Abstract
The capacity to reason about complex information is a central characteristic of human cognition. An important component of many reasoning tasks is the need to integrate multiple mental relations. Several researchers have argued that rostrolateral prefrontal cortex (RLPFC) plays a key role in relational integration. If this hypothesis is correct, then RLPFC should play a key role in transitive inference, which requires the integration of multiple relations to reach a conclusion. Thus far, however, neuroscientific research on transitive inference has focused primarily on the hippocampus. In this fMRI study, we sought to compare the roles of RLPFC and the hippocampus on a novel transitive inference paradigm. Four relations between colored balls were presented on the screen together with a target relation. Participants were asked to decide whether the target relation was correct, given the other indicated relations between balls. RLPFC, but not the hippocampus, exhibited stronger activation on trials that required relational integration as compared with trials that involved relational encoding without integration. In contrast, the hippocampus exhibited a pattern consistent with a role in relational encoding, with stronger activation on trials requiring encoding of relational predicate–argument structure as compared with trials requiring encoding of item–item associations. Functional connectivity analyses give rise to the hypothesis that RLPFC draws on hippocampal representations of mental relations during the process of relational integration.
INTRODUCTION
The capacity to reason with complex information and to solve novel problems, often referred to as fluid reasoning, is a central characteristic of human cognition (Markman & Gentner, 2001; Halford, Wilson, & Phillips, 1998; Holyoak & Thagard, 1997; Catell, 1987). Understanding this most complex of human abilities provides a daunting but compelling challenge. Relational integration, or the ability to jointly consider multiple relations between mental representations, is a key component of fluid reasoning and higher level cognition (Robin & Holyoak, 1995).
An earlier neuropsychological investigation of relational integration ability showed that structural damage in prefrontal but not temporal cortex in patients with fronto-temporal dementia was associated with severe deficits in relational integration (Waltz et al., 1999). fMRI research has implicated anterior PFC—in particular rostrolateral prefrontal cortex (RLPFC; lateral Brodmann’s area 10)—in this function (Ramnani & Owen, 2004; Christoff & Gabrieli, 2000). For example, it has been shown that RLPFC is specifically engaged by comparison of relations in propositional analogy tasks (Wright, Matlen, Baym, Ferrer, & Bunge, 2008; Wendelken, Nakhabenko, Donohue, Carter, & Bunge, 2007; Green, Fugelsang, Kraemer, Shamosh, & Dunbar, 2006; Bunge, Wendelken, Badre, & Wagner, 2005), by the combination of relational patterns on the Raven’s progressive matrices task (Crone, Wendelken, Leijenhorst, Honomichl, & Bunge, 2009; Kroger et al., 2002; Christoff et al., 2001) and by the requirement to perform a second-order matching across pairs of relations (Smith, Keramatian, & Christoff, 2007; Christoff, Ream, Geddes, & Gabrieli, 2003).
On the basis of the evidence that RLPFC is involved in relational integration, we hypothesized that this region should play a key role in transitive inference, which requires the joint consideration of multiple relations to reach a conclusion (e.g., if B → C and A → B, then A → C). By contrast, we reasoned, if RLPFC’s role in relational processing is not limited to relational integration, then it should not be differentially engaged by a transitive inference problem relative to a well-matched problem in which participants consider multiple relations without integrating them.
Previous neuroscientific studies of transitive inference have focused primarily on the role of the hippocampus, a region implicated in relational memory (Cohen, Poldrack, & Eichenbaum, 1997; Eichenbaum, Otto, & Cohen, 1992). In a key study, the hippocampus was implicated in transitive inference over odor relationships in rats (Dusek & Eichenbaum, 1997). After being well trained on a set of four overlapping odor relationships (e.g., A > B, B > C, C > D, and D > E), rats with hippocampal–cortical disconnection were—unlike control rats—unable to make correct transitive inferences about odors that were unpaired during training (e.g., B > D). On the basis of these and other findings, the authors argued that hippocampus is required for the flexible access to memory required when inferring across relations.
The association between transitive inference and hippocampus has been extended to humans. A PET imaging study involving the memorization of ordered relations between pairs of faces demonstrated increased hippocampal activation for the memorization of “bridging pair” relations that enabled transitive inference (Nagode & Pardo, 2002). In an fMRI study (Heckers, Zalesak, Weiss, Ditman, & Titone, 2004), subjects were trained to identify a preference relationship between selected pairs of pattern stimuli before scanning. The hippocampus was engaged whenever subjects had to infer across multiple learned pairs to choose a preferred pattern. A third human imaging study replicated both of these findings, demonstrating both increased hippocampal activation during memorization of “bridging pairs” and increased hippocampal activation for the test-time performance of transitive inference relative to direct recall (Greene, Gross, Elsinger, & Rao, 2006). Notably, all of the studies that have implicated the hippocampus in transitive inference involved retrieval of previously learned relations as a prerequisite for inference.
We hypothesized that the role of the hippocampus in these transitive inference tasks is related to relational memory requirements rather than to relational integration demands. Although the hippocampus is required for the flexible access to long-term memory that is needed when inferring across memorized relations, this does not mean that it is involved in bridging across two relations to infer a relationship between previously unrelated items (see also Lipton & Eichenbaum, 2007). We consider this latter process to be the key component of transitive inference (c.f. Penn, Holyoak, & Povinelli, 2008; Hummel & Holyoak, 2001; Waltz et al., 1999). To test whether the hippocampus would be involved in transitive inference even in the absence of explicit long-term memory demands, we designed a task in which multiple novel visuospatial relations were displayed for the duration of a trial and compared trials on which responding required or did not require an inference.
