Abstract
Direct interaction of unactivated primary monocytes with endothelial cells induces a mitogenic effect in subconfluent, injured endothelial monolayers through activation of endothelial Met. We now report that monocytes' contact-dependent mitogenicity is controlled by activation-mediated regulation of hepatocyte growth factor. Direct interaction of unactivated monocytes with subconfluent endothelial cells for 12 hours resulted in 9- and 120-fold increase in monocyte tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β) mRNA levels and bitemporal spike in hepatocyte growth factor that closely correlates with endothelial Met and extracellular signal-related kinase (ERK) phosphorylation. Once activated, monocytes cannot induce a second wave of endothelial cell proliferation and endothelial Met phosphorylation and soluble hepatocyte growth factor levels fall off. Monocyte-induced proliferation is dose dependent and limited to the induction of a single cell cycle. Monocytes retain their ability to activate other endothelial cells for up to 8 hours after initial interaction, after which they are committed to the specific cell. There is therefore a profoundly sophisticated mode of vascular repair. Confluent endothelial cells ensure vascular quiescence, whereas subconfluence promotes vessel activation. Simultaneously, circulating monocytes stimulate endothelial cell proliferation, but lose this potential once activated. Such a system provides for the fine balance that can restore vascular and endothelial homeostasis with minimal overcompensation.
Introduction
Vascular health is cell-density specific. Confluent endothelial cells control thrombosis, inflammation, vascular cell proliferation, and matrix remodeling—subconfluent endothelial cells (ECs) promote these events.1 Monocyte interaction with the vascular endothelium is a primary event in vessel healing, and typically culminates in tissue localization as resident macrophages. Monocyte infiltration depends upon the health of the EC; the greater the endothelial injury, the greater monocyte infiltration and macrophage transformation.2 Monocytes play an active role in vascular remodeling, angiogenesis, and inflammation and after the response to the transient and chronic vascular injuries that accompany percutaneous vascular intervention and atherosclerosis.3–6 Rapid accumulation of monocytes is observed after ischemic events and was previously reported to play a critical role in tissue revascularization through angiogenesis.7,8 In cancer biology, monocyte accumulation and activation are associated with tumor angiogenesis and growth.9–12
Recently we reported that primary unactivated monocytes induce mitogenesis in ECs upon direct contact through a mechanism mediated in part by endothelial hepatocyte growth factor receptor (Met).13 This effect is distinct from the paracrine mitogenesis induced by activated macrophages and represents a new form of regulation over EC proliferation. In the present study, we explore the mechanism through which activation of monocytes regulates their mitogenic potential. We characterized EC proliferation kinetics after interaction with primary unactivated monocytes, the dependency of monocyte mitogenic potential on monocyte activation state, and the basic characteristics of monocyte-induced EC proliferation.
Methods
Cells
Primary human umbilical vein ECs (HUVECs) were cultured in endothelial growth medium (EGM; both from Lonza Walkersville). Primary monocytes were separated before each assay from blood drawn from healthy male subjects under formal protocols sanctioned by the MIT Committee on Use of Humans in Experimental Sciences, and separated using a negative isolation method (Miltenyi Biotec). Monocyte separation yield was more than 90% and validated using fluorescence-activated cell sorting analysis with anti–CD14-R-phycoerythrin (RPE) antibodies (DakoCytomation). Monocyte viability after negative selection separation was more than 98% as measured by trypan blue staining.
Thymidine incorporation
HUVECs were grown to subconfluence in 24-well plates (5 × 104 cells/cm2) in Dulbecco modified Eagle medium (DMEM) 10% fetal bovine serum (FBS) followed by mild starvation (DMEM 5% FBS, 24 hours). HUVECs were exposed to proportional numbers of primary monocytes, and at the indicated time points cultures were pulsed with 3H-thymidine (1 μCi [0.037 MBq]/mL; Perkin Elmer Life Sciences) for the indicated periods. Cultures were washed twice with 2 mL of ice-cold phosphate-buffered saline (PBS) followed by 30-minute incubation in 5% wt/vol trichloroacetic acid (TCA). TCA was washed twice with cold PBS, followed by lysis with 0.4 mL of 0.5% sodium dodecyl sulfate, 0.5 N NaOH. The TCA-insoluble radioactivity was counted in a liquid scintillation counter (Packard 25000-TR).
