Abstract
Uncontrolled combustion due to garbage recycling is a widespread activity among slum dwellers in distressed economy countries and has been indicated as a major source of dioxin contamination. However, because of the high cost and complexity of gas chromatography/high-resolution mass spectrometry (GC-HRMS) analysis, the magnitude of the problem remains largely unknown. The present study describes a first approach toward the use of a dioxin antibody-based enzyme-linked immunosorbent assay (ELISA) as the basis for a sustainable, simple, and low-cost monitoring program to assess the toxicological impact of uncontrolled combustion in slums. A panel of 16 samples was analyzed by GC-HRMS and ELISA on split extracts. Close to 20% of the analyzed samples showed dioxin concentrations up to almost twice the guidance level for residential soil in several countries, pointing out the need for performing a large-scale monitoring program. Despite the potential for variations in dioxin congener distribution due to the mixed nature of the incinerated material, there was a good correlation between the toxic equivalents as determined by GC-HRMS and ELISA. Furthermore, an interlaboratory ELISA validation showed that the capacity to perform the dioxin ELISA was successfully transferred between laboratories. It was concluded that the ELISA method performed very well as a screening tool to prioritize samples for instrumental analysis, which allows cutting down costs significantly.
Keywords: Polychlorinated dibenzo-p-dioxins, Polychlorinated dibenzo-p-furans, Immunoassays, Dioxin, Developing countries
INTRODUCTION
Dioxin is a generic term for polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofurans (PCDF). Together they represent a family of compounds comprising 210 chemically related molecules (congeners). These molecules are very toxic and persistent organic compounds produced unintentionally as a by-product of combustion of different materials such as municipal waste, coal and wood in power plants, and wood in forest fires as well as in certain industrial processes. Seven PCDDs and 10 PCDFs, all with chlorine in lateral positions (i.e., 2, 3, 7, and 8), have been assigned toxic equivalency factors (TEFs) [1,2]. They all act through a common mechanism, initiated by binding to the aryl hydrocarbon receptor in living cells, and exhibit toxic characteristics similar to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most toxic of the dioxin congeners [1,2].
Dioxins tend to bioaccumulate in the environment and in the food chain. They have been associated with an increased risk of cancer, developmental and reproductive problems, and other adverse health effects [3–11]. As a consequence of the Stockholm Convention on Persistent Organic Pollutants, 23 countries have reported PCDD/PCDFs release inventories using harmonized methodology [12]. These reports identify open fires from different sources, including open burning of wastes, as major sources of PCDD/PCDFs worldwide. In the United States and other developed countries, because of strong regulation and control efforts, dioxin emissions have decreased in the past decades [13]. However, in most of the Third World countries, the actual level of emissions to the environment and to the food chain is largely ignored.
The reference method for the determination of PCDD/PCDFs is gas chromatography/high-resolution mass spectrometry (GC-HRMS) [14,15]. However, there are very few laboratories performing this analysis in South America (none in Uruguay). Because of the high cost and complexity of this methodology, the environmental dispersion of these compounds remains unknown. This is an important issue because in less economically developed countries, a significant percentage of the population lives in slums (at least one billion persons in the whole world) with little or no services and a marginal economy (http://www.citymayors.com/report/slums.html). In these areas many people subsist by recycling different materials from garbage and copper from stolen electric wires. The materials with little or no value, as well as the plastic cover of the electrical wires, are disposed of by burning in areas that are generally close to or even inside these slum areas. According to the national inventory of emissions of dioxin (2002–2003), uncontrolled combustion is estimated to be the greatest source of dioxin pollution in Uruguay and accounts for two-thirds of the total emissions for the country (http://www.nip.gub.uy/docu/inventario2003.pdf). As much as 5% of the total urban solid waste residues generated in Montevideo, the capital city of Uruguay, is processed by informal recyclers, and the leftovers (about one-third) are burnt in open fires at low temperature. Dioxin contamination is thus likely to occur, so affordable monitoring programs are urgently needed to assess the actual risk of this activity.
As an analytical alternative to GC-HRMS, immunoassays offer the possibility to analyze many samples simultaneously under simple experimental conditions, facilitating rapid and cost-effective results [16,17]. A highly sensitive polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for dioxins has been developed by Sugawara et al. [18]. The method uses 2,3,7-trichloro-8-methyl-dibenzo-p-dioxin (TMDD) as a surrogate standard, which has the advantage of a lower toxicity than TCDD [18]. The antibody used in the assay has strong cross-reactivity with the most toxic PCDD/PCDF congeners. It has been demonstrated that the ELISA readout can be used as an estimate of the toxic equivalency (TEQ) value for biological and environmental samples (fish, eggs, soils, and sediments) [19–22].
The aim of the present study was to evaluate the ELISA method as a screening tool to estimate the occurrence of toxic PCDD/PCDF in slum soils where there have been small-scale uncontrolled combustion of diverse materials and to perform an interlaboratory validation of the immunochemical dioxin analysis of these soils. Thus, the present study is a first approach to a low-cost sustainable monitoring program to study the possible contamination by PCDD/PCDF in slum soils. Toward this end, we analyzed a set of 16 soil samples originating from uncontrolled combustion in different slums of Monte-video, Uruguay. The samples were analyzed by GC-HRMS, and the results were compared to those obtained by ELISA on split extracts. It should be noted that the study objective represented a very challenging demand for the ELISA method since the congener profile of the samples, as well as the interfering compounds present, could be quite variable.
MATERIALS AND METHODS
Chemicals
The solvents used for extraction and cleanup (acetone, toluene, n-hexane, dichloromethane, and methanol) were of glass-distilled grade from Honeywell Burdick and Jackson (Muskegon, MI, USA). Dimethylsulfoxide (DMSO; analytical grade) was from Scharlau (Barcelona, Spain) and tetradecane (olefin free) from Fluka (Buchs, Switzerland). Silica (Kieselgel 60) and anhydrous sodium sulfate were from Merck (Darmstadt, Germany) and diatomaceous earth (Celite 545) from Fluka. The activated carbon (AX-21) originated from Anderson Development (Adrian, MI, USA) but is not currently commercially available.
