Abstract
The condensing action of cholesterol has been compared with that of a structural isomer having its hydroxyl group located at the C-25 position (i.e., 25-OH’); that is, an isomer favoring an “upside down” orientation in lipid membranes. Surface pressure-area isotherms of mixed monolayers made from 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC)/cholesterol and DMPC/25-OH’ have established that 25-OH’ has a weaker condensing effect than cholesterol. Nearest-neighbor recognition measurements in liposomes made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) have also shown that 25-OH’ has a weaker condensing effect in the physiologically-relevant fluid bilayer state. These findings provide support for surface occupancy playing a role in the condensing action of cholesterol.
The role that cholesterol plays in controlling the conformation and function of integral membrane proteins as well as lipids in cell membranes continues to be a subject of considerable interest.1,2 Studies with model membranes have already shown that certain high-melting phospholipids can combine with cholesterol to form “condensed complexes” as well as a “liquid-ordered” phase.3,4 Although the ability of cholesterol to condense fluid phospholipids was demonstrated more than 80 years ago, the mechanism of its condensing action remains to be established.5 One model that has been proposed (i.e., the “umbrella model”) considers that both acyl chains of a phospholipid and a neighboring cholesterol molecule are required to share limited space beneath the phospholipid's head group, and that the hydrophobic nature of cholesterol forces it together with these acyl chains.6 Alternatively, with the “template model”, the flexible acyl chains of the phospholipid are able to complement, perfectly, the planar nucleus of a neighboring cholesterol molecule, resulting in a high number of hydrophobic contacts and tight packing.7 In both of these models, hydrophobic interactions have been proposed to play a major role.
In the present study, we have tested whether surface occupancy plays a role in cholesterol's condensing action. We define “surface occupancy” as the taking up of space in the interphase region of phospholipid membranes; that is, the region where water makes direct contact with exposed CH2 groups. Since phospholipid head groups occupy only about half of the surface area of liquid phospholipid bilayers, the remaining surface is occupied by partially hydrated CH2 groups. In principle, by replacing the space occupied by these wet CH2 groups, the sterol nucleus of cholesterol should release them into the hydrophobic interior of the membrane, resulting in a partial straightening of the acyl chains, stronger chain-chain interactions and tighter packing.
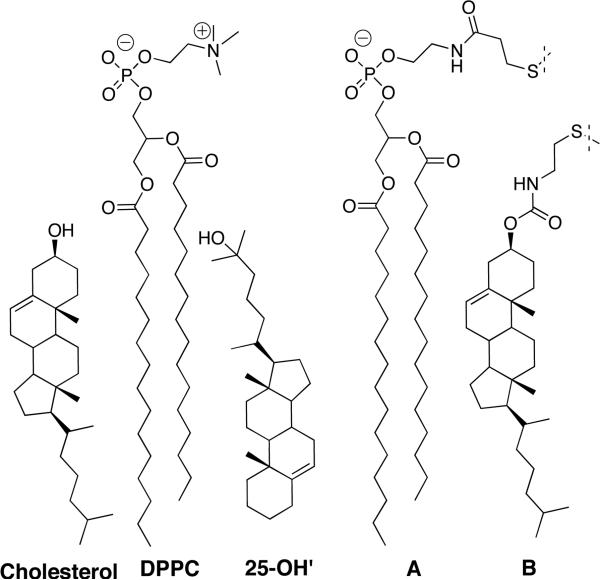
As a critical test of this surface occupancy hypothesis, we compared cholesterol's condensing effect with that of a structural isomer, 25-OH’ (Chart 1). For the latter, only an “upside down” orientation can anchor the sterol to the surface, such that the bulky sterol nucleus is free to penetrate deeper into the membrane. It should be noted that 25-OH’ was specifically chosen for this investigation because only the location of the hydroxyl group has been changed. Although other head groups could be considered (e.g., substituting a larger sulfate or a smaller carbonyl oxygen for cholesterol's hydroxyl group), such a change would not only alter head group size but also the sterol's hydrophilic/lipophilic balance, and interpretations of relative condensing power would be less certain.
Chart 1.
In Scheme 1, we show the synthetic approach that was used to prepare 25-OH’ starting from 25-hydroxycholesterol (25-OH). In brief, regioselective tosylation of the C-3 hydroxyl group of 25-OH, followed by reduction with Zn and NaI in DME/H2O afforded the requisite “upside down” isomer, 25-OH’.8-10
Scheme 1.
