93) | 5.54 (3.48–8.81) |
| CHD | |||||
| CKD-EPI | 0.83 (0.61–1.12) | (ref) | 1.01 (0.91–1.12) | 1.46 (1.08–1.97) | 2.20 (0.90–5.34) |
| MDRD | 1.06 (0.88–1.29) | (ref) | 1.00 (0.90–1.11) | 1.29 (0.99–1.68) | 2.25 (0.92–5.46) |
| Stroke | |||||
| CKD-EPI | 1.12 (0.80–1.58) | (ref) | 1.17 (0.98–1.40) | 1.71 (1.09–2.67) | 1.77 (0.56–5.61) |
| MDRD | 1.14 (0.87–1.49) | (ref) | 1.16 (0.97–1.38) | 1.88 (1.29–2.75) | 1.81 (0.57–5.76) |
Note: Adjusted for following covariates: age, race, gender, level of education, systolic blood pressure, antihypertensive medication, diabetes, smoking, body mass index, low- and high -density lipoprotein cholesterol, left ventricular hypertrophy, and carotid atherosclerosis.
Abbreviations: eGFR: estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; ESRD, end-stage renal disease; CHD, coronary heart disease; ref, reference.
Participants included in the fully adjusted analysis.
Although the adjusted relative risks are very similar, there was substantial reclassification of individual participants to different eGFR groups. Specifically, the size of the reference group with eGFR 90–119 changed dramatically from 4,830 by the MDRD Study equation to 8,357 with the CKD-EPI equation. However, the risk of these groups is similar since the absolute risk of individuals with eGFRCKD-EPI 90–119 but eGFRMDRD 60–89 or ≥120 is very similar with individuals with eGFR 90–119 by both equations (Table 3).
Table 3.
Crude Incidence Rates (95% CIs) (per 1,000 Person-Years) of Outcomes according to eGFR Categories Determined by CKD-EPI and MDRD Study Equations
| eGFRCKD-EPI, ml/min/1.73m2 | ||||||
|---|---|---|---|---|---|---|
| eGFRMDRD, ml/min/1.73m2 |
>=120 | 90–119 | 60–89 | 30–59 | <30 | reclassifi cation |
| >=120 | ||||||
| n | 716 | 723 | 0 | 0 | 0 | 50.2% |
| ESRD | 1.5 (0.9– 2.4) |
0.6 (0.3– 1.4) |
- | - | - | |
| All-cause mortality |
12.4 (10.5– 14.6) |
12.7 (10.8– 14.9) |
- | - | - | |
| CHD | 4.7 (3.6– 6.2) |
8.7 (7.1– 10.6) |
- | - | - | |
| Stroke | 4.2 (3.2– 5.6) |
3.3 (2.4– 4.5) |
- | - | - | |
| 90–119 | ||||||
| n | 0 | 5,233 | 0 | 0 | 0 | 0% |
| ESRD | - | 0.4 (0.3 to 0.6) |
- | - | - | |
| All-cause mortality |
- | 11.4 (10.7– 12.2) |
- | - | - | |
| CHD | - | 8.7 (8.1– 9.4) |
- | - | - | |
| Stroke | - | 3.1 (2.8– 3.5) |
- | - | - | |
| 60–89 | ||||||
| n | 0 | 3,079 | 3,780 | 0 | 0 | 44.9% |
| ESRD | - | 0.4 (0.2 to 0.6) |
1.1 (0.8 to 1.4) |
- | - | |
| All-cause mortality |
- | 7.4 (6.7 to 8.2) |
12.1 (11.3 to 13.1) |
- | - | |
| CHD | - | 7.5 (6.7– 8.3) |
10.4 (9.6– 11.2) |
- | - | |
| Stroke | - | 2.2 (1.8– 2.7) |
3.6 (3.1–4.1) | - | - | |
| 30–59 | ||||||
| n | 0 | 0 | 151 | 196 | 0 | 43.5% |
| ESRD | - | - | 1.3 (0.4 to 4.1) |
13.1 (9.3 to 18.6) |
- | |
| All-cause mortality |
- | - | 10.0 (6.7– 14.9) |
32.8 (26.6– 40.4) |
- | |
| CHD | - | - | 7.7 (4.8– 12.2) |
21.5 (16.4– 28.1) |
- | |
| Stroke | - | - | 4.7 (2.6–8.4) | 9.4 (6.3– 14.0) |
- | |
| <30 | ||||||
| n | 0 | 0 | 0 | 0 | 27 | 0% |
| ESRD | - | - | - | - | 260.0 (169.5– 398.8) |
|
| All-cause mortality |
- | - | - | - | 150.6 (101.8 to 222.9) |
|
| CHD | - | - | - | - | 44.0 (21.0–92.4) | |
| Stroke | - | - | - | - | 19.2 (6.2–59.6) | |
Abbreviations: eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; ESRD, end-stage renal disease; CHD, coronary heart disease.