Bovine serum albumin (BSA), goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase, 3,3′,5,5′-tetramethylbenzidine, and polysorbate 20 (Tween 20) used in the ELISA experiments were from Sigma (St. Louis, MO, USA). Phosphate-buffered saline (PBS) solution (pH 7.5) was prepared by dissolving 8 g NaCl, 0.2 g KH2PO4, 2 g Na2HPO4·7 H2O, and 0.2 g KCl/L deionized water, and PBS was added with 0.05% Tween 20 (PBST). The coating buffer (pH 9.6) was 1.6 g Na2CO3 and 2.9 g NaHCO3/L deionized water. The substrate solution was 400 μl of 0.6% 3,3′,5,5′-tetramethylbenzidine in DMSO and 100 μl of 1% H2O2 in 25 ml of 100 mM citrate/acetate buffer (pH 5.5). High-binding 96-well microtiter plates were from Nunc (Roskilde, Denmark). Development of the coating antigen (III-BSA) and the antibody (7598) have been described elsewhere [18,19]. The synthesis of the surrogate standard, TMDD, used in the ELISA has been previously reported [23]. The isotopically labeled recovery and internal standards used for GC-HRMS analysis were obtained from Cambridge Isotope Laboratories (Andover, MA, USA). A standard solution containing the 17 native PCDD/PCDFs congeners and polychlorinated biphenyls (PCBs) 77, 81, 126, and 169, supplied by Wellington Laboratories (ON, Canada), was used to quantify target analytes.
Samples
Sixteen samples from sites showing visual signs of small-scale combustion were collected from eight different slums in Montevideo. In all cases the sampling spots were close to the combustion trace (1–2 m). The samples were superficial (depth 0–5 cm) and were collected with a thoroughly cleaned stainless-steel scoop. Rocks, vegetation, and debris were removed. Each sample was dried overnight at 100°C, crushed and sieved through a 2-mm screen, thoroughly homogenized, and weighed before extraction.
Sample preparation
The soil samples were Soxhlet extracted for 15 h with toluene at the Department of Chemistry, Umeå University (Umeå, Sweden). The resulting extracts were split, and isotopically labeled internal standards were added to one part to allow for correction of the GC-HRMS analysis for analyte losses during cleanup. The extracts were purified in parallel in accordance with protocols described by Liljelind et al. [24]. Briefly, consecutive columns were used for cleanup. Initially, the extracts were applied to a multilayer silica column (packed with 35% KOH-silica [w/w], activated silica, 40% sulfuric acid-silica [w/w], and Na2SO4 on top) and eluted with hexane. Then a carbon column was utilized that contained AX-21/celite (1:12, w/w). Interfering compounds were eluted with dichloromethane/n-hexane (1:1, v/v). The carbon column was then inverted to elute target analytes with toluene. As a final step, a miniaturized multilayer silica column was used and eluted with n-hexane. The solvent of the spiked purified extracts containing internal standards was changed to tetradecane prior to GC-HRMS analysis. The solvent of the nonspiked purified extracts was changed to DMSO, and the resulting samples were split in two parts for ELISA analysis; one sample was sent to the University of California, Davis (UC Davis, Davis, CA, USA) and the second to the Laboratory of Environmental Quality, Intendencia Municipal de Montevideo (Montevideo, Uruguay).
GC-HRMS analysis
The instrumental analysis by GC-HRMS was done as previously described according to European Union standard methods [24,25]. The selected ion-monitoring mode and a resolution of 8,000 to 10,000 were used for the quantification of the dioxin congeners with the isotope dilution technique. The GC-HRMS results for the 17 target PCDD/PCDF congeners were converted to a single dioxin TEQ value for each sample according to the TEF classification of the World Health Organization (WHO) [2]. Congener acronyms are defined as follows: 2378-tetrachloro dibenzo-p-furan (TCDF) (1), 2378-tetra-chloro dibenzo-p-dioxin (TCDD) (2), 12378-pentachloro diben-zo-p-furan (PeCDF) (3), 23478-PeCDF (4), 12378-pentachloro dibenzo-p-dioxin (PeCDD) (5), 234678-hexachloro dibenzo-p-furan HxCDF (6), 123478-HxCDF (7), 123678-HxCDF (8), 123789-HxCDF (9), 123478-hexachloro dibenzo-p-dioxin (HxCDD) (10), 123789-HxCDD (11), 123678-HxCDD (12), 1234789-heptachloro dibenzo-p-furan (HpCDF) (13), 1234678-HpCDF (14), 1234678-heptachloro dibenzo-p-dioxin (HpCDD) (15), octachloro dibenzo-p-furan (OCDF) (16), and octachloro dibenzo-p-dioxin (OCDD) (17).
The concentration of non-ortho polychlorinated biphenyls (PCBs 77, 81, 126, and 169) were determined in order to evaluate possible interferences or contributions to the ELISA results.
Principal component analysis
The software SIMCA-P+™ version 11 (Umetrics, Umeå, Sweden) was used to evaluate relationships among the samples with regard to congeneri profiles by principal component analysis (PCA) [26]. Prior to PCA, data describing the relative abundance of non-ortho PCBs and/or PCDD/PCDF congeners in each sample were log transformed, scaled to unit variance, and mean-centered. Two principal components were calculated.
