Abstract
The objective of this cohort study was to determine the incidence of Parkinson’s disease (PD) and the effects of race/ethnicity, other demographic characteristics, geography, and healthcare utilization on probability of diagnosis. The authors used the Pennsylvania state Medicaid claims dataset from 1999 to 2003 to identify newly diagnosed cases of PD among the 182,271 Medicaid enrolled adults age 40–65; 319 incident cases of PD were identified. The 4-year cumulative incidence of PD was 45 per 100,000; 54 per 100,000 among whites, 23 per 100,000 among African-Americans and 40 per 100,000 among Latinos (P < 0.0001), corresponding to a relative risk (RR) of PD of 0.43 for African-Americans (P < 0.0001) compared with whites. After adjusting for age, sex, geography, reason for Medicaid eligibility, and average number of visits, African-Americans were still half as likely to be diagnosed with PD as whites (RR 0.45, P < 0.0001). Older age, more healthcare visits and Medicaid eligibility because of income alone also were significantly associated with PD diagnosis, while male sex was not. Observed racial differences in incidence of PD are not explained by differences in age, sex, income, insurance or healthcare utilization but still may be explained by biological differences or other factors such as education or aging beliefs. Better understanding of the complex biological and social determinants of these disparities is critical to improve PD care.
Keywords: African Americans, epidemiology, healthcare disparities, Medicaid, Parkinson’s disease
INTRODUCTION
The relationship between race and Parkinson’s disease (PD) risk is controversial. Most prevalence studies suggest that African-Americans are less likely to have PD than whites.1-3 Prevalence, however, is a poor proxy for risk.4 Studies of incidence better represent risk. The only two incidence studies conducted in the United States that examine the relationship between race and incident PD had contradictory findings.5,6
Differences in methods and samples may partly explain these findings. Mayeux et al.,5 developed a community registry of patients with PD in Northern Manhattan and found that the incidence of PD was more than two times greater in African-American men than white men. The authors’ calculated incidence using US Census data, but census numbers may underestimate minority populations,7 which would have inflated the observed incidence of PD among African-Americans. Van Den Eaden’s study of a large group practice in Northern California concluded that African-Americans were at decreased risk of PD.6 This study had the advantage of reducing bias related to healthcare access since all subjects had the same insurance coverage. These individuals were on average wealthier and better educated than the general population, however, which may affect the generalizability of the results. In addition, the authors did not account for other access-related factors that could affect risk of PD identification such as healthcare utilization and geography.
Both of these studies relied on the initial identification of PD by community clinicians. It may be that geographic and financial barriers to access to healthcare among African-Americans lead to undetected PD among this underserved minority. Undetected PD can lead to additional morbidity and mortality, as it has for many other health conditions such as heart disease and cancer.8 Better understanding of the extent of undetected PD and associated factors can lead to policies and interventions that improve the timely and appropriate diagnosis of PD among at-risk populations. Alternatively, it is possible that there are genetic risk factors related to both race and PD. In this case, understanding the biological determinants of PD will help in the development of future therapies.
The goal of the current study was to estimate racial differences in the incidence of PD. The secondary aim was to determine the role of factors related to healthcare access including geography and healthcare utilization in the diagnosis of PD. We used Medicaid billing claims to determine the diagnosed incidence of PD among a large, racially and ethnically diverse cohort of middle-aged adults with similar socioeconomic status and insurance coverage.
METHODS
The Institutional Review Board at the University of Pennsylvania reviewed and approved the study protocol.
Data Source and Sample
Data were abstracted from Pennsylvania Medicaid adjudicated claims. These data include information on all health services billed through Medicaid insurance, including the date, type, location, provider, and associated ICD-9 code for each individual service. Patient information includes date of birth, sex, self-identified race and ethnicity, zip code and reason for Medicaid eligibility.
The study period was from January 1, 1999 to December 31, 2003 and the sample included 182,271 Medicaid eligible adults ages 40 to 65 years who did not meet the study definition of PD in the year before the start of the study. Vascular parkinsonism associated with cerebrovascular disease and treatment with neuroleptics for schizophrenia or bipolar disorder are two common causes of secondary parkinsonism.9 Because of the concern of misdiagnosis or misclassification, all individuals with at least one claim for conditions that are common causes of secondary parkinsonism – stroke (ICD-9: 433–436), schizophrenia (ICD-9: 295) and bipolar disorder (ICD-9: 296.4–296.9) – were excluded from the cohort. Individuals were censored from the cohort once (1) they met criteria for PD (see below) (2) in a given calendar year they were not Medicaid-enrolled for at least 9 of 12 months or (3) the end of the study period was reached.
