Abstract
Human embryonic stem cells (hESC) have the potential to revolutionize certain medical treatments, including T-cell-based therapies. However, optimal approaches to develop T cells from hESC are lacking. In this report, we show that T-cell progenitors can be derived from hESC cultured as embryoid bodies (EBs). These EB-derived T-cell progenitors give rise to phenotypically and functionally normal cells of the T lineage when transferred into human thymic tissue implanted in immunocompromised mice, suggesting that introduction of these progenitors into patients may also yield functional T cells. Moreover, hematopoietic progenitors demonstrating T-cell potential appeared to be CD45+/CD34+, resembling those found in normal bone marrow. In contrast to T cells developed from hESC cocultured on murine stromal cells, the EB-derived T cells also expressed normal levels of CD45. Importantly, the EB system eliminates the previous need for murine cocultures, a key impediment to developing a protocol for T-cell progenitor derivation suitable for clinical use. Furthermore, following lentiviral-mediated introduction of a vector expressing enhanced green fluorescent protein into hESC, stable transgene expression was maintained throughout differentiation, suggesting a potential for gene therapy approaches aimed at the augmentation of T-cell function or treatment of T-cell disorders.
Keywords: Human embryonic stem cells, Embryoid bodies, Hematopoiesis, T-cell development, Gene therapy
Introduction
In recent years, a number of protocols have been successfully developed to coerce human embryonic stem cells (hESC) to differentiate into cells of different lineages [1], including those of the hematopoietic system [2– 8]. Previously, we demonstrated that hESC-derived T-cell progenitors could be generated by coculture with the murine bone marrow stromal cell line OP9 [9]. However, the resulting thymocytes expressed only very low levels of CD45, bringing into question their functional capacity. Furthermore, the presence of murine cells made this system incompatible with clinical applications. We therefore investigated alternative methods of T-cell differentiation from hESC. The embryoid body system has been used to differentiate hESC into a variety of different cell types [10]. In this method intact hESC colonies are detached from the culture plates by enzymatic treatment and transferred to low-attachment, feeder-free plates, where they form EBs. At a low rate, EBs spontaneously differentiate into cells of all three germline layers; however, differentiation toward the desired lineage can be potentiated by addition of the appropriate cytokines [10]. hESC-derived EB cultures have been used previously to derive hematopoietic progenitors and cells of myeloid and erythroid lineages in the absence of mouse stromal cells [3, 5–7]. However, this system has never been tested for its potential to generate T-cell progenitors. In this report, we demonstrate that an EB culture system can generate hematopoietic progenitors capable of developing into cells of the T lineage, which express normal levels of CD45 and other T-cell markers and respond to TCR ligation.
Materials and Methods
Cell Culture
hESC line H1 was cultured on irradiated CF-1 mouse embryonic fibroblast feeders in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 medium (F12) containing 20% Serum Replacer (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), 2 mM L-glutamine, 100 μM nonessential amino acids, 0.1 mM β2-mercaptoethanol, and 10 ng/ml basic fibroblast growth factor (Invitrogen). The cells were passaged on a weekly basis using collagenase IV (Invitrogen). All work with hESC was approved by the UCLA Embryonic Stem Cell Research Oversight committee.
Vectors
To generate enhanced green fluorescent protein (EGFP)-expressing hESC we used lentiviral vectors pSIN18.cPPT.hEF1α-EGFP.WPRE [11] and pFG12-green fluorescent protein (GFP)-IRES-NEO. The FG12-GFP-IRES-NEO lentiviral vector was constructed by first polymerase chain reaction (PCR) amplifying the IRES-NEO cassette from pTOPO-IRES-NEO using the sense primer CGAGCTGTACAAGTAAACGCCCTTTACCTGCAGGCGACGAC and the antisense primer TCTGTACATAGCTAGCCCTTTAGTCGACGCTCAGAAGAAC (BsrGI restriction sites are indicated). The BsrGI-digested PCR fragment was then inserted into the unique BsrGI site present at the end of the EGFP coding sequence in lentiviral vector FG12 [12] (kind gift from Dr. David Baltimore). The sequence of the final clone is available upon request. The protocol for transduction of H1 cells with pSIN18.cPPT.hEF1α-EGFP.WPRE, and the selection of the transduced cells was described previously [9]. H1 cells transduced with pFG12-GFP-IRES-NEO were grown for 4 weeks on irradiated DR4 mouse embryonic fibroblast feeders under selection of 200 μg/ml G418. These cells were used in the experiments presented in Figures 5 and 6.
Figure 5.