There is recent evidence that the hippocampus is involved in basic relational encoding during the viewing of visual scenes, even in the absence of explicit long-term memory requirements. For example, patients with hippocampal damage have been shown to exhibit deficits on short-term relational memory tasks (Hannula, Tranel, & Cohen, 2006; Olson, Page, Moore, Chatterjee, & Verfaellie, 2006), and engagement of hippocampus during relational short-term memory has been demonstrated with fMRI (Hannula & Ranganath, 2008). Thus, we predicted that the hippocampus would be engaged by relational encoding demands, although not by the need to integrate multiple relations.
In the current study, we used fMRI to test the hypothesis that the RLPFC and the hippocampus would contribute differentially to transitive inference and relational encoding. Our 2 × 2 factorial design (see Figure 1) crossed a manipulation of the type of judgment required (direct vs. inference), with a manipulation of the type of relations being considered (general vs. specific relations). On each trial, participants viewed four source relations and one target relation and were asked to indicate via button press if the target relation was valid, given the four source relations. Relations were depicted using pairs of colored balls, where balls in a pair were related to one another in a manner specified by a simple icon.
Figure 1.
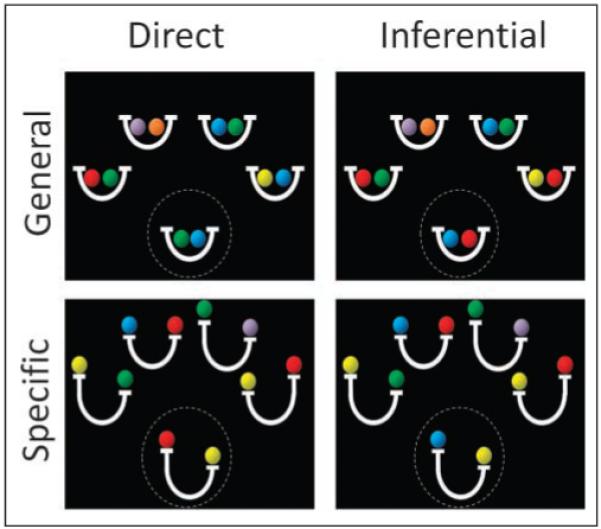
Sample valid stimuli for each of the task conditions. The participant’s task was to judge the validity of the target relation (circled here for illustrative purposes) on the basis of the four source relations. For general relation trials, the basket icon indicated the existence of an equality relationship. For specific relation trials, the balance scale icon indicated the existence of an inequality relationship. For the examples shown, invalid trials would be created either by replacing the blue ball in the target relation with an orange ball (general relations) or by switching the position of the two balls in the target relation (specific relations).
Inference trials, unlike direct trials, required participants to integrate across two source relations to evaluate the target relation. We reasoned that the contrast between inference trials and direct trials would yield brain activation related to transitive inference. At first glance, direct and inference trials appear identical; in fact, it is possible to replace the color of one ball in the target relation to convert a direct trial into an inference trial and vice versa. These well-matched conditions were intermixed without warning, and therefore participants were given no indication that they should allocate more attention to one problem than to another. We consider that this manipulation of inference versus direct trials provides the best test to date of the relational integration hypothesis of RLPFC function.
On specific relation trials, participants encoded ordered balance relations between items. Each relation consisted of two balls (e.g., red and green) on an iconic balance scale (Figure 1), with the following possible relationships: (1) the red and green balls could be equally heavy, (2) the red ball could be heavier than the green ball, or (3) the green ball could be heavier than the red ball. In contrast, on general relation trials, participants encoded bidirectional item–item associations; that is, if a red ball was associated with a green ball, it was equally true that the green ball was associated with the red ball. These bidirecgtional associations were depicted as pairs of balls in iconic “baskets,” where the “baskets” were visually similar to the balance scales used for the specific relation trials (see Figure 1). Mathematically, general relation trials involved only equality relations, whereas specific relation trials also involved inequality relations. Thus, on specific relation trials, participants had to encode predicate–argument structure, that is, the specific relational predicates and the ordering of items within each relation. The general relation trials, by contrast, can be solved without reference to the specific relational predicate and thus in the absence of any encoding of predicate–argument structure (although encoding of such a structure is not precluded). The contrast between specific and general trials was used to identify brain regions that process predicate–argument structure, a key aspect of relational encoding (Hurford, 2003).
We predicted a double-dissociation between the RLPFC and the hippocampus, such that RLPFC would be differentially engaged by inference > direct judgments, consistent with a role in relational integration, and the hippocampus would be differentially engaged by specific > general relations, consistent with a role in relational encoding.
METHODS
Experimental Task
As described in the Introduction, the ball task included four conditions: general direct, specific direct, general inference, and specific inference trials (Figure 1). On general trials, each equality relation was depicted as a pair of colored balls in an iconic basket, whereas on specific trials, each inequality relation was depicted as a pair of colored balls on an iconic balance scale (Figure 1). Direct trials could be solved by considering only one of the four source relations, whereas inference trials required consideration of two source relations.
Each trial began with a 1-sec presentation of four source relations, followed by presentation of the source relations together with a target relation. Participants were required to indicate by pressing one of two buttons whether each target relation was valid, given the source relations. Half of the trials were valid, requiring a “yes” response, and the remaining trials were invalid. A new stimulus array was randomly generated on every trial, and valid and invalid trials were randomly intermixed. The full stimulus array remained onscreen until the participant responded, up to a maximum of 5 sec. A blank screen separated each pair of trials; the duration of this ITI was jittered to optimize efficiency (Dale, 1999). There were 48 trials in each of the four conditions across four 6-min scan sessions. The four trial types were randomly intermixed, with no cues as to the nature of the upcoming trial. On every trial, three of four source relations contained at least one item from the target relation, and the target relation was never an exact visual match for any of the source relations.
