Abstract
Although adolescent ethanol (EtOH) exposure has been associated with long-lasting changes in brain function, little is known as to whether EtOH exposure during adolescence alters sleep and cortical arousal. This study examined protracted alterations in sleep in adult rats exposed to EtOH during adolescence. Adolescent male Wistar rats were exposed to EtOH vapor for 12 hr/day for five weeks. Cortical electroencephalograms (EEGs) were obtained during 4-hr recording sessions after five weeks of withdrawal from EtOH. Adolescent EtOH exposure significantly reduced the mean duration of slow-wave sleep (SWS) episodes and the total amount of time spent in SWS in EtOH-exposed rats, compared to controls. Spectral analysis revealed that adolescent EtOH exposure significantly increased cortical peak frequencies during SWS in the 2-4 Hz, 4-6 Hz and 6-8 Hz bands. Taken together, our findings suggest that chronic EtOH exposure in adolescent rats reduces measures of SWS, an effect also seen as part of normal aging. Although the cellular and molecular mechanisms mediating the consequences of EtOH exposure on the aging process are not known, the similarities between adolescent EtOH exposure and aging merits further investigation.
Keywords: Adolescent, Cortex, EEG, Ethanol, Sleep, Slow-wave sleep
Introduction
Disturbances in sleep architecture are symptoms commonly observed in alcohol-dependent individuals. Understanding the mechanisms mediating the effects of ethanol (EtOH) on sleep patterns and cortical arousal is a research topic of considerable importance, in view of the evidence supporting a relationship between sleep disturbances and relapse to alcohol drinking (Brower et al., 1998; Clark et al., 1998, 1999; Drummond et al., 1998; Foster & Peters, 1999; Gillen et al., 1994). Deficits in sleep architecture in adult alcoholics during EtOH withdrawal include increased latency to sleep onset, increased rapid eye movement (REM) sleep and reduced slow-wave sleep (SWS) activity (Gillen et al., 1994; Williams & Rundell Jr., 1981). Studies in animal models have also shown that chronic EtOH exposure produces alterations in sleep patterns and on cortical EEG power (Ehlers & Slawecki, 2000; Kubota et al., 2002). Results from our previous studies have demonstrated that chronic EtOH exposure in rats results in significantly reduced spectral power in the δ (2-4 Hz), θ (4-8 Hz), and β (16-32 Hz) frequencies (Ehlers & Slawecki, 2000). These effects were also found to persist after five weeks of EtOH withdrawal (Ehlers & Slawecki, 2000). Chronic EtOH exposure has been also shown to reduce the circadian variation of REM sleep and increase non-REM sleep in rats (Kubota et al., 2002). These findings provide the basis for the development of future studies characterizing the neural and cellular mechanisms mediating changes in sleep patterns following chronic EtOH exposure.
Despite these findings, many questions remain unanswered regarding the consequences of chronic EtOH on sleep and cortical arousal. As a result of the increasing reports of adolescent EtOH consumption, one question that needs to be addressed is whether adolescent EtOH exposure also produces alterations in sleep. Alcohol is one of the most abused drugs in adolescents (U.S. Dept. Health and Human Services, 1999) and it has been shown to have detrimental effects on brain development (Crews et al., 2007). Neurodevelopmental changes during adolescence may confer increased vulnerability to insult by drug use and/or abuse (reviewed by, Barron et al., 2005; Crews et al., 2007). Consistent with those findings, early onset of drinking has been associated with the development of alcohol-related problems during adulthood (Ehlers et al., 2006; Grant et al., 2001; Hawkins et al, 1997).
There is evidence to suggest that EtOH produces age-related effects on behavioral and physiological responses that could influence and/or interact with mechanisms regulating sleep and arousal. For instance, studies have shown that adolescent rats are less sensitive than adult rats to acute EtOH-induced motor incoordination, sedation, and hypothermia (Little et al., 1996, Pian et al., 2008; Silveri & Spear, 1998; Varlinskaya & Spear, 2002). Adolescent rats also show greater tolerance than adult rats to the acute effects of high doses of EtOH on sleep times, the righting reflex, and the expression of anxiogenic signs during acute EtOH withdrawal (Doremus et al., 2003; Silveri & Spear, 1998). These age-related differences may lead to higher consumption and tolerance to EtOH than adults and may enhance the risk for alcohol dependence in adolescents (Ehlers et al., 2006; Spear, 2000; Witt, 1994).
