Abstract
Transcripts encoding 5-HT2C receptors are modified posttranscriptionally by RNA editing, generating up to 24 protein isoforms. In recombinant cells, the fully edited isoform, 5-HT2C-VGV, exhibits blunted G-protein coupling and reduced constitutive activity. The present studies examine the signal transduction properties of 5-HT2C-VGV receptors in brain to determine the in vivo consequences of altered editing. Using mice solely expressing the 5-HT2C-VGV receptor (VGV/Y), we demonstrate reduced G-protein coupling efficiency and high-affinity agonist binding of brain 5-HT2C-VGV receptors. However, enhanced behavioral sensitivity to a 5-HT2C receptor agonist was also seen in mice expressing 5-HT2C-VGV receptors, an unexpected finding given the blunted G-protein coupling. In addition, mice expressing 5-HT2C-VGV receptors had greater sensitivity to a 5-HT2C inverse agonist/antagonist enhancement of dopamine turnover relative to wild-type mice. These behavioral and biochemical results are most likely explained by increases in 5-HT2C receptor binding sites in the brains of mice solely expressing 5-HT2C-VGV receptors. We conclude that 5-HT2C-VGV receptor signaling in brain is blunted, but this deficiency is masked by a marked increase in 5-HT2C receptor binding site density in mice solely expressing the VGV isoform. These findings suggest that RNA editing may regulate the density of 5-HT2C receptor binding sites in brain. We further caution that the pattern of 5-HT2C receptor RNA isoforms may not reflect the pattern of protein isoforms, and hence the inferred overall function of the receptor.
Keywords: serotonin, 5-HT2C, RNA editing, signal transduction, behavior, DA turnover, 5-HT2C inverse agonist, 5-HT2C agonist
Introduction
The serotonin 2C (5-HT2C) receptor modulates a number of neurophysiological functions including appetite, mood, sexual behavior, and locomotion (Buhot, 1997; Olivier et al., 1998; Giorgetti and Tecott, 2004; Millan, 2005), and is implicated in the etiology of psychiatric disorders such as depression, anxiety, anorexia-nervosa, and obsessive-compulsive disorder (Griebel, 1995; Delgado and Moreno, 1998; Berg et al., 2008; Flaisher-Grinberg et al., 2008). Consequently, the 5-HT2C receptor has received considerable attention as a target for pharmacological treatment of these conditions (Meltzer et al., 2003; Serretti et al., 2004; Millan, 2005; Halford et al., 2007; Morabito and Emeson, 2009).
A distinctive feature of the G-protein coupled 5-HT2C receptor is its ability to be modified posttranscriptionally by RNA editing (Burns et al., 1997). RNA editing is an enzymatic process that converts adenosine to inosine at five nucleotide positions in the 5th exon of the 5-HT2C receptor RNA transcript, encoding the second intracellular loop, a region important for G-protein coupling. Selective RNA editing can generate up to 32 mRNA isoforms, predicting 24 protein isoforms of the 5-HT2C receptor, potentially adding immense diversity to the function of the receptor in vivo. Furthermore, recent studies using animal models suggest that RNA editing of the 5-HT2C receptor is a dynamic process. For example, chronic stress increases the degree of RNA editing of the 5-HT2C receptor in both mice (Englander et al., 2005) and rats (Iwamoto et al., 2005). Variation in 5-HT2C receptor RNA editing has also been implicated in a variety of neuropsychiatric disorders, with the most reproducible finding being an increase in RNA editing in brains of suicide victims (Niswender et al., 2001; Gurevich et al., 2002; Dracheva et al., 2008a,b). Despite these findings, defining the in vivo functional consequences of 5-HT2C receptor RNA editing has been limited due to technical constraints presented by the immense diversity of receptor RNA isoforms in brain.
In vitro studies in cultured cells transfected with cDNA encoding a single receptor isoform show that differentially edited 5-HT2C receptor isoforms have unique signaling features, with increased editing generally leading to decreased function. For example, the edited isoform valine-serine-valine (at amino acid positions 157, 159, 161 in humans) has four-fold reduced constitutive activity and four- to five-fold reduced serotonin potency to activate phospholipase C (PLC) relative to the non-edited isoleucine- asparagine- isoleucine isoform (INI); the function of the fully edited valine-glycine-valine (VGV) isoform is reduced even further (Fitzgerald et al., 1999; Herrick-Davis et al., 1999; Niswender et al., 1999). These studies suggest dramatic alterations will accompany changes in RNA editing of the 5-HT2C receptor; however, it is widely accepted that functional properties demonstrated in cell culture studies of cloned receptors may not be reproduced in vivo.
Since the majority of 5-HT2C receptors in brain are edited (Burns et al., 1997), studies of the in vivo functional consequences of 5-HT2C receptor RNA editing are critically needed. Here we take advantage of mutant mice solely expressing a single isoform, the fully-edited 5-HT2C-VGV receptor, to characterize for the first time the signal transduction consequences of RNA editing of the 5-HT2C receptor in vivo. We show that the proximal event in cell signaling, receptor:G-protein coupling, is nearly abolished for the fully edited 5-HT2C-VGV receptor in brain. We further show that changes in the degree of editing significantly alters the density of receptor binding sites within the brain, suggesting that the pattern of protein isoforms, and hence the inferred overall function of the receptor protein, may not reflect the pattern of RNA isoforms. This finding has significant ramifications for basic as well as clinical studies of disease-related alterations in RNA editing of the 5-HT2C receptor.
Materials and Methods
Animals
All experiments involving animals were approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Naive adult mice, 3–6 months of age, were used for all experiments. Mice were housed in groups of 2–5 in a temperature controlled, colony room (ambient temperature 22–23°C, 12:12 light: dark cycle). Food and water were available ad libitum, and all testing occurred between 1200 and 1700 during the light phase. Unless otherwise indicated, male mice were used.
Mutant mice solely expressing 5-HT2C-VGV receptors were generated by homologous recombination using a replacement-type targeting vector with the five edited adenosine residues of the 5-HT2C receptor gene mutated to guanosine to mimic the base- pairing properties of inosine (Morabito et al., 2007). Mice were maintained on a mixed JAX® 129S1/Taconic® 129S6 background. Four genotypes of mice were used in the experiments examining functional characteristics of the in vivo expressed 5-HT2C-VGV receptor: wild-type males and females (WT), heterozygous females (VGV/X), and hemizygous (the 5-HT2C receptor is X-linked) males (VGV/Y), expressing solely the VGV isoform. The genotypes of mice were determined by PCR analysis of genomic DNA from tail samples using the RED Extract-N-Amp tissue PCR kit (Sigma, St. Louis, MO, USA), with the following primers: 5′GGG CAA ATA TTC TGA AAA GAT GTT 3′ (reverse) and 5′AAT ATC AAT AGG TAA TTA TAC C 3′ (forward).
