Abstract
The mammalian soluble epoxide hydrolase (sEH) is a multidomain enzyme composed of C- and N-terminal regions that contain active sites for epoxide hydrolase (EH) and phosphatase activities, respectively. We report the cloning of two 60 kDa multidomain enzymes from the purple sea urchin Strongylocentrotus purpuratus displaying significant sequence similarity to both the N- and C-terminal domains of the mammalian sEH. While one urchin enzyme did not exhibit EH activity, the second enzyme hydrolyzed several lipid messenger molecules metabolized by the mammalian sEH, including the epoxyeicosatrienoic acids. Neither of the urchin enzymes displayed phosphatase activity. The urchin EH was inhibited by small molecule inhibitors of the mammalian sEH and is the likely ancestor of the enzyme. Sequence comparisons suggest that the urchin sEH homologs are the result of a gene fusion event between a gene encoding for an EH and a gene for an enzyme of undetermined function. This fusion event was followed by a duplication event to produce the urchin enzymes.
Introduction
Soluble epoxide hydrolase (sEH) coverts epoxides to their corresponding vicinal diols through the addition of water (Gill and Hammock, 1980; Morisseau and Hammock, 2005). In mammals, sEH has been shown to play a role in the regulation of blood pressure, pain, and inflammation in numerous disease models (Imig et al., 2005; Schmelzer et al., 2005; Smith et al., 2005). This effect is at least in part due to sEH involvement in the hydrolysis of autocrine and paracrine lipid messenger molecules called epoxyeicosatrienoic acids (EETs) (Yu et al., 2000; Fang et al., 2001).
The mammalian sEH possesses two catalytic activities localized to distinct regions of the enzyme (Cronin et al., 2003; Newman et al., 2003). The epoxide hydrolase (EH) active site is located on the C-terminal region of sEH, while an active site on the N-terminal region has been found to display phosphatase activity using several lipid phosphate substrates, including polyisoprenyl phosphates (Tran et al., 2005). The two distinct catalytic activities have not been placed within a common metabolic pathway, as yet. Fatty acid diol phosphates are hydrolyzed to their corresponding fatty acid diols by the N-terminal phosphatase domain and fatty acid epoxides to the same diols by the C-terminal domain. This means both domains can yield the same product although from different substrates.
The N- and C-terminal regions of sEH are separated by a short linker and belong to two different gene superfamilies. The sEH N-terminal region is a member of the haloacid dehalogenase (HAD) superfamily, while the C-terminal region is a member of the haloalkane dehalogenase (HLD) super-family (Beetham et al., 1995). The spatial separation and differing homologies of the N- and C-terminal regions have led to the hypothesis that the full-length mammalian enzyme is the result of a gene fusion event between two ancestral genes (Beetham et al., 1995).
Previously, we reported two EHs in Caenorhabditis elegans with significant sequence similarity to sEH (Harris et al., 2008). These HLD superfamily enzymes displayed EH activity when assayed with common mammalian sEH substrates and were inhibited by small-molecule sEH inhibitors. When compared to the mammalian enzyme, they aligned with the C-terminal region. The genome of C. elegans also contains three genes that display significant sequence similarity to the N-terminal domain of sEH, all belonging to the HAD superfamily. There are no predicted enzymes corresponding to a full-length sEH, containing both a C- and N-terminal domain, in the genome of C. elegans. Genes encoding for full-length enzymes can be found in the genome of the amphibians Xenopus laevis (African clawed frog) and Xenopus tropicalis (Western clawed frog). This suggests that the fusion event occurred in the higher invertebrates or lower chordates.
Strongylocentrotus purpuratus (purple sea urchin) is an echinoderm widely used as a model organism. Its genome contains many of the enzymes and cytokines implicated in the mammalian inflammatory response, as well as the enzymes and precursors involved in the production of the EETs (Decker and Kinsey, 1983; Goldstone et al., 2006; Hibino et al., 2006; Rast et al., 2006). Phylogenetically, the organism is more closely related to the chordates than other commonly used invertebrate model organisms such as C. elegans and Drosophila melanogaster (Lee, 2003). Study of sEH homologs in this organism offers a number of advantages. The urchin genomic database will allow analysis of gene structure and in silico searches to identify other EHs. The wealth of knowledge concerning the biology of S. purpuratus will aid in the investigation of potential sEH homologs.
We report the identification of two sEH homologs in the genomic database of S. purpuratus. The mRNA transcripts have been experimentally verified, and found to encode for full-length enzymes, containing both a C- and N-terminal domain. Characterization of these urchin enzymes will provide information concerning the functional context of the hypothesized fusion event, and physiological role of sEH homologs in invertebrates.