The electroencephalogram (EEG) and event-related potentials (ERPs) have been used to study neurophysiologic brain activity associated with age-related changes during development and with neuroelectric endophenotypes associated with the risk of alcoholism (Dustman et al., 1996; Porjesz & Begleiter, 1991; Porjesz et al., 2005). Electrophysiological studies have shown that repeated exposure to EtOH during adolescence produce long-lasting changes in cortical and hippocampal EEG and ERP activity of adult rats (Slawecki, 2002; Slawecki et al., 2001, 2006). EtOH exposure during adolescence is also associated with an increase in the mean frequency of the EEG in the 1-2 Hz range, in the parietal cortex of adult rats. Moreover, we have recently shown that, following administration of acute intoxicating doses of EtOH, adolescent rats exhibited shorter sleeping time and higher blood EtOH levels after regaining reflex than adult rats (Pian et al., 2008). Our findings also suggested that acute EtOH significantly increased the parietal EEG power of slow-wave frequency bands in adult, but not adolescent rats. These data suggest age-related differences in the effects of EtOH on cortical EEG and behavior. However, whether chronic EtOH exposure during adolescence produces long-lasting effects on sleep is not known. The present study assessed whether changes in the frequency content of the sleep EEG is altered in adult rats following adolescent EtOH exposure. In these experiments, rats were exposed to EtOH vapor for five weeks during adolescence. The initial onset and duration of slow-wave sleep (SWS) episodes and EEG spectral analyses were evaluated in adult rats five weeks after EtOH withdrawal to determine whether adolescent EtOH exposure had long-lasting effects on cortical sleep EEG.
Materials and methods
Subjects
Male Wistar rats (n= 40) were used in these experiments (Charles River, Wilmington, MA). Rats were housed three per cage in standard plastic cages [25 cm (w) × 20 cm (h) × 45 cm (l)] and maintained under a 12 h light-dark cycle (lights on at 9 AM) with ad libitum food/water. The Scripps Research institutional IACUC committee approved the animal use protocol. Animal care was in accordance with NIH guidelines.
EtOH vapor exposure
The EtOH vapor inhalation procedure and the chambers used in this study were previously described (Rogers et al., 1979, Slawecki, 2002, Slawecki et al., 2001). In brief, rats were divided into two groups (EtOH-exposed, n = 22; control, n = 18). EtOH-exposed rats were housed in sealed chambers, which were infused with vaporized 95% EtOH for 12 h/day during the dark cycle (from 9 pm to 9 am). For the remaining 12 h of the day, EtOH vapor was not infused into the chamber. This EtOH exposure regimen continued for five weeks. At the start of the EtOH exposure, adolescent rats were 24 days old and continued until rats were 60 days old. Age-matched controls were handled identically to EtOH-exposed rats. Food and water were always available. Blood samples were collected from the tip of the tail once per week to assess blood EtOH assessment (target: 175-225 mg/dl). BALs were determined using the Analox micro-statGM7 (Analox Instr. Ltd, Lunenberg, MA). When exposure ended, all rats were maintained in the Scripps vivarium and maintained under a 12 h light-dark cycle (lights on at 12 PM) for the rest of the study. After two weeks, recording electrodes were implanted.
Surgical procedure
Rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally). Atropine (24 μg, subcutaneously) coadministration minimized respiratory suppression. Screw electrodes were placed in the skull overlying the frontal cortex (AP: + 1.5 mm, ML: ± 3.0 mm) and parietal cortex (AP: −4.5 mm, ML: ± 4.5 mm) (Paxinos & Watson, 1986). A midline screw electrode was placed posterior to lambda in the skull overlying the cerebellum. The tooth bar was set at −3.3 mm. Electrode connections were made to an Amphenol 5-pin connector, and the assembly was anchored to the skull with dental acrylic and anchor screws. A two-week recovery period was provided before the beginning of electrophysiological studies.
Electrophysiological recording procedures
EEG was collected from freely moving rats, in a sound-attenuated and electrically grounded BRS/LVE recording chamber [(90 cm (w) × 90 cm (h) × 85 cm (d)], equipped with a single house light and an exhaust fan. The light was turned on during the recording session. All rats were habituated to the testing apparatus before the first test day. Rats were habituated two days preceding the recording session. Rats were placed in the recording chamber and attached to the recording cable each day for 15 minutes. The day of the recording session, a 5-10 min habituation period was followed by the EEG recording session that lasted 4 h (11 AM - 3 PM). The EEG was recorded from two monopolar leads referenced to cerebellum ground (i.e., frontal cortex and parietal cortex) on a Sensorium preamplifier/amplifier unit (Shelburne, VT). Raw EEG signals were amplified (50% gain), band-pass filtered (0.53 - 70 Hz), digitized at a rate of 256 Hz and then transferred to an IBM compatible PC.
A Fourier transform of 4 s epochs was used to generate the power spectrum. Mean power density was quantified in μV2/octave and peak frequency was calculated in Hz. Individual spectra were averaged and compressed into seven frequency bands: 1-2 and 2-4 Hz frequency bands to assess slow waves, 4-6 and 6-8 Hz to assess θ activity, 8-16 Hz to assess high frequency spindling, 16-32 Hz to assess β activity, and a 32-50 Hz frequency band to assess γ activity. Frequencies greater than 50 Hz were not assessed to minimize potential interference from 60 Hz AC line current. EEG spectra were identified as containing artifact when average cortical power was > 2000 μV2/octave. Artifact epochs were excluded only after visual analysis of the raw EEG and spectral distributions.
Mean spectral power and peak frequency within each band were calculated from the entire 4 hr recording session and from the recording time spent in SWS. Mean spectral power was defined as the measure of the amplitude of the EEG; and, peak frequency was defined as the measure of the predominant frequency of the EEG. These analysis procedures have been described previously (Ehlers & Havstad, 1982).