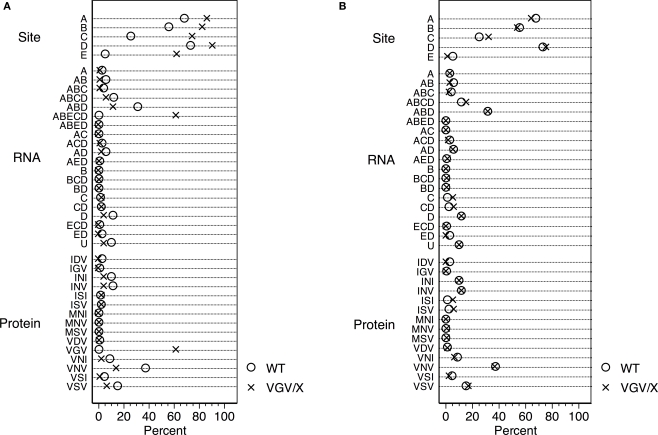
To assess the possible contribution of gene dosing, heterozygous mice expressing a mixture of receptor isoforms enriched with the mutant allele were used; these studies were performed in female mice since the 5-HT2C receptor is X-linked and it is not possible to generate heterozygote males. Using the method of pyrosequencing (Canal et al., 2009), we verified that heterozygous VGV/X females had VGV as the major isoform,while control mice (N = 4, average of 60 sequences per mouse) had less than 1% VGV isoforms. VGV/X females (N = 4, average of 60 sequences per mouse) had an average of 62% of RNA isoforms that translate to VGV (Figure 1A); although suggestive of biased X-inactivation, this value was not significantly different from the expected 50% (one sample t-test, t = 3.0949, df = 3, p > 0.05). The finding that the frequency of VGV isoform in the heterozygous VGV/X mice is about 50%, combined with subsequent results showing that receptor density in VGV/X mice is about halfway between WT and VGV/Y mice, suggests that there is not significant bias in X-inactivation. We also verified that the increase in the percentage of the VGV isoform in brains of VGV/X mice did not alter the remaining distribution of isoforms (Figure 1B).
Figure 1.
Distribution of 5-HT2C RNA isoforms. (A) Percentage distribution of Htr2c RNA editing in cortex of wild type (WT, O) control female mice and heterozygous VGV/X (X) transgenic mice, for editing sites (top), observed RNA sequences (middle), and predicted receptor protein isoforms (bottom). Differences in other proportions (e.g., ABD, VNV) reflect inclusion of VGV in the calculation of the editing measures (%). Heterozygous VGV/X females expressed the 5-HT2C-VGV isoform at a quantity (mean = 61.9%, 95% CI 49.7–74.1%) that was significantly higher than the rare expression observed in wild type litter mate females (two sample t-test, t = 15.89, df = 6, p < 0.0001) and significantly lower than the 100% expression seen in VGV homozygous females and hemizygous VGV/Y males (one sample t-test, t = −9.92, df = 3, p = 0.002). (B) Percentage distribution of Htr2c RNA excluding the fully edited sequences (ABECD or VGV) shows that the remaining distribution is unaltered by the presence of a VGV transgenc (Fischer's exact Chi-square test p = 0.37).
Drugs and radioligands
MK212 hydrochloride [6-Chloro-2-(1-piperazinyl) pyrazine hydrochloride], purchased from Tocris Bioscience (Ellisville, MO, USA), was dissolved in deionized water, and injected subcutaneously (s.c.) at a volume of 10 ml/kg. [N6-methyl-3H]-Mesulergine ([3H]-mesulergine) was purchased from GE Healthcare/Amersham (Buckinghamshire, UK). (±)-1-(2,5,-dimethoxy-4-[125I]iodophenyl)-2-aminopropane ([125I]-DOI) was purchased from Perkin Elmer (Boston, MA, USA). Spiperone and methysergide were purchased from Sigma-Aldrich (St. Louis, MO, USA); SB206553 was purchased from Tocris Bioscience.
GTP-sensitive high-affinity agonist binding in membranes
Agonists, such as DOI, bind preferentially to 5-HT2C receptors existing in a high-affinity state, a receptor conformation that is coupled to G-protein. In vitro, 5-HT2C-VGV receptors show no basal coupling to G-proteins (Herrick-Davis et al., 1999; Niswender et al., 1999). We tested whether this recapitulates in brain. Mice were briefly anesthetized using isoflurane and decapitated. Unless otherwise indicated, brains were removed, placed in a slicing mold, and sliced coronally at the level of the optic chiasm. Tissue anterior to the cut, defined here as forebrain, was assayed. Tissue was homogenized in 20 ml of ice cold binding buffer (50 mM Tris, 10 mM MgCl2, 0.1% ascorbic acid, pH 7.4). Homogenates were centrifuged at 15,000 × g for 20 min. at 4°C. Supernatant was decanted, and the pellet was re-suspended in fresh binding buffer and incubated at 37°C for 15 min to dissociate endogenous ligand from receptors. After a second centrifugation, supernatant was decanted, and the pellet re-suspended in fresh, cold binding buffer. Binding assays were run on the same day as the membrane preparation to avoid a decrease in signal due to freezing.
[125I]-DOI saturation binding was performed on membranes from VGV/Y and WT mice to test potential differences in the proportion of receptors in the agonist high-affinity conformation. Assays were carried out in triplicate and incubated for 90 min in a 37°C water bath at final volume of 600 μl, consisting of 500 μl of membrane suspension, 1 μM spiperone to mask 5-HT2A and 5-HT7 binding sites and increasing concentrations of [125I]-DOI. Non-specific binding was determined in the presence of 100 μM methysergide. Data were analyzed using nonlinear regression (GraphPad Prism 5.02, Graphpad Inc., USA). In a separate experiment, GTP sensitivity was evaluated using a single concentration of [125I]-DOI (1 nM) in the absence and presence of 200 μM guanylyl-5′- imidodiphosphate (Gpp(NH)p), a non-hydrolyzable analog of GTP that prevents G-protein coupling. Total density was estimated in each membrane preparation using a single saturating concentration (30 nM) of [3H]-mesulergine.
Samples were incubated for 90 min in a 37°C water bath, and the reactions were terminated by the addition of ice-cold 50 mM Tris buffer. Membranes were collected by vacuum filtration (Brandel harvester) using Whatman GF/B glass fiber filters presoaked in 0.3% polyethyleneimine. Filters were rinsed three times with cold Tris buffer, and the bound radioactivity was determined by liquid scintillation spectrometry after soaking filters overnight in scintillation cocktail (Aquasol-2, PerkinElmer, Boston, MA, USA).
[125I]-DOI and [3H]-mesulergine autoradiography
Mice were anesthetized with isoflurane inhalation, followed by decapitation. Brains were quickly removed and frozen in 2-methylbutane on dry ice, dried briefly with a Kimwipe (Kimberly-Clark, Roswell, GA, USA), wrapped in aluminum foil and stored at −80°C. Brains were brought to −20°C in a cryostat (Leica, Wetzlar, Germany), sectioned at 20 μm and thaw mounted onto Superfrost glass slides (Fisher, USA), then stored at −80°C until the autoradiographic assay.
For [125I]-DOI autoradiography, slides were thawed at room temperature for 30 min, followed by incubation for 30 min in assay buffer containing 50 mM Tris, 5 mM MgSO4, 4 mM CaCl2 and 0.5 mM EDTA. Slides were then transferred to assay buffer that contained one of four drug treatments: (1) 0.14 nM [125I]-DOI to label 5-HT2A and 5-HT2C receptors; (2) 0.14 nM [125I]-DOI plus 100 nM spiperone to identify the 5-HT2C receptor; (3) 0.14 nM [125I]-DOI plus 100 μM GTPγS to identify GTP-sensitive high affinity [125I]-DOI binding (Appel et al., 1990); (4) 0.14 nM [125I]-DOI plus 30 μM methysergide to define non-specific binding. Slides were incubated with radioligand at room temperature for 60 min, then washed 4 × 10 min in ice-cold 50 mM Tris buffer, pH 7.4 and dried with a steady stream of dehumidified air.