Data Collection
Sixteen right-handed young adults (12 women) were scanned on a Siemens 3T Trio at the UCSF Neuroscience Imaging Center. High-resolution anatomical images (MPRAGE, Siemens AG, Münich, Germany) were acquired first from each subject, followed by acquisition of echo-planar functional images during performance of the task. For the functional images, thirty-three 3.45-mm axial slices (3 mm plus .45 mm gap) were collected with repetition time = 2 sec, echo time = 25 msec, field of view = 230 mm, and 128 × 128 voxels. Visual stimuli were displayed on a monitor that participants were able to view by means of a mirror. Subjects responded by pressing one of two buttons on a button box that was held in the right hand. Stimulus presentation and response acquisition were controlled by the Presentation software system (Neurobehavioral Systems, Inc.; Albany, CA).
Data Analysis
Data were preprocessed and analyzed using SPM5 (Wellcome Department of Cognitive Neurology, London). Functional images were corrected for differences in slice acquisition timing and were realigned to the first volume by means of rigid body motion correction with sinc interpolation. Structural images were coregistered to the functional images and then spatially normalized to SPM5’s T1 template. These normalization parameters were then applied to the functional images. Functional images were spatially smoothed with an 8-mm FWHM isotropic Gaussian kernel. The data were then high-pass filtered with a limit of 120 sec and submitted to statistical analyses.
Whole-brain exploratory analysis was performed using a general linear model (GLM) that incorporated task effects, session effects, and a general linear trend. Task effects were modeled via zero-duration event regressors, located at query stimulus onsets (i.e., at the appearance of the final relation picture in each trial) that were convolved with SPM’s canonical hemodynamic response function. In a secondary analysis, task effects were modeled as epochs with durations equal to the response times (RTs) on each trial. There were regressors for each of the four experimental conditions as well as a separate regressor for all incorrect trials. This GLM was used to compute the least-squares parameter estimate of the height of the best-fitting synthetic response function for each condition at each voxel. Parameter estimates associated with each experimental condition were combined to produce contrast images for target contrasts. Group-level t tests were performed on these contrast images to produce group activation maps. Activation clusters that survived a voxel-level threshold of p < .001 (uncorrected) with 10-voxel extent threshold, roughly corresponding to acluster p value of .05 (Poline, Worsley, Evans, & Friston, 1997), are reported as significant.
ROI analyses were performed using Marsbar (http://marsbar.sourceforge.net). The specific regions utilized are described in the Results section. Functionally defined ROIs were obtained from activation clusters identified in the whole-brain contrasts. Anatomical template regions were obtained from the Anatomical Automatic Labeling repository (Tzourio-Mazoyer et al., 2002), included with the Marsbar distribution. ROIs based on results from the literature were created as 5-mm spheres around specific coordinates. The mean signal across all voxels in a defined region was submitted to the GLM analysis as described above to produce an ROI parameter estimate for each experimental condition for each subject. These ROI parameter estimates were then submitted to repeated measures ANOVA in SPSS.
Correlated activity between an ROI and other brain regions was assessed via the beta correlation method (Rissman, Gazzaley, & D’Esposito, 2004), implemented via SPM5 and custom Matlab (Mathworks, Natick, MA) scripts. SPM’s canonical hemodynamic response function was fit to each occurrence of each condition, and the resulting parameter estimates (betas) were sorted according to condition to produce a condition-specific beta series for each voxel. The beta series associated with a functional ROI seed were correlated with voxels across the brain to produce beta correlation images. Contrasts between beta correlation images were subjected to an arc-hyperbolic tangent transform to allow for statistical inference based on the correlation magnitudes. Group-level t tests were performed on the resulting subject contrast images to produce group correlation contrast maps.
RESULTS
Behavioral Performance
Both experimental manipulations affected task performance (see Table 1). As revealed by ANOVA, participants were slower (4.3 vs. 2.9 sec, F = 119, p < .001) and less accurate (84% vs. 96%, F = 12.3, p = .007) on inference trials than on direct trials. Additionally, participants were slower (3.71 vs. 3.43 sec, F = 5.6, p = .04) and less accurate (87% vs. 93%, F = 35.4, p < .001) on specific relative to general relation trials. For RTs, there was a significant interaction between inference demand and relational encoding demand (F = 36.5, p < .001), such that participants responded more slowly to specific than to general relations on inference trials, but not on direct trials.
Table 1.
Behavioral Results for Each Experimental Condition
| Condition | Accuracy (%) | RT (sec) |
|---|---|---|
| General direct | 98.5 ± 1 | 3.0 ± 0.13 |
| General inference | 87.6 ± 3.7 | 3.9 ± 0.19 |
| Specific direct | 93.1 ± 2.6 | 2.8 ± 0.09 |
| Specific inference | 80.1 ± 3.2 | 4.7 ± 0.15 |
Valus are presented as mean ± SE.
Transitive Inference: Inference > Direct
The main effect of transitive inference was tested via examination of the contrast between inference trials and direct trials. Consistent with our prediction, this primary contrast pointed to right RLPFC (BA 10; 33, 54, 6) as the most significant locus of activation (Figure 2A, blue, and Table 2A). There were two additional, but less prominent, loci of activation for this contrast in right premotor cortex and medial frontal gyrus.
Figure 2.