Case Definition
To be considered an incident case, subjects must have been: (1) Medicaid-enrolled, (2) have no Medicaid reimbursed claim associated with a diagnosis of PD (ICD-9 332.0) or parkinsonism (ICD-9 332.1) in the 12 months prior to the index claim, (3) and not have received medication indicated for PD in those 12 months. PD medications included levodopa (Larodopa), l-dopa/carbidopa (Atamet, Sinemet, Sinemet CR), l-dopa/ carbidopa/ entacapone (Stalevo), bromocriptine (Parlodel), pergolide (Permax), pramipexole (Mirapex), ropinorole (Requip), and selegiline (Eldepryl, Zelapar).
Independent Variables
Age, sex, racial/ethnic group, county, number of physician visits, and reason for Medicaid eligibility were abstracted from the claims data. Because both age and physician visits were not normally distributed, they were categorized for further data analysis. Age was categorized into two groups: 40–49 and ≥50 years. The average number of yearly physician visits were categorized by quartiles and used as a measure of healthcare utilization and co-morbid conditions. A location of care variable was created based on the urbanization spectrum for counties from the National Center for Health Statistics’ urban–rural classification scheme.10 Medicaid eligibility was either based on income alone or disability.
Data Analysis
Demographic and clinical characteristics were compared among whites, African-American and Latinos using non-parametric ANOVA (age, average number of yearly visits) and Chi-squared test (gender, location of care, incident PD, reason for Medicaid eligibility). Time to PD diagnosis was calculated using Kaplan–Meier survival estimates. Cox regression was used to estimate the adjusted association between independent variables and probability of PD diagnosis. All reported P-values are two-sided. All analyses were performed using SAS.
RESULTS
Of the sample, 68% were white, 28% were African-American, and 4% were Latino (Table 1). Although all demographic variables were statistically significantly different among the three racial/ethnic groups, only location of care and incidence of PD showed differences of any clinically significant magnitude. African Americans were more likely to receive care in urban areas (89%) than Latinos (65%) and whites (44%).
TABLE 1. Demographic and clinical characteristics of Pennsylvania state Medicaid-eligible middle-aged adults, 1999–2003.
| Non-Hispanic White; N = 123,489 | Black; N = 50,808 | Hispanic; N = 7,974 | P | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age (SD) | 54.9 (7.3) | 54.1 (7.3) | 55.5 (7.1) | <0.0001 |
| Female (%) | 73,655 (59.6) | 32,493 (64.0) | 4,834 (60.6) | <0.0001 |
| Clinical | ||||
| Urban location of care (%) | 54,448 (44.1) | 45,374 (89.3) | 5,057 (65.1) | <0.0001 |
| Incident PD (%) | 264 (0.21) | 43 (0.08) | 12 (0.15) | <0.0001 |
| Average number of visits/year (SD) | 4.0 (5.6) | 2.9 (4.1) | 3.3 (4.2) | <0.0001 |
| Medicaid eligibility due to disability (%) | 85,072 (68.9) | 31,113 (61.2) | 5,830 (73.1) | <0.0001 |
Three hundred nineteen incident PD cases were identified. The 4-year incidence of PD was 54 per 100,000 person-years for whites, 23 per 100,000 person-years for African-Americans and 40 per 100,000 person-years for Latinos. This translates to a 4-year cumulative risk of PD that was 0.18%. For whites the risk was 0.21%, for African-Americans 0.08% and Latinos 0.15% (P-value < 0.0001). The Kaplan–Meier curve for time to PD diagnosis by race/ethnicity is shown in the Figure 1. The unadjusted relative risk of PD among African-Americans was 0.43 (95% confidence interval: 0.31, 0.60, P-value < 0.0001) and among Latinos was 0.74 (95% confidence interval: 0.41, 1.31, P-value = 0.30) compared with whites (Table 2).
FIG. 1.