Materials and Methods
Total RNA extraction
Gonads from freshly harvested specimens of S. purpuratus were dissected, and frozen at −80°C. Tissue (0.03 g) was placed in 0.75 mL TRIzol (Invitrogen, Carlsbad, CA) and homogenized using an Ultra-Turrax T8 roto-stator grinder (IKA Works, Wilmington, NC) rotating at 25,000 rpm for three 30 s bursts separated with 1 min rests on ice. Total RNA was then extracted according to the TRIzol manufacturer's suggestions.
Rapid amplification of cDNA ends
Soluble epoxide hydrolase–like protein 1 (SPEH1) 3′ RACE experiments were performed on the total RNA sample with the 3′ RACE System for Rapid Amplification of cDNA Ends kit (Invitrogen) using the nested primers 3SPEH11: 5′–CGGTC ACGACTGGGGTGGTT–3′, 3SPEH12:5′–CGCCAGAAGCCGAGATCGAA–3′, and 3SPEH13: 5′–CCCCTTTCTTTCCTGCTAATGA–3′. The remaining RACE experiments were performed on the total RNA sample with the SMART RACE cDNA Amplification kit (Clontech, Palo Alto, CA). Soluble epoxide hydrolase–like protein 2 (SPEH2) 3′RACE experiments were performed using the nested primers 3SPEH21: 5′–CCAAAGGATGTTCCAGACGTCAG–3′,3SPEH22: 5′–CAATCAGTTCCCCTGCCTTAAGGGG–3′, followed by a second PCR with 3SPEH21 and 3SPEH23: 5′–CCCATTTTTGATAATGTATTG GCAGTTTGTCC–3′. SPEH1 5′ RACE experiments were performed using the nested primers 5SPEH11: 5′–TCGATCTCGGCTTCTGGCGGTCCCACTT–3′ and 5SPEH12: 5′–AGCATA GGAATGAGGGACCCTCTCATGA–3′.SPEH25′ RACE experiments were performed using the nested primers 5SPEH21: 5′–GTATCGGGTATGACGACAGAGGGCGATG–3′ and 5SP EH22: 5′–GACGTAACTGTGTGTGACTTCATCCGGG–3′.
Cloning
Primers for SPEH1 were designed to add BglII endonuclease sites on both ends, and a six histidine tag on the 3′ end of the coding sequence. Primers for SPEH2 were designed to add XhoI and NotI endonuclease sites to the 5′ and 3′ ends, respectively, and a six histidine tag on the 3′ end of the coding sequence. The primer pair for SPEH1 was 5′–AGATCTATGGCCCAAAATATGAAGAAGAAAGCTGTG–3′ and 5′–AGATCTCTAGTGATGGTGATGGTGATGCAGACTGGAA GGGAAGATTGGTC–3′. The primer pair for SPEH2 was 5′–CTCGAGATGATAGACAAGAAAGTTGTGCTGTTC–3′ and 5′–GCGGCCGCTCAGTGATGGTGATGGTGATGCATCGG CATAAGAGGTGTATG–3′. The PCR was performed with KOD polymerase (EMD Chemicals, San Diego, CA) on first-strand cDNA from the RACE experiments with the following thermocycler settings: 95°C for 3 min; 35 cycles of 95°C for 30 s; 60°Cfor 1 min;72°Cfor 2 min;72°Cfor 10 min. The PCR products were gel purified and inserted into the cloning vector pCR-Blunt II-TOPO (Invitrogen), and then excised and ligated into the baculovirus transfer vector pACUW21 or pBacPAK8 (BD Biosciences, San Jose, CA). Proper orientation and nucleotide sequence were verified.
Baculovirus expression
Recombinant baculoviruses harboring the SPEH1 or SPEH2 cDNA sequence were generated by cotransfection of Spodoptera frugiperda–derived Sf21 cells with the recombinant transfer vector plasmid and Bsu36I-cleaved BacPAK6 viral DNA (Clontech) as previously described (Merrington et al., 1999). For expression, a 100 mL culture of High Five cells derived from Trichoplusia ni were infected at 0.1 MOI and incubated for 1h at 28°C, and then 400 mL of ESF921 media (Expression Systems, Woodland, CA) supplemented with 1 × penicillin–streptomycin solution (Sigma-Aldrich, St. Louis, MO) was added to the infected cells and the culture was incubated for 72 h at 28°C.