(A) Significant areas of activation for both the inference > direct contrast (blue) and for the specific > general contrast (yellow), displayed on a rendered brain. Inset right: RLPFC activation for inference > direct from the RT-convolved analysis. Inset left: SPL activation for specific > general from the RT-convolved analysis. (B) Inference > direct contrasts for general relations (shown in red on the coronal section) and for specific relations (shown in yellow). ROI graphs for RLPFC regions identified from these contrasts are shown on both sides of the coronal section.
Table 2.
Activation Clusters from the Main Whole-brain Contrasts
| Region | x, y, z | T (Peak Voxel) | No. Voxels (p < .001) | Cluster p Value |
|---|---|---|---|---|
| A. Transitive inference: inference > direct | ||||
| Right RLPFC (BA 10) | 33, 54, 6 | 5.37 | 28 | .004 |
| Right middle FG (BA 6) | 30, 18, 60 | 3.92 | 11 | .049 |
| Medial FG (BA 8) | −9, 45, 45 | 4.60 | 14 | .029 |
| B. Relational encoding: specific relations > general relations | ||||
| Right middle FG (BA 6) | 30, 6, 60 | 5.54 | 20 | .018 |
| Right SPL/IPL (BA 7, 40) | 42, −39, 60 | 5.27 | 79 | <.001 |
| Right SPL (BA 7) | 21, −69, 51 | 5.28 | 30 | .005 |
| Left SPL/IPL/precuneus (BA 7, 40) | −9, −51, 60 | 8.02 | 145 | <.001 |
| Right MOG (BA 19) | 30, −84, 6 | 5.52 | 58 | <.001 |
| Left MOG (BA 19) | −27, −93, 15 | 4.91 | 21 | .015 |
| Thalamus | 15, −15, 9 | 9.02 | 61 | <.001 |
| Culmen of Cb, PHG (BA 19, 30) | 15, −42, −9 | 6.63 | 55 | <.001 |
Activations maps were thresholded at p < .001 (uncorrected) with a 10-voxel cluster-size minimum. FG = frontal gyrus; IPL = inferior parietal lobe; MOG = middle occipital gyrus; PHG = parahippocampal gyrus; Cb = cerebellum.
To rule out the possibility that RLPFC was more active on inference than direct trials simply because participants took longer to respond on inference trials, we performed a secondary analysis in which RTs for each trial were incorporated into the GLM. Even when controlling differences in RTs between conditions, right RLPFC was the most prominent activation for the inference > direct contrast (Figure 2B).
Because inference processing could differ as a function of the type of relation being handled, we also examined the inference > direct whole-brain contrast separately for the general and the specific trial types. When this contrast was limited to general relation trials, we observed bilateral RLPFC, including a right-side region (30, 54, 6) similar to that identified in the main effect contrast, as well as a more ventral cluster on the left side (−30, 48, −3). When the contrast was limited to specific relation trials, only a right-side RLPFC activation was present; this region was located laterally to the previously identified cluster (45, 57, 9). ANOVAs across conditions within each of these clusters revealed a significant Region × Inference × Relation interaction (F = 17.6, p = .001), indicating that different types of relations may be integrated by different populations of neurons in RLPFC. It remains to be seen whether this unexpected finding will generalize to other studies.
Despite the prior literature focusing on the role of the hippocampus in transitive inference, no hippocampal voxels were activated by any of the above contrasts, even at a relaxed statistical threshold (p < .005). As described below, ROI analyses in the hippocampus confirmed that this structure was not modulated by transitive inference requirements per se. Consistent with our predictions, only RLPFC showed robust modulation by relational integration demands.
Relational Encoding: Specific > General Relations
Relational encoding demand was examined by contrasting specific and general relation trials. This contrast revealed prominent activation in bilateral posterior parietal cortex (BA 7, 40) as well as in a cluster that extended from the cerebellum to the parahippocampal gyrus (Figure 2A, yellow, and Table 2B). This cluster did not include activation in the hippocampus proper, although we tested for an effect of specific > general relations in hippocampal ROIs, as discussed below. The finding that the superior parietal lobule (SPL) is implicated in the encoding of predicate–argument structure for the visuospatial relationships presented in this task is consistent with previous demonstrations of SPL involvement in relational reasoning (Goel & Dolan, 2001).
Because specific relation trials were associated with longer RTs than general relation trials, we examined the same specific > general relation contrast for the secondary analysis in which RTs were factored into the GLM. Significant activation was observed in the SPL (BA 7), as previously. In addition, significant activation was observed in left DLPFC (BA 9; −48, 6, 30), consistent with previous studies that have linked this region to relational encoding (Wendelken, Bunge, & Carter, 2008; Murray & Ranganath, 2007).
ROI Analysis: Hippocampus
Hippocampus was not significantly activated in the wholebrain exploratory analyses for either the transitive inference or the relational encoding contrasts. However, the role of the hippocampus in our task was of particular interest because of the prior evidence of participation by this region in transitive inference as well as relational encoding. To test our predictions about the hippocampus, we examined several hippocampal ROIs based on coordinates from prior fMRI studies (see Figure 3A). One spherical ROI, centered at (34, −14, −16) in right anterior hippocampus, was based on fMRI activation observed during transitive inference (Heckers et al., 2004). Another spherical ROI, centered at (−27, −33, 3) in left posterior hippocampus, was based on activation observed during relational encoding (Hannula & Ranganath, 2008). In addition to these literature-based ROIs, we also examined anatomical ROIs constructed from a hippocampal template (Tzourio-Mazoyer et al., 2002), collapsing across left and right hemispheres. Because prior studies have found functional distinctions between relatively more anterior and posterior portions of the hippocampus (Schacter & Wagner, 1999; Lepage, Habib, & Tulving, 1998; Moser & Moser, 1998), we divided the hippocampal template into an anterior part (y > −20) and a posterior part (y < −20; see Lepage et al., 1998).