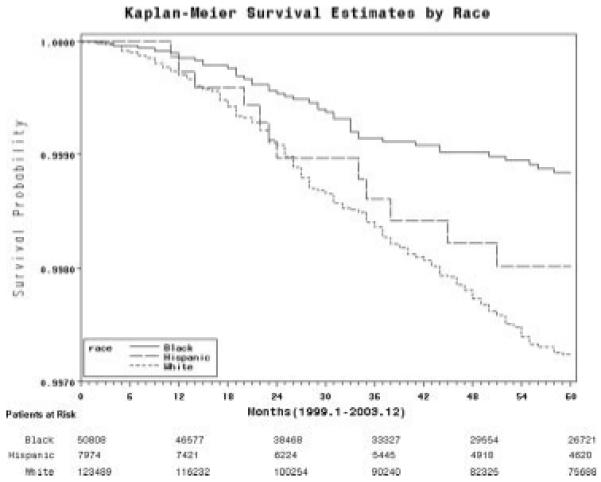
Kaplan–Meier curve showing time to PD diagnosis among Pennsylvania state Medicaid-eligible middle-aged adults, 1999–2003.
TABLE 2. Unadjusted analysis of predictors of PD risk among Pennsylvania state Medicaid-eligible middle-aged adults, 1999–2003.
Reference category non-Hispanic White Americans.
Table 3 shows the results of the Cox regression predicting PD diagnosis. Adjusting for all other demographic and clinical characteristics did not significantly affect the risk associated with race. Sex and location of care were not significantly associated with PD diagnosis. Older age (≥50 years) was associated with a RR of 1.6 (95% confidence interval: 1.21, 2.10, P-value < 0.001) compared with the younger age group (40–49). There was a linear trend in the increase in probability of PD diagnosis with each successive quartile of average annual physician visits. Individuals eligible for Medicaid due to disability had a RR of 0.43 (95% confidence interval: 0.30, 0.62) when compared with those eligible due to income alone.
TABLE 3. Adjusted analysis of predictors of PD risk among Pennsylvania state Medicaid-eligible middle-aged adults, 1999–2003.
| Relative risk |
95% CI | P | |
|---|---|---|---|
| Black racea | 0.45 | 0.32, 0.64 | <0.0001 |
| Hispanic ethnicitya | 0.68 | 0.38, 1.22 | 0.20 |
| Age ≥50 years | 1.44 | 1.09, 1.89 | 0.01 |
| Male | 1.22 | 0.97, 1.53 | 0.09 |
| Urban area of care | 0.95 | 0.75, 1.19 | 0.64 |
| Average no. of visits/yearb | |||
| 2nd quartile | 1.52 | 0.90, 2.57 | 0.12 |
| 3rd quartile | 2.74 | 1.67, 4.49 | <0.0001 |
| 4th quartile | 4.35 | 2.69, 7.03 | <0.0001 |
| Medicaid eligibility due to disability | 0.43 | 0.30, 0.62 | <0.0001 |
Reference category non-Hispanic white Americans.
Quartiles for yearly visits: 1st quartile (reference category) = 0 visits; 2nd quartile = 0.2–1.8 visits; 3rd quartile = 1.9–4.9 visits; 4th quartile = ≥5 visits.
To address concerns related to disproportionate censoring by race/ethnicity and its potential effect on observed differences in the diagnosis of PD, we calculated the proportion of the sample in each racial/ethnic group that was censored. African-Americans were most likely to be censored (37.1%) followed by Latinos (36.8%) and then whites (32.5%).
DISCUSSION
Although the overall incidence of PD among this 40–65-year-old Medicaid-enrolled sample was similar to that found in other studies,11 African-Americans were diagnosed with PD at half the rate of whites, even when controlling for age, sex, location of care, healthcare use, and reason for Medicaid eligibility.
Older age was associated with increased PD risk as in multiple other studies; however, our study did not show an increased PD risk among men.5,6,11 The association between male sex and PD diagnosis was marginally significant (RR 1.2, P-value = 0.09). The lack of statistical significance in our findings may have been due to at least three reasons: (1) Gender differences may only be apparent in patients over the age of 65. Although there was no statistically significant interaction between age and sex in our sample, we only studied individuals younger than 65. An earlier study has shown that differences in PD diagnosis between men and women varied by age, and these differences were observed at ages greater than 65-years;6 (2) As gender inequities in healthcare are addressed gender differences will narrow; and (3) Chance may have resulted in a disproportionate sample of women with PD in our sample thus inflating the risk of PD compared with the general population.
This study also found that greater use of healthcare, as measured by annual physician visits, increased the probability of PD diagnosis, suggesting the importance of healthcare access. The finding that individuals who qualified for Medicaid due to disability had a decreased probability of PD is likely related to our investigation of only incident PD, when functional disability is minimal.