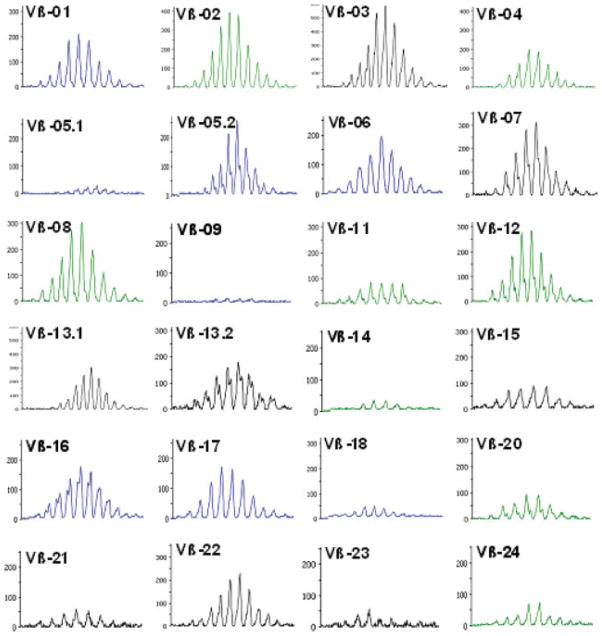
Human embryonic stem cell (hESC)-derived thymocytes undergo normal V(D)J recombination in the thymic implants during T-cell development. Spectratyping analysis of CDR3 length distributions of 24 Vβ families in the sorted EGFP+ hESC-derived thymocytes showed random utilization of Vβ fragments. The expected deletions and additions of nucleotides at the recombination junctions were also observed, as manifested by a normal gaussian-like distribution of the polymerase chain reaction amplicons in each Vβ family.
Figure 6.
Human embryonic stem cell- and EB-derived EGFP+ thymocytes respond to CD3-mediated signaling in vitro. Cells derived from Thy/Liv implants injected with EB-derived EGFP+ hematopoietic progenitors were cultured in medium alone (unstimulated) or in the presence of anti-CD3 and anti-CD28 monoclonal antibodies (costimulated), and EGFP+ cells were analyzed for expression of CD25 by flow cytometry. The figure represents data from one of five implants analyzed, all of which contained EGFP+ cells equally responsive to the costimulation.
Formation and Differentiation of EBs
Two days before regular passage (day 5), undifferentiated hESC at confluence in six-well plates were treated with 0.5 mg/ml Dispase (Invitrogen) in DMEM/F12 for 20 minutes at 37°C. The hESC colonies were detached by gentle pipetting, washed twice, and transferred to six-well low-attachment plates (Corning Enterprises, Corning, NY, http://www.corning.com) to allow for EB formation by overnight incubation in differentiation medium. The differentiation medium consisted of Iscove’s modified Dulbecco’s medium supplemented with 15% non-heat-inactivated defined fetal bovine serum (HyClone, Logan, UT, http://www.hyclone.com), 1% nonessential amino acids, 1 mM L-glutamine, 0.1 mM β2-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin. The medium was exchanged every 2 days, and starting at day 4 the EB cultures were supplemented with 10 ng/ml bone morphogenetic protein-4 (BMP-4) (R&D Systems Inc., Minneapolis, http://www.rndsystems.com), 300 ng/ml stem cell factor (Amgen, Thousand Oaks, CA, http://www.amgen.com), and 20 ng/ml Flt-3 ligand (Invitrogen). BMP-4 was removed from the differentiation medium on day 12. To obtain a single-cell suspension, the EB cultures were harvested at different time points, washed twice in 1× phosphate-buffered saline, and treated with trypsin-EDTA (0.25%) supplemented with 2% chick serum (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) for 30 minutes at 37°C with periodic gentle agitation. The resulting suspension was washed twice, filtered through a 40-μm mesh to remove cell debris, and subsequently used for phenotypic analysis, methylcellulose assays, and the T-cell reconstitution experiments in SCID-hu mice.
Methylcellulose Assay
To evaluate their hematopoietic potential, 5 × 104 of the total EB-derived cells were plated per plate in MethoCult GF+ H4435 (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) methylcellulose-based medium and incubated in a humidified atmosphere at 37°C and 5% CO2 for 2 weeks, at which point the colonies were enumerated on the basis of their morphological characteristics. Cells were plated in quadruplicates.
In Vivo T-Cell Development
SCID-hu mice were generated as previously described [13]. Briefly, small pieces of human fetal liver and thymus were inserted under the renal capsule of severe combined immunodeficient (SCID) mice and allowed to develop into a thymus-like organoid called a Thy/Liv implant. SCID-hu mice were irradiated at 300 RADs and injected with either 7.5 × 105 or 1.7 × 106 purified CD34+ cells directly into Thy/Liv implants. For the fractionation experiment, implants of SCID-hu mice were injected with either 5 × 104 (three or four mice) or 2.5 × 105 (three or four mice) cells for each of the four purified subsets of cells (CD34+/CD45+, CD34+/CD45−, CD34−/CD133+, and CD34−/CD133−). The Thy/Liv implant biopsies were performed at various time points as specified in the text. Single cell suspensions were obtained, and the cells were analyzed for the expression of EGFP and T-cell developmental markers. All immunodeficient mouse work was approved by the UCLA Animal Research Committee, and the use of human fetal tissues was approved by the UCLA Institutional Review Board.