ELISA analysis
The ELISA method was performed as previously reported [19,22]. High binding microtiter plates were coated overnight at 4°C with 100 μl per well of III-BSA coating antigen (0.05 μg/ml in coating buffer). After washing with PBST, 200 μl of blocking solution (0.5% BSA in PBS) were added, and the plates were incubated for 30 min at room temperature and subsequently washed three times with PBST. An aliquot of 50 μl per well of antibody 7598 diluted 1/3,500 in PBS with 0.2% BSA and 50 μl per well of sample or standard (TMDD prepared in DMSO containing 0.01% Triton X-100-PBS 1:1, v/v) were added. The plates were incubated for 90 min and then washed five times with PBST. Goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (diluted 1:3,000 in PBST, 100 μl per well) was added, and the plates were incubated for 60 min at room temperature. After another washing step with PBST (five times), the substrate solution (100 μl per well) was added. The blue color development was stopped with 2 M sulfuric acid (50 μl per well) after 10 to 20 min, and absorbances were measured at two wavelengths: 450 and 650 nm. Standard curves were obtained by plotting absorbance (A450–A650) against the logarithm of TMDD concentration, which were fitted to a four-parameter logistic equation: y = {(A − D)/[1 + (x/C)B]} + D, where A is the maximum absorbance at no analyte, B is the curve slope at the inflection point, C is the concentration of analyte giving 50% inhibition, and D is the minimum absorbance at infinite concentration. For the immunoassay, a surrogate standard, TMDD, was used [23]. Soil samples were analyzed by ELISA with the TMDD standard curve, and the results were expressed in TCDD equivalents using the cross-reactivity factor for TMDD (130%). All sample extracts and standards were analyzed in triplicate. The accuracy was evaluated on the basis of the recoveries obtained from TMDD spiked extracts and the precision as the percent relative standard deviation (% RSD) between intra- and interplate replicate analyses. Sample extracts were analyzed using at least three different dilutions.
RESULTS AND DISCUSSION
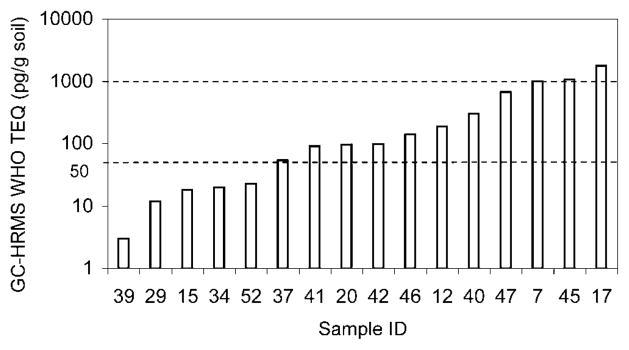
Because of the high cost of the GC-HRMS analysis, the number of samples was limited to 16. These soil samples from slum sites where uncontrolled combustion had occurred contained significant amounts of PCDD/PCDF, as determined by GC-HRMS (Fig. 1). Three of the samples (19%) showed dioxin concentrations above the guidance level of 1,000 pg TEQ/g for residential soil (according to criteria of the U.S. Environmental Protection Agency, Germany, and Japan; http://www.mfe.govt.nz/publications/hazardous/taranaki-dioxin-report-sep02/appendix-d-sep02.pdf). Actual values observed for these three samples were 1,005, 1,070, and 1,790 pg/g by GC-HRMS analysis.
Fig. 1.
Gas chromatography/high-resolution mass spectrometry (GC-HRMS) analysis of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzo-p-furans for the 16 soil samples. The results for the 17 target polychlorinated dibenzo-p-dioxin/furan congeners were converted to a single dioxin toxic equivalent value (TEQ) for each sample according to the toxic equivalency factors of the World Health Organization (WHO). The dotted line at 50 pg TEQ/g shows the upper limit of the screening level of the Agency for Toxic Substances and Disease Registry, and the line at 1,000 pg TEQ/g shows the lower limit of the Agency for Toxic Substances and Disease Registry action level [27].
The Agency for Toxic Substances and Disease Registry has established three different guidance levels for residential soil contamination with PCDD/PCDFs: screening level (TEQ value ≤50 pg TEQ/g), evaluation level (TEQ value between 51 and 999 pg TEQ/g), and action level (TEQ value ≥1,000 pg TEQ/g) [27]. If one or more soil samples exceed the screening level, further site-specific evaluations are needed, and if the concentration in residential soil exceeds 1,000 pg TEQ/g, the agency’s health assessors should consider site-specific public health recommendations/actions to prevent or interdict exposures.
Approximately 70% of the samples were above the screening level (Fig. 1), which highlights the magnitude of the problem. In Montevideo alone, a more comprehensive study would require the analysis of hundreds of samples, which would be an unaffordable task using GC-HRMS. Therefore, the question is whether ELISA may be used as a low-cost screening technique to identify burning sites that contain more than the action level of 1,000 pg TEQ/g.
ELISA/GC-HRMS validation
Congener profile and principal component analysis
The ELISA used in the present study has been shown to correlate with the TEQs as determined by GC-HRMS in sediments, soils, and biological matrices [19,22]. However, this correlation depends on the congener profile of the samples, and major sample-to-sample variations would require the use of ELISA correction factors to estimate the toxicity equivalents, limiting the usefulness of the technique [22]. Since this can be particularly critical in the case of the uncontrolled combustion of miscellaneous waste material, we initially used GC-HRMS results to study the inherent variability of the PCDD/PCDF composition in our sample panel.
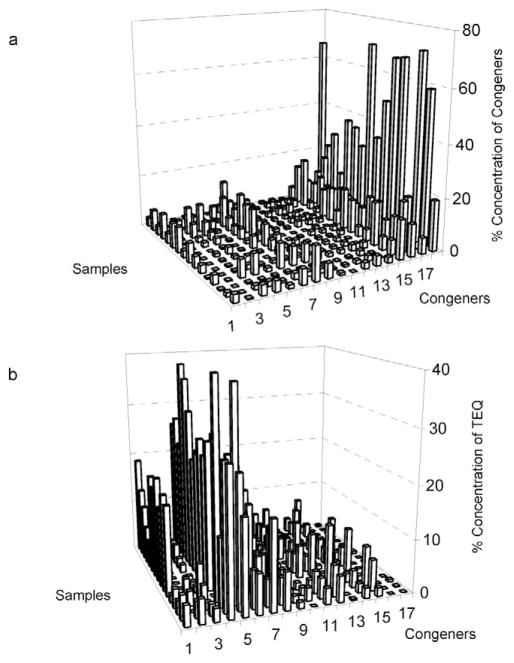
The PCDD/PCDF congener profiles of the 16 soil samples are presented in Figure 2. The relative abundance of each congener is obtained by dividing its concentration by the total concentration of the 17 congeners analyzed. In agreement with previous reports on congener profiles from diverse combustions [28], the most abundant compounds in our samples were the highest chlorinated dioxins (Fig. 2a). In terms of toxicity, tetra- and penta-CDD/Fs are the most relevant, while the more highly chlorinated compounds are less important (Fig. 2b). Figure 2 shows a similar congener profile for all samples. To reveal possible differences and groupings among them, PCA was used.