Conversion factor for eGFR in ml/min/1.73m2 to mL/s/1.73 m2, ×0.01667.
Cross-Tabulated Incidence Rates According to eGFR by Both Equations
To directly compare both equations, we computed the crude incidence rates of each outcome for cross-categories of eGFRCKD-EPI and eGFRMDRD (Table 3). Of 1,439 participants with eGFRMDRD ≥120 ml/min/1.73m2, 50.2% (n=723) were reclassified downward to the category of eGFR 90–119 ml/min/1.73m2 by the CKD-EPI equation. No one was reclassified by the CKD-EPI equation among participants with eGFRMDRD of 90–119 or <30 ml/min/1.73m2. In contrast, 44.9% (n=3,079) and 43.5% (n=151) of participants with eGFRMDRD of 60–89 ml/min/1.73m2 and of 30–59 ml/min/1.73m2, respectively, were reclassified upward to a higher eGFR category, lowering the CKD prevalence based on eGFR in our population from 2.7% to 1.6%.
Importantly, participants who were reclassified upward to a higher eGFR category consistently had lower risk of all outcomes than those who remained unchanged in the same eGFR category. For participants with eGFRMDRD 30–59 who were reclassified to eGFRCKD-EPI of 60–89, the incidence rate ratios compared to those who stayed in the lower category was: 0.10 (95% CI, 0.03 to 0.33) for ESRD (1.3 vs. 13.1 per 1000 person years in Table 3), 0.30 (0.19 to 0.48) for all-cause mortality, 0.36 (0.21 to 0.61) for CHD and 0.50 (0.24 to 1.01) for stroke. Similar results were observed for participants with eGFRMDRD 60–89 (ESRD incidence rate ratio, 0.35 [95% CI, 0.20–0.58], all-cause mortality, 0.61 [0.54–0.69], CHD, 0.72 [0.63–0.82], stroke, 0.62 [0.49–0.78]). The pattern was less consistent for participants with eGFRMDRD ≥120 ml/min/1.73m2 who were reclassified downward to eGFRCKD-EPI of 90–119.
Subsequently, we compared age-, gender-, and race-adjusted incidence rates and found that participants reclassified upward by the CKD-EPI equation still tended to have lower risk as compared to those who were not reclassified, although this comparison was only statistically significant for ESRD (ESRD incidence rate ratio, 0.55 [95% CI, 0.31–0.95] in eGFRMDRD of 60–89 ml/min/1.73m2 and 0.13 [0.04–0.47] in eGFRMDRD of 30–59 ml/min/1.73m2; all-cause mortality, 0.94 [0.82–1.08] and 0.72 [0.42–1.23]; CHD, 0.94 [0.82–1.08] and 0.78 [0.41–1.47]; stroke, 0.95 [0.74–1.21] and 0.95 [0.40–2.23], respectively).
Net Reclassification Improvement
The analysis of net reclassification improvement based on eGFR categories was conducted after excluding participants with either eGFRCKD-EPI or eGFRMDRD ≥120 ml/min/1.73m2, since re-classification to lower values from this group has a different meaning due to the J-shaped associations of eGFR with risk. The detailed table for calculating net reclassification improvement for incident ESRD is shown in Table 4 (Tables S2 to S4 for the other outcomes). Of 170 participants who had incident ESRD during follow-up, 12.4% (n=21) were reclassified incorrectly to a lower risk (higher eGFR) category by the CKD-EPI equation. On the other hand, among 12,230 participants who were free of ESRD during follow-up, 26.1% (n=3,193 participants) were reclassified correctly to a lower risk (higher eGFR) group. In sum, net reclassification improvement for ESRD by the CKD-EPI equation was 0.138 (P<0.001). Overall, the CKD-EPI equation was associated with a significantly positive net reclassification improvement for all outcomes (Table S5). Similar results were obtained in subgroups by race, gender, and age (45–54 or 55–64 y), though blacks tended to have lower net reclassification improvements for all outcomes than whites. net reclassification improvements based on 10-year risk including participants with eGFR ≥120 were generally smaller than those based on eGFR categories, particularly when adjusted for covariates or where eGFR was modeled as a spline (Table S6).
Table 4.