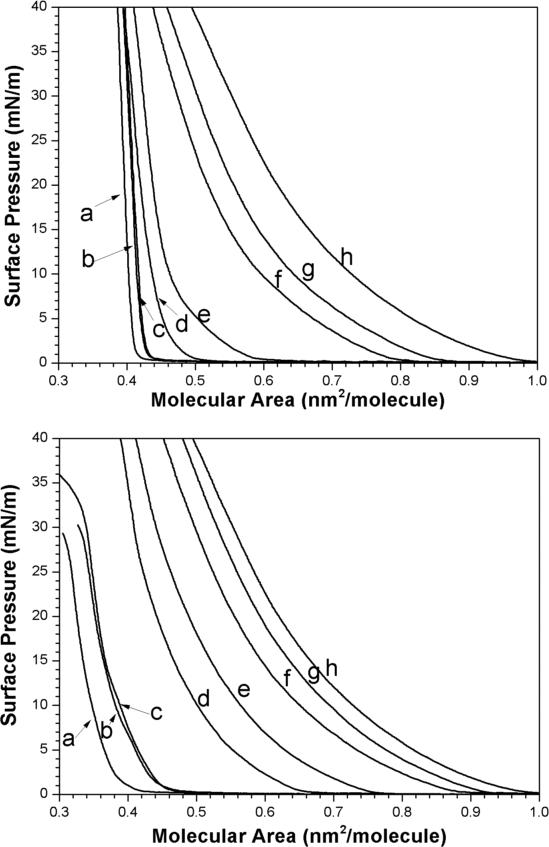
To judge the relative condensing power of cholesterol versus 25-OH’ in the monolayer state, surface pressure-area isotherms were recorded for varying mixtures of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC)/25-OH’ and DMPC/cholesterol at the air/water interface (Figure 1). Here, the slightly shorter DMPC phospholipid was used instead of DPPC because of its lower gel to liquid-crystalline phase transition temperature (i.e., 23°C), which affords the liquid state at moderate subphase temperatures (i.e., 25°C). Compared with cholesterol, 25-OH’ was found to be more compressible. This fact is fully consistent with an upside down orientation, where the sterol is now anchored to the membrane surface by its side chain.
Figure 1.
Surface pressure-area isotherms for (top) DMPC/cholesterol and (bottom) DMPC/25-OH’ over a Tris buffer (pH 7.4) at 25°C. The mole fractions of DMPC used were (a) 0.0, (b) 0.1, (c) 0.2, (d) 0.5, (e) 0.6, (f) 0.8, (g) 0.9, and (h) 1.0.
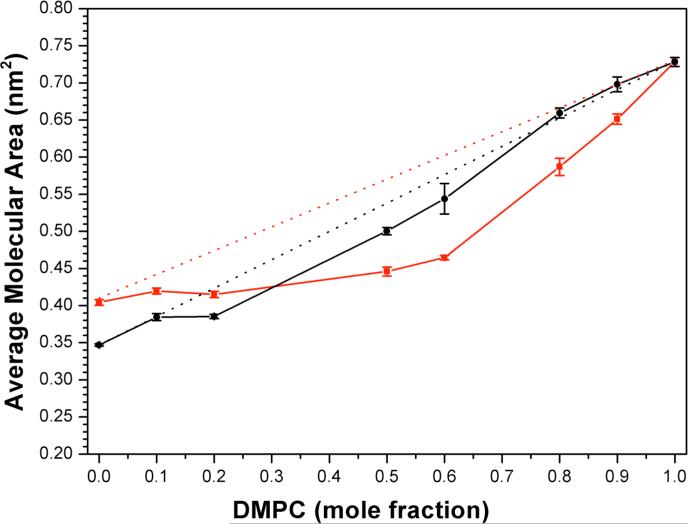
Plots of average molecular areas for both binary systems as a function of the mole fraction of DMPC at 10 mN·m−1 are shown in Figure 2. The straight lines that are drawn represent ideal additivity. As is apparent, both cholesterol and 25-OH’ have a condensing effect on DMPC, which is reflected by a negative deviation from ideality.11 The fact that the negative deviation is greater for cholesterol further indicates that its condensing power is stronger than that of 25-OH’.11 A similar plot that was made from data obtained at 25 mN·m−1, which is close to the internal pressure that has been estimated for fluid bilayers (31-33 mN·m−1), showed the same behavior (Supporting Information).12
Figure 2.
Area-additivity curves for (□) DMPC/cholesterol and (○) DMPC/25-OH’ with a surface pressure of 10 mN/m. Ideal additivities are shown for (---) DMPC/cholesterol and (—) DMPC/25-OH’. Error bars that are not visible lie within the symbols themselves.