EC growth kinetics
EC growth kinetics was measured from time-lapse phase microscopy images recorded over 28 hours. HUVECs seeded to subconfluence (3 × 104 cells/cm2) in 6-cm plates in DMEM 10% FBS followed by mild starvation (DMEM 5% FBS, 24 hours). Six plates were exposed to proportional numbers of primary monocytes in DMEM 2.5% FBS, and 6 plates were treated with DMEM 2.5% FBS only. Cell number was determined from phase-contrast images taken every 4 hours over 28 hours using a semiautomated computer directed grid (20 random squares of a 96-square grid). Unbound cells were removed by gentle wash with DMEM 10% FBS before imaging, and monocytes were excluded from cell counts based on cell size and shape (primary unactivated monocytes are smaller then ECs and gain volume as they differentiate into macrophages14).
Antibodies and cytokines
Phosphorylated extracellular signal-related kinase 1/2 (ERK1/2), phosphorylated Met (pMet), and β-actin antibodies were obtained from Cell Signaling Technology. Tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β) were obtained from Chemicon International. Lipopolysaccharide (LPS) was obtained from Sigma Chemical.
Western blot
Whole-cell extracts of ECs and monocytes were harvested from cocultures by separation of monocytes from the coculture with repeated washes (PBS, 2.5mM EDTA [ethylenediaminetetraacetic acid], 2 minutes) as previously described.13,15 The purity of separated monocytes and ECs was verified by fluorescence-activated cell sorting analysis as previously described.13 Whole-cell protein samples were extracted using Nonidet P40 cell lysis buffer (Invitrogen), supplemented with complete protease inhibitor tablet (Roche Molecular Biochemicals) and 0.5mM sodium orthovanadate. Proteins were separated on Tris-glycine–sodium dodecyl sulfate gels, transferred to polyvinylidene fluoride membranes, and immunoblotted with the indicated antibodies. Ten minutes before cell harvesting, cultures were treated with 0.5mM sodium orthovanadate to preserve phosphorylation. Blots were scanned and bands quantified using ImageJ software (National Institutes of Health).
Reverse-transcription–polymerase chain reaction
Endothelial-monocyte cocultures were separated13 by incubation in PBS, EDTA 5mM. RNA from ECs and monocytes was extracted using the RNeasy Mini Kit (QIAGEN), and RNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TNFα, and IL-1β were measured using the following primers: TNFα: TCCTTCAGACACCCTCAACC, AGGCCCCAGTTTGAATTCTT; IL-1β: GGGCCTCAAGGAAAAGAATC, TTCTGCTTGAGAGGTGCTGA; and GAPDH: AGGGCCCTGACAACTCTTTT, AGGGGTCTACATGGCAACTG. RNA levels were standardized to GAPDH.
Data analysis
Data represent a minimum of 3 assay repetitions. All growth kinetics assays were performed in minimum of duplicate and most as triplicates. Data are presented as mean (± SD). When applicable, values were compared by Student t test or analysis of variance. P value less than .05 was considered statistically significant.
Results
Monocytes induce a single cell cycle in ECs
To understand better the EC cycle kinetics after direct interaction with primary monocytes, thymidine incorporation was evaluated for 109 hours after monocyte addition in proportional numbers to subconfluent, serum-starved ECs.