Cell Sorting and Flow Cytometry
H1-GFP EB-derived progenitors were sorted by magnetic-activated cell sorting as previously described [9]. For multiple subset cell sorting, cells were first labeled with anti-CD34 multisort microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com), and the positive and negative fractions were collected following two-column sorting (POSSEL_d2 program) on an AutoMACS cell sorter (Miltenyi Biotec). The magnetic beads were then cleaved off of the positive fraction, according to manufacturer’s instructions, and cells were then labeled with anti-CD45 magnetic beads. The CD34-negative fraction was labeled with anti-CD133 microbeads (Miltenyi Biotec), and subsequent positive and negative fractions were collected following another AutoMACS sort (POSSEL_d2 program). As determined by postsort flow cytometry analysis there was typically a greater than 90% enrichment for the respective sorted populations. H1-GFP hESC cultured in vitro and cells derived from Thy/Liv implants were stained with monoclonal antibodies to CD3, CD4, CD5, CD7, CD8, CD10, CD11c, CD19, CD31, CD34, CD43, CD45, CD56, CD127 (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com), CD117, CXCR4 (eBioscience), HLA-A2 (Serotec Ltd., Oxford, U.K., http://www.serotec.com), TCR, CD235 (BD Pharmingen, San Diego, http://www.bdbiosciences.com/index_us.shtml), and CD133 (Miltenyi Biotec) conjugated to either phycoerythrin, ECD, allophycocyanin (APC), PC5, PC7, Pacific Blue, or APC-Alexa750. Cells were analyzed by flow cytometry using a Coulter FC500 flow cytometer or a Becton Dickinson FACSAria (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com), and the phenotype was determined using FlowJo software (Tree Star, Ashland, OR, http://www.treestar.com).
T-Cell Receptor Vβ-Complementarity-Determining Region 3 Distribution Analysis
hESC-derived thymocytes were sorted on a FACSAria sorter (Becton Dickinson) on the basis of EGFP expression; EGFP+ cell purity was greater than 99% (data not shown). Total RNA was isolated from 3.5 × 105 sorted thymocytes by Trizol reagent (Invitrogen), and then 20 μl of total RNA was reverse-transcribed with random primers in a 40-μl reaction using high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). To determine the diversity of complementarity-determining region 3 (CDR3) repertoire, TCR-Vβ-specific PCRs were performed for 24 Vβ families as described earlier [14]. In short, each reaction has one Vβ-specific forward primer, as reported previously [15], along with a fluorescent-labeled (either 6-FAM, VIC, or NED) TCRβ constant region (Cβ-R: 5′-CTTCTGATGGCTCAAACAC-3′) reverse primer to amplify the CDR3 region. These amplified fragments with the fluorescent label on each amplicon were run through the capillary-electrophoresis system on an ABI-3130 genetic analyzer, and the TCRVβ-CDR3 length distribution was analyzed by calculating the fluorescent intensity and the length of nucleotide fragment using Genemapper software (Applied Biosystems).
Cell Stimulation
Thymocytes from Thy/Liv implants injected with EB-derived EGFP+ cells and thymocytes from control animals were harvested 4 weeks postinjection and cultured in RPMI 1640 medium containing 10% humanAB serum (Gemini Bio-Products, West Sacramento, CA, http://www.gembio.com), 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich). A fraction of the cells from each biopsy was costimulated with anti-CD3 (1 μg/ml) (Ortho Biotech, Bridgewater, NJ, http://www.orthobiotech.com) and cross-linked to the plate with goat anti-mouse IgG and soluble anti-CD28 (100 ng/ml) (Beckman Coulter). After 3 days of costimulation the cells were assessed for CD25 expression by flow cytometry.
Results
To asses the T-cell potential of EB-derived cells we used the H1 hESC line transduced with a EGFP-expressing lentiviral vector [9]. H1 cells were differentiated in EBs according to the protocol of Chadwick et al. [3], with the exception of an altered cytokine differentiation cocktail composed of BMP-4, stem cell factor (SCF), and Flt-3L (Fig. 1). The EBs generated in this way exhibited a typical appearance and maintained the expression of EGFP throughout the culture period (Fig. 2A). Importantly, this protocol resulted in the development of CD34+ cells, most of which also expressed markers associated with early hematopoietic progenitors, such as CD45, CD43, and CD133 (Fig. 2B). CD38, a marker of differentiating hematopoietic progenitors, was also coexpressed on a subset of CD34+ cells. Some of the CD34+ cells also expressed CD184 (CXCR4), a known chemokine receptor critical for progenitor cell migration during thymopoiesis [16] (Fig. 2B). To better define the potential of the EB system to support hematopoietic differentiation, we performed kinetic studies of hematopoietic marker expression and hematopoietic progenitor formation (Fig. 2C, 2D). The expression of CD34 was detectable by flow cytometry at the earliest time point tested (day 8). However, expression of CD45 lagged and was not observed until day 15 (Fig. 2C), indicating that the hematopoietic activity detected in EB cultures at earlier times (described below) precedes CD45 expression. We then assayed the hematopoietic potential of cells cultured as EBs for up to 39 days in a standard methylcellulose-based colony-forming assay. As shown in Figure 2D, the first hematopoietic activity was associated with day 10 of culture. Hematopoietic activity continued to increase until two and half weeks of EB culture, although some myeloid progenitors persisted up to day 39. At earlier time points erythroid colony-forming potential of EB-derived cells was observed, but this diminished over time and was completely lost by day 20. These data suggest that the cytokine combination used in our system supports development of multilineage hematopoietic progenitors.
Figure 1.
