Abstract
A mass balance study was performed under controlled field conditions to investigate the phytoremediation of perchloroethylene (PCE) by hybrid poplar trees. Water containing 7–14 mg L−1 PCE was added to the test bed. Perchloroethylene, trichloroethylene, and cis-dichloroethylene were detected in the effluent at an average of 0.12 mg L−1, 3.9 mg L−1, and 1.9 mg L−1, respectively. The total mass of chlorinated ethenes in the water was reduced by 99%. Over 95% of the recovered chlorine was as free chloride in the soil, indicating near-complete dehalogenation of the PCE. Transpiration, volatilization, and accumulation in the trees were all found to be minor loss mechanisms. In contrast, 98% of PCE applied to an unplanted soil chamber was recovered as PCE in the effluent water or volatilized into the air. These results suggest that phytoremediation can be an effective method for treating PCE-contaminated groundwater in field applications.
Keywords: Phytoremediation, Perchloroethylene, Mass balance
1. Introduction
Due to its widespread use, perchloroethylene (PCE) is one of the most common environmental pollutants found at National Priority List and Superfund sites (United States Environmental Protection Agency, 2007). A recent survey of groundwater in the United States found that PCE was detected in 11% of the samples analyzed (Moran et al., 2007). Perchloroethylene is associated with health risks such as liver and kidney damage, spontaneous abortions, and is listed as a probable human carcinogen (ATSDR, 1997). Although peak production occurred in 1980, it continues to be used in nearly 30 000 dry cleaning operations in the United States, presenting an ongoing risk of accidental releases (Doherty, 2000). As such, finding inexpensive and reliable remediation methods is an ongoing priority.
The phytoremediation of PCE is potentially advantageous based on economics and aesthetics. Several studies have demonstrated the effectiveness of phytoremediation against PCE in the laboratory (Nzengung and Jeffers, 2001; Nzengung et al., 2003) and other chlorinated hydrocarbons such as trichloroethylene (TCE) and carbon tetrachloride (CT) in both field and laboratory settings (Anderson et al., 1993; Anderson and Walton, 1995; Newman et al., 1999; Shaw and Burns, 2003; Walton and Anderson, 1990; Wang et al., 2004). However, field studies on the phytoremediation of PCE are rare. Recent field studies have focused on the utility of tree sampling for subsurface chloroethene plume identification (Gopalakrishnan et al., 2007; Larsen et al., 2008; Sorek et al., 2008) or evaluated various tree species for potential phytoremediation application (Stanhope et al., 2008). The fate of PCE in a field setting has not been investigated. Plant-related processes for attenuation of halogenated organic compounds may include rhizosphere degradation and plant uptake followed by oxidative or reductive transformation, sequestration, or volatilization (Nzengung and Jeffers, 2001). Uptake and volatilization may be an important mechanism for loss of chlorinated ethenes (Ma and Burken, 2003; Struckhoff et al., 2005) though there have been no previous attempts to compare the relative importance of these mechanisms for the phytoremediation of PCE.
In this study, we perform a chlorine mass balance in a phytoremediation test bed dosed with PCE-contaminated water to allow a comparison of loss pathways. The following parameters were monitored: 1) influent and effluent volatile organic compound (VOC) and chloride ion concentrations, 2) volatilization of VOCs from soil, 3) volatilization of VOCs from tree trunks and leaves, 4) accumulation of PCE and metabolites, and halides, in tree tissue, and 5) accumulation of chloride ions in the soil. Based on the measurements, the proportion of loss from each mechanism was quantified and compared. Here we report the first systematic mass balance approach applied to the phytoremediation of PCE.
2. Materials and methods
2.1. Field site
The field site was located at the University of Washington Phytoremediation Field Facility. The test bed was 1.5 m deep by 3.0 m wide by 5.7 m long and was filled with 0.3 m of coarse sand overlain by 1.1 m of Sultan silty clay loam topsoil. The test bed was lined with twin 60 mil polyethylene liners and had influent and effluent wells consisting of perforated T-pipes allowing the controlled application of water and PCE. The bottom of the bed was sloped at 1:40 to facilitate flow.
The test bed contained 12 hybrid poplars OP 367 (Populus deltoides × Populus nigra) that were planted in spring 2002. Tree height was estimated to be 7 m. Average trunk circumference at 1.37 m height was 22.2 cm. The approximate mass density of the trees was 10 kg m3. All trees appeared healthy and showed vigorous growth.
2.2. Water supply and chemical dosing
Water was added or removed from the test bed with the goal of maintaining a water depth of 20–25 cm in the effluent well, roughly coinciding to the depth of the sand layer. Water levels were measured daily by inserting a graduated rod into the well. Water was supplied either directly through the influent well, or through surface watering. Water supplied in the influent well contained PCE; irrigation water was obtained from an on-site supply or a municipal source and did not contain PCE.