Protein purification
A 100 mL culture of infected High Five cells expressing SPEH1 or SPEH2 was homogenized with an Ultra-Turrax homogenizer. Talon metal affinity resin (Clontech) was used for purification according to manufacturer's directions. The eluent was concentrated in a 30 kDa cut Centricon centrifugal filter unit (Millipore, Billerica, MA), desalted with a 5mL desalting column (Amersham, Piscataway, NJ), and stored at −80°C for future use.
Protein analysis
SDS-PAGE was performed using precast NUPAGE gels and SeeBlue Plus 2 protein standards (Invitrogen). Isoelectric focusing was performed using precast Novex pH 3–10 gels and SERVA IEF 3–10 pH markers (Invitrogen). Protein concentrations were determined using the BCA reagent (Pierce, Rockford, IL) according to manufacturer's directions. Protein purity was estimated from an SDS-PAGE gel stained with Coomassie Brilliant Blue with the public domain ImageJ software v1.33 (http://rsb.info.nih.gov/ij/).
Radiometric assays
Assays with tritium-labeled t-DPPO were performed as previously described, with the following exceptions (Borhan et al., 1995). An enzyme concentration of 0.3 μg/mL was used, and the assays were terminated at 10 min.
Fluorescent assays
Assays with (3-phenyl-oxiranyl)-acetic acid cyano-(6-methoxy-naphthalen-2-yl)-methyl ester (PHOME) were performed and analyzed as previously described (Jones et al., 2005), with the following exceptions: an enzyme concentration of 0.9 μg/mL was used, and the assay was terminated at 10 min. For the IC50 assays, the enzyme was incubated with the inhibitors for 5 min at 30°C prior to substrate introduction. By definition, IC50 is the concentration of inhibitor that reduces enzyme activity by 50%.
Nonfluorescent and nonradiometric assays
Assays with the EETs and EpOMEs were performed and analyzed by LC-MS/MS, as described previously (Harris et al., 2008), with the following exceptions: an enzyme concentration of 3.4 μg/mL was used with a 10 min incubation.
Results
A database of EST sequences from S. purpuratus (Spur20050718-glean3_nucleotide at Baylor College of Medicine, http://www.hgsc.bcm.tmc.edu) was searched with TBLASTX using cDNA sequences of sEH homologs from Gallus gallus and C. elegans. ESTs containing sequences corresponding to predicted exons in gene loci LOC590376 and LOC579596 in the NCBI genome database from S. purpuratus (http://www.ncbi.nlm.nih.gov/genome/guide/sea_urchin) were retrieved and used to design primers for 3′ and 5′ RACE experiments. cDNA was prepared from sea urchin gonads, and the 5′ and 3′ UTRs of two sEH homologs were experimentally determined. Primers to clone the cDNA were designed based on these sequences.
The full-length transcripts corresponded to two potential sEH homologs (Figs. 1 and 2). Both contained the C-terminal EH and N-terminal phosphatase domains, and were called SPEH1 and SPEH2.
FIG. 1.
Nucleotide sequence of the SPEH1 cDNA with translation. GenBank accession no. EU642645.
FIG. 2.
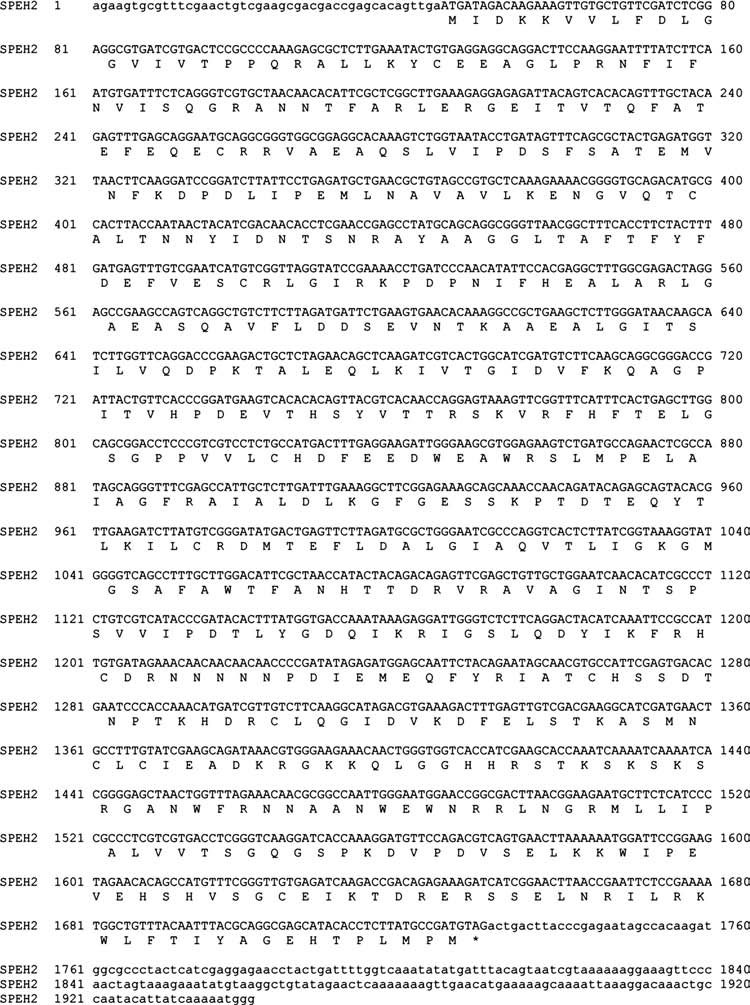
Nucleotide sequence of the SPEH2 cDNA with translation. GenBank accession no. EU642646.