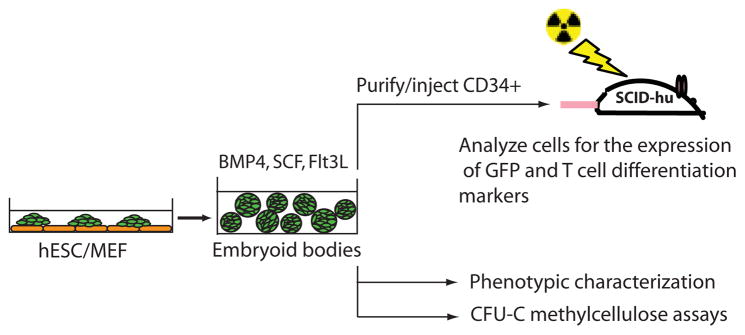
A diagram of our experimental approach to test the hematopoietic potential of the hESC- and EB-derived cells. EGFP+ hESC were coerced to form EBs and cultured in the presence of cytokines for various periods of time. At different time points the EB-derived cells were assayed for the expression of hematopoietic markers, the presence of hematopoietic progenitors as measured by colony-forming unit assay, and their ability to give rise to cells of the T lineage, when injected into Thy/Liv implants of SCID-hu mice. Abbreviations: BMP, bone morphogenetic protein; GFP, green fluorescent protein; hESC, human embryonic stem cell; MEF, mouse embryonic fibroblast; SCF, stem cell factor.
Figure 2.
Human embryonic stem cell (hESC) differentiation toward hematopoietic lineage in EB cultures. (A): EBs formed by detachment of H1-green fluorescent protein hESC colonies maintained the expression of EGFP throughout the culture period. (B): Cells derived from 17-day EB cultures were analyzed by flow cytometry first for the expression of CD34 and EGFP (left panel) and then for the expression of CXCR4 and hematopoietic markers CD45, CD43, CD113, and CD38 (right panels). Kinetic analyses of hematopoietic marker expression and hematopoietic progenitor formation in EB cultures were performed by harvesting EBs at the indicated time points and assaying total EB-derived cells either for the expression of CD34, CD45, CD117, and CXCR4 by flow cytometry (C) or for their ability to form myeloid and erythroid colonies in a standard methylcellulose assay (D). Results from two independent time course experiments are presented. All platings in methylcellulose were done in quadruplicate, and colonies were enumerated 2 weeks postplating. Abbreviation: EGFP, enhanced green fluorescent protein.
To assess the T lymphoid potential of the EB-derived hematopoietic progenitors we used the SCID-hu mouse. In this animal model small pieces of human fetal liver and thymus are implanted under the renal capsule of SCID mice, where they develop into a thymus-like organ, the Thy/Liv implant [13, 17, 18]. This conjoint organ provides a microenvironment for long-term T-lineage differentiation. Importantly, we and others have previously shown that direct injection of exogenous CD34+ human hematopoietic progenitor cells into Thy/Liv implants in sublethally irradiated SCID-hu mice results in engraftment and T-lymphoid differentiation of the exogenous cells [9, 19 –21].
In two independent experiments, we purified CD34+ cells from 8-, 10-, and 12-day EB cultures (experiment 1) or from 12-, 15-, 18-, and 21-day EB cultures (experiment 2) and injected these into Thy/Liv implants of irradiated SCID-hu mice. The implants were biopsied at different time points, starting at 3 weeks postinjection, and analyzed for the presence of EGFP+ thymocytes that would be derived from hESC (Table 1; supporting information Table 1). Engraftment of the hESC-derived EGFP+ cells was seen in 17 of 38 injected animals and ranged from 0.015% to 23.9% of human cells (supporting information Table 1). Given the inherent variation in implant sizes, it is misleading to use the percentages of EGFP+ cells following progenitor cell transfer as an absolute measure of the reconstitution efficiency in the transferred population. Therefore, we used the frequency of engraftment as a means of assessing T progenitor potential. Our data indicate that the T-cell reconstitution potential increased with the duration of the EB culture up to day 15, at which point we observed a maximum engraftment frequency of 66%. Interestingly, the T-cell potential seemed to be completely lost by day 21 of EB culture, indicating the transient nature of T-cell progenitor development in this system. However, the presence of EGFP+ thymocytes clearly demonstrates the ability of the EB culture system to generate T lymphoid progenitors.
Table 1.
Kinetic and phenotypic assessment of T cell progenitor development in EB cultures using the SCID-hu system
| Experimental condition | Engraftment frequency (no. of mice with GFP+ cells) |
|---|---|
| Days in EB culturea | |
| Experiment 1 | |
| 8 | 2/4 (engrafted weakly) |
| 10 | 1/3 |
| 12 | 5/7 |
| Experiment 2 | |
| 12 | 3/6 |
| 15 | 4/6 |
| 18 | 2/6 |
| 21 | 0/6 |
| Sorted progenitor subsetb | |
| CD34+CD45+ | 7/8 |
| CD34+CD45− | 1/8 |
| CD34−CD133+ | 0/6 |
| CD34−CD133− | 0/6 |
For the time course experiments, 7.5 × 105 purified CD34+ cells derived from EBs cultured for the indicated times were introduced into Thy/Liv implants of SCID-hu mice. The only exceptions were two animals in experiment 1, which were injected with 1.7 × 106 of the day 12 EB-derived cells (supporting information Table 1). The T cell-forming potential of these cells was determined by their ability to generate CD45+CD3+ enhanced green fluorescent protein (EGFP) + thymocytes (distinguishing H1-EGFP derived cells from endogenous cells). Experiment 1 assessed early time points. Experiment 2 was subsequently performed to test later time points.