Before measuring the relative condensing power of cholesterol and 25-OH’ in the bilayer state, we determined whether the phase properties of cholesterol-rich liposomal membranes of DPPC would be significantly altered by incremental replacement with 25-OH’. With this aim in mind, we measured the fluorescence of a phase-sensitive probe, Laurdan, in a series of liposomal dispersions and determined generalized polarization (GP) values as a function of temperature.13 Here, GP= (I440-I490)/(I440+I490), and I440 and I490 are the emission intensities at these wavelengths (λex=350 nm). As disscussed elsewhere, these GP values reflect the polarity surrounding the Laurdan moiety and are very sensitive to changes in phase.13
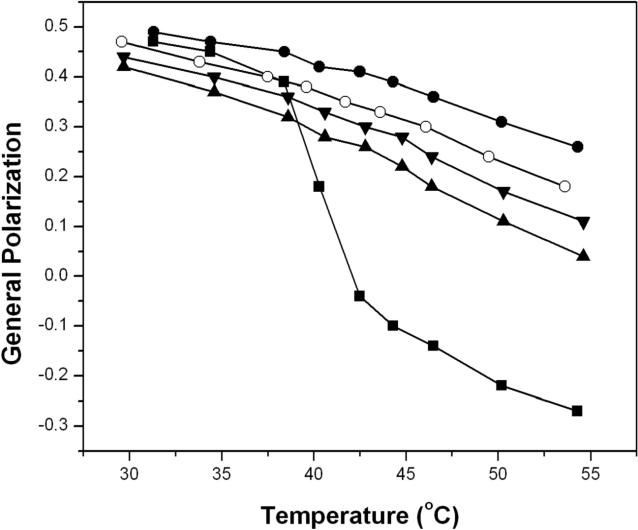
At low cholesterol concentrations (2.5 mol%), a well-defined gel to fluid phase transition was clearly evident with a melting temperature of ca. 41°C (Figure 3). In contrast, when 40 mol% cholesterol was included in the membrane, which is known to maintain the lo phase from 30°C to 55°C, the GP values were found to decrease modestly and almost linearly with increasing temperatures.14 Incremental replacement with 25-OH’ showed this same “signature” for the lo phase, except that the GP values were lower at each temperature, implying that these bilayers are less compact and allow for greater penetration of water. In addition, the slight increase in the slope that is apparent with increasing concentrations of 25-OH’ further suggests that some “softening” of the membrane has occurred.
Figure 3.
Plot of General Polarization versus temperature in liposomes made from DPPC/DPPG/cholesterol/25-OH’ with the following molar percentages: ( ● ) 57.5/2.5/40/0; (○)57.5/2.5/30/10; (▼)57.5/2.5/20/20; (▲) 57.5/2.5/10/30 (■) 95/2.5/2.5/0.
To probe the relative condensing power of cholesterol versus 25-OH’ in the fluid bilayer state, we examined their effects on membrane compactness using the nearest-neighbor recognition (NNR) method.7,15,16 In brief, NNR measurements take molecular-level snapshots of bilayer organization by detecting and quantifying the thermodynamic tendency of exchangeable monomers to become nearest-neighbors of one another. Typically, two lipids of interest (A and B) are converted into exchangeable dimers (homodimers AA and BB, and heterodimer AB), which are then allowed to undergo monomer interchange via thiolate-disulfide exchange (Chart 1). The resulting equilibrium that is established is governed by an equilibrium constant, K= [AB]2/([AA][BB]). When monomers A and B mix ideally, this is reflected by an equilibrium constant that equals 4.0. When hetero-associations are favored, the equilibrium constant is greater than 4.0; favored homo-associations are indicated by a value that is less than 4.0. As shown previously, NNR measurements that use low concentrations of exchangeable lipids A and B (Chart 1) in host membranes made from DPPC/cholesterol reflect changes in the state of compactness of the host membrane; that is, the exchangeable lipids act as a chemical sensor.15 Thus, the transition from the liquid-ordered (lo) to the liquid-disordered phase (ld), is revealed by a steady decrease in K. Three advantages of the use of the NNR method over classic monolayer measurements for investigating the condensing action of sterols are: (i) it provides quantitative insight into changes in lipid packing, (ii) it is highly sensitive and can detect differences in membrane compactness that correspond to tens of calories per mole, and (iii) it probes the physiologically-relevant fluid bilayer state.