[3H]-mesulergine autoradiography procedures were identical to [125I]-DOI autoradiography with the following exceptions. The assay buffer for [3H]-mesulergine was 50 mM Tris, 10 mM MgCl2, and 0.1 mM EDTA. The binding conditions were: (1) 3 nM [3H]-mesulergine; (2) 3 nM [3H]-mesulergine plus 100 nM spiperone; (3) 3 nM [3H]-mesulergine plus 30 μM methysergide to define non-specific binding. The addition of 300 nM SB204741, a 5-HT2B antagonist, did not alter [3H]-mesulergine binding in WT or VGV/Y brain slices.
Slides were incubated for 120 min at room temperature, then washed 4 × 10 min in ice-cold 50 mM Tris buffer, pH 7.4 and dried with a steady stream of dehumidified air.
After air drying for an additional 90 min, slides were placed in autoradiography cassettes and exposed to Kodak Biomax MR film (Carestream Health, Inc., USA) for 24–48 h for [125I]-DOI or 6–8 weeks for 3H-mesulergine prior to developing (shorter exposures were required for brain sections from VGV/Y mice to accommodate an increased density of 5-HT2C receptors). Film was scanned (CanoScan 4400F, Canon, USA) onto a PC, and images were analyzed with Image J software (Abramoff et al., 2004). The average of the minimum gray value (darkest area) from three brain slices containing brain area of interest was converted to μCi/g protein using 14C standards (ARC, Inc., St. Louis, MO) for statistical comparisons of specific [125I]-DOI binding, and 3H standards for statistical comparisons of specific [3H]-mesulergine. Data were compared using 2-tailed unpaired t-tests.
5-HT2C receptor agonist impact on locomotor activity in VGV/Y and VGV/X
Dose-response studies were conducted to assess the locomotor effects of MK212, a preferential 5-HT2C receptor agonist (Fone et al., 1998; Gleason et al., 2001; Stiedl et al., 2007; Fletcher et al., 2009) in WT, hemizygous VGV/Y and heterozygous VGV/X mice. Locomotor activity was measured in Plexiglas activity chambers (11 × 11 × 11 in; LxWxH; Med Associates St. Albans, Vermont), equipped with 16 infrared beams to monitor and record beam breaks in the x–y–z coordinates. VGV/Y and VGV/X mice were tested with saline and MK212 at doses of 0.01, 0.03, 0.1, 0.3 and 1.0 mg/kg. Twenty minutes after s.c. injection, individual mice were placed inside the activity chamber and distance traveled was recorded for 10 min using Activity Monitor software version 5 (Med Associates, St. Albans, Vermont, USA). Dose-response data were analyzed by two-way ANOVA for independent groups.
Biogenic amine turnover in VGV/Y mice
Dopamine (DA), serotonin (5-HT) and metabolites were quantified by high-performance liquid chromatography (HPLC) electrochemical detection methods (Vanderbilt Neurochemistry Core). Animals were anesthetized with isoflurane and rapidly decapitated after cervical dislocation. Brains were removed and placed in a chilled stainless steel mold for dissection – the tissue slice 1.7–3.6 relative to bregma is referred to as frontal cortex; striatum represents slice 1.70 to −0.82 relative to bregma with the cortex removed by freehand dissection. Tissue was frozen rapidly on dry ice and stored at −80°C until assay. Thawed samples were homogenized in 250 μl acetonitrile and centrifuged at 13,000 × g for 30 min. The acetonitrile fraction was transferred to a clean tube, washed twice with 125 μl heptane and then evaporated using a stream of nitrogen. The sample was suspended in 75 μl of the HPLC mobile phase (37.5 mmol H3PO4, pH 8.5) and 50 μl was injected into the equilibrated HPLC. DA turnover was measured by the ratio DOPAC/DA and HVA/DA; 5-HT turnover was measured by the ratio 5-HIAA/5-HT. Each measure was predicted by a mixed ANOVA model that incorporated between-subject fixed effects for genotype (VGV/Y or WT) and drug (saline or SB206553), a within-subject fixed effect for brain area (frontal cortex or striatum), and a random effect to account for repeated measures in each mouse.
[3H]-mesulergine saturation binding in membranes
The procedures for [3H]-mesulergine saturation binding were essentially identical to [125I]-DOI binding in membranes. Membrane homogenates, binding buffer, spiperone at a final concentration of 1 μM, and increasing concentrations of [3H]-mesulergine up to 36 nM were added. Non-specific binding was determined in the presence of 100 μM methysergide.
Results
5-HT2C-VGV receptors in brain exhibit reduced high-affinity agonist binding: membrane binding
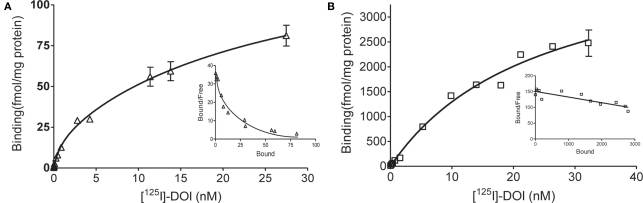
In saturation binding assays of membranes from WT littermates, the binding of the radiolabeled agonist [125I]-DOI was best fit by a two-site equation, as illustrated in the Scatchard plot (Figure 2A). Approximately 10% of the binding sites were in a high affinity state (Kd = 0.51 nM).The low-affinity site had a Kd (20 nM) and density of 137 fmol/mg protein. In contrast, [125I]-DOI binding in membranes from VGV/Y mice was best fit by a one-site equation, as illustrated in the Scatchard plot (Figure 2B) with a Kd (29 nM) that was consistent with the Kd of the low affinity site in WT mice. The extremely high density of binding sites in VGV/Y mice was confirmed in [3H]-mesulergine binding experiments (see below).
Figure 2.
Agonist high affinity binding: Membranes from WT and VGV/Y mice. Representative saturation plots of specific [125I]-DOI binding are shown for membranes from WT (A), and VGV/Y mice (B). Increasing concentrations of [125I]-DOI are plotted on the x-axis and density (fmol/mg protein) is plotted on the y-axis. Data were tested for best fit to one- or two-site models (GraphPad Prism 5.02). Insets show Scatchard transformation of the saturation binding data, bound/free (y-axis) versus bound (x-axis). Scatchard plots illustrate the absence of high-affinity agonist binding sites in VGV/Y mice.
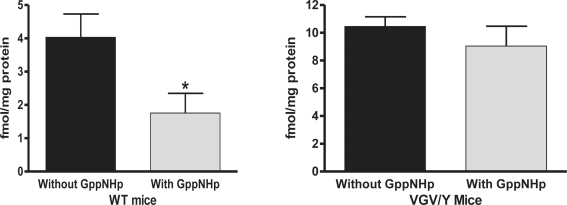
To corroborate the finding of decreased high-affinity binding sites in brains of VGV/Y mice and to estimate the percentage of G-protein coupled receptors, the binding of a single concentration of [125I]-DOI was determined in the absence and presence of Gpp(NH)p to uncouple the receptor:G-protein complex (Figure 3). Fifty-seven percent of the [125I]-DOI binding in membranes from WT mice was eliminated by addition of Gpp(NH)p; in contrast [125I]-DOI binding in membranes from VGV/Y mice was Gpp(NH)p-insensitive (Figure 3).