Sleep analysis
SWS was visually identified as synchronized slow-wave activity with voltage higher than 50 μV during the 4-h EEG recording session. Increases in the EEG power of at least twice the amplitude of the baseline EEG power were counted as episodes of SWS. Sleep patterns were analyzed by: (1) calculating the total amount of time spent in SWS in the frontal cortex; and, (2) determining the duration and onset latency of the first episode of SWS in the frontal cortex. The duration of each individual SWS episode was added to determine total sleep time. The onset latency of the first SWS episode lasting at least 8 s was determined from the raw EEG. The onset of the first SWS episode in the EEG was identified as the first transition from low-amplitude high-frequency EEG to SWS (high-amplitude low-frequency EEG).
Statistical analysis
Statistical analyses were performed by using SPSS for the Macintosh (SPSS, Inc., Chicago, IL). Brain regions were assessed independently. EEG data analyses consisted of mean spectral power and peak frequency for each frequency band. The effects of treatment (EtOH-exposed vs. control) on body weight, total sleep time, sleep episodes, the onset and duration of the first sleep episode and EEG spectra were assessed using a one-way analysis of variance (ANOVA) with a group as a between subject factor (P < 0.05 for significance). To determine whether the effects of adolescent EtOH exposure were consistent across the 4-hr recording session, EEG spectra were reduced into 1-hr time blocks, and a two-way ANOVA with repeated measures (group × interval) was used, when appropriate. For all repeated measures analyses, Greenhouse-Geisser corrected P-values are reported (P < 0.05 for significance). When appropriate, post hoc assessment of adolescent EtOH exposure effects for each interval consisted of one-way ANOVA (vs. control group; P < 0.05 for significance).
Results
At the time of SWS assessment, one rat from the EtOH-exposed group had to be removed from the study because it lost the Amphenol connector that was anchored to the skull. In addition, due to initial problems with the software, four rats from the EtOH-exposed group and four rats from the control group were not included in the spectral analysis.
Body weight and blood EtOH assessment
Blood alcohol levels during the exposure period averaged 194 ± 6 mg/dl. At the start of treatment, body weight averaged 54 ± 1 g in EtOH-exposed rats and 53 ± 1 g in controls [F(1,38) = 0.8, P > 0.05]. At the end of treatment, body weight was lower in EtOH-exposed rats (296 ± 5 g; n = 22) relative to controls (317 ± 4 g; n = 18) [F(1,38) = 11.3, P < 0.005]. At the end of the sleep study, no weight differences were found between EtOH-exposed rats (464 ± 7 g; n = 21) and controls (478 ± 8 g; n = 18) [F(1,38) = 1.7, P > 0.05].
Effects of adolescent EtOH exposure on SWS
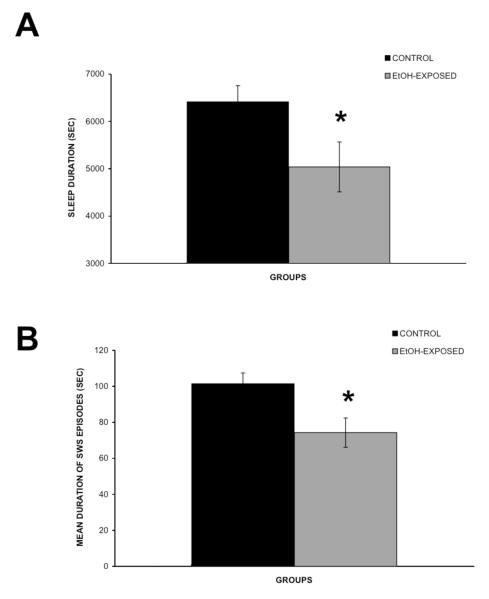
There were no significant differences in duration or time to onset of the first SWS episode or in the total number of sleep episodes between the control and the EtOH-exposed group. However, the mean duration of SWS episodes [F(1,38) = 6.7, P < 0.05] and total SWS duration [F(1,30) = 4.4, P < 0.05] were significantly reduced in EtOH-exposed rats, compared to controls (Figure 1A-B).
Figure 1.
Estimated sleep duration of the 4 h recording session (A) and mean durationof SWS episodes (B) in the control (n = 14) and EtOH-exposed (n = 17) groups. Error bars represent the SEM. * Represent statistically significant difference between treatment groups, P < 0.05.
Effects of adolescent EtOH exposure on EEG spectral analysis: Total recording session
EEG spectral analysis of the entire 4-h recording session showed that EtOH exposure during adolescence had no effect on EEG power in the frontal cortex [F’s (1,30) < 2.0, P’s > 0.05] and parietal cortex [F’s (1,30) < 2.4, P’s > 0.05]. Whereas, adolescent EtOH exposure increased peak frontal EEG frequency in the 4-6 Hz band [Control: 4.78 ± 0.01 Hz; n = 14; EtOH-exposed: 4.81 ± 0.01 Hz; n = 17; F(1,30) =9.9, P < 0.05], it had no effect on other frequency bands in the frontal cortical EEG [F’s (1,30) < 2.5, P’s > 0.05] (Data not shown). Adolescent EtOH exposure produced no significant changes on parietal EEG frequency [F’s (1,30) < 3.8, P’s > 0.05] (Data not shown).