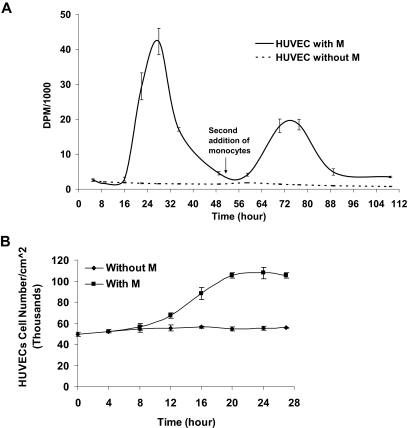
HUVECs first entered S-phase 10 to 16 hours after the addition of monocytes, with peak incorporation at 28 hours (Figure 1A). The thymidine incorporation phase ended after the first 40 hours and was followed by a slow decrease in cell proliferation. To study the ability of monocytes to induce repetitive cell cycles, HUVECs that underwent 1 cell cycle induced by monocytes were gently washed with medium, without removing adherent monocytes, before the addition of a second group of primary monocytes at 51 hours. Sequential pulsing with 3H-thymidine 1 μCi (0.037 MBq)/mL confirmed the reoccurrence of a second synchronized cell cycle within HUVECs exhibiting similar kinetics.
Figure 1.
Monocytes induce a single cell cycle in ECs. (A) Monocytes added to subconfluent human umbilical vein endothelial cells (HUVECs) at time 0 induce a single cycle of 3H-thymidine incorporation in HUVECs. A second addition of monocytes at time 51 hours induces an additional cycle of EC proliferation. (B) At 27 hours after monocyte addition, EC number nearly doubles as measured by image analysis from phase-contrast microscopy (n ≥ 3).
Whereas monocytes induced a sharp increase in EC thymidine incorporation, untreated HUVECs underwent gradual decrease in thymidine incorporation, representative of the decreasing proliferation rate under prolonged starvation conditions.
To measure the effect of primary monocytes on EC growth kinetics, HUVECs seeded in 6-well plates to subconfluence (5 × 104 cells/cm2) and mildly starved (DMEM 5% FBS, 24 hours) were treated with proportional numbers of primary unactivated monocytes. EC growth kinetics was measured from time-lapse phase microscopy as described in “EC growth kinetics.” Similar to measured thymidine incorporation, HUVECs went through a single wave of proliferation resulting in 2-fold increase in cell density (Figure 1B).
MIECP is monocyte dose dependent and EC density dependent
Monocyte recruitment to injured endothelium varies in number and location. To study dose dependency, monocytes were added to subconfluent HUVECs (5 × 104 cells/cm2) at different relative ratios and pulsed 20 hours later with 3H-thymidine 1 μCi (0.037 MBq)/mL for 2 hours. Monocytes induced a density-dependent increase in EC thymidine incorporation, with an optimal effect observed when monocyte to HUVEC ratio ranged between 1 and 2 (Figure 2A). Interestingly, the effect of monocyte number on HUVEC proliferation was not linear and required a minimum ratio of more than 0.5.
Figure 2.
Monocyte-induced proliferation in ECs is dose dependent and EC density dependent. (A) 3H-thymidine incorporation (disintegrations per minute [DPM]) is dependent on the relative ratio of monocyte to EC density. (B) Monocytes added in proportional numbers to ECs, seeded in increasing densities, exhibit a decreasing mitogenic effect as ECs approach confluence (n ≥ 3).
MIECP was similarly dependent on EC density in a manner resembling EC response to soluble growth factors. HUVECs were seeded in 24-well plates ranging from 13 000 to 80 000 cell/cm2 and mildly starved (DMEM 5% FBS, 24 hours) before addition of primary unactivated monocytes in proportional numbers. MIECP was limited as ECs approached confluence (Figure 2B).