Figure 3.
GTP sensitive agonist binding: Membranes from WT and VGV/Y. Gpp(NH)p (200 μM) was added to assay buffer containing 1 nM [125I]-DOI to eliminate high affinity binding; the [125I]-DOI binding in the absence and presence of Gpp(NH)p is plotted. Membranes from VGV/Y show no detectable GTP-sensitive [125I]-DOI binding to 5-HT2C receptors. A significant effect of genotype was observed (F1,43 = 54.66, p < 0.0001).
5-HT2C-VGV receptors in vivo exhibit reduced GTP-sensitive high affinity agonist binding: [125I]-DOI autoradiography
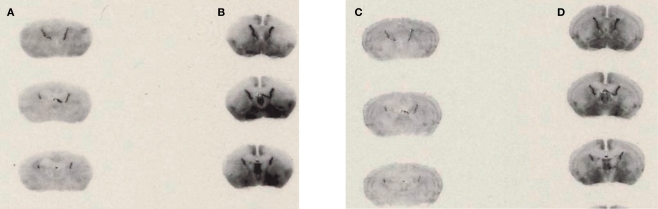
[125I]-DOI binding to 5-HT2C receptors was clearly visible in the choroid plexus in brain slices from WT and VGV/Y mice; thus the choroid plexus was used for analysis of GTP sensitivity of 5-HT2C receptor binding. As illustrated in Figure 4, the addition of GTPγS to WT sections incubated with [125I]-DOI decreased specific binding in choroid plexus (45.7 ± 6.52% decrease; N = 5; p < 0.05). However, GTPγS did not significantly alter [125I]-DOI binding in choroid plexus of VGV/Y sections (5.5 ± 3.9% decrease; N = 6; p = 0.15), confirming greatly reduced high-affinity, GTP-sensitive, agonist binding at 5-HT2C-VGV receptors.
Figure 4.
Agonist high affinity binding: autoradiography of brain slices from WT and VGV/Y mice. Representative [125I]-DOI autoradiography. (A) WT sections incubated in 0.14 nM [125I]-DOI plus 1 μM spiperone. (B) VGV/Y sections incubated in 0.14 nM [125I]-DOI plus 1 μM spiperone. (C) WT sections incubated in 0.14 nM [125I]-DOI plus 100 μM GTPγS. (D) VGV/Y sections incubated in 0.14 nM [125I]-DOI plus 100 μM GTPγS. The addition of GTPγS reduced binding in choroid plexus of sections from WT, but not VGV/Y mice.
VGV/Y mice have increased sensitivity to 5-HT2C receptor agonist: locomotor activity
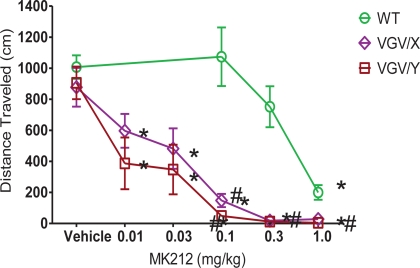
Injection of the 5-HT2C agonist, MK212, dose-dependently decreased total distance traveled in all genotypes, yet the potency of MK212 was greater in mutant mice relative to their respective WT littermates. For WT, female VGV/X and VGV/Y mice (Figure 5), a 2-way ANOVA revealed main effects of dose (F5,135 = 31.77, p < 0.0001) and genotype (F2,135 = 42.46, p < 0.0001). The potency of MK212 was 10-fold higher in VGV/Y and VGV/X mice relative to WT's (Figure 5; N = 10). For example, 0.1 mg/kg MK212 essentially eliminated locomotor activity in VGV/Y mice (post-hoc Fisher least significant difference (LSD) test, saline vs. 0.1 mg/kg, p < 0.001), whereas this dose did not reduce locomotor activity in WT mice (post-hoc Fisher LSD test, saline vs. 0.1 mg/kg, p = 0.58). There were no gender differences in the motor effects of MK212 in WT mice (data not shown).
Figure 5.
MK212 dose-dependent induction of hypolocomotion. Locomotor activity of WT, VGV/X, and VGV/Y mice after single subcutaneous injection of saline or MK2I2 (0.01, 0.03, 0.1, 0.3 or 1.0 mg/kg). *Denotes significantly different from saline within group. #Denotes significantly different from WT.
VGV/Y mice have increased sensitivity to 5-HT2C receptor inverse agonist: DA turnover
Given that the 5-HT2C receptor has been reported to exert an inhibitory influence on DA release, we asked if the DA turnover is decreased in VGV/Y mice and further if SB206553 differentially altered DA turnover. Twenty minutes after treatment with saline or 3 mg/kg of the inverse agonist SB206553, biogenic amine levels were determined in striatum and cortex of VGV/Y and WT male mice (Table 1). DA turnover was measured by the ratio DOPAC/DA and the ratio HVA/DA, and 5-HT turnover was measured by the ratio 5-HIAA/5-HT. Each measure was predicted by a mixed ANOVA model that incorporated between-subject fixed effects for genotype (VGV/Y or WT) and drug (saline or SB206553), a within-subject fixed effect for brain area (cortex or striatum), and a random effect to account for repeated measures in each mouse.
Table 1.
Biogenic amines.
| Amine/metabolite | DOPAC | Dopamine | 5-HIAA | 5-HT | HVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | WT | VGV | WT | VGV | WT | VGV | WT | VGV | WT | VGV |
| FRONTAL CORTEX | ||||||||||
| Saline | 7.1 ± 1.8 | 10.2 ± 1.8 | 31.5 ± 10.4 | 68.2 ± 11.4* | 10.4 ± 0.88 | 13.6 ± 1.7 | 25.9 ± 1.2 | 30.8 ± 2.2 | 6.7 ± 1.6 | 11.2 ± 1.8 |
| SB206553 | 6.6 ± 0.8 | 15.6 ± 1.7 | 28.9 ± 6.7 | 34.2 ± 6.3 | 9.1 ± 0.7 | 16.0 ± 0.8 | 24.5 ± 2.4 | 20.0 ± 0.9 | 6.0 ± 0.7 | 13.9 ± 1.3 |
| STRIATUM | ||||||||||
| Saline | 15.3 ± 1.3 | 11.8 ± 0.9 | 111.9 ± 5.6 | 116.6 ± 7.5 | 19.4 ± 3.1 | 16.9 ± 1.8 | 26.6 ± 1.6 | 24.8 ± 1.4 | 17.1 ± 1.2 | 27.5 ± 1.5 |
| SB206553 | 14.39 ± 1.4 | 24.1 ± 1.0 | 110.8 ± 10.7 | 95.0 ± 15.1 | 18.9 ± 0.7 | 26.2 ± 1.1 | 25.9 ± 1.8 | 25.4 ± 0.6 | 16.2 ± 1.3 | 16.1 ± 1.1 |
The results are presented as means ± SEM (n = 5). *Significantly different from WT, p < 0.01. Data were analyzed with ANOVAs.