Recombinant enzymes with six histidine tags were produced in a baculovirus expression system and purified on cobalt chelation resin. Eluted recombinant SPEH1 and SPEH2 were estimated to be at most 90% and 80% pure, respectively, after analysis of a Coomassie-stained SDS-PAGE gel using the NIH software ImageJ (http://rsb.info.nih.gov/ij/). The molecular weights of the recombinant SPEH1 and SPEH2 were 63.8 and 64.2 kDa, respectively, very close to the predicted molecular weights of 63.6 and 64.4kDa.
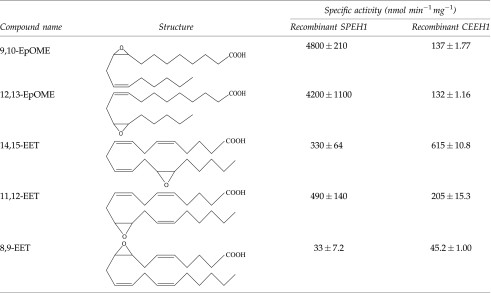
t-DPPO is a commonly used tritiated surrogate substrate for the mammalian sEH. When assayed with t-DPPO, SPEH1 possessed approximately the same activity as the nematode enzyme, while SPEH2 was not active under assay conditions (Table 1).SPEH1 possessed a Km of 35 μM and a kcat of 44 s−1 with t-DPPO. The SPEH1 EH activity displayed a half-life of approximately 2 h at 37°C, between 1 and 2 days at room temperature, and over a week at 4°C. The optimal pH for SPEH1 EH activity was 7.4. SPEH1 and SPEH2 did not display phosphatase activity when assayed with AttoPhos (Promega, Madison, WI), a substrate used to assay human sEH N-terminal phosphatase activity (Tran et al., 2005). SPEH1 also hydrolyzed proposed endogenous substrates of the mammalian sEH (Table 2).These included the EET regioisomers, as well as 9,10-epoxy-12-octadecenoate (called leukotoxin, coronaric acid, or 9,10-EpOME) and 12,13-epoxy-9-octadecenoate (called isoleukotoxin, vernolic acid, or 12,13-EpOME).
Table 1.
Kinetic Parameters with t-DPPO as Substrate
Recombinant SPEH1 was partially purified as described. Assay conditions are described in the Materials and Methods section. For SPEH1, results are presented as the mean ± standard deviation of two or three separate experiments performed in triplicate. Specific activity adjusted for estimated purity. Values for the human enzyme are from Morisseau et al. (2000). Values for the nematode enzyme are from Harris et al. (2008).
Table 2.
Specific Activity with Natural Substrates
Recombinant SPEH1 was partially purified as described. Assay conditions are described in the Materials and Methods section. For SPEH1, results are presented as the mean ± standard deviation of an experiment performed in triplicate. Values for CEEH1 are from Harris et al. (2008). Specific activity adjusted for estimated purity.
Next, sea urchin extract was assayed for sEH-like EH activity. Specimens of S. purpuratus were obtained and an extract prepared from dissected gonads. t-DPPO was used to assay EH activity. t-DPPO can be turned over by glutathione S-transferase, but the assay can be corrected for GST activity by measuring the amount of glutathione adduct. No GST activity on t-DPPO was detected in these samples. Of the activity detected in the crude extract, 85% was contained in the 10,000 g supernatant, while 15% was contained in the pellet.