Fig. 2.
Polychlorinated dibenzo-p-dioxins and polychlorinated dibenzo-p-furans (PCDD/PCDF) congener profiles for 16 soil samples determined by gas chromatography/high-resolution mass spectrometry (GC-HRMS). The different samples are presented in the z-axis, in the following order, from back to front: 7, 12, 15, 17, 20, 29, 34, 37, 39, 40, 41, 42, 45, 46, 47, and 52. (a) The relative abundance of each congener obtained by dividing its concentration by the sum of concentrations of the 17 quantified congeners in each sample. (b) The percentage of toxic equivalent value (TEQ) contributed by each congener to the total TEQ value for each sample. Congeners: 2378-TCDF (1), 2378-TCDD (2), 12378-PeCDF (3), 23478-PeCDF (4), 12378-PeCDD (5), 234678-HxCDF (6), 123478-HxCDF (7), 123678-Hx-CDF (8), 123789-HxCDF (9), 123478-HxCDD (10), 123789-HxCDD (11), 123678-HxCDD (12), 1234789-HpCDF (13), 1234678-Hp-CDF(14), 1234678-HpCDD (15), OCDF (16), and OCDD (17). Congener acronym definitions are presented in the Materials and Methods section under GC-HRMS Analysis.
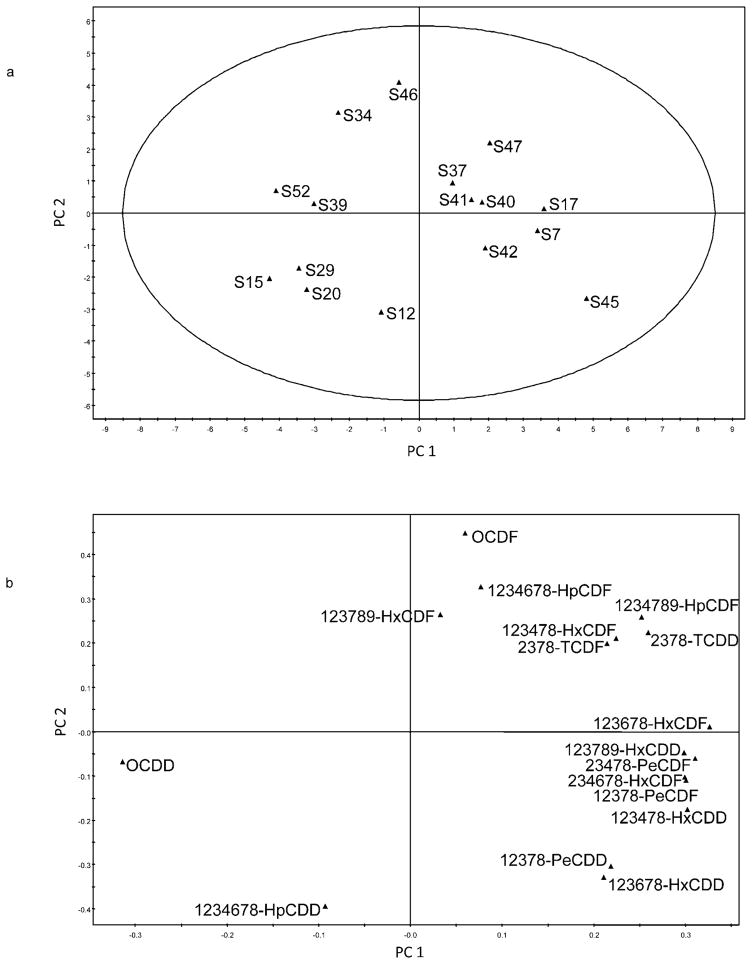
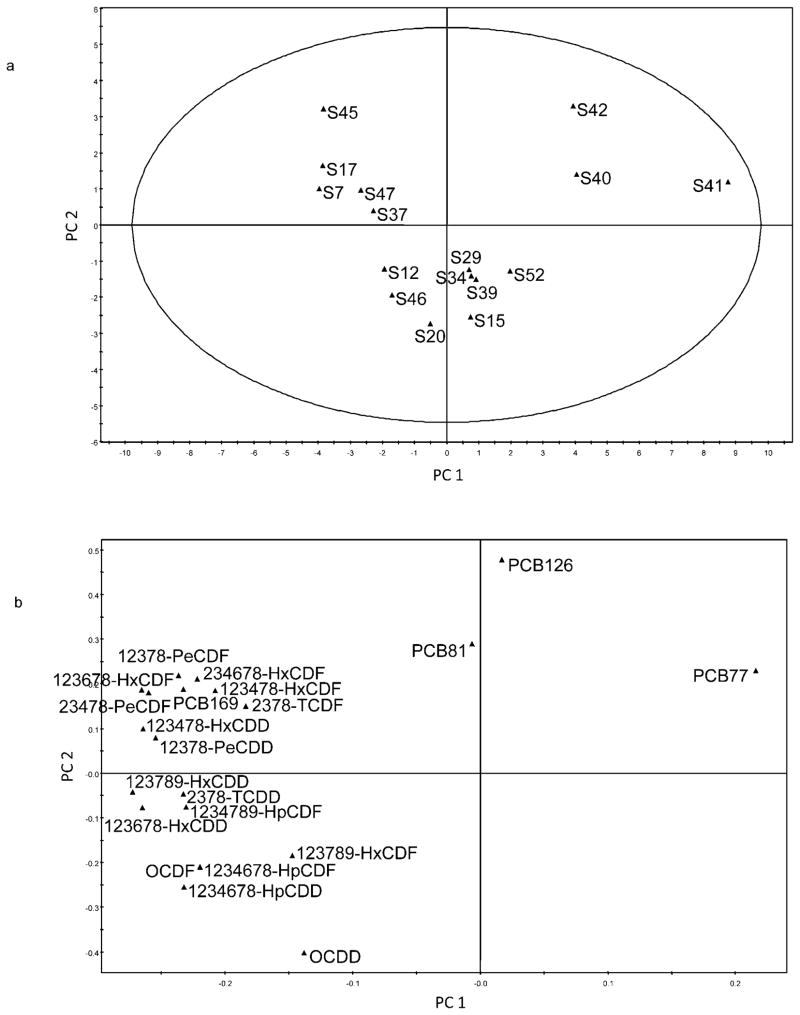
Principal component analysis was applied to study both the congeners of PCDD/PCDFs as well as those of PCDD/PCDFs, including non-ortho PCBs. The relative abundance of each congener was obtained by dividing its concentration by the total concentration of the quantified target congeners, and the first principal component (PC 1) was related to this relative abundance (Figs. 3 and 4). The first two components of the models (PC 1 and PC 2) explained 79% of the variance, both with and without the non-ortho PCBs. Principal component 1 captured slightly more than half the overall variance for PCDD/PCDFs or approximately two-thirds of the overall variance when both PCDD/PCDFFs and non-ortho PCBs are considered. The score plots in Figures 3a and 4a, illustrate how the different objects (samples) were related to each other. The positions of the variables (congeners) in the loading plots (Figs. 3b and 4b) were related to the positions of the objects that can be used to interpret groupings in the score plot.