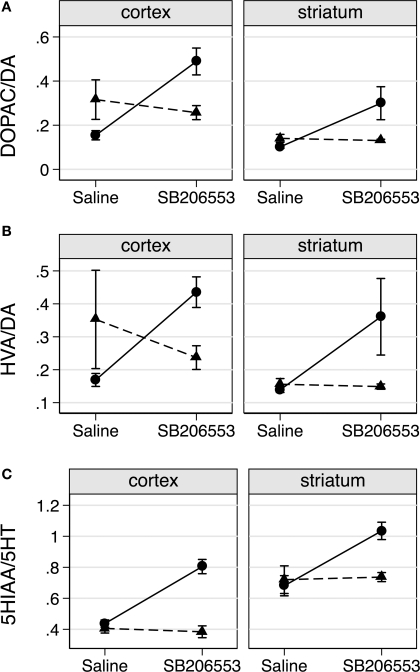
Although basal DA turnover (saline treated) was reduced in cortex of VGV/Y mice (p = 0.039 for DOPAC/DA, p = 0.063 for HVA/DA, least-squared difference post-hoc tests, Figures 6A,B), this effect was small and not reproduced in the striatum. A more striking dopamine phenotype occurred in both cortex and striatum following SB206553 challenge. Significant genotype by drug interactions were observed for the ratios DOPAC/DA (Figure 6A), HVA/DA (Figure 6B), and for 5-HIAA/5-HT (Figure 6C). Similar interaction profiles of the marginal means were observed for each turnover measure (Figure 6), where VGV/Y mice showed significantly increased turnover after SB206553 challenge compared to saline, but WT mice revealed a relatively flatter profile across saline and SB206553 conditions.
Figure 6.
SB206553 increases DA and 5-HT turnover in VGV/Y mice. (A) DA turnover (DOPAC/DA) following administration of saline or SB206553 (3 mg/kg) in WT (▲) and VGV/Y (•) mice; cortex (left), striatum (right). (B) HVA/DA and (C) 5-HT turnover (5-HIAA/5-HT) after the same conditions. Significant genotype by drug interactions were observed for the ratio DOPAC/DA (F1,16 = 20.39, p = 0.0004), for HVA/DA (F1,16 = 8.10, p = 0.0117) and for 5-HlAA/5-HT (Fl,16 = 19.42, p = 0.0004).
VGV/Y mice show large increases in 5-HT2C receptor density: [3H]-mesulergine saturation binding
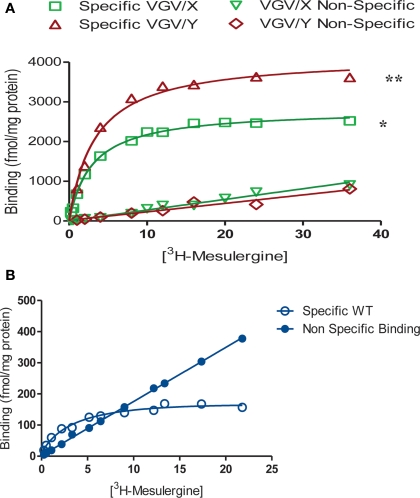
[3H]-Mesulergine saturation binding was performed to determine the maximum density of 5-HT2C receptor binding sites in brains of WT, VGV/Y and VGV/X mice (Figure 7). All plots were best fit by a single site equation. The density of 5-HT2C receptors in the forebrain was increased by 25-fold in VGV/Y mice relative to WT mice (Bmax = 163.3 ± 5.9 fmol/mg protein in WT vs. 4266 ± 260 in VGV/Y; N = 4). Heterozygous VGV/X mice displayed an approximately 10-fold increase in the density of [3H]-mesulergine binding sites (Bmax = 1754 ± 149 fmol/mg protein; N = 8), which was intermediate to that observed for WT and VGV/Y mice. The Kd values for [3H]-mesulergine were identical in all three genotypes. Furthermore, in competition binding experiments, the Ki values for MK212, SB206553 and spiperone were not altered in membranes from VGV/Y vs WT mice (data not shown).
Figure 7.
5-HT2C receptor density in WT, VGV/X, and VGV/Y mice. Mesulergine saturation binding analyses are shown for VGV/Y and VGV/X mice (A), and WT mice (B), The data are means of triplicate determinations and represent four to eight separate experiments. Mean values were as follows. Bmax (fmol/mg protein) ± SEM: VGV/ Y, 4266 ± 259.9 **(p < 0.001 compared to WT and VGV/ X); VGV/X, I754 ± 149 *(p < 0.001 compared to WT); WT, 163.3 ± 5.9. Kd (nM) ± SEM: VGV/Y. 3.18 ± 0.23; VGV/X, 2.49 ± 0.30; WT, 2.13 ± 0.26. Differences between means were determined by a one-way ANOVA followed by a Tukey test.
VGV/Y mice show large increases in 5-HT2C receptor density: [3H]-mesulergine autoradiography
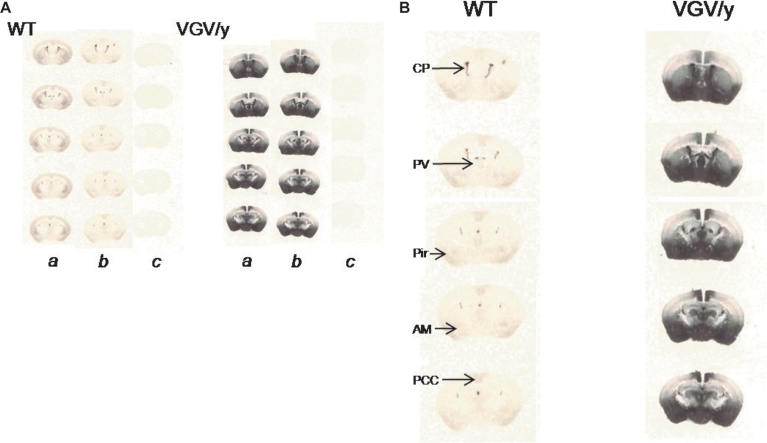
Similar to results of saturation binding experiments, [3H]- mesulergine autoradiograms showed tremendous increases in 5-HT2C receptor density in VGV/Y mice (Figure 8). WT specific binding versus VGV/Y specific binding was significantly increased (p < 0.05) in all regions examined [e.g., 89% increase in nucleus accumbens of VGV/Y mice (N = 6) relative to WT (N = 5)]. The widespread increase in 5-HT2C receptor binding in thalamus, amygdala, posterior cingulate cortex, piriform cortex (Figure 8) appears to reproduce the distribution in WT mice, suggesting that overexpression in the mutant mice does not reflect an aberrant distribution of the 5-HT2C receptor.
Figure 8.
5-HT2C receptor density and distribution: [3H]-mesulergine autoradiography in brain slices. (A) Representative [3H]-mesulergine autoradiography of brain sections from WT and VGV/Y mice. (a) Sections incubated in 3 nM [3H]-mesulergine: (b) plus 1 μM spiperone: (c) plus 30 μM methysergide. (B) Representative [3H]-mesulergine autoradiographs of brain sections displaying 5-H2C receptors in WT, and VGV/Y mice. Increases in 5-HT2C-receptor expression in VGV/Y mice are observed in several brain structures. Abbreviations: CP, choroid plexus; PV, paraventricular thalamic nucleus; Pir, piriform cortex; Am, amygdala; PCC, posterior cingulate cortex.