Effects of adolescent EtOH exposure on EEG spectral analysis: SWS
Adolescent EtOH exposure had no effect on EEG power in the frontal cortex [F’s (1,30) < 2.0, P’s > 0.05] and parietal cortex [F’s (1,30) < 1.8, P’s > 0.05] (Data not shown). In contrast, EtOH exposure during adolescence produced significant shifts in cortical EEG frequency during SWS. Adolescent EtOH exposure significantly increased EEG frequency in the 2-4 Hz, 4-6 Hz and 6-8 Hz bands in the frontal and parietal cortices (Table 1). To determine whether this increase in EEG frequency following adolescent EtOH exposure was consistent across the 4-hr recording session, EEG spectra were reduced into 1-hr time blocks, and a two-way ANOVA with repeated measures was used for the analyses. Two-way ANOVA revealed significant group × interval interactions on parietal EEG frequency in the 4-6 Hz band [F(1.6,56.6) = 4.9, P < 0.05 ]. Post hoc assessment showed that adolescent EtOH exposure increased EEG frequency in the fourth 1-hr time block [EtOH-exposed rats (4.69 ± 0.01 Hz; n = 20) and controls (4.65 ± 0.01 Hz; n = 17)]. There was also a trend toward an increase in the third 1-hr time block, but this was not significant (Data not shown). Statistical analyses of the EEG sleep spectral data showed that the effects of adolescent EtOH exposure on the parietal EEG frequency were not different from controls when reduced into 1-hr time blocks in the 2-4 Hz [F(2,70) = 0.9, P > 0.05] and 6-8 Hz [F(1.6,56.9) = 0.1, P > 0.05] and in the frontal EEG frequency in the 2-4 Hz [F(1.2,42.5) = 0.2, P > 0.05], 4-6 Hz [F(1.4, 47.4) = 2.1, P > 0.05] and 6-8 Hz bands [F(1.8,63.3) = 0.1, P > 0.05] (Data not shown).
Table 1.
Effects of Adolescent EtOH Exposure on Mean Peak Frequency during SWS
| Control | Ethanol-Exposed | Treatment effects | |
|---|---|---|---|
| Frontal Cortex | |||
| 1-2 Hz | 1.37 ± 0.004 | 1.37 ± 0.006 | F (1,30) = 0.0, p > 0.05 |
| 2-4 Hz | 2.63 ± 0.009 | 2.67 ± 0.014 | F (1,30) = 5.2, p < 0.05* |
| 4-6 Hz | 4.70 ± 0.009 | 4.73 ± 0.008 | F (1,30) = 7.7, p < 0.05* |
| 6-8 Hz | 6.72 ± 0.010 | 6.76 ± 0.010 | F (1,30) = 4.9, p < 0.05* |
| 8-16 Hz | 9.68 ± 0.056 | 9.65 ± 0.055 | F (1,30) = 0.2, p > 0.05 |
| 16-32 Hz | 17.53 ± 0.034 | 17.56 ± 0.035 | F (1,30) = 0.4, p > 0.05 |
| 32-50 Hz | 35.73 ± 0.052 | 35.85 ± 0.105 | F (1,30) = 0.9, p > 0.05 |
| Parietal Cortex | |||
| 1-2 Hz | 1.38 ± 0.005 | 1.38 ± 0.004 | F (1,30) = 0.0, p > 0.05 |
| 2-4 Hz | 2.54 ± 0.010 | 2.59 ± 0.015 | F (1,30) = 5.1, p < 0.05* |
| 4-6 Hz | 4.65 ± 0.013 | 4.68 ± 0.007 | F (1,30) = 6.0, p < 0.05* |
| 6-8 Hz | 6.72 ± 0.008 | 6.75 ± 0.009 | F (1,30) = 4.9, p < 0.05* |
| 8-16 Hz | 9.58 ± 0.042 | 9.55 ± 0.039 | F (1,30) = 0.2, p > 0.05 |
| 16-32 Hz | 17.67 ± 0.026 | 17.69 ± 0.024 | F (1,30) = 0.4, p > 0.05 |
| 32-50 Hz | 35.63 ± 0.042 | 35.74 ± 0.055 | F (1,30) = 2.4, p > 0.05 |
Values represent mean ± SEM.
Represent statistically significant difference between treatment groups, p < 0.05. EtOH-exposed (n = 17) and control (n = 14) rats
Discussion
The present investigation extends our previous studies by determining whether repeated exposure to EtOH during adolescence produces long-lasting changes in SWS and in the spectral power and frequency of the cortical EEG. We previously demonstrated that adult rats exposed to chronic EtOH exhibited a reduction in the spectral power in the δ and θ frequencies five weeks after EtOH withdrawal (Ehlers & Slawecki, 2000). Consistent with our previous observations, the present study found that adolescent EtOH exposure produced alterations in cortical sleep EEG activity. Adult EtOH exposure produced no effect on the total time spent in SWS or on cortical EEG frequency after EtOH withdrawal (Ehlers & Slawecki, 2000), whereas, adolescent EtOH exposure significantly reduced the duration of SWS episodes and the total amount of time spent in SWS in adult rats. Adolescent EtOH exposure also increased cortical EEG frequency during SWS in both frontal and parietal cortices, while having no effect on cortical EEG power. These data suggest no differences in the sensitivity of these cortical regions to the long-term consequences of adolescent EtOH exposure. The mechanisms mediating the long-term consequences of adolescent EtOH exposure on cortical EEG peak frequency during SWS are not well understood. However, taking in consideration our initial results characterizing the effects of chronic EtOH on sleep in adult rats (Ehlers & Slawecki, 2000), our findings suggest that the effects of chronic EtOH exposure on SWS are dependent upon the developmental stage when the chronic EtOH was administered.