Fig. 3.
Score plot (a) showing relations between the 16 slum soil samples (S7–S52) and loading plot (b) showing the corresponding relationships among target dioxin congeners. Congener acronym definitions are presented in the Materials and Methods section under GC-HRMS Analysis. PC = principal component.
Fig. 4.
Score plot (a) showing relations between the 16 slum soil samples (S7–S52) and loading plot (b) showing the corresponding relationships among target dioxin and polychlorinated biphenyls (PCBs) congeners. Congener acronym definitions are presented in the Materials and Methods section under GC-HRMS Analysis. PC = principal component.
The PCA model for PCDD/PCDF congeners (Fig. 3) did not show any clear groupings. Yet it is observed that samples 15, 20, 29, 34, 39, and 52 (to the left) contained mostly octachloro-dibenzo-dioxin. In samples 7, 17, 37, 40, 41, 42, 45, and 47 (to the right), the PCDD/PCDF congeners appear to be more evenly distributed. When the non-ortho PCBs were included in the model, it was evident that samples 40, 41, and 42 contained a large proportion of PCB 77 (Fig. 4).
Hence, even though the soil samples originated from different uncontrolled combustion sites, the composition of congeners observed are not expected to produce divergent ELISA responses because PCB 77 has a very low cross-reactivity and octachloro-dibenzo-dioxin does not cross-react at all with the antibody used [19]. Therefore, it is expected that the immunochemical method can be used as a screening tool for dioxin assessment of the uncontrolled combustion sites included in the study.
Correlations between ELISA and WHO-TEQs
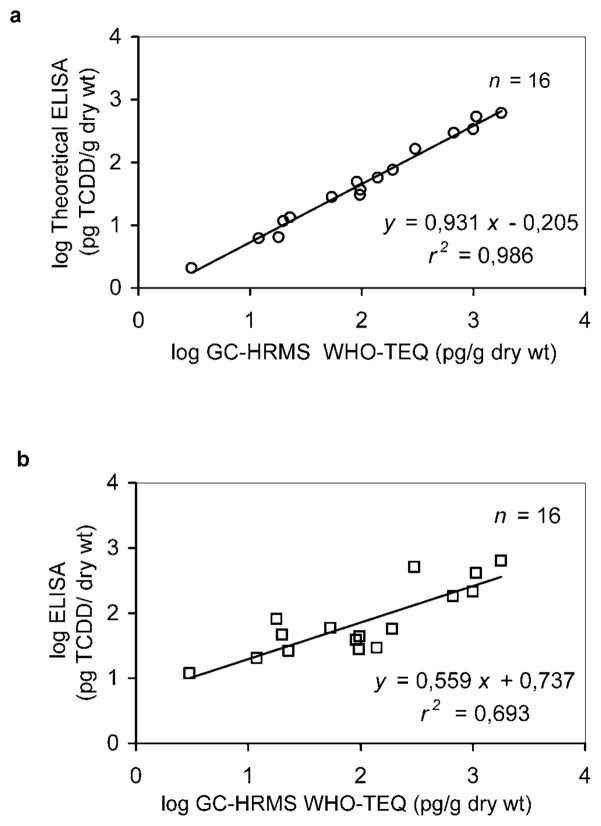
The comparison between theoretical and experimental ELISA TCDD equivalents (ELISA determination performed at UC Davis) with total GC-HRMS WHO-TEQs is shown in Figure 5. The theoretical ELISA value is computed as the sum of the concentrations of each congener (determined by GC-HRMS) multiplied by the corresponding cross-reactivity factor.
Fig. 5.
Correlation between enzyme-linked immunosorbent assay (ELISA) for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) equivalents and gas chromatography/high-resolution mass spectrometry (GC-HRMS) toxic equivalents (TEQs) for 16 slum soil samples. (a) The theoretical ELISA values (TCDD equivalents), computed as the sum of the concentration of each congener (determined by GC-HRMS) multiplied by the corresponding cross-reactivity versus GC-HRMS TEQ values. (b) Experimental ELISA (University of California, Davis, UC Davis, CA, USA)-determined TCDD equivalents versus GC-HRMS TEQ values. WHO = World Health Organization.