Reclassification of eGFR Categories by the CKD-EPI and the MDRD Study Equations, Stratified according to Incident ESRD (yes or no) during the Follow-Up
| eGFRCKD-EPI, ml/min/1.73m2 |
|||||
|---|---|---|---|---|---|
| 90–119 | 60–89 | 30–59 | <30 | Total no. | |
| Participants who had incident ESRD | |||||
| eGFRMDRD, ml/min/1.73m2 | |||||
| 90–119 | 33 | 0 | 0 | 0 | 33 |
| 60–89 | 18 | 63 | 0 | 0 | 81 |
| 30–59 | 0 | 3 | 32 | 0 | 35 |
| <30 | 0 | 0 | 0 | 21 | 21 |
| Total no. | 51 | 66 | 32 | 21 | 170 |
| Participants who did not have incident ESRD | |||||
| eGFRMDRD, ml/min/1.73m2 | |||||
| 90–119 | 5,167 | 0 | 0 | 0 | 5,167 |
| 60–89 | 3,046 | 3,700 | 0 | 0 | 6,746 |
| 30–59 | 0 | 147 | 164 | 0 | 311 |
| <30 | 0 | 0 | 0 | 6 | 6 |
| Total no. | 8,213 | 3,847 | 164 | 6 | 12,230 |
Note: Net Reclassification Improvement (NRI) was calculated as follows: clinically correct reclassification (proportion of participats reclassified upward among those did not have ESRD: [3,046 + 147]/12,230) - clinically incorrect reclassification (proportion of participats reclassified upward among those had ESRD: [18 + 3]/170) = 0.138 (P<0.001)
Abbreviations: eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; ESRD, end-stage renal disease.
Conversion factor for eGFR in ml/min/1.73m2 to mL/s/1.73 m2, ×0.01667.
DISCUSSION
Overall, our results suggest that categorization of kidney function using the CKD-EPI equation more appropriately stratifies middle-aged individuals according to risk of important clinical outcomes as compared to the conventional MDRD Study equation. The prevalence of CKD stage 3 (eGFR 30–59 ml/min/1.73m2) at baseline was reduced from 2.5% (n=347) to 1.4% (n=196) when comparing the CKD-EPI and MDRD study equations in a large, community-based middle-aged population. Importantly, participants who were reclassified upward from CKD stage 3 based on the MDRD Study equation to mildly reduced eGFR (from <60 to 60–89 ml/min/1.73m2) by the CKD-EPI equation had lower risks of all clinical outcomes as compared to those who were not reclassified.
Improved risk stratification using categories of eGFRCKD-EPI is partially a function of inherent properties of the equation: lower risk populations, i.e., female, whites, and younger,3, 28 are systematically assigned to a higher eGFR category as compared to use of the MDRD Study equation. Indeed, the significantly lower risk for all-cause mortality, CHD, and stroke among persons who were reclassified upward as compared with those who were not reclassified was attenuated by the adjustment for age, gender, and race. The coefficients of age, gender, and race in the survival model may compensate for the different age, gender, and race terms in the CKD-EPI and MDRD study equations. Nevertheless, these findings suggest that the total information content in serum creatinine and demographics is only marginally improved if one calculates the eGFRCKD-EPI vs. eGFRMDRD and uses them in a risk equation along with the same demographics. Indeed, risk reclassification improvement based on 10-year risk by the CKD-EPI equation was marginal when adjusted for other risk factors.
The gain by the CKD-EPI equation was also limited when spline terms of eGFR were implemented in the models. Again, it seems that coefficients of multiple spline terms can compensate for differences between the MDRD and CKD-EPI study equations. Predicted risk by spline models allows for a different risk association across GFR, limiting differences between eGFR equations which use identical variables. Nevertheless, since clinical decisions and guidelines are based on eGFR categories, the improvement in risk prediction with eGFR categories is clinically important.
Importantly, for ESRD, an outcome directly linked to decreased eGFR, more accurate risk reclassification based on eGFR categories by the CKD-EPI equation remained statistically significant even after the adjustment for demographic variables. Furthermore, it is of note that net reclassification improvement was significantly positive for all outcomes in most subgroups according to age, gender, or race (Table S5). These data indicate that the CKD-EPI GFR estimate is more closely related to risk, classifying a smaller and higher risk subgroup as having CKD stage 3. Thus, in a middle-aged population, CKD-EPI eGFR focuses the attention of clinicians on a subgroup that is more likely to benefit from interventions.