The importance of studying the consequences of adolescent EtOH exposure on mean EEG spectral power is based on the evidence that EEG power is considered a measure of cortical information processing (reviewed by Klimesch, 1999) and a neuroelectric endophenotype associated with the risk of alcoholism (Porjesz & Begleiter, 1991; Porjesz et al., 2005). The EEG, which is produced by postsynaptic potentials, consists of complex rhythms and oscillations generated by different brain regions (Steriade, 2005). SWS is characterized by high-amplitude (> 50μV) synchronized slow-wave activity and by spindle oscillations (Jones, 2005). While slow-wave activity predominates in SWS, multiple frequency bands have been shown to emerge during SWS including δ (1-4 Hz), θ (4-8 Hz), α (8-16 Hz), β (16-32 Hz) and γ (32-50). It has been suggested that EEG oscillations or rhythms are basic forms of information transmission in the brain (Klimesch, 1996). Despite considerable amount of progress, the functional role of brain oscillations in the sleep-wake cycle remains unclear (Steriade, 2003).
The present study assessed the mean spectral power within each EEG frequency band during SWS. This study also assessed the peak frequency for each EEG band during SWS. Assessing the mean peak frequency allowed us to determine whether adolescent EtOH exposure produced shifts in the predominant frequency for each of the EEG bands studied. The present findings demonstrated that adolescent EtOH exposure significantly increased cortical peak frequencies in the θ band. Studies characterizing the functional role of θ oscillations during sustained wakefulness have suggested that brain oscillations in the θ band are associated with the ability to encode new information (Klimesch, 1999). However, whether the long-term effects of adolescent EtOH exposure on cortical peak frequencies in the θ band during SWS are associated with alterations in cognitive processes remains to be determined.
Findings from our earlier electrophysiological studies suggest that adolescent EtOH exposure produce changes in adult cortical and hippocampal event-related potential (ERPs) associated with learning and memory function. Specifically, adolescent EtOH exposure decreased hippocampal P3 amplitude and decreased cortical and hippocampal P2 amplitudes (Slawecki, et al., 2001). Since decreases in the amplitude of P3 are associated with impaired cognitive processing (reviewed by Polich & Criado, 2006), the decrease in P3 amplitude observed after adolescent EtOH exposure may partially contribute to deficits in hippocampal-dependent learning and memory observed after chronic EtOH exposure (Arendt et al., 1989; Beracochea et al., 1992). Moreover, we also showed a reduction in P2 amplitude when reinforcement contingencies associated with a conditioned stimulus is extinguished (Ehlers et al., 1998; Slawecki, et al., 2000a). Taken together, the long-term effects of adolescent EtOH exposure on P2 and P3 amplitudes may be considered neurophysiological indices of EtOH-induced learning and memory deficits.
The relationship between the effects of adolescent EtOH on SWS and these cognitive ERPs is presently unclear. Further research is also needed to determine whether the deficits in SWS duration and the increase in cortical peak frequencies in the δ and θ bands observed in the present study play a role on the long-term behavioral consequences of adolescent EtOH exposure. For instance, we previously reported behavioral data suggesting that adolescent EtOH exposure increased prepulse inhibition in adult rats, which is a neurobehavioral index of sensorimotor gating, and produced a more pronounced EtOH withdrawal-associated hypoactivity (Slawecki & Ehlers, 2005; Slawecki & Roth, 2004). The relationship among these neurophysiological and behavioral consequences of adolescent EtOH exposure is still not well understood.
The present study found that adolescent EtOH exposure produced a significant increase in cortical peak frequencies in the 2-4 Hz, 4-6 Hz and 6-8 Hz bands during SWS in adult rats. While these statistically significant differences between groups appeared to be small, previous studies have consistently shown that changes in these tightly regulated peak EEG frequencies are functionally significant. There is considerable evidence to suggest that variations in cortical peak frequencies correlate with cognitive performance and have been associated with attentional demands and arousal (Angelakis et al., 2004, 2007; Klimesch et al., 1990). We have also demonstrated that acute EtOH consumption is associated with a reduction in the frontal peak frequency in the θ and β bands in humans (Ehlers, et al., 1989). Moreover, differences in cortical peak frequencies have been shown in rats and mice models of high EtOH consumption compared to their low drinking counterparts (e.g., Katner, et al., 2002; Morzorati, et. al., 1994; Robledo, et al., 1994; Slawecki et. al., 2000b, 2001, 2003). Further research is needed to determine the functional significance of the increase in cortical peak frequencies in the δ and θ bands observed in the present study.