In agreement with the previously mentioned hypothesis generated by PCA, there is an outstanding correlation (Spearman’s R = 0.99) between the theoretical ELISA result and the corresponding WHO-TEQ values, and the linear regression curve for the log-transformed data has an excellent regression coefficient (r2) of 0.99 (Fig. 5a). This further supports the result that all samples have a similar relative concentration of the most relevant toxic congeners. The moderate tendency of the ELISA to, in theory, underestimate the GC-HRMS TEQ value is probably due to differences between the cross-reactivity and the TEF of the individual PCDD/PCDFs congeners present in the samples.
Figure 5b shows the relationship between experimental ELISA results analyzed at UC Davis and WHO-TEQ results obtained by GC/HRMS at Umeå University. Although ELISA appears to overestimate the values lower than 100 pg WHO-TEQ/g dry weight, the overall correlation is good (Spearman’s R = 0.76), and the linear regression curve for the log-transformed data has an r2 of 0.69. We can also note that the log-log scale used in Figure 5b makes it difficult to appreciate that there are two samples slightly above the action level by GC-HRMS of ≥1,000 pg TEQ/g with values of 1,005 and 1,070 pg/g and only one sample that is grossly contaminated (1,790 pg/g). The ELISA technique, by itself, correctly found this to be the most contaminated sample by a large margin. Thus, the possibility of guiding the decision-making process for prioritization of site cleanup based on ELISA analysis alone may also be worthy of consideration if future studies demonstrate such a correlation to be a consistent finding.
Definition of cutoff values for screening PCDD/PCDFs with ELISA
In order to find out whether ELISA may be used as a low-cost screening technique to identify burning sites that contain more than the action level of 1,000 pg TEQ/g, we had to define a threshold ELISA value.
With the present results, we decided to propose a preliminary cutoff level for ELISA that would be used for a starting point in a larger-scale screening phase so that only those samples above the cutoff limit would be analyzed by GC/HRMS. Because of the limited sample size of the present study, we needed to start with a very conservative boundary in order to select practically all positive samples.
As shown in Table 1, we found that by setting an ELISA cutoff of 100 pg TCDD/g soil, we can identify all samples above 1,000 pg TEQ/g soil and two samples with less than 1,000 pg TEQ/g soil (false positives). Actually, the same ELISA cutoff value could be used to detect a 250-pg TEQ/g soil (the limit for residential soils in Sweden) with zero false negatives and zero false positives (Table 1). These figures are very satisfactory; however, we cannot assume that they represent the actual rate of false positives and false negatives that would be found in a larger-scale study. In order to estimate the expected rate, we need to build a statistical model. Unfortunately, this has been an impossible task with the present results since two different statistical models (logarithmic and linear) that show similar agreement with the experimental data display discordant estimates of the false-negative rates. Therefore, the model should be refined in the next phase of the present study. In the Conclusions section, we present more details on how the next phase will proceed. We find the lack of false negatives reassuring, as we will be able to cut costs significantly by screening while still remaining protective of public health.
Table 1.
Enzyme-linked immunosorbent assay (ELISA) cutoff value for the detection of toxic equivalents (TEQs) determined by gas chromatography/high-resolution mass spectrometry (GC-HRMS). The ELISA result is expressed in 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents (TCDD)a
| Detection of samples above 1,000 pg TEQ/g soil | |||||
|---|---|---|---|---|---|
| GC-HRMS (pg TEQ/g) |
|||||
| ELISA (pg TCDD/g) | >1,000 | <1,000 | Total | ||
| >100 | (True +) | 3 | (False +) | 2 | 5 |
| <100 | (False −) | 0 | (True −) | 11 | 11 |
| Total | 3 | 13 | 16 | ||
| Detection of samples above 250 pg TEQ/g soil | |||||
| GC-HRMS (pg TEQ/g) |
|||||
| ELISA (pg TCDD/g) | >250 | <250 | Total | ||
| >100 | (True +) | 5 | (False +) | 0 | 5 |
| <100 | (False −) | 0 | (True −) | 11 | 11 |
| Total | 5 | 11 | 16 | ||
True positive: Samples higher than the defined GC-HRMS criteria that were correctly predicted by ELISA (ELISA result higher than 100 pg TCDD/g soil); true negative: Samples lower than the defined GC-HRMS criteria that were correctly predicted by ELISA (ELISA result lower than 100 pg TCDD/g soil); false negative: Samples higher than the defined GC-HRMS criteria that were incorrectly predicted by ELISA (ELISA result lower than 100 pg TCDD/g soil); false positive: Samples lower than the defined GC-HRMS criteria that were incorrectly predicted by ELISA (ELISA result higher than 100 pg TCDD/g soil).
The overall savings by using ELISA as a screening tool would be proportionally greater if the population of samples to be tested had a lower rate of PCDD/PCDF contamination and lesser if the samples had a higher rate of contamination. In the case of these 16 samples, we would have saved approximately 70% of the instrumental analysis costs since we would have selected only five samples for GC-HRMS analysis. This is very important, especially because the cost of using this noncommercial test is insignificant in comparison with the instrumental analysis. The most important cost associated with noncommercial ELISA testing is hand labor, and, provided that proper training is available, this should not be an obstacle in low-income countries.
Interlaboratory validation of the dioxin ELISA
In the course of six months, the average parameter values of 15 ELISA calibration curves performed in Uruguay were midpoint 25.3 ± 4.7 pg TCDD/ml, and the limit of detection (defined as the concentration giving 80% of the maximum response) was 10.9 ± 2.3 pg TCDD/ml. To demonstrate that we had consistent calibration curves whether the ELISA was performed in Davis [19] or in Montevideo, we performed a two-sample Student’s t test, with a null hypothesis that the midpoints for the two populations were the same. We found a p value of 0.2, so we do not reject the null hypothesis and can conclude that these values are not significantly different from each other.