There are some potential limitations to this study. The accuracy of the ICD-9 codes associated with PD in Medicaid claims is unknown. A recent study using Medicare data found the highest specificity (>99%) and positive predictive value (73%) when using ICD-9 332.0 alone.12 To minimize the number of false positives included as cases, we used only this ICD-9 code. Additionally, we excluded those individuals who were at increased risk for secondary parkinsonism based on a history of stroke, bipolar disorder, or schizophrenia. There is still the potential for additional unmeasured factors leading to misclassification bias. However, this misclassification would need to differ between African-Americans and whites to confound any observed differences.
A second limitation is that the generalizability of results may be limited. The sample was relatively young, poor and disabled. However, having a large, diverse cohort with a complete record of health service use offers a valuable resource to approach questions related to the epidemiology of less common disorders and the study of healthcare access. Furthermore, this sample represents a particularly vulnerable group of adults whose needs are important to understand for intervention and policy development.
Lastly, there was unequal dropout because of either death or change in Medicaid eligibility from the study. Although African-Americans were more likely to be censored from the analysis than whites, this is unlikely to completely explain the observed racial differences in the probability of PD diagnosis. For the incidence of PD in African-Americans to equal whites, 2% of censored African-American subjects would have to develop PD which is 10 times greater than the risk we found in our study.
Despite these limitations, there are important implications of these findings. This study is among the few that have examined racial differences in PD incidence.5,6 Our findings of a lower diagnosed incidence of PD among African-Americans than whites are similar to the only other study of PD incidence among a population of adults with the same insurance access.6 Our study had an even lower relative risk of PD among African Americans (RR 0.45) than Van Den Eaden et al. (RR 0.75). This may be explained by differences in our samples (e.g. lower income and lower education in a Medicaid population) and case ascertainment methods, and may suggest that among lower income adults, racial disparities are exacerbated. We cannot compare our results to the second PD incidence study because that study identified no cases among white men under the age of 65.5
To evaluate the association between healthcare access and PD risk we assessed the average number of physician visits per year. Although average yearly visits were independently associated with the identification of PD, they did not affect the relationship between race and PD risk. The number of visits may reflect the level of co-morbid conditions or the ability to negotiate medical bureaucracy to arrange visits. One other study found no contribution of co-morbid conditions in racial differences in rates of PD diagnosis.5 Although we similarly observed lower service use among African-Americans than whites,13 this did not correlate with differences in probability of PD diagnosis.
Some researchers have argued that geographic variations in healthcare may be an important explanation for racial differences in healthcare.14-16 Variations in health systems and healthcare providers can affect the quality of care that patients receive. As African-Americans and Latinos tend to live in different areas than whites, these geographic differences in quality of care may exacerbate racial/ethnic differences. Our study did not find that location of care affected racial differences in PD identification; however, our measure of urbanization may not have captured the geographical variation in quality of care that may be better seen when comparing private, teaching or high-volume centers.
The observed racial differences in diagnosis of PD were not explained by insurance, income, location of care, or healthcare utilization. Biological factors may contribute to observed racial differences in PD. However, in the only US population-based study of PD prevalence, there were no significant racial differences when both post-encephalitic and idiopathic PD cases were counted and less stringent diagnostic criteria than used today were applied to cases.17 Furthermore, this door-to-door study found that almost twice as many African-Americans than whites with PD had been undiagnosed before the study. This finding substantiates the conclusion that the racial differences presented in the current study are due to racial disparities, that is, differences that are not due to clinical needs or appropriateness of care, but may be due to discrimination or bias.
Many other causes of the observed racial disparities should be considered. Patient, physician, and system-level factors may all contribute. Patient-level factors may include education, culture, aging beliefs, trust, and stigma. For example, research on dementia has found that expectations regarding aging vary among people of different racial/ethnic backgrounds and these expectations delay healthcare seeking for symptoms of cognitive decline in ethnic minorities.18 Provider-level factors such as clinician biases, stereotyping and medical uncertainty also play a role in racial disparities. This is evident in studies of depression care in which researchers have shown racial differences in the quality of physician–patient communication.19 Healthcare system factors include financing, accessibility, fragmentation of health coverage and legal policy can all influence racial disparities as well.20
Alternatively, despite a similar burden of PD between racial/ ethnic groups, different clinical phenotypes by race/ethnicity may prevent the appropriate identification of PD in non-white groups because consensus diagnostic criteria are based on evaluation of only white patients.21 It also possible that genetic or environmental risk factors may differ by race/ethnicity and affect the true incidence of PD in non-white populations.