Window of MIECP interaction
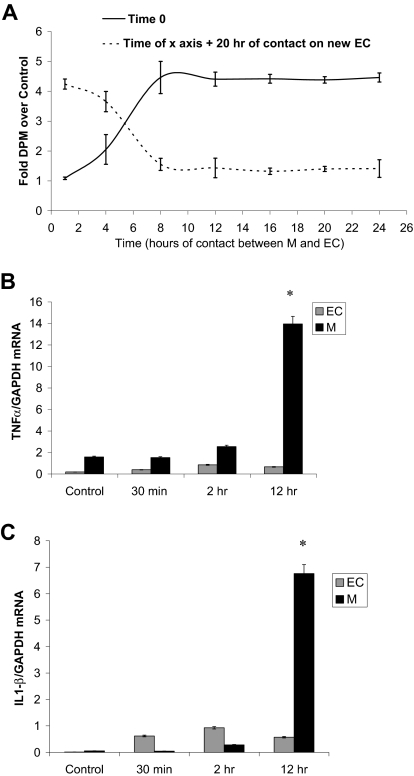
The contact time with monocytes required to induce EC proliferation and the ability of monocytes to repeat the effect in additional EC culture was studied in unactivated monocytes seeded over subconfluent starved HUVECs (5 × 104 cells/cm2, DMEM 5% FBS). (coculture A; Figure 3A). Monocytes were removed at variable times after addition by gentle wash with PBS, EDTA 2.5mM13 and introduced to fresh EC cultures (coculture B). Twenty hours after the addition of monocytes to either coculture system, cells were pulsed with 3H-thymidine 1 μCi (0.037 MBq)/mL for 2 hours and thymidine incorporation was measured. Eight hours of interaction between ECs and primary monocytes are sufficient to induce maximum EC proliferation (Figure 3A). If monocytes are left in 1 culture system for this complete time, all of the proliferation induction is expended there. If the monocytes are removed before this time, they retain the residual ability to stimulate new cells.
Figure 3.
Monocytes lose their mitogenic potency as they interact with ECs. (A) Primary monocytes added to subconfluent HUVECs (solid line) were removed at different time points and placed over new subconfluent HUVECs (broken line). The potentiated proliferation was measured as 3H-thymidine incorporation 20 hours after the addition of monocytes. (B) mRNA levels of TNFα in untreated monocytes and monocytes separated from monocyte-HUVEC coculture, standardized to GADPH mRNA. (C) mRNA levels of IL-1β in untreated monocytes and monocytes separated from monocyte-HUVEC coculture, standardized to GADPH mRNA (n ≥ 3, *P < .001).
Monocytes lose the potency for contact-induced proliferation as they become activated
The transient nature of MIECP and the loss of mitogenic potency soon after initial contact with ECs suggest that monocyte induction of proliferation is associated with monocyte activation state. TNFα and IL-1β mRNA levels served as measures of monocyte activation after initial contact of unactivated monocytes with subconfluent ECs. mRNA extracted from monocytes collected from cocultures was tested by reverse-transcription–polymerase chain reaction for GAPDH, TNFα, and IL-1β mRNA using specific primers. Twelve hours after interaction of primary unactivated monocytes with subconfluent ECs, TNFα and IL-1β mRNA levels increased up to 9- and 120-fold over untreated monocyte control, respectively (Figure 3B-C), indicating a correlation between the activation state of monocytes and MIECP.
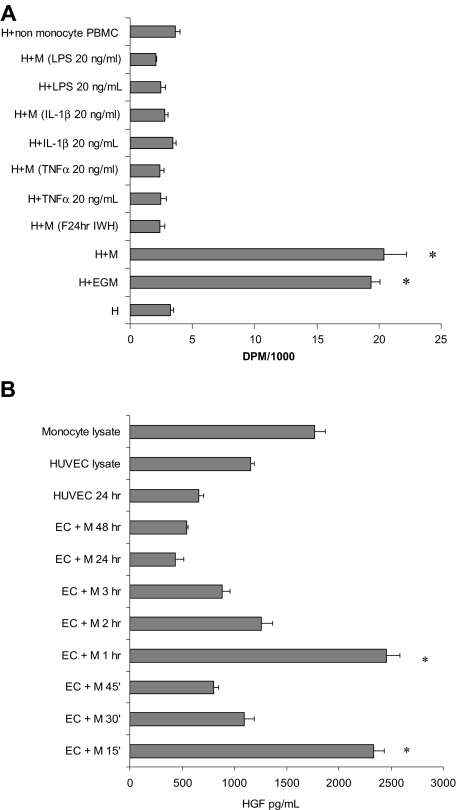
The effect of cytokine activation on monocyte potency to induce proliferation in ECs was tested by pretreatment of monocytes with TNFα, IL-1β, and LPS before their addition to subconfluent HUVECs (5 × 104 cells/cm2) in proportional numbers. Twenty hours after the addition of monocytes, cocultures were pulsed with 3H-thymidine 1 μCi (0.037 MBq)/mL for 2 hours, and the insoluble radioactivity was measured. Addition of unactivated monocytes or conditioning in full medium (EGM) induced 7.3- and 6.2-fold increases in 3H-thymidine incorporation, respectively (Figure 4A). Any form of monocyte activation resulted in complete elimination of MIECP.