Both the MDRD and CKD-EPI study equations are limited by the information available in serum creatinine and demographics. Further improvements are likely to require additional markers such as serum cystatin C, which improves risk prediction29 and, when added to creatinine, GFR estimation.2 Cystatin C standardization across methods and laboratories is lagging that of serum creatinine. Thus, it is anticipated that eGFR based on serum creatinine will continue to be used in most clinical practice settings.12
Clinical guidelines recommend that clinical laboratories should report eGFR using the MDRD Study equation whenever serum creatinine measurement is requested.3, 4 Indeed, about 70% of laboratories are currently reporting eGFR along with serum creatinine results.6 Although false-positive CKD due to underestimation of GFR by the MDRD Study equation is a concern,30 the original CKD-EPI and our results suggest the new CKD-EPI equation reduce this false-positive rate.
The present study also raises important interpretative issues about the CKD-EPI equation. In blacks, eGFRCKD-EPI does not differ as much from eGFRMDRD as in whites and, consequently, there were lower net reclassification improvements for all outcomes in blacks as compared to whites. The majority of blacks in the study population used to develop the CKD-EPI equation had CKD with reduced GFR.12 Therefore, the CKD-EPI equation may lack precision in GFR estimation ≥60 ml/min/1.73m2 but the risk relationship with all outcomes was at least as strong as in whites (data not shown). Further studies are needed to evaluate the accuracy of the CKD-EPI equation among individuals of different race and ethnicity groups with mildly decreased or normal GFR levels.
Participants with eGFRCKD-EPI or eGFRMDRD ≥ 120 ml/min/1.73m2 had statistically significantly higher risks for all-cause mortality and ESRD. The result for all-cause mortality was not unexpected, as high eGFR can result from low serum creatinine due to muscle wasting secondary to ill health, reflecting inherent limitations of all serum creatinine-based GFR equations.
Why eGFR ≥120 ml/min/1.73m2 by both equations was associated with incident ESRD is unclear. Participants in this category by both equations were likely to be black, to have higher body mass index, and to have diabetes at baseline as compared to the reference group (Table 1 and Table S1). These results suggest that the high eGFR group in this study over-represents diabetic and obese persons with hyperfiltration at risk for progression to CKD. The over-representation of blacks might also contribute to this finding. Blacks are known to have higher risk of ESRD and are at risk for a more rapid decline in GFR as compared to whites.31 Indeed, persons with eGFR ≥120 ml/min/1.73m2 who had incident ESRD in our study were mostly black (16 of 16 for eGFRCKD-EPI and 20 of 23 for eGFRMDRD).
Reliability of eGFR at high values is another important issue. Individuals, particularly blacks, with measured GFR ≥120 ml/min/1.73m2 were under-represented in the populations, from which the CKD-EPI and MDRD study equations were derived,7, 12 limiting the ability to quantify hyperfiltration and its progression. Indeed, GFR estimates by both equations have lower precision at higher GFRs.8, 9, 12
Some limitations of the present study should be mentioned. First, since the ARIC study consists of a middle-aged, bi-ethnic community-based population of the US, additional studies are needed in younger populations, the elderly, or other ethnicities. Second, there were relatively few participants with eGFR of 30–59 ml/min/1.73m2, the range where eGFR alone is used to define CKD and risk relationships become steeper. Finally, albuminuria was not measured at baseline. Thus, we could not evaluate eGFR and albuminuria simultaneously along with other factors which are important for the most accurate risk prediction.
In conclusion, the CKD-EPI equation was recently developed through a large collaborative effort to reduce bias and improve precision and accuracy in estimating measured GFR. The equation uses the same variables (serum creatinine, age, gender and race) as the MDRD Study equation, facilitating its implementation in computerized algorithms to estimate GFR in clinical practice and laboratories. This study shows that in a large community-based middle-age population the CKD-EPI equation more appropriately classified individuals with respect to risk of ESRD, mortality, CHD and stroke as compared to the MDRD Study equation. This demonstrates that the improved accuracy in estimating GFR by the CKD-EPI equation translated to improved risk prediction and greater clinical utility among middle-aged individuals.
Descriptive Text for Online Delivery.