Figure 3.

(A) Hippocampal ROIs derived from previous studies of transitive inference (left) and relational encoding (right) are depicted as light blue circles overlaid on the dark blue hippocampal template ROI. There was no effect of inference or relational encoding in right anterior hippocampus. In the left posterior region, there was a significant effect of relational encoding. Similar results were obtained from bilateral anterior and posterior template regions, separated on the graphs by a dashed line. (B) Results of a functional connectivity (beta series correlation) analysis that utilized right RLPFC as a seed region revealed strong correlation across frontal and parietal cortices during inference trials relative to baseline (left) and increased functional connectivity between RLPFC and right hippocampus for inference trials relative to direct trials (right; thresholded at p < .001 and >10 voxels).
None of the four hippocampal ROIs exhibited a main effect of, or interaction involving, transitive inference (all ps > .2). However, both posterior hippocampal ROIs exhibited a main effect of relational encoding, with increased activation for specific trials relative to general trials (template: F = 4.1, p = .04; spherical: F = 5.2, p = .02, respectively). In summary, current evidence supports involvement of posterior hippocampus in relational encoding but shows that the hippocampus is not engaged by transitive inference demands per se.
Functional Connectivity during Inference
The present data implicate RLPFC as the primary locus of transitive inference processing, although naturally this region does not operate in isolation. As such, we sought to use functional connectivity analysis methods to identify brain regions that were engaged in concert with RLPFC during task performance. The right RLPFC activation cluster identified in the primary inference > direct contrast was used as a seed in two beta series correlation analyses (Rissman et al., 2004)
First, we probed for regions that were functionally correlated with right RLPFC during performance of inference trials, relative to the resting baseline (Figure 3B, left panel, and Table 3A). This analysis revealed a swath of activation extending back from RLPFC, including dorsolateral PFC (BA 9, 46) and posterior parietal cortex (BA 7, 40). Thus, as expected, a number of prefrontal and parietal regions appear to work together during transitive inference.
Table 3.
Clusters That Exhibited Significant Functional Connectivity with Right RLPFC, for (A) Inference > Baseline, Thresholded at p < .0001 (Uncorrected), and (B) Inference > Direct Thresholded at p < .001 (Uncorrected)
| Region | x, y, z | T (Peak Voxel) | No. Voxels (p < .001) | Cluster p Value |
|---|---|---|---|---|
| A. Correlation with right RLPFC: inference > baseline | ||||
| Right RLPFC, DLPFC (BA 10, 46) | 33, 54, 6 | 23.14 | 491 | <.001 |
| Left RLPFC (BA 10) | −36, 54, −3 | 5.87 | 43 | <.001 |
| Left DLPFC, VLPFC (BA 9, 45) | −60, 12, 21 | 5.61 | 41 | <.001 |
| Medial FG, ACC (BA 9, 6, 32) | 6, 39, 42 | 7.42 | 98 | <.001 |
| Left postcentral gyrus | −57, −21, 27 | 7.8 | 131 | <.001 |
| Right postcentral gyrus | 45, −15, 60 | 7.14 | 61 | <.001 |
| Right SPL, IPL (BA 7, 40) | 42, −51, 54 | 5.88 | 52 | <.001 |
| Left SPL (BA 7) | −30, −72, 36 | 5.79 | 33 | <.001 |
| Left MTG (BA 39, 19) | −39, −75, 21 | 6.94 | 80 | <.001 |
| Lingual gyrus (BA 18) | 3, −78, 0 | 6.77 | 37 | <.001 |
| Right MOG, lingual gyrus (BA 18) | 21, −96, −9 | 5.56 | 33 | <.001 |
| Left MOG (BA 18) | −27, −87, 0 | 5.89 | 24 | <.001 |
| Thalamus | 12, −9, 9 | 5.93 | 46 | <.001 |
| B. Correlation with right RLPFC: inference > direct | ||||
| Right hippocampus and PHG | 27, −21, 12 | 4.69 | 11 | .016 |
For the more general contrast of inference > baseline, clusters smaller than 20 voxels are not listed. DLPFC = dorsolateral PFC; VLPFC = ventrolateral PFC; FG = frontal gyrus; IPL = inferior parietal lobe; MOG = middle occipital gyrus; PHG = parahippocampal gyrus.
Second, we probed for regions that were differentially correlated with right RLPFC during performance of inference vs. direct trials (Figure 3B, right panel, and Table 3B). Only one cluster demonstrated this pattern (at p < .001); this cluster consisted of voxels in right hippocampus (27, −18, −15) and parahippocampal gyrus (27, −21, −12). An ROI analysis of this cluster revealed no effect of either transitive inference or relational encoding. Thus, activation in right hippocampus was more strongly correlated with right RLPFC during transitive inference than during direct judgments, although the hippocampus was equally engaged on inference and direct trials. This finding indicates that RLPFC may draw preferentially on hippocampal relational representations on trials during relational integration.
DISCUSSION
RLPFC Supports Inference across Multiple Relations
The current findings provide evidence that RLPFC contributes to transitive inference by integrating previously unlinked mental relations to form a novel relation. At the same time, the current finding that RLPFC was engaged during performance of transitive inference problems relative to well-matched direct judgment problems is arguably the best evidence to date in favor of the relational integration hypothesis of RLPFC function (Smith et al., 2007; Wendelken et al., 2007; Green et al., 2006; Bunge et al., 2005; Christoff et al., 2001). The current study employs a transitive inference task that is novel to the investigation of relational integration, and thus the finding that RLPFC is the most prominent locus of activation during transitive inference provides important independent confirmation of the relational integration hypothesis.