Figure 4.
Activated monocytes do not induce proliferation in ECs. (A) Monocytes activated with TNFα, IL-1β, or LPS or by previous interaction with ECs lose their mitogenic potential as measured by 3H-thymidine incorporation. (B) HGF levels in the medium spiked 15 minutes and again 60 minutes after interaction of unactivated monocytes with subconfluent HUVECs. M indicates monocytes; H, HUVECs; PBMC, peripheral blood mononuclear cells; LPS, lipopolysaccharides; IL, interleukin; and EGM, endothelial growth medium (n ≥ 3, *P < .001).
The levels of hepatocyte growth factor (HGF) released after the initial interaction of ECs with monocytes and the kinetics of HGF release were followed in medium sampled from subconfluent ECs cocultured with primary unactivated monocytes (Figure 4B). HGF levels in cell lysates and media cultured over 5 × 104 ECs alone or with monocytes served as controls. All cells began experiments in fresh DMEM with 1% FBS with no detectable HGF. HGF levels in the coculture peaked at the 15- and 60-minute time points and declined thereafter.
Only monocytes that were not activated induce the phosphorylation of endothelial Met upon direct contact
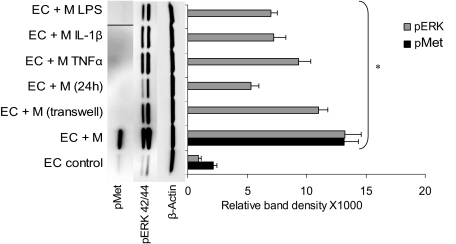
EC Met phosphorylation was determined 1 hour after interaction of subconfluent starved HUVECs (5 × 104 cells/cm2, DMEM 1% FBS, 24 hours) with proportional numbers of unactivated or activated monocytes. The latter was achieved with monocyte pre-exposure to TNFα, IL-1β, or LPS (20 ng/mL, 30 minutes). Monocytes were removed 1 hour after addition to ECs by gentle wash with PBS, EDTA 2.5mM, 0.5mM sodium orthovanadate (SOV),13 and EC cultures were lysed and tested for pMet by Western blot analysis. Only unactivated primary monocytes induced endothelial Met phosphorylation, raising levels more than 6-fold (Figure 5). Preactivated monocytes treated with TNFα, IL-1β, or LPS failed to induce EC Met phosphorylation and practically abolished the basal level of pMet observed in the untreated control. Monocytes removed from previous 24-hour coculture with ECs also failed to induce EC Met phosphorylation.
Figure 5.
Endothelial Met is phosphorylated only upon interaction with unactivated monocytes. Monocytes preactivated with TNFα, IL-1β, or LPS or by previous interaction with ECs fail to activate endothelial Met but do activate endothelial ERK42/44. pMet band of monocyte activation by LPS was inserted by splicing from the same blot. Figure showing representative blot with SD calculated from different assays (n ≥ 3, *P < .001).