What has been learned in other fields of medicine can be applied to PD. It is possible that PD is under-recognized in African-Americans based on this data, but it remains uncertain why. To develop targeted interventions to improve the appropriate diagnosis of PD among underserved minorities, we will need to use a population-based approach in our research and gain a better understanding of the complex biological and social interactions in creating disparities in neurodegenerative diseases.
Acknowledgments
We would like to thank both The Robert Wood Johnson Foundation Clinical Scholars Program and the Philadelphia VA Medical Center for the research support provided.
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Kessler II. Epidemiologic studies of Parkinson’s diseae II. A hospital-based survey. Am J Epidemiol. 1972;95:308–318. doi: 10.1093/oxfordjournals.aje.a121399. [DOI] [PubMed] [Google Scholar]
- 2.Kessler II. Epidemiologic studies of Parkinson’s disease III. A community-based survey. Am J Epidemiol. 1972;96:242–254. doi: 10.1093/oxfordjournals.aje.a121455. [DOI] [PubMed] [Google Scholar]
- 3.Paddison RM, Griffith RP. Occurrence of Parkinson’s disease in black patients at Charity Hospital in New Orleans. Neurology. 1974;24:688–690. doi: 10.1212/wnl.24.7.688. [DOI] [PubMed] [Google Scholar]
- 4.Gordis L. Epidemiology. 3rd ed. Elsevier, Inc.; Philadelphia: 2004. [Google Scholar]
- 5.Mayeux R, Marder K, Cote LJ, et al. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in Northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 6.Van Den Eaden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 7.Anderson M, Fienberg SE. Race and ethnicity and the controversy over the US Census. Curr Sociol. 2000;48:87–110. [Google Scholar]
- 8.Smedley BD, Stith AY, Nelson AR. Unequal treatment: confronting racial and ethnic disparities in healthcare. The National Academies Press; Washington DC: 2003. [PubMed] [Google Scholar]
- 9.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5:75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- 10.NCHS . National Center for Health Statistics: urban–rural classification scheme for counties. 2006. [Google Scholar]
- 11.Elbaz A, Bower JH, Maraganore DM, et al. Risk tables for Parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55:25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 12.Noyes K, Liu H, Holloway R, Dick AW. Accuracy of medicare claims data in identifying Parkinsonism cases: comparison with the medicare current beneficiary survey. Mov Disord. 2007;22:509–514. doi: 10.1002/mds.21299. [DOI] [PubMed] [Google Scholar]
- 13.Tai-Seale M, Freund D, LoSasso A. Racial disparities in service use among Medicaid beneficiaries after mandatory enrollment in managed care: a difference-in-differences approach. Inquiry. 2001;38:49–59. doi: 10.5034/inquiryjrnl_38.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48(1 Suppl):S42–S53. [PubMed] [Google Scholar]
- 15.Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349:1350–1359. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- 16.Leape LL, Hilborne LH, Bell R, Kamberg C, Brook RH. Underuse of cardiac procedures: do women, ethnic minorities, and the uninsured fail to receive needed revascularization? Ann Intern Med. 1999;130:183–192. doi: 10.7326/0003-4819-130-3-199902020-00003. [DOI] [PubMed] [Google Scholar]
- 17.Schoenberg BS, Anderson DW, Haerer AF. Prevalence of Parkinson’s disease in the biracial population of Copiah County, Mississippi. Neurology. 1985;35:841–845. doi: 10.1212/wnl.35.6.841. [DOI] [PubMed] [Google Scholar]
- 18.Dilworth-Anderson P, Gibson BE. The cultural influence of values, norms, meanings and perceptions in understanding dementia in ethnic minorities. Alzheimer Dis Assoc Disord. 2002;16:S56–S63. doi: 10.1097/00002093-200200002-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ghods BK, Roter DL, Ford DE, Larson S, Arbelaez JJ, Cooper LA. Patient–physician communication in the primary care visits of African Americans and Whites with depression. J Gen Intern Med. 2008;23:600–606. doi: 10.1007/s11606-008-0539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leigh WA, Lillie-Blanton M, Martinez RM, Collins KS. Managed care in three states: experiences of low-income African Americans and Hispanics. Inquiry. 1999;36:318–331. [PubMed] [Google Scholar]
- 21.Hardy J, Singleton A, Gwinn-Hardy K. Ethnic differences and disease phenotypes. Science. 2003;300:738–739. doi: 10.1126/science.300.5620.739. [DOI] [PubMed] [Google Scholar]