Previous neuroimaging studies have demonstrated engagement of pFC by transitive inference but have not implicated RLPFC (Heckers et al., 2004; Acuna, Eliassen, Donoghue, & Sanes, 2002). Heckers et al. (2004) reported activation in left inferior frontal gyrus (BA 47), near RLPFC, whereas although Acuna et al. (2002) report activation of bilateral dorsolateral PFC. Unlike the current study, these previous studies involved transitive inference over memorized relations. It may be that the demand for on-line relational integration is reduced when subjects have the opportunity, through repeated exposure, to develop efficient mnemonic representations of an underlying sequence.
The finding that right but not left RLPFC was most clearly implicated in relational integration in this study may be due at least in part to the visuospatial nature of the task. In previous studies involving verbal propositional analogies, left RLPFC was most strongly implicated in the integration of semantic relations (Wendelken et al., 2007; Bunge et al., 2005; Christoff et al., 2001). In most prior studies of relational integration, left and right RLPFC exhibit fairly similar patterns of activation, although one side or the other may be more strongly modulated by a particular task manipulation. It may be that left and right RLPFC carry out the same basic functions, but that one side is preferentially engaged for a particular task basedon privileged access to task-specific representations in the same hemisphere.
It has been argued that relational reasoning ability, of which relational integration is a key component, is one of the few cognitive capacities in which humans are profoundly different than other animals (Penn et al., 2008). Similarly, anterior PFC (BA 10), including RLPFC, has been shown to be the region that is most expanded in humans relative to other primates (Semendeferi, Armstrong, Schleicher, Zilles, & Van Hoesen, 2001). Taken together, these findings are suggestive of the intriguing possibility that a relational integration function mediated by RLPFC may be unique to humans.
It must be noted that relational integration is but one of several competing accounts of anterior prefrontal function. Anterior PFC, including RLPFC, has been hypothesized to integrate information of all kinds and not merely relations (De Pisapia, Slomski, & Braver, 2007; Reynolds, McDermott, & Braver, 2006; Ramnani & Owen, 2004). Given that anterior PFC is well-connected to multimodal regions that represent complex information (Petrides & Pandya, 2007), it is quite possible that this region does support integration of complex representations, whether these are strictly relational.
Another set of accounts of anterior PFC function focuses on the distinction between external (stimulus-dependent) and internal (stimulus-independent, self-generated) representations, suggesting that RLPFC processes internal representations (Burgess, Simons, Dumontheil, & Gilbert, 2006; Christoff et al., 2003; Christoff & Gabrieli, 2002). Relations are internal representations, so relational integration is an example of internal processing and the current results are not inconsistent with the hypothesis that RLPFC supports this more general function. However, in an earlier study of analogical problem solving, we observed that RLPFC was less active when participants had to complete an analogy (e.g., “writer is to pen as painter is to…?”) than when they had to evaluate a complete analogy (e.g., “writer is to pen as painter is to brush?”), despite the fact that the former problem places greater demands on self-generation (Wendelken et al., 2007). This prior finding argues against the idea that RLPFC is broadly engaged by internal processing, suggesting instead that it is involved in the comparison or integration of disparate mental relations.
Yet another set of accounts emphasizes the role of anterior PFC in planning and/or coordinating task performance (Sakai & Passingham, 2006; Gilbert, Frith, & Burgess, 2005; Braver & Bongiolatti, 2002; Burgess, Veitch, de Lacy Costello, & Shallice, 2000; Koechlin, Basso, Pietrini, Panzer, & Grafman, 1999). The current results, as well as a number of other studies from our laboratory, show that RLPFC is active in the absence of explicit task coordination requirements (Crone et al., 2009; Wendelken et al., 2007; Bunge et al., 2005), and a meta-analysis from Gilbert et al. (2006) implicates not the RLPFC (lateral BA 10) but rather the frontal pole (medial BA 10) in this function.
Finally, multiple studies have pointed to a role for RLPFC in episodic retrieval, and in particular in the monitoring of retrieved content (McDermott, Jones, Petersen, Lageman, & Roediger, 2000; Ranganath, Johnson, & D’Esposito, 2000; Rugg, Fletcher, Frith, Frackowiak, & Dolan, 1996). The retrieval monitoring hypothesis cannot explain the present results; rather, it is likely that the relational integration hypothesis explains the association between RLPFC and retrieval monitoring, insofar as monitoring involves comparison (integration) of a retrieved representation with a template (Dobbins & Han, 2006).
Hippocampus Encodes Relations and Is Functionally Correlated with RLPFC during Inference
The current results indicate that the hippocampus does not perform the integration of relations in working memory that is essential to transitive inference over novel relations because it was no more engaged for inference trials than for direct trials. That posterior hippocampus demonstrated positive activation values across all conditions (Figure 3A, right) may indicate that this region plays a more general role in relational processing. However, the hippocampus was more strongly correlated with RLPFC during inference than during direct judgments, suggesting that the representations in this region are called upon for transitive inference. We propose that the hippocampus encodes the individual visuospatial relations presented on the screen, consistent with a general role in relational encoding, and that the outcome of this hippocampal operation is accessed by RLPFC during relational integration. This proposal is consistent with a growing body of literature that describes the differing roles of hippocampus and frontal cortex in relational processing (Ryan, Moses, & Villate, 2009; Ryan & Cohen, 2004). By this account, the hippocampus supports transitive inference, but the inference itself is made at a later stage in neural processing.