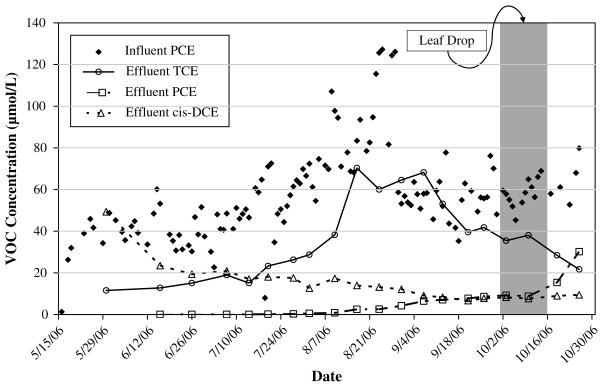
Chemical dosing was performed by mixing from 2 to 4 L of PCE stock solution with 95 L water in a 100 L polyethylene drum connected to the influent well in order to achieve desired influent concentration. Chemical dosing began on May 16, 2006. Perchloroethylene was supplied at an average concentration of 7.0 mg L−1 through July 29, 2006. The influent concentration was increased to an average of 14.3 mg L−1 from July 30, 2006 through August 29, 2006, after which it decreased to an average of 9.5 mg L−1 through October 30, 2006 (Fig. 1). The influent water contained trace levels of TCE with an average concentration less than 0.1 mg L−1.
Fig. 1.
Chlorinated ethene concentration in test bed influent and effluent water over the growing season. VC and 1,1- and trans-DCE were measured at below 1 μmol L−1 and not shown.
2.3. Water sampling and analysis
Influent water samples were collected daily from a sampling port located between the feed drum and the influent well. Effluent samples were collected each time water was pumped from the test beds, or at least weekly. Prior to collecting effluent samples, approximately 19 L of water were pumped from the effluent well. Effluent samples were collected as described in Newman et al. (1999). Irrigation water from the municipal supply was sampled directly from the on-site source. Rainwater samples were collected during the rain events in wide-mouth glass jars. All samples were stored at 4 °C until analysis.
Samples were analyzed according to EPA Method 8260A for PCE, TCE, cis-, trans-and 1,1-dichloroethylene (DCE), and vinyl chloride (VC). Analysis for PCE, TCE, and the DCE isomers was performed using a Perkin Elmer AutoSystem XL GC-electron capture detector (ECD) with a Supelco VOCOL fused silica capillary column (60 m, 0.53 mm ID, 3.00 μm film thickness). GC oven temperature program was 40 °C for 1 min, ramped at 10 °C min−1 for 19 min, and held at 230 °C for 4 min. The GC-ECD was linked to a Tekmar Precept II Purge and Trap and Tekmar Purge and Trap Concentrator. Liquid samples were purged with helium for 11 min at 30 °C onto the concentrator. Samples were desorbed off the concentrator at 225 °C for 4 min into the GC for analysis. Analytical limits for PCE, TCE, and DCE were approximately 0.5 μg L−1, 1 μg L−1, and 15 μg L−1, respectively.
Analysis for VC was performed by collecting headspace samples from the effluent sample vials after they had been analyzed with the GC-ECD. Sample vials were allowed to reach equilibrium at 23 °C at least 3 h prior to analysis. A headspace air sample (150 μL) was removed through the septa using a 500 μL gas-tight glass syringe (Hamilton, Reno, NV) and injected manually into an SRI 8610C GC-FID with a Supelco Alumina Sulfate Plot column (50 m, 0.53 mm ID). All sampling was performed in triplicate. Analytical limit for VC was approximately 20 μg L−1.
Trichloroethanol (TCOH) analysis was performed on selected samples. An 8 mL aliquot was combined with 4 mL of 1 N H2SO4/10% NaCl solution in a 25 mL Cornex centrifuge tube, sealed with a screw cap with a Teflon-lined septa, and shaken for 1 min. Methyl tert-butyl ether (MTBE; 10 mL) was added and mixed vigorously for 2 min. The MTBE layer was withdrawn and injected into a sealed, glass-amber vial which contained 2 g Na2SO4 and held for 1 h. One mL of MTBE was removed and placed in autosampler vials for analysis with Perkin Elmer AutoSystem XL GC-ECD with a PTE-5 column (30 m, 0.32 mm ID, 0.32 μm film thickness). The GC oven temperature program was as described above. Analytical limit for TCOH in water was approximately 1.5 μg L−1.
Chloride analysis was performed with a Dionex AS40 Automated Sampler connected to a Dionex DX-120 ion chromatograph (IC) with a Dionex IonPac AS14 4 × 250 mm anion exchange column; eluent was 3.5 mM Na2CO3/1 mM NaHCO3 in degassed, deionized water. Analytical limit was approximately 0.1 mg L−1.
Concentrations of the chlorinated ethenes and ions were calculated based on external standards.
2.4. Soil sampling and analysis
Eight manual push core samples were collected approximately monthly on a 2 × 4 grid evenly distributed across the test bed area, at 4 depths (0–25 cm, 25–50 cm, 50–75 cm, 75–100 cm) per location. Chloride was extracted from the soil samples as described previously (Wang et al., 2004).
Soil chloride accumulation in the unsaturated portion of the test bed was calculated by summing the product of the soil chloride concentration for each sample, by the portion of the overall bed volume represented by each sample. The chloride accumulation in the saturated layer was calculated by multiplying the chloride concentration of the effluent water by the volume of water in the test bed.
2.5. Soil volatilization
Volatilization was measured using a soil flux chamber as described previously (Tillman et al., 2003). Sampling time was 4 h, though one sample was collected for 12 h and two for 18 h.