Evidence from human studies has provided insight into the consequences of chronic EtOH exposure on sleep. Earlier studies have shown that sleep-related deficits in alcohol-dependent patients could last for up to almost two years after cessation of EtOH consumption (e.g., Williams & Rundell Jr., 1981). Consistent with the present findings, a previous study found an increase in the EEG peak frequencies in alcohol-dependent individuals (Irwin et al., 2000). Moreover, we have previously shown greater slow-wave frequencies as a normal function of aging (Ehlers & Kupfer, 1989). The present results together with those earlier findings indicate that the aging process may be accelerated as a consequence of adolescent chronic EtOH exposure. The similarities between adolescent EtOH exposure and aging merits further investigation.
We have previously shown that the effects of chronic EtOH on cortical spectral EEG power were attenuated five weeks after EtOH withdrawal, compared to its actions immediately after termination of treatment (Ehlers & Slawecki, 2000). The present study only assessed the animal’s SWS and cortical EEG activity five weeks after EtOH withdrawal. We previously demonstrated that neurophysiological changes following chronic EtOH exposure are transient and subtle (Ehlers & Chaplin, 1991; Slawecki et al., 1999). In fact, in those studies the chronic effects of EtOH on EEG normalized in only two weeks following four weeks of continuous EtOH exposure (Ehlers & Chaplin, 1991). Whether the increase in cortical peak frequencies during SWS in the δ and θ bands and the reduction in the total amount spent in SWS observed in the present study were triggered during adolescent EtOH exposure or during the prolonged withdrawal period remains unknown.
There is evidence to suggest that the neurophysiological consequences of adolescent EtOH exposure on adult SWS could have been triggered during the EtOH withdrawal period. In fact, consistent with the present findings, we previously demonstrated that intracerebroventricular administration of the neuropeptide corticotrophin-releasing factor (CRF) significantly reduced the amount of time spent in SWS and increased EEG and behavioral signs of arousal (Ehlers et al., 1986, 1997). CRF, a potent anxiogenic agent, appears to play an important role in the regulation of the stress response and the negative affective state associated with EtOH withdrawal (reviewed by Valdez & Koob, 2004). While further studies are needed to determine the role of CRF on the long-term effects of adolescent EtOH exposure on adult SWS, recent studies have provided support for a direct role of CRF-containing neurons on circuits regulating sleep-wake states (Winsky-Sommerer, et al., 2004). Therefore, we speculate that the increase in cortical peak frequencies in the δ and θ frequency ranges and reduction in the amount of time spent in SWS could be triggered during the EtOH withdrawal period and mediated by the increase in CRF activity.
There are two important factors to take in consideration when interpreting the present findings. First, as recently discussed by Veatch (2006), several brain regions and networks implicated in the regulation of sleep are also sensitive to the effects of EtOH. For instance, neurons from the basal forebrain, the preoptic area, the brainstem and the diffuse thalamocortical projection relay have been shown to play a role in the regulation of sleep-wake states (Jones, 2005; Pace-Schott & Hobson, 2002) and are also sensitive to the effects of EtOH (Fadda & Rossetti, 1998). Secondly, neurotransmitter systems known to be sensitive to EtOH exposure such as glutamate, norepinephrine, dopamine, serotonin, GABA and acetylcholine (Fadda & Rossetti, 1998) have been shown to play a role in the generation and maintenance of sleep-wake states (Jones, 2005; Siegel, 2004).
However, despite the multiple networks and neurotransmitter systems that could partially account for the present findings, evidence from studies in adolescent rats suggest an important role for glutamate transmission on the lasting compensatory effects of adolescent EtOH exposure on SWS. In fact, studies characterizing the cellular and molecular mechanisms mediating the enhanced vulnerability to EtOH exposure during adolescence have focused on studying glutamatergic neurotransmission (Crews et al, 2002; Fadda & Rossetti, 1998), in particular, the N-methyl-D-aspartate (NMDA) type of glutamate receptor. Findings from a substantial number of studies have provided a better understanding of the interactions between NMDA receptors and EtOH during adolescence. Adolescence is characterized by a transient overproduction of NMDA receptors in the developing brain (e.g., Insel et al, 1990). Cortical and hippocampal NMDA receptors play an important role regulating cognitive processes that are impaired by adolescent EtOH exposure (Carpenter-Hyland & Chandler, 2007; Crews et al., 2007; Krystal et al, 2003; Robbins & Murphy, 2006). Electrophysiological studies have demonstrated age-related differences in the sensitivity of NMDA receptor function to the acute and chronic effects of EtOH. Those studies have shown that the inhibitory effects of EtOH on NMDA-mediated cortical post-synaptic currents and hippocampal long-term potentiation and excitatory post-synaptic potentials are more pronounced in adolescent rats compared to adult rats (Li et al., 2002; Pyapali et al., 1999; Swartzwelder et al., 1995). Moreover, there is also evidence of compensatory upregulation of NMDA systems after prolonged EtOH exposure and withdrawal (Carpenter-Hyland & Chandler, 2007).