H1-EGFP EB-derived cells were cultured for 17 days and sorted into CD34+CD45+, CD34+CD45−, CD34−CD133+, and CD34−CD133− populations by magnetic-activated cell sorting (purity was greater than 95% for each population) (not shown) and tested for their T-cell potential by injecting the implants of SCID-hu mice with either 5 × 104 (three or four mice) or 2.5 × 105 (three or four mice) cells for each of the four purified subsets of cells. The CD34+CD45+ population at day 17 following EB formation appears to have the greatest potential to undergo T-lineage hematopoiesis.
Abbreviation: GFP, green fluorescent protein.
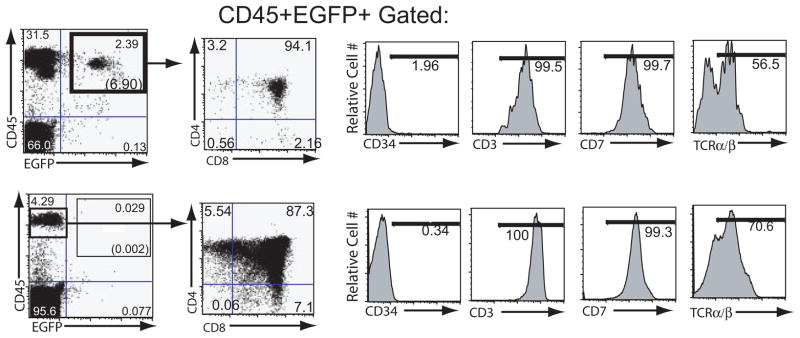
The implants receiving EB-derived progenitors, analyzed 4 weeks postinjection, displayed a distinct population of CD45+ EGFP+ cells, which was not detected in the control animals (Fig. 3). Most of these cells exhibited a CD4/CD8 double-positive phenotype, indicating an early T-cell developmental stage. This population of cells also expressed T-cell markers CD3, CD5, CD7, and TCRαβ at levels comparable to those found on control thymocytes (Fig. 4). CD45+ EGFP+ cells were still present in the same implants 8 –9 weeks postinjection (Fig. 3, right panels). However, at this time their CD4/CD8 profile changed dramatically and consisted mainly of more mature single-positive CD4 or CD8 cells. This marked decrease in the percentage of CD4/CD8 double-positive cells as a function of time suggests limited self-renewal of T-cell progenitors in this system. These data either imply that the T-cell reconstitution by EB-derived progenitors is transient due to a limited self-renewal capacity of the cells or reflect the limited ability of the SCID-hu system to provide access to an environment needed to support continued stem cell renewal and differentiation. However, as later time points were not assessed, it also remains possible that additional waves of thymopoiesis may occur. It would be of considerable interest to directly compare, in future parallel studies, the longevity of T-lymphoid reconstitution between hESC-derived hematopoietic progenitors and those isolated from fetal liver, cord blood, or mobilized peripheral blood to determine whether any qualitative or quantitative differences between these progenitors exist.
Figure 3.
In vivo T lymphoid differentiation of EB-derived hematopoietic progenitor cells expressing eGFP. Shown are flow cytometry profiles of cells derived from irradiated SCID-hu Thy/Liv mice 4 weeks (denoted as week 4) and 8 weeks (denoted as week 8) after transplantation with either no cells (mock; top panels) or sorted CD34 progenitor cells (middle and bottom panels) derived from H1 cells maintained in EB culture for 18 days. SCID-hu mice were biopsied on two successive occasions, and thymocytes were analyzed first for expression of eGFP and CD45 and then for expression of CD4 and CD8 by multicolor flow cytometry. The gates drawn through the upper right quadrants denote eGFP cells, with the percentage of total cells given within each gate. Numbers in parentheses indicate percentages of CD45+eGFP+ cells. Abbreviation: eGFP, enhanced green fluorescent protein.
Figure 4.
Phenotypic characterization of human embryonic stem cell (hESC)-derived CD45+/EGFP+ cells. EB-derived CD45+/EGFP+ cells (gated cells in the upper right quadrant of the top left panel, and other top panels) were compared with the control CD45+ cells (gated cells in the left quadrant of the lower left panel, and other bottom panels) for the expression of CD34 and T lineage markers CD3, CD7, and TCR α/β receptor. Region markers are based on isotype controls. hESC-derived and control cells displayed comparable levels of expression of T-cell markers. Abbreviation: EGFP, enhanced green fluorescent protein; TCR, T cell receptor.