Volatile organic compounds were extracted from the sample carbon tubes as described previously, with modifications (Wang et al., 2004). The carbon was placed in 4 mL amber glass vials with 3 mL of 1:1 hexane, acetone mix, agitated on a shaker table for 30 min and held at room temperature for 4 h. Analysis for VOCs employed a Perkin Elmer AutoSystem GC-ECD, as described above. Analytical limit for PCE, TCE, and DCE in vapor was approximately 0.1, 0.6, and 6 μg m−3.
There were no exposed roots in the test bed. Any volatilization of VOCs from the roots was captured in the soil volatilization analysis.
2.6. Volatilization and transpiration from trees
Trunk volatilization was measured with air chambers attached to the trunk as described previously (Wang et al., 2004). Leaf transpiration was measured by placing a set of leaves were placed inside a Tedlar® bag modified with two sample ports. The bag was sealed around the base of the stem. Activated carbon sampling tubes were placed in the influent and effluent sampling ports and air was pulled across the leaves at 2 L min−1 using a vacuum pump. The effluent sampling tube was heated slightly with heating tape to minimize the effects of high humidity on VOC adsorption by the activated carbon (Wang et al., 2004). Carbon from the sample tubes was analyzed by GC-ECD, as described above. The leaves that had been placed inside the Tedlar® bag were collected to measure the transpiration area for each sample set. The total leaf area of the trees in the test beds was determined by calculating the product of the average leaf area (71 samples), the average leaf count per branch (10 samples), and the average branch count per tree (4 samples). Total leaf area was used to calculate the total transpiration losses of VOCs from the test beds. The average leaf area was 32.7 cm2; the total leaf area of all of the trees was 3.2 × 106 ± 1.6 × 105 cm2.
2.7. VOC in trunk and leaf tissue
Leaf and core samples were collected to determine the concentration of PCE and metabolites that accumulated in the tissues. Samples were collected and immediately flash frozen on-site in liquid nitrogen. The frozen samples were homogenized and stored in capped 50 mL centrifuge vials on dry ice for transport. Samples were stored at −80 °C until analysis. Core samples were collected from trees located in the middle of the test bed and from trees not exposed to PCE. Analysis for TCOH-glucoside was per Shang et al. (2001). Analysis for PCE and free metabolites was per Newman et al. (1997) with analysis on GC-ECD as described above. Analytical limits for PCE and TCE were approximately 0.01 and 0.05 μg g−1 tissue, respectively.
Analysis for di- and trichloroacetic acid (DCAA, TCAA) was performed by adding a 1 mL aliquot of the MTBE supernatant from the tissue extraction with 2 mL methanol and 0.1 ml 50% H2SO4, and incubating at 50 °C for 1 h. Five mL 10% Na2SO4 solution and 1 mL MTBE were added and the mixture shaken for 2 min. The mixture was held for 10 min and an aliquot of the MTBE layer removed for analysis on GC-ECD. Analytical limits for TCAA and DCAA were approximately 0.1 and 0.05 μg g−1 tissue, respectively.
The accumulation of VOCs in the tree and leaf tissues was calculated by multiplying the average molar concentration in the tissues by the mass of tissues. The leaf mass was calculated as described above except that the average leaf mass (0.785 g) was used instead of the average leaf area. The total wood mass was calculated based on trunk measurements (Balatinecz and Kretschmann, 2001).
2.8. Organic halide analysis in tree tissue
Leaf and core samples from trees in the test bed and an undosed control bed were collected as described above and analyzed by Spectra Laboratories, Tacoma, WA for total organic halide (TOX) analysis by EPA Manual SW-846, Method 9076.
2.9. Unplanted control chamber
An unplanted, laboratory chamber was established as a control. The chamber was glass with dimensions 90 cm × 30 cm × 45 cm deep. It was filled with a 5 cm sand layer overlain by 30 cm of soil obtained from the test beds at the UW Phytoremediation Field Facility. The soil was roughly screened to remove root material. The chamber had influent and effluent T-wells of perforated PVC and was sloped at approximately 1:40. Chemical dosing and sample collection were performed with peristaltic pumps. The chamber was operated for seven months. It was dosed with water containing PCE at approximately 1 mg L−1 for two months, held for three months, and dosed again for two months. Water sampling occurred during the dosing periods. Volatilization was measured by sealing the top of the chamber, except for influent and effluent air ports, and collecting samples on activated carbon tubes for approximately 4 h at a flow rate of 1.7 L min−1.
2.10. Data analysis
A Monte Carlo method was used to estimate the uncertainty of each mass balance component which allowed the estimation of the uncertainty of the total mass balance, and the contribution of uncertainty from each component (Hoffman and Hammonds, 1994). The formulae used for calculating each mass balance component are shown in Table S1. Random number sets were created for each factor of each component assuming a normally distributed population; means were determined from field data and standard deviations were either estimated from method analysis, or were calculated directly (Table S1). The resulting random number sets from each mass balance component were utilized to create total mass balance distribution.