Discussion
The integrity of the vascular endothelium is critical for its function as a barrier between the blood and the underlining vessel wall and tissue. Although the endothelium is characterized by quiescence and low cell turnover, it is fragile and prone to disruptions by mechanical shear stress,16 chemical injury,6 and vascular remodeling. Minor discontinuities in the endothelial monolayer close rapidly with rapid mobilization, migration, proliferation, and spread of EC at the wound edge.17 More significant endothelial loss can be observed after the mechanical injury of catheter-based endovascular interventions.18,19 The pace of endothelial regeneration dictates the extent of the thrombotic and intimal hyperplastic reactions with concomitant local inflammation and smooth muscle cell accumulation.18,20,21 Monocytes are among the first cells to arrive to the site of the damaged endothelium and may play a critical role in endothelial restoration. In a previous study, we reported on the ability unactivated monocytes to induce a limited round of EC proliferation through a contact-mediated activation of endothelial Met.13 In the present work, we explore the molecular signals that arise after the interaction of unactivated monocytes with subconfluent ECs and the way this unique sequence of signaling results in tight regulation of endothelial growth.
This effect of monocytes represents an added layer of complexity in an already tightly regulated homeostatic system and a novel general form of cell-cell interaction. Endothelial state governs vascular homeostasis. An intact endothelial monolayer imposes quiescence, and an injured or noncontiguous layer stimulates the cascade of vascular healing. Nonconfluent ECs draw monocytes to sites of endothelial injury. The former induce proliferation of the latter until they are themselves transformed to an activated state by the interaction.
Cell-cycle kinetics
Upon direct contact, monocytes initiate a single cell-cycle doubling of EC number, with an optimal dose response ranging from 1 to 2 monocytes per EC. The single cell-cycle mode of proliferation presented by monocyte binding to ECs (Figures 1A, 2A) may indicate the existence of a precise mechanism of control over EC proliferation. As long as monocyte recruitment continues, and ECs retain their growth potential, recruited monocytes will induce EC proliferation. For yet unaffected ECs to proliferate, newly recruited monocytes are required (Figure 1A). Yet, there is another exciting aspect of monocyte-EC modulation. The single cell-cycle commitment allows the system to sense endothelial state before initiating another round of endothelial proliferation. Indeed, the mitogenic effect of monocytes is reduced as ECs approach confluence (Figure 2B).
Monocyte activation regulates HGF secretion and Met signaling in MIECP
HGF is a key regulator of endothelial and vascular function through activation of Met tyrosine kinase, promoting EC motility, growth, and survival.22,23 Appearance of soluble HGF correlates closely with vascular activation and monocyte recruitment, as demonstrated by the elevated levels of serum HGF after acute myocardial infraction.24,25 We now report that HGF is secreted after the early stages of monocyte interaction with ECs, showing a unique biphasic appearance at 15 and 60 minutes. The secretion of HGF after interaction of primary unactivated monocytes with ECs and the consequent phosphorylation of endothelial Met may be part of the mechanism through which monocytes regulate EC proliferation.13 The transient decline of HGF levels and loss of Met phosphorylation after monocyte activation suggests that HGF may be readily available for secretion within circulating monocytes and at least the initial spike in HGF level is from secretion upon monocyte contact with ECs. The need for 2 successive steps of endothelial Met activation within a short period of time suggests a possible role for Met in maintaining endothelial confluence after monocyte transendothelial migration.
Only unactivated primary monocytes are capable of inducing Met phosphorylation. Activation of monocytes with TNFα, IL-1β, or LPS as well as monocytes seeded in transwell inserts raised over ECs cannot induce Met phosphorylation (Figure 5). These findings may indicate that the specific mitogenic potential carried by unactivated circulating monocytes is conditioned by their activation state and may be regulated even before monocytes interact with the endothelium. Interestingly, TNFα was recently demonstrated to inhibit HGF-mediated Met phosphorylation through a protein tyrosine phosphatase–mediated mechanism.26 The increase in TNFα levels after monocyte-EC interaction may therefore be part of this timely signaling cascade.