Our previous studies using OP9 coculture of hESC could not distinguish the phenotype of the T progenitor cells [9]. This was largely due to low levels of CD34+ and CD45+ cells in these cocultures. Therefore, to further characterize the phenotype of the EB-derived T-cell progenitors, we purified cells from day 17 EB cultures on the basis of their expression of the hematopoietic differentiation markers CD34, CD45, and CD133. Four purified subsets of cells (CD34+/CD45+, CD34+/CD45−, CD34−/CD133+, and CD34−/CD133−) were assayed for their T-lymphoid potential. Either 5 × 104 or 2.5 × 105 cells of each subset were introduced into Thy/Liv implants of SCID-hu mice. As shown in Table 1, our data clearly indicate that the T-cell potential of the cells derived from the EBs cultured for 17 days resides exclusively within the CD34+/CD45+ subset of cells. Implants of seven of eight mice injected with this population of cells contained EGFP+ cells, and these cells also exhibited normal thymocyte profiles of CD4 and CD8 expression. In contrast to this, only 1 (injected with 2.5 × 105 purified CD34+/CD45− cells) of the other 14 animals was positive for hESC-derived thymocytes (Table 1). These results also demonstrate the dose-dependent nature of the reconstitution assay. In humans, bone marrow-derived T-cell progenitors have been shown to express both CD34 and CD45 (reviewed in [22]). It appears that the hESC-derived hematopoietic progenitor cells generated in this culture system are more similar to bone marrow-derived progenitors than are those derived from hESC cocultured on OP9 [9]. We conclude that the EB culture system supplemented with BMP-4, SCF, and Flt-3L promotes development of T-cell progenitors from hESC and that by day 17 these progenitors exclusively reside within the CD34+CD45+ population of cells.
To assess whether normal T-cell receptor rearrangement takes place in hESC-derived cells, we examined the frequency of usage of different Vβ gene fragments during the process of V(D)J recombination, as another measure of normal thymocyte development. hESC-derived thymocytes from several Thy/Liv implants were sorted on the basis of EGFP expression, pooled together, and subjected to spectratyping as previously described [14]. Our data clearly establish that all 24 Vβ families tested have been used for generation of TCRs within the population of hESC-derived EGFP+ thymocytes (Fig. 5). Furthermore, TCR β-chain CDR3 length distribution in each Vβ family showed normal gaussian-like distribution, suggesting that the expected deletions and additions of nucleotides at the recombination junctions also take place during the process of V(D)J recombination in these cells. These data establish that hESC-derived thymocytes undergo a normal process of random V(D)J recombination in the thymic implants during T-cell development. This situation was comparable to that observed in the EGFP-control thymocytes (data not shown) and was similar to that reported in peripheral lymphocytes of healthy individuals [23].
Finally, to test the ability of EB-derived thymocytes to respond to TCR-mediated signals, we stimulated these cells with antibodies against CD3 and CD28 in vitro. We have shown previously that these costimulating conditions induce the expression of the high-affinity interleukin-2 receptor (CD25) on thymocytes derived from both normal human thymus and from thymocytes obtained from progenitors following OP9 stromal cells cocultures and injections into Thy/Liv implants [9, 24]. The EB-derived EGFP+ cells from five of five Thy/Liv implants tested responded to the costimulation by expressing CD25 on their surface (Fig. 6), suggesting that the hESC-derived cells were functional and able to respond to TCR-mediated signals.
Discussion
Previously, our group demonstrated that hESC can differentiate through the T-lymphoid lineage by sequential in vitro coculture on the murine bone marrow stromal cell line OP9 and subsequent engraftment into human Thy/Liv implants in SCID-hu mice [9]. However, the hESC-derived hematopoietic progenitors and the resulting thymocytes expressed unusually low levels of the hematopoietic marker CD45, a molecule known to be involved in normal T-cell signaling. Although those hESC-derived thymocytes responded to antibody-mediated costimulation in vitro, we could not exclude the possibility that the low levels of CD45 might affect their function in vivo. Here we show that the EB-derived T-cell progenitors give rise to T lineage cells with normal expression of CD45, indicating that the EB system is superior to murine stromal cell coculture for generating T progenitor cells. Interestingly, we found that the CD34+ cells purified from EBs cultured for shorter periods of time have low to undetectable (by flow cytometry) surface expression of CD45, similar to what was observed in the OP9 cocultures. Nevertheless, the resulting thymocytes expressed levels of CD45 comparable to those seen on endogenous thymocytes from the Thy/Liv implants (not shown). These data suggest that the EB and thymic microenvironments are capable of providing necessary signals for the controlled expression of CD45 and highlight another difference between OP9-derived progenitors and those derived from EB cultures. Clearly, different systems for hESC differentiation predispose the resulting hematopoietic progenitors to respond to the same signals from the thymic microenvironment in different ways. These data also suggest that the low level of CD45 expression seen in OP9 coculture experiments is not an intrinsic property of hESC-derived hematopoietic progenitors and T cells but rather an effect of the OP9 coculture system. It is possible that, depending on the culture conditions, the CD45 locus may attain different epigenetic footprints regulating expression, and in the case of hESC/OP9-derived progenitors this level of control cannot be fully overridden by intrathymic signals.
Interestingly, in our previous studies using OP9 cocultures [9], we did not observe a high frequency of CD34+/CD45+ cells, suggesting that the hematopoietic developmental process was not yet completed. We subsequently noted T-cell progenitor activity in two subsets, CD34+ and CD34−/CD133+ cell populations, at relatively early time points of coculture, suggesting that cells in both subsets had the capacity to respond to thymic signals. We did not explore later time points in that study. In our current studies using EB cultures, by day 17 we observed a relatively robust generation of CD34+/CD45+ cells. This subset contained all of the T progenitor activity, whereas CD34−CD133+ cells did not exhibit any T lymphoid potential, suggesting that we had fully differentiated all immature progenitor subsets. However, at earlier time points in the current study, CD34+/CD45-negative cells appeared capable of T-cell progenitor activity, perhaps reflecting a more immature progenitor phenotype. Since CD133 is a marker found on very immature progenitor cells of several lineages, it would be of interest to determine whether CD34−CD133+ cells would give rise to T lineage cells if taken from EB cultures at a time point earlier than day 17.