Results from the ROI analyses for posterior hippocampus in the current study support prior evidence that the hippocampus is involved in relational encoding, even in the absence of explicit long-term memory demands. For example, Hannula and Ranganath (2008) showed that hippocampus is engaged when subjects must maintain a set of visual relationships over a short delay period. Earlier electrophysiologial and neuroimaging studies had shown that hippocampus is involved in the short-term maintenance of novel information (Ranganath & D’Esposito, 2001; Miyashita & Chang, 1968). In addition, studies of patients with hippocampal damage have demonstrated that damage to this region is associated with deficits on shortterm relational memory tasks (Hannula et al., 2006; Olson et al., 2006), suggesting that hippocampal activation is not merely incidental to the short-term retention of relations. Our results confirm the involvement of the hippocampus in relational encoding over short intervals and further demonstrate that this region plays only a supporting role in relational integration.
It has been suggested that the contribution of the hippocampus to transitive inference is related to the creation of a highly structured memory for a set of learned items (Fortin, Agster, & Eichenbaum, 2002) or possibly to the establishment of a simpler magnitude representation (ranking) of learned items (Penn et al., 2008; Van Elzakker, O’Reilly, & Rudy, 2003). Recent evidence from the study of relational memory and sleep suggests that the formation of the higher order memory structure that would support inference is a slow process: Time and especially sleep demonstrably improved the ability of subjects to perform transitive inference across memorized relations (Ellenbogen, Hu, Payne, Titone, & Walker, 2007). Thus, although the hippocampus is involved in the integration of memories over an extended period, we argue that this hippocampal-dependent memory integration is fundamentally different from the fast and temporary integration of relational representations in working memory that was supported by RLPFC. We propose that the hippocampus plays a supporting role in transitive inference by encoding relations between items and that it is RLPFC that performs the essential integrative function for transitive inference in working memory. This interpretation of hippocampal function during transitive inference is, we believe, fundamentally consistent with the original interpretation of Dusek and Eichenbaum (1997), who argued that hippocampus is critical for the “development or flexible expression of a representation of orderly relations among stimulus items.” Moreover, one recent study involving excitotoxic hippocampal lesions in mice lends strong support to this view of hippocampus (Van der Jeugd et al., 2009). In this study, mice that underwent surgery before training on a set of relations were impaired on a subsequent transitive inference task, but mice that underwent surgery after training but before the inference task were not impaired.
An open question is the nature of the anatomical connection between RLPFC and hippocampus that could support the observed pattern of functional connectivity. In the macaque, there are no known direct projections between the anterior PFC and the hippocampus (Petrides & Pandya, 2007). However, it is possible that direct projections between these regions exist in the human: RLPFC is disproportionately larger in humans than in even our closest primate relatives (Semendeferi et al., 2001), and recent electrophysiological recordings from anterior PFC in macaques suggest that this region may be more akin to the frontal pole than to RLPFC in humans (see Tsujimoto, Genovesio, & Wise, 2008). There are also multiple indirect pathways that are candidates for communication between the RLPFC and the hippocampus (e.g., via DLPFC); however, the current results do not point clearly to any one path.
A number of prior studies have pointed toward a functional differentiation between more anterior and more posterior regions of the hippocampus, although the nature this difference remains unclear (Schacter & Wagner, 1999; Lepage et al., 1998; Moser & Moser, 1998). In the current study, only posterior hippocampus was associated with encoding visuospatial relations. It was a cluster in a relatively anterior portion of the hippocampus, however, that was functionally connected with RLPFC more strongly during inference than during direct judgments. It has been suggested that episodic or spatial memory is specifically subserved by posterior hippocampus (Greicius et al., 2003; Moser & Moser, 1998). Both episodic and spatial forms of memory are characterized by the need to encode predicate–argument structure; for example, a remembered episode involves specific agents, items, and locations in specific relations with one another. Spatial memory requires knowledge of specific relationships between objects, for example, the circle is above the square (and not vice versa). Thus, our finding of greater posterior hippocampal activation during the encoding of specific relative to general relations is broadly consistent with this literature. In contrast to hypotheses about the function of posterior hippocampus, it has been argued that anterior hippocampus is critical for associative encoding, as evidenced by increased activation for memorization of item–item associations (Chua, Schacter, Rand-Giovannetti, & Sperling, 2007; Giovanello, Schnyer, & Verfaellie, 2004). To the extent that item–item associations are encoded in both general and specific relation trials in this study, this could account for the finding that anterior hippocampus was not differentially engaged by these conditions.
Summary
The present study demonstrates that RLPFC is the primary locus of transitive inference processing and thereby provides strong support for the relational integration hypothesis of RLPFC function. Despite a strong focus on the hippocampus in the transitive inference literature, the current results demonstrate that hippocampus is not specifically engaged by transitive inference in the absence of explicit long-term memory demands. Hippocampus does appear to play a supporting role, however, given both its sensitivity to the manipulation of relational encoding demands and its increased functional connectivity to RLPFC during inference. These results give rise to the hypothesis that RLPFC draws on hippocampal as well as parietal representations of visuospatial relations during the process of relational integration.
Acknowledgments
The authors thank Chris Blais, Craig Brozinsky, Sandeep Sabhlok, and Gary Turner for their help. This work was supported by an NINDS grant (R01 NS157046-01) and by an NIH program project grant (P01 NS40813-06).