Discussion
5-HT2C receptor RNA transcripts undergo five adenosine to inosine RNA editing events, resulting in the generation of distinct amino acids in the second intracellular loop of the protein, a region critical for G-protein coupling (Moro et al., 1993; Ballesteros et al., 1998; Gaborik et al., 2003). Combinations of the five edited nucleotides predict 24 different protein isoforms (Burns et al., 1997); many isoforms display altered signaling properties in transfection experiments in cell lines. The fully-edited VGV isoform, where all five sites are edited, shows the most dramatic decrease in agonist potency, as well as silencing of receptor constitutive activity and elimination of high-affinity, Gpp(NH)p sensitive agonist binding (Niswender et al., 1999). The current set of experiments takes advantage of genetically modified mice solely expressing the VGV isoform to show, for the first time in brain, that RNA editing of the 5-HT2C receptor alters the proximal event in cell signaling, receptor:G-protein coupling.
Several techniques were used to highlight the in vivo properties of the 5-HT2C-VGV receptor including [3H]-mesulergine saturation binding, [3H]-mesulergine autoradiography, [125I]-DOI saturation binding, and [125I]-DOI GTP-sensitive high affinity binding in membranes and in autoradiography. Nonlinear regression analysis of [125I]-DOI saturation binding revealed two saturable binding sites in membranes from brains of WT mice, with the high affinity site representing nearly 10% of the total binding. In contrast, nonlinear regression analysis of [125I]-DOI saturation binding to membranes from mice expressing the VGV isoform reveals only a single, low-affinity site, even though receptor density is increased dramatically. Based on the extended ternary complex model of receptor:G-protein coupling (Samama et al., 1993), it is likely that the loss of high affinity binding reflects reduced G-protein coupling. To test this further, [125I]-DOI binding was carried out in the presence and absence of a GTP analog. The addition of Gpp(NH)p reduces the binding of [125I]-DOI in brain membranes from WT mice, but has essentially no effect on [125I]-DOI binding in membranes from VGV/Y mice, suggesting that the G-protein coupling capacity is compromised in brains of mice solely expressing the 5-HT2C-VGV receptors. An unexpected finding was the marked increase in total [125I]-DOI binding in brains of VGV/Y mice relative to WT, with a binding site density comparable to that found in the choroid plexus (Conn and Sanders-Bush, 1986). We considered the possibility that Gq/11 protein may become rate-limiting in the case of the large increase in receptor density in brains of VGV/Y mice. However, quantitative studies of Milligan (1993) show that the level of Gq protein in rat frontal cortex is 17 pmol/mg protein and combined Gq/G11, 25 pmol/mg protein, which is five-fold higher than receptor density in VGV/Y mice. Furthermore, we have previously shown that the levels of Gq and G11 protein are not altered in VGV/Y mice (Canal et al., 2009). These findings suggest that Gq/11protein is not rate limiting in the brains of VGV/Y and that the reduction in GTP-sensitivity reflects reduced coupling capacity of the VGV isoform.
Consistent with a reduction in G-protein coupling capacity found in membranes prepared from brains of VGV/Y mice, receptor autoradiography in intact brain slices reveals that, although total 3H-mesulergine binding is increased, the proportion of GTP- sensitive high affinity [125I]-DOI binding is reduced nearly eight-fold in choroid plexus of VGV/Y mice. These experiments confirm that the blunted G-protein coupling of the VGV isoform described in cell lines is reproduced in a native setting. We conclude therefore that it is valid to interpret in vivo increases in the degree of RNA editing of the 5-HT2C receptor as a loss of function at the cellular level. An attempt to evaluate the classical effector pathway, Gq mediated-PLC activation, was unsuccessful; 5-HT failed to elicit a PLC signal in cortical tissue from wild-type or VGV/Y mice. Previous studies of 5-HT2C receptor-mediated PLC activation in rat brain were performed in choroid plexus (Conn and Sanders-Bush, 1986), but this was not feasible in mice.
Our studies also suggest that changes in the proportion of edited isoforms alter the density of 5-HT2C receptor binding sites within the brain. VGV/Y mice, solely expressing the VGV isoform, have a 25-fold increase in 5-HT2C receptor density relative to WT mice, similar to that found by Morabito et al. (2007). In heterozygous VGV/X mice, the percentage of RNA isoform encoding VGV and the density of 5-HT2C receptor binding sites is approximately halfway between WT and VGV/Y mice. This gene-dose dependent increase in binding site density supports the notion that the increases are not the result of erroneous genetic manipulation. In addition, receptor autoradiography suggests that the increased expression of 5-HT2C receptor in VGV/Y mice does not reflect aberrant distribution of the receptor. It is possible that the increased density is a compensatory response to reduced cellular function of the edited receptor. In support of this conclusion, transgenic mice solely expressing the non-edited, highly functional, 5-HT2C-INI receptor have reduced receptor density (unpublished results).
The molecular mechanism of the substantial density alteration in mutant mice is unknown. In the initial characterization of the VGV/Y mouse phenotype, Morabito et al. (2007) found no change in the steady state levels of 5-HT2C receptor RNA transcripts was detected. Other possible mechanisms include differences in translation efficiency or stability of different protein isoforms. The latter is suggested by studies in cultured cells showing that the VGV isoform has diminished ability to bind to beta-arrestin and translocate into intracellular compartments (Marion et al., 2004). It is important to point out that the RNA isoform distribution, even in heterozygous mice where the VGV isoform accounts for approximately 50%, is markedly different from the native situation where the VGV isoform represents less than 5%. It is possible that some aberrant regulatory mechanism, which is not engaged under normal circumstances, is responsible for the marked increase in 5-HT2C-VGV receptor density in the mutant mice. This question can only be definitively answered when a method becomes available for isolation and quantification of individual protein isoforms.
Although our pharmacological studies in brains of mice expressing the VGV isoform document reduced G-protein coupling capacity, the marked increase in 5-HT2C receptor site density may mitigate this diminished cellular signal in the intact animal. To investigate the functional consequences in vivo of these opposing effects, we employed a behavioral assay of 5-HT2C receptor activation. 5-HT2C receptor agonists, such as MK212, have long been known to induce hypolocomotion (Fone et al., 1998; Gleason and Shannon, 1998; Gleason et al., 2001; Stiedl et al., 2007), although there is debate about their specificity. Recent studies in 5-HT2C receptor knockout mice (Fletcher et al., 2009), showing that MK212-induced hypolocomotion is eliminated, suggest that, in mice, this behavior is a valid measure of in vivo 5-HT2C receptor activation. We found that MK212 elicits a dose-dependent decrease in locomotor activity in both WT and VGV/Y mice, but the sensitivity to MK212 is dramatically increased in VGV/Y mice. This observation confirms the work of Kawahara et al. (2008), and importantly shows that the marked behavioral sensitization is reproduced on another background strain. These data suggest that the large increase in receptor density overcomes the reduced G-protein coupling of the VGV receptor. The finding that 5-HT2C antagonists increase locomotor activity in VGV/Y mice (Kawahara et al., 2008; data not shown) agrees with this interpretation. Given emerging evidence suggesting that the 5-HT2C receptor attenuates DA release and 5-HT2C receptor antagonists augment the action of dopamine releasing agents such as cocaine (Filip and Cunningham, 2003; De Deurwaerdere et al., 2004; Navailles et al., 2004; Alex and Pehek, 2007), we examined DA turnover as another index of in vivo 5-HT2C receptor function. 5-HT2C receptor regulation of DA release is complex, involving direct and indirect pathways; microdialysis studies suggest that the mechanism of regulation differs in the nigrostriatal versus mesocorticolimbic DA systems (Di Matteo et al., 1999; Di Giovanni et al., 2000; see Di Matteo et al., 2008 for review). This may explain the regional difference seen in saline-treated VGV/Y mice, where DA turnover in the mutant mice is decreased in frontal cortex but not in striatum. Microdialysis experiments combined with direct microinjection would be valuable in pursuing this question. The finding that SB206553 increases DA turnover in frontal cortex and striatum of VGV/Y mice is consistent with the evidence that 5-HT2C receptors inhibit DA release in both areas. The ability of SB206553 to increase DA release has been proposed to reflect constitutive activity of the 5-HT2C receptor (De Deurwaerdere et al., 2004); however, such an explanation is less plausible in mice solely expressing the VGV isoform, since in vitro data in transfected cells shows that this isoform is essentially devoid of constitutive activity (Herrick-Davis et al., 1999; Niswender et al., 1999; Wang et al., 2000; Berg et al., 2008) and the present studies in brain are consistent with that interpretation. An alternative explanation is suggested by our finding of increased 5-HT turnover in VGV/Y mice given SB206553; enhanced release of 5-HT onto the up-regulated 5-HT2C receptors may play a role in the SB206553 modulation of DA release in VGV/Y mice.