The interlaboratory comparison between UC Davis and Uruguay was studied using a subset of 11 extracts in DMSO (five of the samples of the original sample panel were unfortunately lost during international transportation) that were independently analyzed in the UC Davis and Uruguay laboratories. The correlation between ELISA TCDD equivalents and WHO-TEQ equivalents by GC-HRMS measured by the Uruguayan group (Spearman’s R = 0.79) was very similar to that obtained at the UC Davis laboratory (Spearman’s R = 0.76; see Fig. 5b). Furthermore, the ELISA TCDD data obtained by both laboratories showed an excellent correlation (Spearman’s R = 0.87), showing the reproducibility of the ELISA method.
In both laboratories, the precision of the ELISA method, calculated as the % RSD between both intra- and interplate replicates, was very similar, with an intra-plate % RSD between 1 and 13% and an inter-plate RSD in the range of 3 to 35%. In addition, accuracy, as calculated on the basis of the recoveries of spiking cleaned-up extracts with TMDD, was similar in both laboratories and in the range of 72 to 140%. These values are acceptable according to the European Commission requirements for the control of dioxins by screening methods (recoveries in the range of 30–140%) [29].
These results show that the capacity to perform noncommercial dioxin ELISAs was successfully transferred to a laboratory overseas. The limit of detection as well as the accuracy and precision parameters obtained can be considered highly adequate for screening purposes [14,17].
CONCLUSIONS
To our knowledge, we are reporting the first data of PCDD/PCDFs in slum soils from small-scale uncontrolled combustions in South America. It is noteworthy that the successful application of ELISA in this case critically depends on the sample preparation procedures. Our attempts to simplify the sample cleanup procedure by omitting the carbon column have been unsuccessful; the extracts showed important matrix effects, and the results did not correlate with the corresponding GC-HRMS TEQ values (data not shown). Thus, a thorough extract cleanup method including multilayered silica and carbon is necessary to obtain a high correlation with GC-HRMS. Fortunately, this laborious procedure required is not expensive and can be manually performed in laboratories with only simple equipment available, so it does not represent an impediment for using ELISA as a screening tool in poor countries.
To follow up on the present study and to refine the ELISA threshold, we have several tasks to perform. To reduce costs, only those samples with ELISA values above 100 pg TCDD/g soil will be tested by GC-HRMS. If many samples with 100 to 120 pg TCDD/g soil test as positive for high dioxin levels by GC-HRMS, this will be evidence that we have set our threshold too high, and we would have to redefine our cutoff value. Assuming that this does not happen, we will learn about the relationship between GC-HRMS and ELISA levels near our threshold of interest. We will then have empirical rather than model-based evidence from which to select the desired trade-off between false positives and false negatives to pass from the ELISA to the GC-HRMS.
The significant percentage of samples (19%) showing PCDD/Fs above the criteria for residential soil according to the U.S. Environmental Protection Agency confirms the need for performing a larger-scale monitoring program in uncontrolled combustion slum soils in order to define critical areas and to perform a proper risk assessment.
Because of the high cost of instrumental analysis, the number of samples in the present study is not high enough to allow the determination of the rate of false positives and false negatives with precision. However, the use of an ELISA cutoff level of 100 pg TCDD equivalents/g soil made possible the detection of all samples containing more than 250 pg/g TEQ equivalents, a value considerably lower than the guidance level of the U.S. Environmental Protection Agency for residential soils, which could be used as a starting point cutoff value to prioritize samples to be tested for instrumental analysis, thus cutting costs significantly. It is possible that with a larger database, we will be able to raise this ELISA cutoff level and achieve further economies of analysis without compromising human health.
Despite the inherent complexity of the samples, the ELISA used in the present study has shown itself to be promising for screening purposes and easily transferable to laboratories in different countries. Therefore, it can be of great help to make a larger-scale screening program viable, even in countries with distressed economies.
Acknowledgments
The present study was supported by the National Institutes of Health, Fogarty International Center training grant (TW-05718; International Training Program in Environmental Immunoassay), under its International Training and Research Program in Environmental and Occupational Health Program and the Fulbright Commission (Grant ASJ 4-1362 from the Bureau of Educational Affairs of the U.S. Department of State) and the Program for the Development of Basic Sciences, United Nations, Uruguay. Work conducted at the University of California, Davis, was supported in part by the National Institute of Environmental Health Sciences Superfund Basic Research Program P42 ES04699. The work at Umeå University was carried out within the framework of the North Sweden Soil Remediation Centre and the Swedish Environmental Protection Agency Sustainable Remediation and was funded by the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning and the Swedish Association of Graduate Engineers. We acknowledge the excellent technical assistance of Jonas Nording in sample preparation.