Hyperlink: Supplementary Table S1
About: Characteristics of Participants According to Clinical Categories of eGFRMDRD
Hyperlink: Supplementary Table S2
About: Reclassification of eGFR Categories by the CKD-EPI and the MDRD Study Equations, Stratified According to All-Cause Mortality (yes or no) During Follow-up
Hyperlink: Supplementary Table S3
About: Reclassification of eGFR Categories by the CKD-EPI and MDRD Study Equations, Stratified According to Incident CHD (yes or no) During Follow-up
Hyperlink: Supplementary Table S4
About: Reclassification of eGFR categories by the CKD-EPI and MDRD Study Equations, Stratified According to Incident Stroke (yes or no) During Follow-up
Hyperlink: Supplementary Table S5
About: Net Reclassification Improvement by the CKD-EPI Equation Among Participants With eGFR < 120 mL/min/1.73 m2 by Both Equations
Hyperlink: Supplementary Table S6
About: Net Reclassification Improvement by the CKD-EPI Equation based on 10-year risk categories (<5%, 5-<10%, 10-<20%, and≥20%)
Supplementary Material
Characteristics of Participants According to Clinical Categories of eGFRMDRD
Reclassification of eGFR Categories by the CKD-EPI and the MDRD Study Equations, Stratified According to All-Cause Mortality (yes or no) During Follow-up
Reclassification of eGFR Categories by the CKD-EPI and MDRD Study Equations, Stratified According to Incident CHD (yes or no) During Follow-up
Reclassification of eGFR categories by the CKD-EPI and MDRD Study Equations, Stratified According to Incident Stroke (yes or no) During Follow-up
Net Reclassification Improvement by the CKD-EPI Equation Among Participants With eGFR < 120 mL/min/1.73 m2 by Both Equations
Net Reclassification Improvement by the CKD-EPI Equation Based on 10-year Risk Categories (<5%, 5-<10%, 10-<20%, and ≥20%)
ACKNOWLDEGEMENTS
The authors thank the staff and participants of the ARIC Study for their important contributions.
Support: The ARIC Study is carried out as a collaborative study supported by National Institute of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. Dr Matsushita was supported by a grant from the Japan Society for the Promotion of Science. Dr Selvin was supported by the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K01DK076595. Dr Bash was supported by NIH/NHLBI grant T32HL07024. Drs Astor and Coresh were supported by NIH/NIDDK grant R01DK076770 and a KDOQI research initiative grant of the National Kidney Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Note: The supplementary material accompanying this article (doi:___) is available at www.ajkd.org.
REFERENCES
- 1.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50(2):169–180. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 4.The National Institute for Health and Clinical Excellence. [Accessed July 27th, 2009];Chronic kidney disease: early identification and management of chronic kidney disease in adults in primary and secondary care. http://www.nice.org.uk/nicemedia/pdf/CG073NICEGuideline.pdf. [PubMed]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 6.Miller WG. Reporting estimated GFR: a laboratory perspective. Am J Kidney Dis. 2008;52(4):645–648. doi: 10.1053/j.ajkd.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 9.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43(1):112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Coresh J, Greene T, Levey AS. Assessing Kidney Function -- Measured and Estimated Glomerular Filtration Rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 11.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 14.Operations Manual 7: Blood Collection and Processing. Bethesda: National Heart, Lung and Blood Institute. National Heart Lung and Blood Institute Atherosclerosis Risk in Communities (ARIC) Study; 1987. [Google Scholar]
- 15.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75(3):565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 16.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 17.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 18.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 19.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 21.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 22.Rathore SS, Hinn AR, Cooper LS, Tyroler HA, Rosamond WD. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke. 2002;33(11):2718–2721. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 24.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18(2):629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 28.Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. Journal of Clinical Epidemiology. 2003;56(9):880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 29.Shlipak MGab, Praught MLb, Sarnak MJc. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Current Opinion in Nephrology & Hypertension. 2006;15(3):270–275. doi: 10.1097/01.mnh.0000222694.07336.92. [DOI] [PubMed] [Google Scholar]
- 30.Rainey PM. Automatic Reporting of Estimated Glomerular Filtration Rate--Jumping the Gun? Clin Chem. 2006;52(12):2184–2187. doi: 10.1373/clinchem.2006.069732. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of Participants According to Clinical Categories of eGFRMDRD
Reclassification of eGFR Categories by the CKD-EPI and the MDRD Study Equations, Stratified According to All-Cause Mortality (yes or no) During Follow-up
Reclassification of eGFR Categories by the CKD-EPI and MDRD Study Equations, Stratified According to Incident CHD (yes or no) During Follow-up
Reclassification of eGFR categories by the CKD-EPI and MDRD Study Equations, Stratified According to Incident Stroke (yes or no) During Follow-up
Net Reclassification Improvement by the CKD-EPI Equation Among Participants With eGFR < 120 mL/min/1.73 m2 by Both Equations
Net Reclassification Improvement by the CKD-EPI Equation Based on 10-year Risk Categories (<5%, 5-<10%, 10-<20%, and ≥20%)