In summary, in vitro studies in transfected cell lines have shown that RNA editing of 5-HT2C receptor RNA transcripts profoundly alters the signaling properties of the 5-HT2C receptor protein. The current studies utilizing genetically modified mice solely expressing the most extensively edited isoform of the 5-HT2C receptor show, for the first time, that the G-protein coupling capacity in the brain is altered, i.e., expression of fully edited 5-HT2C-VGV receptor isoform within the brain blunts receptor:G-protein coupling. We further show that changes in the degree of editing significantly alters the density of receptor binding sites within the brain, suggesting that the pattern of protein isoforms, and hence the inferred overall function of the receptor protein, may not reflect the pattern of RNA isoforms. This conclusion points to a critical need for a method that differentiates 5-HT2C receptor protein isoforms in brain tissue. Until such a method becomes available, clinical and laboratory investigations must continue to rely on RNA isoform distribution to infer function. Our studies emphasize the need for caution in these interpretations.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the technical assistance of Kathleen Patterson and Erin E. Watt. This work was supported by National Institute of Health training grants [T32 MH065782 (U.B.OD), T32 MH065215 (C.E.C.)]; National Institute of Health research grants [R01 MH34007 (E.S.B.), R21 MH77942 (D.C.A.), and P50 MH078028 (E.S.B., R.B.E.)].
Abbreviations
5-HT, serotonin; DA, dopamine; [125I]DOI, (±)-1-(2,5,-dimethoxy-4-[125I]iodophenyl)-2-aminopropane; INI, isoleucine-asparagine-isoleucine; MK212, 6-Chloro-2-(1-piperazinyl) pyrazine hydrochloride; PLC, phospholipase C; VGV, valine-glycine-valine; WT, wild-type.
References
- Abramoff M. D., Magelhaes P. J., Ram S. J. (2004). Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- Alex K. D., Pehek E. A. (2007). Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 113, 296–320 10.1016/j.pharmthera.2006.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel N. M., Mitchell W. M., Garlick R. K., Glennon R. A., Teitler M., De Souza E. B. (1990). Autoradiographic characterization of (+-)-1-(2,5-dimethoxy-4-[125I] iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J. Pharmacol. Exp. Ther. 255, 843–857 [PubMed] [Google Scholar]
- Ballesteros J., Kitanovic S., Guarnieri F., Davies P., Fromme B. J., Konvicka K., Chi L., Millar R. P., Davidson J. S., Weinstein H., Sealfon S. C. (1998). Functional microdomains in G-protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J. Biol. Chem. 273, 10445–10453 10.1074/jbc.273.17.10445 [DOI] [PubMed] [Google Scholar]
- Berg K. A., Harvey J. A., Spampinato U., Clarke W. P. (2008). Physiological and therapeutic relevance of constitutive activity of 5-HT 2A and 5-HT 2C receptors for the treatment of depression. Prog. Brain Res. 172, 287–305 10.1016/S0079-6123(08)00914-X [DOI] [PubMed] [Google Scholar]
- Buhot M. C. (1997). Serotonin receptors in cognitive behaviors. Curr. Opin. Neurobiol. 7, 243–254 10.1016/S0959-4388(97)80013-X [DOI] [PubMed] [Google Scholar]
- Burns C. M., Chu H., Rueter S. M., Hutchinson L. K., Canton H., Sanders-Bush E., Emeson R. B. (1997). Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387, 303–308 10.1038/387303a0 [DOI] [PubMed] [Google Scholar]
- Canal C. E., Mahautmr K. C., Cao C., Sanders-Bush E., Airey D. C. (2009). RNA editing of the serotonin 2C receptor and expression of Galpha protein: genetic mouse models do not support a role for regulation or compensation. J. Neurochem. 108, 1136–1142 10.1111/j.1471-4159.2008.05852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P. J., Sanders-Bush E. (1986). Agonist-induced phosphoinositide hydrolysis in choroid plexus. J. Neurochem. 47, 1754–1760 10.1111/j.1471-4159.1986.tb13085.x [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P., Navailles S., Berg K. A., Clarke W. P., Spampinato U. (2004). Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J. Neurosci. 24, 3235–3241 10.1523/JNEUROSCI.0112-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado P. L., Moreno F. A. (1998). Hallucinogens, serotonin and obsessive-compulsive disorder. J. Psychoactive Drugs 30, 359–366 [DOI] [PubMed] [Google Scholar]
- Di Giovanni G., Di Matteo V., Di Mascio M., Esposito E. (2000). Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin(2C/2B) receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse 35, 53–61 [DOI] [PubMed] [Google Scholar]
- Di Matteo V., Di Giovanni G., Di Mascio M., Esposito E. (1999). SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology 38, 1195–1205 10.1016/S0028-3908(99)00047-7 [DOI] [PubMed] [Google Scholar]
- Di Matteo V., Di Giovanni G., Pierucci M., Esposito E. (2008). Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog. Brain Res. 172, 7–44 10.1016/S0079-6123(08)00902-3 [DOI] [PubMed] [Google Scholar]
- Dracheva S., Chin B., Haroutunian V. (2008a). Altered serotonin 2C receptor RNA splicing in suicide: association with editing. Neuroreport 19, 379–382 10.1097/WNR.0b013e3282f556d2 [DOI] [PubMed] [Google Scholar]
- Dracheva S., Patel N., Woo D. A., Marcus S. M., Siever L. J., Haroutunian V. (2008b). Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Mol. Psychiatry 13, 1001–1010 10.1038/sj.mp.4002081 [DOI] [PubMed] [Google Scholar]
- Englander M. T., Dulawa S. C., Bhansali P., Schmauss C. (2005). How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J. Neurosci. 25, 648–651 10.1523/JNEUROSCI.3895-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M., Cunningham K. A. (2003). Hyperlocomotive and discriminative stimulus effects of cocaine are under the control of serotonin(2C) (5-HT(2C)) receptors in rat prefrontal cortex. J. Pharmacol. Exp. Ther. 306, 734–743 10.1124/jpet.102.045716 [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. W., Iyer G., Conklin D. S., Krause C. M., Marshall A., Patterson J. P., Tran D. P., Jonak G. J., Hartig P. R. (1999). Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology 21(Suppl. 2), 82S–90S [DOI] [PubMed] [Google Scholar]