3. Results
3.1. Water use and quality
Water quantity values are shown in Table 1. Perchloroethylene was the major chlorinated compound present in the influent water. TCE was present only at trace amounts. Trihalomethanes were not detected. Supplemental irrigation water did not contain chlorinated ethenes. The effluent water contained PCE, TCE, cis-DCE, and VC (Fig. 1 and Table 1). The average PCE concentration was reduced by approximately 90% in the effluent compared to the influent. There was no significant reduction in total VOC concentration; the sum of PCE, TCE, cis-DCE, and VC molar concentrations in the effluent was approximately equal to the influent PCE molar concentration throughout the growing season. Trichloroethylene, cis-DCE, and PCE accounted for an average of 57%, 41%, and 1.3% of the total VOCs measured in the effluent water, respectively. From May through October 2006 influent PCE concentration and effluent TCE concentration were positively correlated (r2 = 0.5). In October 2006, there was a decrease in TCE concentration in the effluent, coinciding with an increase in the PCE concentration. This change coincided with leaf-drop at the end of the growing season. Influent and effluent water quality was monitored following the end of the mass balance period (Figure S1). In November 2006 there was a decrease in effluent VOC concentration coinciding with a period of near-record rainfall.
Table 1.
Total inputs and losses of chlorinated ethenes and water from the test bed during the mass balance period (April 11, 2006–August 24, 2006).
| Inputs | Losses | Difference | |
|---|---|---|---|
| VOC balance | |||
| PCE in water (mol) | 1.15 ± 4.9 × 10−2 | 1.3 × 10−4 ± 5.5 × 10−6 | |
| TCE in water (mol) | 2.2 × 10−2 ± 9.3 × 10−4 | 6.1 × 10−3 ± 2.5 × 10−4 | |
| DCE in water (mol) | ND | 4.2 × 10−3 ± 1.8 × 10−4 | |
| VC in water (mol) | ND | 2.5 × 10−5 ± 1.0 × 10−6 | |
| Total VOCs in water (mol) | 1.17 ± 5.0 × 10−2 | 1.1 × 10−2 ± 4.3 × 10−4 | 1.16 ± 5.0 × 10−2 |
| Soil volatilization (mol) | NA | 1.7 × 10−2 ± 2.0 × 10−2 | |
| Trunk volatilization (mol) | NA | 1.0 × 10−3 ± 7.4 × 10−4 | |
| Leaf volatilization (mol) | NA | 2.8 × 10−3 ± 1.9 × 10−3 | |
| Total VOCs (mol) | 1.17 ± 5.0 × 10−2 | 3.43 × 10−2 ± 2.2 × 10−2 | 1.14 ± 7.2 × 10−2 |
| Water balance | |||
| Water (L) | 19 750 ± 800 | 210 ± 10 | |
| Supplemental irrigation (L) | 2020 ± 100 | NA | |
| Rainwater (L) | 1640 ± 100 | NA | |
| Total water (L) | 23 410 ± 1000 | 210 ± 10 | 23 200 ± 1000 |
ND – not detected.
NA – not applicable.
The mass of VOCs was reduced in by over 99% in the effluent compared to the influent (Table 1); the mass of PCE was reduced by 99.99%.
The average chloride ion concentration in the irrigation water and rainwater was 3.1 ± 0.04 mg L−1 and 0.18 ± 0.17 mg L−1, respectively.
3.2. Volatilization from soil
Fourteen separate measurements were taken to quantify the volatilization of VOCs from the soil matrix, including six measurements at the influent end, four measurements in mid-bed, and four measurements in the effluent end of the test bed (Tables 1 and 2). The average PCE flux from the soil was over an order of magnitude lower than the average TCE flux. There were no significant correlations between flux rates and the sample location, temperature, or date of sampling.
Table 2.
Flux of PCE and TCE measured from the soil, trunk, and leaves. Values reported are mean and standard error for all measurements taken during the mass balance period.
| PCE flux (mol h−1 cm−2) | TCE flux (mol h−1 cm−2) | |
|---|---|---|
| Soil volatilization | 1.2 × 10−12 ± 0.66 × 10−12 | 4.0 × 10−11 ± 1.3 × 10−11 |
| Trunk volatilization | 4.5 × 10−12 ± 1.0 × 10−12 | 1.2 × 10−11 ± 0.20 × 10−11 |
| Leaf transpiration | 1.6 × 10−13 ± 0.73 × 10−13 | 3.7 × 10−13 ± 1.4 × 10−13 |
3.3. Volatilization from trunk
Twelve measurements were made at various trunk heights and locations in the test bed in order to determine the flux of VOCs volatilizing from the trunk tissue (Tables 1 and 2). The flux was greatest near the soil surface and decreased with height. None of the measurements taken at 2 m were above the detection limit, suggesting that the flux at that height was at least an order of magnitude lower than that near the soil surface. A comparison of the measurements taken at the surface and at 1 m height at the influent and effluent ends of the test bed indicated that the rate of volatilization from the tree trunks was greater at the influent end compared to the effluent end for both PCE and TCE (P < 0.05).
3.4. Transpiration from leaves
Five sets of measurements were made over the course of the growing season (Tables 1 and 2). The transpiration losses of TCE and PCE from the leaves were not significantly different.