References
- 1.Van den Berg M, Birnbaum L, Bosveld ATC, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FXR, Liem AKD, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Wærn F, Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundqvist C, Zuurbier M, Leijs M, Johansson C, Ceccatelli S, Saunders M, Schoeters G, ten Tusscher G, Koppe JG. The effects of PCBs and dioxins on child health. Acta Paediatr Suppl. 2006;95:55–64. doi: 10.1080/08035320600886257. [DOI] [PubMed] [Google Scholar]
- 4.Arisawa K, Takeda H, Mikasa H. Background exposure to PCDDs/PCDFs/PCBs and its potential health effects: A review of epidemiologic studies. J Med Invest. 2005;52:10–21. doi: 10.2152/jmi.52.10. [DOI] [PubMed] [Google Scholar]
- 5.Cole P, Trichopoulos D, Pastides H, Starr T, Mandel JS. Dioxin and cancer: A critical review. Regul Toxicol Pharmacol. 2003;38:378–388. doi: 10.1016/j.yrtph.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Greene JF, Hays S, Paustenbach D. Basis for a proposed reference dose (RfD) for dioxin of 1–10 pg/kg-day: A weight of evidence evaluation of the human and animal studies. J Toxicol Environ Health B Crit Rev. 2003;6:115–159. doi: 10.1080/10937400306470. [DOI] [PubMed] [Google Scholar]
- 7.Paustenbach DJ. The U.S. EPA science advisory board evaluation 2001 of the EPA dioxin reassessment. Regul Toxicol Pharmacol. 2002;36:211–219. doi: 10.1006/rtph.2002.1580. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer. IARC Monographs Programme on the Evaluation of Carcinogenic Risks to Humans: Polychlorinated-p-Dibenzodioxins and Polychlorinated Furans. IARC; Lyon, France: 1997. [Google Scholar]
- 9.Safe SH. Comparative toxicology and mechanism of action of polychlorinated-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- 10.Bernes C. Persistent Organic Pollutants: A Swedish View of an International Problem. Naturvårdsverket förlag (Swedish Environmental Protection Agency); Stockholm, Sweden: 1998. [Google Scholar]
- 11.Rodriguez-Pichardo A, Camacho F, Rappe C, Hansson M, Smith AG, Greig JB. Chloracne caused by ingestion of olive oil contaminated with PCDDs and PCDFs. Hum Exp Toxicol. 1991;10:311–322. doi: 10.1177/096032719101000503. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler H. National PCDD/PCDF release inventories under the Stockholm convention on persistent organic pollutants. Chemosphere. 2007;67:S96–S108. doi: 10.1016/j.chemosphere.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Environmental Protection Agency. EPA/600/P-03/002f. Final Report. Washington, DC: 2006. An inventory of sources and environmental releases of dioxin-like compounds in the United States for the years 1987, 1995, and 2000. [Google Scholar]
- 14.European Commission, Directorate General Health and Consumer Protection. Guidance document on residue analytical methods. SANCO/825/00 rev. 6. Brussels, Belgium: 2000. [Google Scholar]
- 15.U.S. Environmental Protection Agency. Method 1613. EPA 821-B-94-005. Washington, DC: 1994. Tetra- through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS. [Google Scholar]
- 16.Harrison RO, Carlson RE. An immunoassay for TEQ screening of dioxin/furan samples: Current status of assay and applications development. Chemosphere. 1997;34:915–928. doi: 10.1016/s0045-6535(97)00395-0. [DOI] [PubMed] [Google Scholar]
- 17.Billets S. CAPE Technologies LLC DF1 Dioxin/Furan Immunoassay Kit, PCB TEQ Immunoassay Kit. EPA/540/R-05/004. U.S. Environmental Protection Agency; Las Vegas, NV: 2005. Innovative technology verification report: Technologies for monitoring and measurement of dioxin and dioxin-like compounds in soil and sediment. [Google Scholar]
- 18.Sugawara Y, Gee SJ, Sanborn JR, Gilman SD, Hammock BD. Development of a highly sensitive enzyme-linked immunosorbent assay based on polyclonal antibodies for the detection of polychlorinated dibenzo-p-dioxins. Anal Chem. 1998;70:1092–1099. doi: 10.1021/ac9708203. [DOI] [PubMed] [Google Scholar]
- 19.Shan G, Leeman WR, Gee SJ, Sanborn JR, Jones AD, Chang DPY, Hammock BD. Highly sensitive dioxin immunoassay and its application to soil and biota samples. Analytica Chimica Acta. 2001;444:169–178. [Google Scholar]
- 20.Sugawara Y, Saito K, Ogawa M, Kobayashi S, Shan G, Sanborn JR, Hammock BD, Nakazawa H, Matsuki Y. Development of dioxin toxicity evaluation method in human milk by enzyme-linked immunosorbent assay—Assay validation for human milk. Chemosphere. 2002;46:1471–1476. doi: 10.1016/s0045-6535(01)00267-3. [DOI] [PubMed] [Google Scholar]
- 21.Nichkova M, Park EK, Koivunen ME, Kamita SG, Gee SJ, Chuang J, Van Emon JM, Hammock BD. Immunochemical determination of dioxins in sediment and serum samples. Talanta. 2004;63:1213–1223. doi: 10.1016/j.talanta.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Nording M, Nichkova M, Spinnel E, Persson Y, Gee SJ, Hammock BD, Haglund P. Rapid screening of dioxin-contaminated soil by accelerated solvent extraction/purification followed by immunochemical detection. Anal Bioanal Chem. 2006;385:357–366. doi: 10.1007/s00216-006-0402-9. [DOI] [PubMed] [Google Scholar]
- 23.Sanborn JR, Gee SJ, Gilman SD, Sugawara Y, Jones AD, Rogers J, Szurdoki F, Stanker LH, Stoutamire DW, Hammock BD. Hapten synthesis and antibody development for polychlorinated dibenzo-p-dioxin immunoassays. J Agric Food Chem. 1998;46:2407–2416. [Google Scholar]
- 24.Liljelind P, Söderström G, Hedman B, Karlsson S, Lundin L, Marklund S. Method for multiresidue determination of halogenated aromatics and PAHs in combustion-related samples. Environ Sci Technol. 2003;37:3680–3686. doi: 10.1021/es0263994. [DOI] [PubMed] [Google Scholar]
- 25.European Committee for Standardization. EN 1948. Brussels, Belgium: 1997. Stationary source emissions—Determination of the mass concentration of PCDDs/PCDFs; pp. 2–3. [Google Scholar]
- 26.Wold S, Esbensen K, Geladi P. Principal component analysis. Chemometrics and Intelligent Laboratory Systems. 1987;2:37–52. [Google Scholar]
- 27.DeRosa CT, Brown D, Dhara R, Garrett W, Hansen H, Holler J, Jones D, Jordan-Izaguirre D, O’Connor R, Pohl H. Dioxin and dioxin-like compounds in soil. Part I. ATSDR interim policy guideline. Toxicol Indust Health. 1999;15:552–557. doi: 10.1177/074823379901500603. [DOI] [PubMed] [Google Scholar]
- 28.Cleverly D, Schaum J, Schweer G, Becker J, Winters D. The congener profiles of anthropogenic sources of chlorinated dibenzo-p-dioxins and chlorinated dibenzofurans in the United States. Organohalogen Compounds. 1997;32:430–435. [Google Scholar]
- 29.European Commission. Commission Directive 2002/69/EC laying down the sampling methods and the methods of analysis for the official control of dioxins and the determination of dioxin-like PCBs in foodstuffs. Official Journal of the European Communities L. 2002;209:5–14. [Google Scholar]