REFERENCES
- Acuna BD, Eliassen JC, Donoghue JP, Sanes JN. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002;12:1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: Evidence for separable retrieval and integration mechanisms. Cerebral Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan J, McLeod P, Philips L, editors. Measuring the mind: Speed, control, and age. Oxford University Press; Oxford: 2006. pp. 215–246. [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Catell RB. Intelligence: Its structure, growth, and action. Elsevier; New York: 1987. [Google Scholar]
- Christoff K, Gabrieli J. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2002;28:168–186. [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behavioral Neuroscience. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Leijenhorst LV, Honomichl RD, Bunge SA. Neurocognitive development of relational reasoning. Developmental Science. 2009;12:55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pisapia N, Slomski JA, Braver TS. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cerebral Cortex. 2007;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Isolating rule- versus evidence-based prefrontal activity during episodic and lexical discrimination: A functional magnetic resonance imaging investigation of detection theory distinctions. Cerebral Cortex. 2006;16:1614–1622. doi: 10.1093/cercor/bhj098. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences, U.S.A. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus—what does it do? Behavioral and Neural Biology. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. European Journal of Neuroscience. 2005;21:1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith C, et al. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Functional neuroanatomy of three-term relational reasoning. Neuropsychologia. 2001;39:901–909. doi: 10.1016/s0028-3932(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN. Frontopolar cortex mediates abstract integration in analogy. Brain Research. 2006;1096:125–137. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. An fMRI analysis of the human hippocampus: Inference, context, and task awareness. Journal of Cognitive Neuroscience. 2006;18:1156–1173. doi: 10.1162/jocn.2006.18.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss A, et al. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Halford GS, Wilson WH, Phillips S. Processing capacity defined by relational complexity: Implications for comparative, developmental, and cognitive psychology. Behavioral and Brain Sciences. 1998;21:803–831. doi: 10.1017/s0140525x98001769. discussion 831–864. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. Journal of Neuroscience. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. Journal of Neuroscience. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Holyoak KJ, Thagard P. The analogical mind. American Psychologist. 1997;52:35–44. [PubMed] [Google Scholar]
- Hummel J, Holyoak K. A process model of human transitive inference. In: Gattis M, editor. Spatial schemas in abstract thought. MIT Press; Cambridge, MA: 2001. [Google Scholar]
- Hurford JR. The neural basis of predicate–argument structure. Behavioral and Brain Sciences. 2003;26:261–283. doi: 10.1017/s0140525x03000074. discussion 283-316. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cerebral Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lipton PA, Eichenbaum H. Binding and organization in the medial temporal lobe. In: Bunge SA, Wallis JD, editors. Neuroscience of rule-guided behavior. Oxford University Press; New York: 2007. pp. 337–363. [Google Scholar]
- Markman AB, Gentner D. Thinking. Annual Review of Psychology. 2001;52:223–247. doi: 10.1146/annurev.psych.52.1.223. [DOI] [PubMed] [Google Scholar]
- McDermott K, Jones T, Petersen S, Lageman S, Roediger H. Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: An event-related fMRI study. Journal of Cognitive Neuroscience. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Chang HS. Neuronal correlate of pictorial short-term memory in the primate temporal cortex. Nature. 1968;331:68–70. doi: 10.1038/331068a0. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagode JC, Pardo JV. Human hippocampal activation during transitive inference. NeuroReport. 2002;13:939–944. doi: 10.1097/00001756-200205240-00008. [DOI] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. Journal of Neuroscience. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: Explaining the discontinuity between human and nonhuman minds. Behavioral and Brain Sciences. 2008;31:109–130. doi: 10.1017/S0140525X08003543. discussion 130-178. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen A. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. Journal of Neuroscience. 2000;20:RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, McDermott KB, Braver TS. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cerebral Cortex. 2006;16:519–528. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Robin N, Holyoak KJ, Gazzaniga MS. The cognitive neurosciences. 1st ed. MIT Press; Cambridge, MA: 1995. Relational complexity and the functions of prefrontal cortex; pp. 987–999. [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RS, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Cohen NJ. Processing and short-term retention of relational information in amnesia. Neuropsychologia. 2004;42:497–511. doi: 10.1016/j.neuropsychologia.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Moses SN, Villate C. Impaired relational organization of propositions, but intact transitive inference, in aging: Implications for understanding underlying neural integrity. Neuropsychologia. 2009;47:338–353. doi: 10.1016/j.neuropsychologia.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. Journal of Neuroscience. 2006;26:1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. American Journal of Physical Anthropology. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Smith R, Keramatian K, Christoff K. Localizing the rostrolateral prefrontal cortex at the individual level. Neuroimage. 2007;36:1387–1396. doi: 10.1016/j.neuroimage.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. The neurophysiology of frontal pole cortex in rhesus monkeys. Paper presented at the Society for Neuroscience; Washington, DC. 2008. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van der Jeugd A, Goddyn H, Laeremans A, Arckens L, D’Hooge R, Verguts T. Hippocampal involvement in the acquisition of relational associations, but not in the expression of a transitive inference task in mice. Behavioral Neuroscience. 2009;123:109–114. doi: 10.1037/a0013990. [DOI] [PubMed] [Google Scholar]
- Van Elzakker M, O’Reilly RC, Rudy JW. Transitivity, flexibility, conjunctive representations, and the hippocampus: I. An empirical analysis. Hippocampus. 2003;13:334–340. doi: 10.1002/hipo.10083. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone KB, Mishkin FS, de Menenzes Santos M, et al. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10:119–125. [Google Scholar]
- Wendelken C, Bunge SA, Carter CS. Maintaining structured information: An investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia. 2008;46:665–678. doi: 10.1016/j.neuropsychologia.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain is to thought as stomach is to??” Investigating the role of rostrolateral prefrontal cortex in relational reasoning. Journal of Cognitive Neuroscience. 2007 doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Wright SB, Matlen BJ, Baym CL, Ferrer E, Bunge SA. Neural correlates of fluid reasoning in children and adults. Frontiers in Human Neuroscience. 2008;1:1–8. doi: 10.3389/neuro.09.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