We have recently found evidence of complex compensatory changes in NMDA receptor function after adolescent EtOH exposure (Criado et al., 2008). Our study found that adolescent EtOH exposure produced no significant effects on parietal EEG power assessed 5 weeks post-EtOH exposure. Systemic administration of MK-801 significantly reduced parietal EEG power in the 4-6, 6-8, 8-16 and 16-32 Hz frequency bands in EtOH-exposed, but not in control rats. These data suggest that a compensatory upregulation of NMDA systems following prolonged adolescent EtOH exposure and withdrawal may regulate the generation of these EEG frequency bands. While the effects of EtOH exposure are likely due to developmental changes in the adolescent brain (Slawecki, et al., 2001), it is likely that NMDA-mediated neuroadaptive changes and neurotoxicity during EtOH withdrawal may also account for some of the findings in the present study. Consistent with those findings, enhanced NMDA activity has been associated with several behavioral and neurophysiological characteristics of EtOH withdrawal, including irritability and anxiety (Erden et al., 1999), increased seizure susceptibility (Morrisett et al, 1990), and neurophysiological hyperarousal (Nelson et al., 1999). These symptoms could mediate the reduction in the duration of total amount of SWS and SWS episodes found in the present study.
It is still unclear whether lasting neuroadaptive changes of NMDA-mediated activity during adolescent EtOH exposure and withdrawal could be mediating their compensatory effects on SWS. The mechanisms by which changes in NMDA receptor function mediate the consequences of adolescent EtOH exposure on SWS are complex and involve multiple neural circuits and neurotransmitters regulating several stages of the sleep-wake cycle. Moreover, glutamate neurotransmission is primarily associated with arousal, rather than sleep promotion. In fact, glutamate-containing neurons are the main component of the cortical-activating and behavioral-arousal systems in the CNS (reviewed by Jones, 2005). Neurons of the caudal pontine and medullary reticular formation (RF), of the thalamocortical projection relay and of the basal forebrain, use glutamate as a neurotransmitter (Fujiyama et al., 2001; Henny & Jones, 2008; Manns et al., 2001). These glutamatergic projections play a variety of roles, from facilitating postural muscle tone to stimulating cortical activation (reviewed by Jones, 2005). However, these arousal-promoting glutamatergic pathways are also regulated by sleep-promoting GABAergic-containing neurons in regions such as the brainstem and thalamus. GABAergic projections have been shown to inhibit glutamate-containing neurons in the brainstem RF and thalamocortical projection relay to suppress cortical activation (Maloney et al., 1999, 2000; Steriade, 1994). Likewise, activation of arousal-promoting pathways blocks the generation of synchronized low-frequency oscillations that are associated with SWS (Steriade, 2003; Steriade & McCarley, 1990). Moreover, there is electrophysiological evidence to suggest an increased sensitivity of GABAergic neurotransmission to EtOH during adolescence (Fleming, et al., 2007; Li, et al., 2003). Therefore, EtOH-induced changes in the regulation of glutamate cortical-activating systems by GABAergic sleep-promoting neurons during adolescence may also partially account for the findings in the present study.
Acknowledgements
Supported in part by National Institute on Alcoholism and Alcohol Abuse (NIAAA) grant AA006059 and AA014339 and by the Stein Endowment fund. The computer programs were written by Dr. James Havstad. The authors thank Phil Lau and Jennifer Roth for assistance in analyses, and Shirley Sanchez for help in editing.
References
- Angelakis E, Lubar JF, Stathopoulou S, Kounios J. Peak alpha frequency: an electroencephalographic measure of cognitive preparedness. Clin Neurophysiol. 2004;115:887–897. doi: 10.1016/j.clinph.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Stathopoulou S, Frymiare JL, Green DL, Lubar JF, Kounios J. EEG neurofeedback: a brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin Neuropsychol. 2007;21:110–129. doi: 10.1080/13854040600744839. [DOI] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, Gray JA. Cholinergic system and memory in the rat: effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience. 1989;33:435–462. doi: 10.1016/0306-4522(89)90397-7. [DOI] [PubMed] [Google Scholar]
- Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res. 2005;29:1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Micheau J, Jaffard R. Memory deficits following chronic alcohol consumption in mice: relationships with hippocampal and cortical cholinergic activities. Pharmacol Biochem Behav. 1992;42:749–753. doi: 10.1016/0091-3057(92)90024-a. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, De Modena A, Smith TL, Danowski S, Irwin M, Schuckit M. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnoses of secondary depression. Biol Psychiatry. 1998;43:601–607. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, De Modena A, Smith TL, Danowski S, Irwin M, Schuckit M. Polysomnography and depressive symptoms in primary alcoholics with and without a lifetime diagnosis of secondary depression and in patients with primary major depression. J Affective Disord. 1999;52:177–185. doi: 10.1016/s0165-0327(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Crews FT, Rudolph JG, Chandler LJ. Glutamate and alcohol-induced neurotoxicity. In: Herman BH, Frankenheim J, Litten RZ, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and addiction. Humana Press; Totowa, N.J: 2002. pp. 357–374. [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008;32(10):1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, De Modena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–802. [PubMed] [Google Scholar]
- Dustman RE, Emmerson RY, Shearer DE. Life span changes in electrophysiological measures of inhibition. Brain Cogn. 1996;30:109–126. doi: 10.1006/brcg.1996.0007. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. EEG and ERP response to chronic ethanol exposure in rats. Psychopharmacology (Berl) 1991;104:67–74. doi: 10.1007/BF02244556. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW. Characterization of drug effects on the EEG by power spectral time series analysis. Psychopharmacol Bull. 1982;18:43–47. [Google Scholar]