It would also be interesting to directly compare the T lymphoid potential of EB- and OP9-derived progenitor cells. This could theoretically be done using the SCID-hu model; however, this model is cumbersome, and the expense and labor associated with generating these mice make this system less attractive for performing detailed quantitative studies. Consequently, a functional in vitro system for T-cell development from hESC would be desirable. However, at the time of this writing a system of this type has not been reported. A recent study by Martin et al. suggests that it may not be possible to differentiate hESC cultured on mouse bone marrow stromal cells into T lineage cells using the standard in vitro models of T-cell development, such as coculture with OP9 expressing the Notch ligand Delta-like 1 or fetal thymic organ culture [25]. It remains to be established whether hESC-derived hematopoietic progenitors generated via EBs would behave the same way.
Our studies also show that T cells developed following EB culture retain expression of a transgene introduced into the original hESC, as the expression of EGFP was maintained throughout the differentiation process. The ability to genetically manipulate T cells or their progenitors could allow for correction of congenital defects affecting T-cell development and function [26], generation of T cells with enhanced roles in immune responses to tumors and pathogens [27], or generation of cells resistant to certain infectious microorganisms, such as HIV [28, 29]. The relevance of these approaches is highlighted by the fact that several clinical trials are currently being conducted using genetically modified T cells or hematopoietic progenitors [28–31] (reviewed in [32]). However, the scope and analysis of such genetic manipulations and the ability to generate large numbers of modified cells are severely limited by the inability to maintain and expand primary T cells and hematopoietic progenitors ex vivo without losing their functional properties. A vast improvement in this regard may be achieved by using hESC. These cells are routinely cultured for long periods of time [33], and they can be genetically manipulated [34–38], expanded to numbers needed for clinical applications, and cryopreserved for future use. Moreover, a detailed genomic analysis of modified hESC can be conducted, possibly avoiding cases of vector insertion-induced oncogenesis [39]. However, to be used in clinical applications, hESC derivatives will have to be developed in systems free of animal products. In this report we demonstrate that the EB culture system can generate hematopoietic progenitors capable of developing into phenotypically normal T lineage cells. This system of differentiation is devoid of animal cells and, with small improvements, could be completely animal product-free, as major advances have recently been made in optimization of xeno-free conditions for hESC derivation and culturing [40]. Furthermore, this technology could be scaled up in an industrial setting to provide sufficient quantities of EB-derived hematopoietic progenitors for downstream applications [41, 42]. In terms of generating T cells, our results suggest that hESC need only be differentiated as far as hematopoietic progenitor cells in vitro and that the thymus of the treated patient would then complete the differentiation program to CD4+ and CD8+ T cells in vivo. It remains to be determined whether the recently described human induced pluripotent stem (iPS) cells [43–46] have hematopoietic potential similar to that of hESC. However, in theory they can be similarly manipulated. With the aforementioned concepts in mind, one can envision that genetically manipulated hESC or iPS cells with well-characterized vector integration sites could be expanded to large numbers under xeno-free conditions and used in the near future for cell-based therapies for treatment of certain hematopoietic disorders or for manipulating T-cell immune responses.
Summary
Human embryonic stem cells hold much promise for transplant medicine, including reconstitution of the immune system. Herein we describe a feeder-free procedure to obtain hESC-derived hematopoietic progenitor cells capable of forming T-lineage progeny. These cells express both CD45 and CD34 and are capable of undergoing robust T-cell receptor rearrangement following introduction into human thymic tissues. Our studies suggest that hESC may have the potential to reconstitute the T-cell arm of the immune system or as a vehicle for gene therapeutic approaches to augment T-cell immunity.