3.5. Accumulation of chlorinated organics in tree tissue
Four sets of leaf tissues and one set of trunk core samples were collected during the growing season. β-glucosidase digestion was performed on two leaf samples and one core sample in order to determine total TCOH (Table 3). Trichloroethanol and TCAA were detected in the leaf tissue with 91% of the TCOH in the conjugated form; PCE and TCE were not detected at levels above the analytical limits. Trichloroethylene was the only compound found in the core samples; PCE, TCOH, and TCAA were not detected at levels above the detection limits.
Table 3.
Concentration of PCE and metabolites measured in leaf and trunk core tissue. Values are reported as mean and standard error for all samples analyzed during the mass balance period. TCAA and DCAA were not measured in trunk core samples.
| PCE (μg g−1) | TCE (μg g−1) | TCOH (μg g−1) | TCOH-glucoside (μg g−1) | TCAA (μg g−1) | DCAA (μg g−1) | TOX (μg g−1) | |
|---|---|---|---|---|---|---|---|
| Leaf | ND | ND | 0.027 ± 0.0074 | 0.31 ± 0.024 | 0.065 ± 0.05 | ND | ND – 10 |
| Trunk core | ND | 9.1 ± 2.6 | ND | ND | NA | NA | ND |
ND – not detected.
NA – not applicable.
Leaf and trunk core samples from the test bed and from trees not exposed to PCE were analyzed for TOX. The results from the samples from the test bed and the unexposed trees were not significantly different. Three leaf samples from the test bed were analyzed for TOX; two were below the detection limit (<5 μg g−1) and one was 10 μg g−1 tissue. Leaf samples from the unexposed trees were all below the detection limit. The core sample from the test bed was below the detection limit; the core sample from the unexposed tree contained 10 μg g−1 tissue.
3.6. Soil chloride concentration
Soil sample sets were collected on April 11, 2006, June 29, 2006, and August 24, 2006 to measure soil chloride over the growing season (Figure S2). The average soil chloride concentration increased from 26 ± 0.79 mg kg−1 on April 11, to 65 ± 2.4 mg kg−1 on August 24, a significant increase (P < 0.05; Figure S3).
The test bed was maintained with a saturated zone, corresponding to the sand layer in the lower 0.3 m, and an overlying unsaturated zone. The increase in soil chloride occurred only in the unsaturated zone. The chloride concentration in the saturated zone decreased over the growing season as the free chloride ions were mobilized and removed in the effluent stream.
3.7. Chlorine mass balance
A summary of the sources and sinks of chlorine during the mass balance period from April 11, 2006 through August 25, 2006 is presented in Table 4 and Figure S4.
Table 4.
Chlorine balance showing total inputs, losses, and accumulation of chlorine during the mass balance period (April 11, 2006–August 24, 2006). Inputs of chloride ion include chloride in the influent water, supplemental irrigation, and rainwater. All values are reported as moles of chlorine.
| Input (mol Cl) |
Out/accumulation (mol Cl) |
|||
|---|---|---|---|---|
| Average | Standard deviation | Average | Standard deviation | |
| Soil chloride | 10.5 | 2.00 | ||
| Water chloride | 4.34 | 0.177 | 0.483 | 1.95 × 10−2 |
| Water PCE | 4.60 | 0.194 | 5.25 × 10−4 | 2.20 × 10−5 |
| Water TCE | 6.75 × 10−2 | 2.78 × 10−3 | 1.82 × 10−2 | 7.55 × 10−4 |
| Water cDCE | 8.42 × 10−3 | 3.55 × 10−4 | ||
| Water VC | 2.48 × 10−5 | 1.04 × 10−6 | ||
| Top water chloride | 0.180 | 1.08 × 10−2 | ||
| Rain water chloride | 9.41 × 10−3 | 7.54 × 10−3 | ||
| Soil volatilization | 5.15 × 10−2 | 6.09 × 10−2 | ||
| Leaf transpiration | 9.65 × 10−3 | 6.93 × 10−3 | ||
| Trunk volatilization | 3.40 × 10−3 | 2.46 × 10−3 | ||
| Leaf TCOH-glucoside | 4.79 × 10−4 | 9.03 × 10−5 | ||
| Trunk TCE | 1.55 × 10−2 | 1.61 × 10−2 | ||
| Total | 9.19 | 11.1 | ||
| Recovery | 121% | |||
The total amount of chlorine added to the test bed as PCE and chloride ion was 9.2 ± 0.27 mol Cl. The total amount of chlorine accumulated or lost through various mechanisms was 11.1 ± 2.0 mol Cl. The net accumulation of free chloride in the test bed soil was 10.5 ± 2.0 mol Cl over the mass balance period, which accounted for approximately 95% of the total chlorine recovered. Other than the free chloride removed with the effluent water, no other loss mechanisms accounted for more than 1% of the total chlorine loss or accumulation.
The mass balance resulted in a 120% recovery of chlorine. Each of the independent measurements of the mass balance was used to create overall distributions for the total input and total loss/recovery by a Monte Carlo analysis (Figure S5). The results of the statistical analysis indicate that the recovery was complete within experimental uncertainty. An analysis of uncertainty of the independent components showed that the soil chloride measurements accounted for approximately 95% of the probable uncertainty in the mass balance.