The initial stage of monocyte differentiation takes place shortly after binding to ECs and continues after trans-endothelial migration, directed by the microenvironment in the specific tissue. By removing primary monocytes from one coculture with subconfluent ECs, and seeding the same monocytes over a different culture of subconfluent ECs, we demonstrated that the initial interaction of primary monocytes with ECs is responsible for the loss of their mitogenic activity (Figure 3A). The increase in TNFα and IL-1β mRNA levels in monocytes after their interaction with subconfluent ECs further supports the role of monocyte activation in the transient nature of MIECP and demonstrates that monocytes indeed respond to interaction with subconfluent ECs by changing their gene expression patterns (Figure 3B-C).
HGF kinetics closely correlates with endothelial Met and ERK phosphorylation after interaction with primary unactivated monocytes. ERK signaling plays a crucial role in receptor tyrosine kinase signaling as well as in cytokine-mediated signaling. Although ERK is activated after monocyte-induced Met phosphorylation (Figure 5), ERK phosphorylation is observed in all modes of monocyte interaction, including indirect interaction through a transwell insert. There may then be a broader role for ERK as a mediator in a wide range of signaling pathways after EC-monocyte interaction. Nevertheless, the intensity of ERK phosphorylation correlates with the kinetics of HGF secretion as well as with endothelial pMet as we previously reported,13 further supporting a role for HGF and Met in monocyte-mediated EC ERK signaling.
Conclusion
Studies of the interaction between endothelium and circulating monocytes have focused on monocyte binding and trans-endothelial migration. Little attention was given to other forms of signaling that may accompany this interaction and affect its outcome. The timely secretion of HGF and phosphorylation of endothelial Met upon direct interaction and its role in the regulation of MIECP adds a novel aspect to the role of monocytes in maintaining vascular homeostasis. These findings infer the existence of 2 distinct mechanisms for regulation of EC proliferation. Whereas one is regulated through endocrine cues initiated at the tissue, the other is paracrine and limited to monocyte binding sites on the endothelium. Prolonged exposure to growth factors and cytokines, therefore, allows for regional and more general response to systemic and regional changes, whereas monocytes through direct contact provide ECs specific and highly localized regulation. Additional research into the early stages of EC-monocyte interaction will be required to elucidate the full extent of the role of monocytes in vascular remodeling and homeostasis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL-49039 and HL-67246; E.R.E.) and the National Institute on Aging (T32-AG023480).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.Y.S. provided overall supervision, designed and performed research, analyzed data, and wrote the paper; A.B. performed research and analyzed data; J.M.-S. performed ELISA technical work and sample collection; and E.R.E. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shai Y. Schubert, Division of Health Sciences and Technology, MIT, 77 Massachusetts Ave, Room E25-438, Cambridge, MA; e-mail: shai@mit.edu.
References
- 1.D'Amore PA. Mechanisms of endothelial growth control. Am J Respir Cell Mol Biol. 1992;6(1):1–8. doi: 10.1165/ajrcmb/6.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73(6):1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- 3.Dickhout JG, Basseri S, Austin RC. Macrophage function and its impact on atherosclerotic lesion composition, progression, and stability: the good, the bad, and the ugly. Arterioscler Thromb Vasc Biol. 2008;28(8):1413–1415. doi: 10.1161/ATVBAHA.108.169144. [DOI] [PubMed] [Google Scholar]
- 4.Cipollone F, Marini M, Fazia M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21(3):327–334. doi: 10.1161/01.atv.21.3.327. [DOI] [PubMed] [Google Scholar]
- 5.Rogers C, Welt FG, Karnovsky MJ, Edelman ER. Monocyte recruitment and neointimal hyperplasia in rabbits: coupled inhibitory effects of heparin. Arterioscler Thromb Vasc Biol. 1996;16(10):1312–1318. doi: 10.1161/01.atv.16.10.1312. [DOI] [PubMed] [Google Scholar]