Supplementary Material
Acknowledgments
We thank Ken Dorshkind and Helen Brown for critically reviewing the manuscript. We also thank Hongying Chen and Jessica Potts for technical assistance. This work was supported by California Institutes for Regenerative Medicine (CIRM) Grants RS1-00203-1 (to Z.G.) and RC1-00149-1 (to J.A.Z.), NIH Grants AI036554, P01-GM081621 (to J.A.Z.), and AI043203 (to O.Y.) and the UCLA Center for AIDS Research. A.S. is supported by a UCLA-CIRM training grant.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author contributions: Z.G.: conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.G.K.: conception and design, collection and assembly of data, data analysis and interpretation; A.S., G.B., and A.K.: collection and assembly of data; M.D.M.: provision of study material; A.B.: collection and assembly of data, data analysis and interpretation; O.Y.: financial support, data analysis and interpretation; J.A.Z.: financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 4.Vodyanik MA, Bork JA, Thomson JA, et al. Human embryonic stem cell-derived CD34+ cells: Efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 5.Ng ES, Davis RP, Azzola L, et al. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 6.Zambidis ET, Peault B, Park TS, et al. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy M, D’Souza SL, Lynch-Kattman M, et al. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pick M, Azzola L, Mossman A, et al. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- 9.Galic Z, Kitchen SG, Kacena A, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev. 2006;27:208–219. doi: 10.1210/er.2005-0016. [DOI] [PubMed] [Google Scholar]
- 11.Gropp M, Itsykson P, Singer O, et al. Stable genetic modification of human embryonic stem cells by lentiviral vectors. Mol Ther. 2003;7:281–287. doi: 10.1016/s1525-0016(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 12.Qin XF, An DS, Chen IS, et al. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldrovandi GM, Feuer G, Gao L, et al. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 14.Killian MS, Sabado RL, Kilpatrick S, et al. Clonal breadth of the HIV-1-specific T-cell receptor repertoire in vivo as determined by subtractive analysis. Aids. 2005;19:887–896. doi: 10.1097/01.aids.0000171402.00372.c2. [DOI] [PubMed] [Google Scholar]
- 15.Currier JR, Deulofeut H, Barron KS, et al. Mitogens, superantigens, and nominal antigens elicit distinctive patterns of TCRB CDR3 diversity. Hum Immunol. 1996;48:39–51. doi: 10.1016/0198-8859(96)00076-6. [DOI] [PubMed] [Google Scholar]
- 16.Plotkin J, Prockop SE, Lepique A, et al. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 17.Namikawa R, Weilbaecher KN, Kaneshima H, et al. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCune JM, Namikawa R, Kaneshima H, et al. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 19.Akkina RK, Rosenblatt JD, Campbell AG, et al. Modeling human lymphoid precursor cell gene therapy in the SCID-hu mouse. Blood. 1994;84:1393–1398. [PubMed] [Google Scholar]
- 20.Amado RG, Symonds G, Jamieson BD, et al. Effects of megakaryocyte growth and development factor on survival and retroviral transduction of T lymphoid progenitor cells. Hum Gene Ther. 1998;9:173–183. doi: 10.1089/hum.1998.9.2-173. [DOI] [PubMed] [Google Scholar]
- 21.Bonyhadi ML, Moss K, Voytovich A, et al. RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem-progenitor cells inhibit human immunodeficiency virus replication. J Virol. 1997;71:4707–4716. doi: 10.1128/jvi.71.6.4707-4716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 23.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson BD, Douek DC, Killian S, et al. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 25.Martin CH, Woll PS, Ni Z, et al. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood. 2008;112:2730–2737. doi: 10.1182/blood-2008-01-133801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavazzana-Calvo M, Lagresle C, Hacein-Bey-Abina S, et al. Gene therapy for severe combined immunodeficiency. Annu Rev Med. 2005;56:585–602. doi: 10.1146/annurev.med.56.090203.104142. [DOI] [PubMed] [Google Scholar]
- 27.Kershaw MH, Teng MW, Smyth MJ, et al. Supernatural T cells: Genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 28.Amado RG, Mitsuyasu RT, Rosenblatt JD, et al. Anti-human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a phase I study: Myeloid and lymphoid reconstitution in human immunodeficiency virus type-1-infected patients. Hum Gene Ther. 2004;15:251–262. doi: 10.1089/104303404322886101. [DOI] [PubMed] [Google Scholar]
- 29.Morgan RA, Walker R, Carter CS, et al. Preferential survival of CD4+ T lymphocytes engineered with anti-human immunodeficiency virus (HIV) genes in HIV-infected individuals. Hum Gene Ther. 2005;16:1065–1074. doi: 10.1089/hum.2005.16.1065. [DOI] [PubMed] [Google Scholar]
- 30.Macpherson JL, Boyd MP, Arndt AJ, et al. Long-term survival and concomitant gene expression of ribozyme-transduced CD4+ T-lymphocytes in HIV-infected patients. J Gene Med. 2005;7:552–564. doi: 10.1002/jgm.705. [DOI] [PubMed] [Google Scholar]
- 31.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J. Building an HIV-proof immune system. Science. 2007;317:612–614. doi: 10.1126/science.317.5838.612. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 34.Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- 35.Urbach A, Schuldiner M, Benvenisty N. Modeling for Lesch-Nyhan disease by gene targeting in human embryonic stem cells. Stem Cells. 2004;22:635–641. doi: 10.1634/stemcells.22-4-635. [DOI] [PubMed] [Google Scholar]
- 36.Costa M, Dottori M, Sourris K, et al. A method for genetic modification of human embryonic stem cells using electroporation. Nat Protoc. 2007;2:792–796. doi: 10.1038/nprot.2007.105. [DOI] [PubMed] [Google Scholar]
- 37.Davis RP, Ng ES, Costa M, et al. Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood. 2008;111:1876–1884. doi: 10.1182/blood-2007-06-093609. [DOI] [PubMed] [Google Scholar]
- 38.Irion S, Luche H, Gadue P, et al. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat Biotechnol. 2007;25:1477–1482. doi: 10.1038/nbt1362. [DOI] [PubMed] [Google Scholar]
- 39.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 40.Lei T, Jacob S, Ajil-Zaraa I, et al. Xeno-free derivation and culture of human embryonic stem cells: Current status, problems and challenges. Cell Res. 2007;17:682–688. doi: 10.1038/cr.2007.61. [DOI] [PubMed] [Google Scholar]
- 41.King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007;11:394–398. doi: 10.1016/j.cbpa.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurosawa H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Lowry WE, Richter L, Yachechko R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.