3.8. Unplanted control chamber
A mass balance of PCE in the unplanted control chamber was performed for a period of one month. The total mass balance recovery was 105%. Perchloroethylene in the effluent water (36%) or volatilized into the air (64%) accounted for 98% of the total VOCs recovered. Trichloroethylene in the effluent water accounted for the remaining 2% of total VOCs recovered. No other VOCs were detected in the water or air. These results indicate that there was very limited transformation of PCE in the unplanted control.
4. Discussion
The results of this study demonstrate that complete dehalogenation is the primary loss mechanism for the phytoremediation of PCE-contaminated water under the conditions tested. To our knowledge, this is the first study to clearly show that PCE loss in phytoremediation is primarily via complete dehalogenation. This conclusion is based on the results of the mass balance, in which we were able to account for all of the chlorine that entered the system. Chlorine recovered in the outputs and accumulations totaled 121% of the combined inputs and a statistical analysis demonstrated that the total inputs and total outputs and recoveries did not differ significantly (Figure S5). The largest single component in overall uncertainty was the variation associated with the soil chloride measurements of soil samples from different locations (Table 4). Complete dehalogenation accounted for approximately 95% of total chloroethene loss across the planted test bed and was the most important loss mechanism in this system (Table 4; Figure S4). The total of all other processes, including volatilization and accumulation of metabolites in the tree tissues, accounted for approximately 5% of the total chlorine recovered.
A second finding of this study is that the presence of trees affects the degradation of PCE in the subsurface. The chloroethene mass was reduced by over 99% in the planted test bed while there was no significant reduction in the unplanted control. Similar reductions in chloroethene mass in planted test beds have been reported for TCE and CT, but not PCE (Newman et al., 1999; Wang et al., 2004). The reduction in mass was primarily due to the reduction in the volume of water in the influent compared to the effluent (Table 1), as the total chloroethene concentration did not decrease significantly in the effluent water compared to the influent water. There was, however, transformation of PCE as it passed through the planted test bed. The concentration of PCE in the influent water was reduced by approximately 90% at the effluent, and could be accounted for by molar-equivalent increases in the TCE and cis-DCE concentrations in the effluent (Fig. 1). A similar transformation was not observed in the unplanted control, nor was it previously reported for TCE- or CT-contaminated water introduced into a similar planted test bed (Newman et al., 1999; Wang et al., 2004). The data suggest that the decrease of PCE concentration was due to reductive dechlorination of PCE mainly to TCE, but also to cis-DCE and VC, which was apparently not an active process in the unplanted control. Field studies have reported the increased transformation of TCE in a planted field site compared to unplanted areas (Eberts et al., 2005). The results suggested that the presence of root exudates led to the eventual creation of sulfate-reducing and methanogenic conditions and an increase in the rate of reductive dechlorination of TCE in the groundwater.
In addition to the presence of trees, the results of this study suggest that chloroethene degradation may also be linked to the metabolic state of the trees. The TCE:PCE molar ratio in the effluent water was used as an indication of PCE transformation (Figure S1). During the growing season, the TCE:PCE molar ratio remained above 10, suggesting a high degree of transformation. Coincident with leaf-drop and the beginning of dormancy, effluent PCE concentration increased and TCE concentration decreased resulting in a TCE:PCE ratio below one. Although the total chloroethene concentration fell in the effluent in November 2006 due to dilution effects associated with heavy rainfall, the TCE:PCE ratio remained relatively constant at 0.8 through the winter. The TCE:PCE ratio began to increase in April 2007, coinciding with leaf emergence, and reached 4.2 in June 2007 when measurements ceased. The co-occurrence of changes of PCE transformation with changes in metabolic state of the trees both at the end of one growing season and the beginning of the next suggests a causative relationship, although other factors such as temperature were likely important as well.
It was not possible to determine whether the dehalogenation reactions were plant- or microbial- mediated. The rhizosphere can be sufficiently reducing to support microbial reductive dehalogenation activity in anaerobic zones created by the combination of plant root–cell metabolism and the aerobic degradation of root exudates in the rhizosphere (Eberts et al., 2005; Hojberg et al., 1999). The presence of cis-DCE and VC in the effluent water, which are known metabolites of microbial dehalogenation, but not plant activity, suggests that microbial dehalogenation was active at our site and contributed to contaminant transformation.
The phytoreduction of PCE to TCE has been reported for axenic parrot feather (Myriophyllum aquaticum) and waterweed (Elodea canadensis) as well as cottonwood (P. deltoides), but not willow (Salix nigra), in laboratory bioreactors (Nzengung and Jeffers, 2001; Nzengung et al., 1999) though endophytic or soil microbial-activity may have contributed to the observed transformations. Similar studies with sterile tobacco (Nicotiana tabacum cv. Xanthii) found no detectable transformation of PCE (James et al., 2008).
Trichloroethylene, TCOH, and TCAA were detected in stem and leaf tissue collected from trees in the test bed, but PCE was not detected. Trichloroethanol, as bound TCOH-glucoside, was the predominate metabolite, with TCAA detected at lower levels. Trichloroethanol and TCAA have been shown to be metabolites of PCE or TCE metabolism in plants (Newman et al., 1999; Nzengung and Jeffers, 2001; Nzengung et al., 1999; Shang et al., 2001; Strycharz and Newman, 2009; Weissflog et al., 2007) suggesting that metabolism of chloroethenes occurred in planta. The data also indicate that TCOH and TCAA were not the final products of metabolism, as the conversion of PCE only to TCE, TCOH, or TCAA would not result in the production of sufficient free chloride to close the chlorine mass balance. We have found that plants exposed to TCE in hydroponics can export TCOH and that TCOH can be completely dehalogenated in aerobic soil microcosms (data not shown).