- Ehlers CL, Kupfer DJ. Effects of age on delta and REM sleep parameters. Electroencephalogr Clin Neurophysiol. 1989;72:118–125. doi: 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone- releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–474. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Seifritz E, Rivier JE. CRF/NPY interactions: a potential role in sleep dysregulation in depression and anxiety. Depress Anxiety. 1997;6:1–9. [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Lopez AL, Robledo P. Long latency event-related potentials in rats: response of amygdala, nucleus accumbens, dorsal hippocampus and frontal cortex to changes in reward characteristics of conditioned stimuli. Brain Res. 1998;780:138–142. doi: 10.1016/s0006-8993(97)01294-8. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr Clin Neurophysiol. 1989;73:179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Erden BF, Ozdemirci S, Yildiran G, Utkan T, Gacar N, Ulak G. Dextromethorphan attenuates ethanol withdrawal syndrome in rats. Pharmacol Biochem Behav. 1999;62:537–541. doi: 10.1016/s0091-3057(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23:1044–1051. [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, De Modena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Irwin M, Miller C, Gillin JC, De Modena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res. 2000;24:1376–1384. [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neurophysiological profiles of replicate line 2 high-alcohol-drinking (HAD-2) and low-alcohol-drinking (LAD-2) rats. Alcohol Clin Exp Res. 2002;26:1669–1677. doi: 10.1097/01.ALC.0000036286.55213.D8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Ladurner G, Pfurtscheller G. Alpha frequency and memory performance. J Psychophysiol. 1990;4:381–390. [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Kubota T, De A, Brown RA, Simasko SM, Krueger JM. Diurnal effects of acute and chronic administration of ethanol on sleep in rats. Alcohol Clin Exp Res. 2002;26:1153–1161. doi: 10.1097/01.ALC.0000024292.05785.03. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Differential effect of ethanol on NMDA EPSCs in pyramidal cells in the posterior cingulate cortex of juvenile and adult rats. J Neurophysiol. 2002;87:705–711. doi: 10.1152/jn.00433.2001. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res. 2003;27:2017–2022. doi: 10.1097/01.ALC.0000108390.62394.71. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci. 1999;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–263. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Rezvani AH, Overstreet D, Janowsky DS, Wilson WA, Swartzwelder HS. MK-801 potently inhibits alcohol withdrawal seizures in rats. Eur J Pharmacol. 1990;176:103–105. doi: 10.1016/0014-2999(90)90138-v. [DOI] [PubMed] [Google Scholar]
- Morzorati S, Breen TE, Lumeng L, Li TK. Comparison of innate EEG parameters in ratlines selected for ethanol preference. Alcohol. 1994;11:253–258. doi: 10.1016/0741-8329(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure alters CA1 synaptic transmission in rat hippocampal slices. Neuroscience. 1999;94:431–442. doi: 10.1016/s0306-4522(99)00336-x. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd. ed Academic Press; Sydney, Australia: 1986. [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Dev Alcohol. 1991;9:53–67. [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neuropathol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27:141–148. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Lumeng L, Li TK, Ehlers CL. Effects of MK 801 and diazepam on the EEG of P and NP rats. Alcohol Clin Exp Res. 1994;18:363–368. doi: 10.1111/j.1530-0277.1994.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Siegel JM. The neurotransmitters of sleep. J Clin Psychiatry. 2004;65(Suppl 16):4–7. [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–1836. doi: 10.1097/01.alc.0000183024.47167.27. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Li TK, Ehlers CL. Neurophysiological findings and drinking levels in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2000b;24:1492–1499. [PubMed] [Google Scholar]
- Slawecki CJ, Grahame NJ, Roth J, Katner SN, Ehlers CL. EEG and ERP profiles in the high alcohol preferring (HAP) and low alcohol preferring (LAP) mice: relationship to ethanol preference. Brain Res. 2003;961:243–254. doi: 10.1016/s0006-8993(02)03959-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170:41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of chronic ethanol exposure on neurophysiological responses to corticotropin-releasing factor and neuropeptide Y. Alcohol Alcohol. 1999;34:289–299. doi: 10.1093/alcalc/34.3.289. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of prolonged ethanol exposure on neurophysiological measures during an associative learning paradigm. Drug Alcohol Depend. 2000a;58:125–132. doi: 10.1016/s0376-8716(99)00072-1. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent period: biological basis of vulnerability to develop alcoholism and other ethanol-mediated behaviors. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 315–333. [Google Scholar]
- Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–d899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- Steriade M. Cellular substrates of brain rhythms. In: Niedermeyer E, da Silva F. H. Lopes, editors. Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 28–75. [Google Scholar]
- Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. Plenum Press; New York: 1990. [Google Scholar]
- Steriade M, Contreras D, Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 1994;17:199–208. doi: 10.1016/0166-2236(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services U. S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: Summary of findings from the National Household Survey on Drug Abuse 1998. 1999
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Veatch LM. Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res. 2006;30:1214–1222. doi: 10.1111/j.1530-0277.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH., Jr. Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Clin Exp Res. 1981;5:318–325. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de LL. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]