- 6.Welt FG, Tso C, Edelman ER, et al. Leukocyte recruitment and expression of chemokines following different forms of vascular injury. Vasc Med. 2003;8(1):1–7. doi: 10.1191/1358863x03vm462oa. [DOI] [PubMed] [Google Scholar]
- 7.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87(5):378–384. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- 8.Arras M, Ito WD, Scholz D, et al. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101(1):40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bando H, Toi M. Tumor angiogenesis, macrophages, and cytokines. Adv Exp Med Biol. 2000;476:267–284. doi: 10.1007/978-1-4615-4221-6_21. [DOI] [PubMed] [Google Scholar]
- 10.Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80(4):705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 11.Sierra JR, Corso S, Caione L, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205(7):1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varney ML, Olsen KJ, Mosley RL, et al. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo. 2002;16(6):471–477. [PubMed] [Google Scholar]
- 13.Schubert SY, Benarroch A, Ostvang J, Edelman ER. Regulation of endothelial cell proliferation by primary monocytes. Arterioscler Thromb Vasc Biol. 2008;28(1):97–104. doi: 10.1161/ATVBAHA.107.157537. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson HC, Katz P, Wright DG, et al. Human blood monocytes: characterization of negatively selected human monocytes and their suspension cell culture derivatives. Scand J Immunol. 1981;14(3):243–256. doi: 10.1111/j.1365-3083.1981.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 15.Kataoka N, Iwaki K, Hashimoto K, et al. Measurements of endothelial cell-to-cell and cell-to-substrate gaps and micromechanical properties of endothelial cells during monocyte adhesion. Proc Natl Acad Sci U S A. 2002;99(24):15638–15643. doi: 10.1073/pnas.242590799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langille BL, Reidy MA, Kline RL. Injury and repair of endothelium at sites of flow disturbances near abdominal aortic coarctations in rabbits. Arteriosclerosis. 1986;6(2):146–154. doi: 10.1161/01.atv.6.2.146. [DOI] [PubMed] [Google Scholar]
- 17.Reidy MA, Schwartz SM. Endothelial regeneration: III, time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981;44(4):301–308. [PubMed] [Google Scholar]
- 18.Kipshidze N, Dangas G, Tsapenko M, et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44(4):733–739. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 19.Versari D, Lerman LO, Lerman A. The importance of reendothelialization after arterial injury. Curr Pharm Des. 2007;13(17):1811–1824. doi: 10.2174/138161207780831239. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Tang JF, Yang PL. [Recovery of endothelium, a new policy of integrative Chinese and Western medicine in prevention and treatment of post-PCI restenosis]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(10):944–946. [PubMed] [Google Scholar]
- 21.Meurice T, Vallet B, Bauters C, et al. Role of endothelial cells in restenosis after coronary angioplasty. Fundam Clin Pharmacol. 1996;10(3):234–242. doi: 10.1111/j.1472-8206.1996.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 22.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119(3):629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagami H, Morishita R, Yamamoto K, et al. Hepatocyte growth factor prevents endothelial cell death through inhibition of bax translocation from cytosol to mitochondrial membrane. Diabetes. 2002;51(8):2604–2611. doi: 10.2337/diabetes.51.8.2604. [DOI] [PubMed] [Google Scholar]
- 24.Shimada Y, Yoshiyama M, Jissho S, et al. Hepatocyte growth factor production may be related to the inflammatory response in patients with acute myocardial infarction. Circ J. 2002;66(3):253–256. doi: 10.1253/circj.66.253. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Hojo Y, Ikeda U, Shimada K. Production of hepatocyte growth factor during acute myocardial infarction. Heart. 2000;83(4):450–455. doi: 10.1136/heart.83.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugano M, Iwasaki Y, Abe M, et al. TNF-alpha employs a protein-tyrosine phosphatase to inhibit activation of hepatocyte growth factor receptor and hepatocyte growth factor-induced endothelial cell proliferation. Mol Cell Biochem. 2009;322(1-2):113–117. doi: 10.1007/s11010-008-9946-7. [DOI] [PubMed] [Google Scholar]