These data suggest that PCE degradation in the phytoremediation environment occurs via at least two distinct metabolic pathways. Overall, the phytoremediation of PCE-contaminated water may be an effective treatment method under the appropriate hydrogeologic conditions.
5. Conclusion
The results presented suggest that the presence of trees may stimulate the transformation of PCE in the subsurface. Influent and effluent VOC and flow measurements indicated that chloroethene mass was reduced by over 99%, while there was only 2% removal in an unplanted control. The chlorine mass balance demonstrated that 95% of the contaminant loss could be accounted for by complete dehalogenation, and that volatilization to the atmosphere of PCE or its products was a minor loss pathway. Thus, phytoremediation of PCE in our test beds was a destructive technology. It is not, however, universally effective. The results indicate that the chloroethenes contained in water taken up by the trees were dehalogenated. Conversely, the chloroethenes contained in the water that passed through the root zone were largely unaffected in terms of total chloroethene concentration, though the data indicate that the transformation rate of PCE in the subsurface may increase through planted zones, potentially leading to more rapid remediation. Treatment effectiveness may be in direct proportion to the fraction of contaminated groundwater captured by the trees; a phytoremediation plantation would need to intercept the entire plume. Finally, though the exact mechanism of PCE degradation is still unknown, it is likely a combination of plant- and microbial-activity. Further studies may elucidate the exact nature of the transformation reactions, allowing optimization and the expanded use of phytoremediation.
Supplementary Material
Acknowledgments
This work was funded by NIEHS grant University of Washington Superfund Basic Research Program, Grant #: > NIEHS P42ES04696 and the Valle Fellowship and Exchange Program at the University of Washington. It was performed with the assistance of Occidental Chemical Corporation. We would like to thank Ms. Julie Horowitz for her careful review of this manuscript.
Appendix. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.envpol.2009.02.033.
Footnotes
A chlorine balance performed on a planted test bed with PCE-contaminated water demonstrated VOC mass reduction of 99% and complete dechlorination.
References
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Tetrachloroethylene. Department of Health and Human Services, P.H.S.; Atlanta, GA: 1997. [PubMed] [Google Scholar]
- Anderson TA, Walton BT. Comparative fate of [C-14] trichloroethylene in the root-zone of plants from a former solvent disposal site. Environmental Toxicology and Chemistry. 1995;14:2041–2047. [Google Scholar]
- Anderson TA, Guthrie EA, Walton BT. Bioremediation in the rhizosphere. Environmental Science and Technology. 1993;27:2630–2636. doi: 10.1021/es00002a036. [DOI] [PubMed] [Google Scholar]
- Balatinecz JJ, Kretschmann DE. Properties and utilization of poplar wood. In: Dickmann DI, Isebrands JG, Eckenwalder JE, Richardson J, editors. Poplar Culture in North America. NRC Research Press; Ottawa, Canada: 2001. pp. 277–291. [Google Scholar]
- Doherty RE. A history of the production and use of carbon tetrachloride, tetrachloroethylene, trichloroethylene and 1,1,1-trichloroethane in the United States: part 1-historical background; carbon tetrachloride and tetrachloroethylene. Environmental Forensics. 2000;1:69–81. [Google Scholar]
- Eberts SM, Jones SA, Braun CL, Harvey GJ. Long-term changes in ground water chemistry at a phytoremediation demonstration site. Ground Water. 2005;43:178–186. doi: 10.1111/j.1745-6584.2005.0018.x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan G, Negri MC, Minsker BS, Werth CJ. Monitoring subsurface contamination using tree branches. Ground Water Monitoring and Remediation. 2007;27:65–74. [Google Scholar]
- Hoffman FO, Hammonds JS. Propagation of uncertainty in risk assessments –the need to distinguish between uncertainty due to lack of knowledge and uncertainty due to variability. Risk Analysis. 1994;14:707–712. doi: 10.1111/j.1539-6924.1994.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Hojberg O, Schnider U, Winteler HV, Sorensen J, Haas D. Oxygen-sensing reporter strain of Pseudomonas fluorescens for monitoring the distribution of low-oxygen habitats in soil. Applied and Environmental Microbiology. 1999;65:4085–4093. doi: 10.1128/aem.65.9.4085-4093.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CA, Xin G, Doty SL, Strand SE. Degradation of low molecular weight volatile organic compounds by plants genetically modified with mammalian cytochrome P450 2E1. Environmental Science and Technology. 2008;42:289–293. doi: 10.1021/es071197z. [DOI] [PubMed] [Google Scholar]