- Flaisher-Grinberg S., Klavir O., Joel D. (2008). The role of 5-HT2A and 5-HT2C receptors in the signal attenuation rat model of obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 11, 811–825 10.1017/S146114570800847X [DOI] [PubMed] [Google Scholar]
- Fletcher P. J., Tampakeras M., Sinyard J., Slassi A., Isaac M., Higgins G. A. (2009). Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology 57, 259–267 10.1016/j.neuropharm.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Fone K. C., Austin R. H., Topham I. A., Kennett G. A., Punhani T. (1998). Effect of chronic m-CPP on locomotion, hypophagia, plasma corticosterone and 5-HT2C receptor levels in the rat. Br. J. Pharmacol. 123, 1707–1715 10.1038/sj.bjp.0701798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaborik Z., Jagadeesh G., Zhang M., Spat A., Catt K. J., Hunyady L. (2003). The role of a conserved region of the second intracellular loop in AT1 angiotensin receptor activation and signaling. Endocrinology 144, 2220–2228 10.1210/en.2002-0135 [DOI] [PubMed] [Google Scholar]
- Giorgetti M., Tecott L. H. (2004). Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur. J. Pharmacol. 488, 1–9 10.1016/j.ejphar.2004.01.036 [DOI] [PubMed] [Google Scholar]
- Gleason S. D., Lucaites V. L., Shannon H. E., Nelson D. L., Leander J. D. (2001). m-CPP hypolocomotion is selectively antagonized by compounds with high affinity for 5-HT(2C) receptors but not 5-HT(2A) or 5-HT(2B) receptors. Behav. Pharmacol. 12, 613–620 [DOI] [PubMed] [Google Scholar]
- Gleason S. D., Shannon H. E. (1998). Meta-chlorophenylpiperazine induced changes in locomotor activity are mediated by 5-HT1 as well as 5-HT2C receptors in mice. Eur. J. Pharmacol. 341, 135–138 10.1016/S0014-2999(97)01474-X [DOI] [PubMed] [Google Scholar]
- Griebel G. (1995). 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol. Ther. 65, 319–395 10.1016/0163-7258(95)98597-J [DOI] [PubMed] [Google Scholar]
- Gurevich I., Tamir H., Arango V., Dwork A. J., Mann J. J., Schmauss C. (2002). Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron 34, 349–356 10.1016/S0896-6273(02)00660-8 [DOI] [PubMed] [Google Scholar]
- Halford J. C., Harrold J. A., Boyland E. J., Lawton C. L., Blundell J. E. (2007). Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs 67, 27–55 10.2165/00003495-200767010-00004 [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K., Grinde E., Niswender C. M. (1999). Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J. Neurochem. 73, 1711–1717 10.1046/j.1471-4159.1999.731711.x [DOI] [PubMed] [Google Scholar]
- Iwamoto K., Nakatani N., Bundo M., Yoshikawa T., Kato T. (2005). Altered RNA editing of serotonin 2C receptor in a rat model of depression. Neurosci. Res. 53, 69–76 10.1016/j.neures.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Kawahara Y., Grimberg A., Teegarden S., Mombereau C., Liu S., Bale T. L., Blendy J. A., Nishikura K. (2008). Dysregulated Editing of Serotonin 2C Receptor mRNAs Results in Energy Dissipation and Loss of Fat Mass. J. Neurosci. 28, 12834–12844 10.1523/JNEUROSCI.3896-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion S., Weiner D. M., Caron M. G. (2004). RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J. Biol. Chem. 279, 2945–2954 10.1074/jbc.M308742200 [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y., Li Z., Kaneda Y., Ichikawa J. (2003). Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 1159–1172 10.1016/j.pnpbp.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Millan M. J. (2005). Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie 60, 441–460 10.2515/therapie:2005065 [DOI] [PubMed] [Google Scholar]
- Milligan G. (1993). Regional distribution and quantitative measurement of the phosphoinositidase C-linked guanine nucleotide binding proteins G11 alpha and Gq alpha in rat brain. J. Neurochem. 61, 845–851 10.1111/j.1471-4159.1993.tb03595.x [DOI] [PubMed] [Google Scholar]
- Morabito M. V., Abbas A., Jacobs M. M., Kump D. S., Resnick J. L., Roth B. L., Nagy T. R., Kesterson R. A., Emeson R. B. (2007). Mice solely expressing the fully edited isoform of the serotonin 2C receptor as an animal model of Prader-Willi Syndrome. Soc. Neurosci. Abstr. 37, 465 [Google Scholar]
- Morabito M. V., Emeson R. B. (2009). RNA editing as a therapeutic target for CNS disorders. Neuropsychopharmacology 34, 246. 10.1038/npp.2008.159 [DOI] [PubMed] [Google Scholar]
- Moro O., Lameh J., Hogger P., Sadee W. (1993). Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 268, 22273–22276 [PubMed] [Google Scholar]
- Navailles S., De Deurwaerdere P., Porras G., Spampinato U. (2004). In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology 29, 319–326 10.1038/sj.npp.1300329 [DOI] [PubMed] [Google Scholar]
- Niswender C. M., Copeland S. C., Herrick-Davis K., Emeson R. B., Sanders-Bush E. (1999). RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J. Biol. Chem. 274, 9472–9478 10.1074/jbc.274.14.9472 [DOI] [PubMed] [Google Scholar]
- Niswender C. M., Herrick-Davis K., Dilley G. E., Meltzer H. Y., Overholser J. C., Stockmeier C. A., Emeson R. B., Sanders-Bush E. (2001). RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology 24, 478–491 10.1016/S0893-133X(00)00223-2 [DOI] [PubMed] [Google Scholar]
- Olivier B., van Oorschot R., Waldinger M. D. (1998). Serotonin, serotonergic receptors, selective serotonin reuptake inhibitors and sexual behaviour. Int. Clin. Psychopharmacol. 13(Suppl. 6), S9–S14 10.1097/00004850-199807006-00003 [DOI] [PubMed] [Google Scholar]
- Samama P., Cotecchia S., Costa T., Lefkowitz R. J. (1993). A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 268, 4625–4636 [PubMed] [Google Scholar]
- Serretti A., Artioli P., De Ronchi D. (2004). The 5-HT2C receptor as a target for mood disorders. Expert. Opin. Ther. Targets 8, 15–23 10.1517/14728222.8.1.15 [DOI] [PubMed] [Google Scholar]
- Stiedl O., Misane I., Koch M., Pattij T., Meyer M., Ogren S. O. (2007). Activation of the brain 5-HT2C receptors causes hypolocomotion without anxiogenic-like cardiovascular adjustments in mice. Neuropharmacology 52, 949–957 10.1016/j.neuropharm.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Wang Q., O'Brien P. J., Chen C. X., Cho D. S., Murray J. M., Nishikura K. (2000). Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J. Neurochem. 74, 1290–1300 [DOI] [PubMed] [Google Scholar]