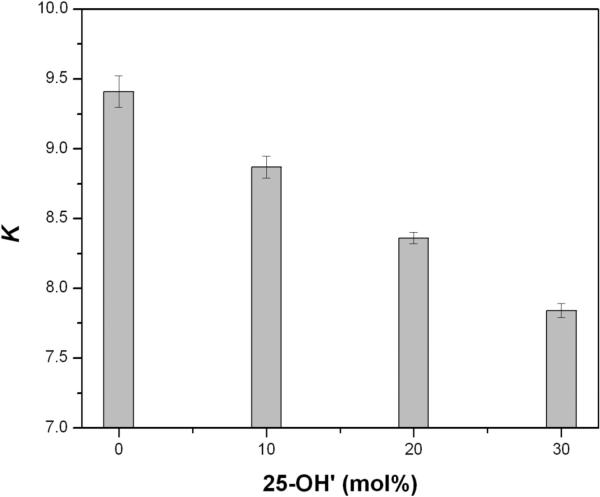
Using procedures described in the Supporting Information, thiolate-disulfide equilibration reactions were carried out at 45°C in liposomes (~200 nm) made from DPPC/cholesterol/25-OH’/AB having the following mole percentages: (a) 57.5/37.5/0/2.5, (b) 57.5/27.5/10/2.5, (c) 57.5/17.5/20/2.5, and (d) 57.5/7.5/30/2.5. As shown in Figure 4, incremental replacement of cholesterol with 25−OH’ resulted in a steady decrease in K, reflecting a decrease in compactness and, hence, a weaker condensing effect by 25-OH’. If one takes statistical considerations into account, nearest-neighbor interaction free energies between A and B are given by ωAB= -1/2 RT ln(K/4).17 Thus, based on these K values for liposomes containing 0, 10, 20 and 30 mol% 25-OH’, the values of ωAB are −270.15 ± 3.66, −251.62 ± 2.81, −232.94 ± 1.34, and −212.39 ± 2.11 cal/mol, respectively.
Figure 4.
Bar graph of K as a function of the mol% of 25-OH’ that is present in ~200 nm diameter liposomes made from DPPC/cholesterol/25-OH’/AB; specific mole percentages of the remaining lipids that listed in the text. Error bars represent one standard deviation.
Taken together, the present findings provide support for surface occupancy playing a role in the condensing action of cholesterol. Whether surface occupancy plays a role in the interaction of other biologically-relevant sterols with fluid phospholipid membranes, however, remains to be established. In a broader context, we believe that the approach that we have described herein (i.e., the use of a combination of monolayer, fluorescence and nearest-neighbor recognition measurements with carefully chosen analogs) could provide valuable insight into the membrane properties of other biologically-relevant sterols. Our efforts in this area are continuing.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by the National Institutes of Health (PHS GM56149).
Footnotes
SUPPORTING INFORMATION. Experimental procedures and tables of data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Lingwood D, Simons K. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 2.Brannigan G, Henin J, Law R, Eckenhoff R, Klein ML. Proc. Natl. Acad. Sci., USA. 2008;2008;105:14418–14423. doi: 10.1073/pnas.0803029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radhakrishnan A, McConnell H. Proc. Natl. Acad. Sci., USA. 2005;102:12662–12666. doi: 10.1073/pnas.0506043102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn PJ, Wolf C. Biochim. Biophys. Acta. 2009;1788:33–46. doi: 10.1016/j.bbamem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Leathes JB. Lancet. 1925;208:853–856. [Google Scholar]
- 6.Huang J, Feigenson GW. Biophys. J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugahara M, Uragami M, Yan X, Regen SL. J. Am. Chem. Soc. 2001;123:7939–7940. doi: 10.1021/ja016199c. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj A, Kondaiah P, Bhattacharya S. Bioconjug. Chem. 2008;19:1640–1651. doi: 10.1021/bc700381v. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto Y, Tatsuno T. Tetrahedron Lett. 1976;37:3325–3326. [Google Scholar]
- 10.Kocovsky P, Cerny V. Collect. Czech. Chem. Commun. 1979;44:246–250. [Google Scholar]
- 11.Kaufmann JM, Westerman PW, Carey MC. J. Lipid Res. 2000;41:991–1003. [PubMed] [Google Scholar]
- 12.Seelig A. Biochim. Biophys. Acta. 1987;899:196–204. doi: 10.1016/0005-2736(87)90400-7. [DOI] [PubMed] [Google Scholar]
- 13.Parassi T, DiStefano M, Loiero M, Ravagnan G, Gratton E. Biophys. J. 1994;66:120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sankaram MB, Thompson TE. Proc. Natl. Acad. Sci., USA. 1991;88:8686–8690. doi: 10.1073/pnas.88.19.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao H, Zhang J, Jing B, Regen SL. J. Am. Chem. Soc. 2005;127:8813–8816. doi: 10.1021/ja0513988. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Jing B, Tokutake N, Regen SL. Biochemistry. 2005;44:3598–3603. doi: 10.1021/bi048258f. [DOI] [PubMed] [Google Scholar]
- 17.Almeida PFF. Biochim. Biophys. Acta. 2009;1788:72–85. doi: 10.1016/j.bbamem.2008.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.