- Larsen M, Burken J, Machackova J, Karlson UG, Trapp S. Using tree core samples to monitor natural attenuation and plume distribution after a PCE spill. Environmental Science and Technology. 2008;42:1711–1717. doi: 10.1021/es0717055. [DOI] [PubMed] [Google Scholar]
- Ma XM, Burken JG. TCE diffusion to the atmosphere in phytoremediation applications. Environmental Science and Technology. 2003;37:2534–2539. doi: 10.1021/es026055d. [DOI] [PubMed] [Google Scholar]
- Moran MJ, Zogorski JS, Squillace PJ. Chlorinated solvents in groundwater of the United States. Environmental Science and Technology. 2007;41:74–81. doi: 10.1021/es061553y. [DOI] [PubMed] [Google Scholar]
- Newman LA, Strand SE, Choe N, Duffy J, Ekuan G, Ruszaj M, Shurtleff BB, Wilmoth J, Heilman P, Gordon MP. Uptake and biotransformation of trichloroethylene by hybrid poplars. Environmental Science and Technology. 1997;31:1062–1067. doi: 10.1289/ehp.98106s41001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Wang XP, Muiznieks IA, Ekuan G, Ruszaj M, Cortellucci R, Domroes D, Karscig G, Newman T, Crampton RS, Hashmonay RA, Yost MG, Heilman PE, Duffy J, Gordon MP, Strand SE. Remediation of trichloroethylene in an artificial aquifer with trees: a controlled field study. Environmental Science and Technology. 1999;33:2257–2265. [Google Scholar]
- Nzengung VA, Jeffers P. Sequestration, phytoreduction, and phytooxidation of halogenated organic chemicals by aquatic and terrestrial plants. International Journal of Phytoremediation. 2001;3:13–40. [Google Scholar]
- Nzengung VA, Wolfe LN, Rennels DE, McCutcheon SC, Wang C. Use of aquatic plants and algae for decontamination of waters polluted with chlorinated alkanes. International Journal of Phytoremediation. 1999;1:203–226. [Google Scholar]
- Nzengung VA, O’Niell WL, McCutcheon SC, Wolfe NL. Sequestration and transformation of water soluble halogenated organic compounds using aquatic plants, algae, and microbial mats. In: McCutcheon SL, Schnoor JL, editors. Phytoremediation: Transformation and Control of Contaminants. Wiley-Inter-science; Hoboken, N.J.: 2003. pp. 499–528. [Google Scholar]
- Shang TQ, Doty SL, Wilson AM, Howald WN, Gordon MP. Trichloroethylene oxidative metabolism in plants: the trichloroethanol pathway. Phytochemistry. 2001;58:1055–1065. doi: 10.1016/s0031-9422(01)00369-7. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Burns RG. Biodegradation of organic pollutants in the rhizosphere. In: Laskin AI, Bennett JW, Gadd G, editors. Advances in Applied Microbiology. Academic Press; San Diego: 2003. pp. 1–60. [DOI] [PubMed] [Google Scholar]
- Sorek A, Atzmon N, Dahan O, Gerstl Z, Kushisin L, Laor Y, Mingelgrin U, Nasser A, Ronen D, Tsechansky L, Weisbrod N, Graber ER. “Phyto-screening”: the use of trees for discovering subsurface contamination by VOCs. Environmental Science and Technology. 2008;42:536–542. doi: 10.1021/es072014b. [DOI] [PubMed] [Google Scholar]
- Stanhope A, Berry CJ, Brigmon RL. Field note: phytoremediation of chlorinated ethenes in seepline sediments: tree selection. International Journal of Phytoremediation. 2008;10:529–546. doi: 10.1080/15226510802115067. [DOI] [PubMed] [Google Scholar]
- Struckhoff GC, Burken JG, Schumacher JG. Vapor-phase exchange of perchloroethene between soil and plants. Environmental Science and Technology. 2005;39:1563–1568. doi: 10.1021/es049411w. [DOI] [PubMed] [Google Scholar]
- Strycharz S, Newman L. USE of native plants for remediation of trichloroethylene: I. deciduous trees. International Journal of Phytoremediation. 2009;11:150–170. doi: 10.1080/15226510802378442. [DOI] [PubMed] [Google Scholar]
- Tillman FD, Choi JW, Smith JA. A comparison of direct measurement and model simulation of total flux of volatile organic compounds from the subsurface to the atmosphere under natural field conditions. Water Resources Research. 2003;39:1284–1295. [Google Scholar]
- United States Environmental Protection Agency. Common chemicals found at superfund sites 2007 [Google Scholar]
- Walton BT, Anderson TA. Microbial-degradation of trichloroethylene in the rhizosphere – potential application to biological remediation of waste sites. Applied and Environmental Microbiology. 1990;56:1012–1016. doi: 10.1128/aem.56.4.1012-1016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XP, Dossett MP, Gordon MP, Strand SE. Fate of carbon tetrachloride during phytoremediation with poplar under controlled field conditions. Environmental Science and Technology. 2004;38:5744–5749. doi: 10.1021/es0499187. [DOI] [PubMed] [Google Scholar]
- Weissflog L, Kruger GHJ, Forczek ST, Lange CA, Kotte K, Pfennigsdorff A, Rohlenova J, Fuksova K, Uhlirova H, Matucha M, Schroder P. Oxidative biodegradation of tetrachloroethene in needles of Norway spruce (Picea abies L.) South African Journal of Botany. 2007;73:89–96. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.