To determine if the EH activity detected in the extract was due to SPEH1, an IEF gel (pH 3–10) was run with the supernatant and recombinant SPEH1 in separate lanes. The gel was then cut into 0.3 cm bands. In the recombinant lane, 100% of the recovered activity was located in the same bands for both the supernatant and recombinant lanes. The peak of activity for the recombinant lane occurred in the band corresponding to 5.9–6.1, while the peak for the supernatant fell in the 5.7–5.9 range. The difference in the pI values is probably due to the six histidine tag on the recombinant enzyme, which should shift the enzyme pI from the predicted value of 5.84 to 6.08. SPEH1 does not contain a known peroxisomal or microsomal targeting sequence, so it is possible that soluble SPEH1 was trapped in the cell debris during centrifugation.
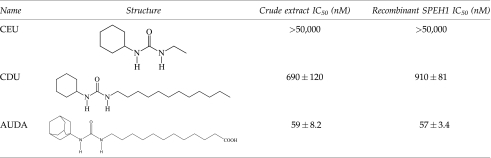
Three inhibitors with low, medium, and high potency with the recombinant enzyme were assayed with crude extract prepared from urchin gonads (Table 3).The crude extract displayed the same pattern of inhibition as the recombinant enzyme, providing additional evidence that the majority of the activity detected is due to SPEH1.
Table 3.
IC50s with Urea-Based Inhibitors and t-DPPO as Substrate
IC50 values for the urea-based inhibitors N-cyclohexyl-N′-ethylurea (CEU), N-cyclohexyl-N′-dodecylurea (CDU), and 12-(3-adamantane-1-yl-ureido)-dodecanoic acid (AUDA). Recombinant CEEH1 was partially purified as described. Assay was performed using [3H] t-DPPO as substrate. Conditions are described in the Materials and Methods section. Error bars represent the standard deviation of two separate experiments performed in triplicate.
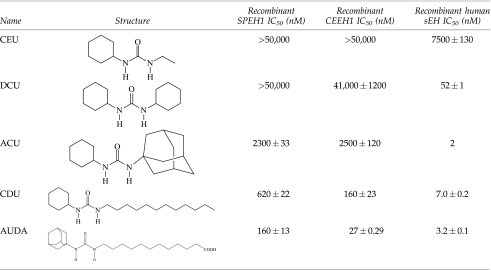
To further characterize the urchin EH activity, inhibitors of mammalian EH activity were assayed with the SPEH1 using the substrate PHOME to compare with previous results obtained with human and nematode sEH homologs (Table 4).When the epoxide moiety in this substrate is hydrolyzed by sEH, the molecules cyclize and free a cyanohydrin leaving group that decomposes into a fluorescent aldehyde. SPEH1 displayed the same pattern of inhibition as the nematode sEH homolog CEEH1.
Table 4.
IC50s with Urea-Based Inhibitors and PHOME as Substrate
IC50 values for the urea-based inhibitors N-cyclohexyl-N′-ethylurea (CEU), N,N′-dicyclohexylurea (DCU), N-cyclohexyl-N′-dodecylurea (CDU), N-adamantyl-N′-cyclohexylurea (ACU), and 12-(3-adamantane-1-yl-ureido)-dodecanoic acid (AUDA). Recombinant SPEH1 was partially purified as described. Values for the human enzyme are from Jones et al. (2005). Values for CEEH1 are from Harris et al. (2008). All values were determined with the fluorescent substrate PHOME. Assay conditions are described in the Materials and Methods section. For SPEH1, the error represents the standard deviation of two separate experiments performed in triplicate.
Discussion
When the SPEH1 and SPEH2 sequences were translated and aligned with vertebrate sEH homologs, a number of interesting features were identified (Fig. 3). First, the C-terminal domains had important differences when residues implicated in sEH EH activity were compared. The catalytic triad of α/β-hydrolases consists of a catalytic aspartate that performs a nucleophilic attack and forms a covalent intermediate with the substrate, and an aspartate-histidine proton shuttle, which activates a molecule of water (marked with circles in Fig. 3) (Pinot et al., 1995; Arand et al., 1996). These residues aligned in SPEH1, which maintained the approximate spacing of the vertebrate enzymes, but SPEH2 contained a mutation in each position, from the aspartates to glycines and from the histidine to isoleucine (Fig. 3). These changes made it unlikely that SPEH2 would hydrolyze epoxides. The N-terminal domain of SPEH1 and SPEH2 both lacked residues thought to be important for sEH phosphatase activity. Aspartate 11 is believed to be involved in the coordination of the Mg2+ atom in the active site while participating in a hydrogen bond with arginine 99 (Gomez et al., 2004). Both SPEH1 and SPEH2 lacked the aspartate and the arginine (marked with triangles in Fig. 3). These same residues are missing in the frog sEH homolog, as well as the chicken homolog, which has been shown to lack phosphatase activity (Harris et al., 2006). Biochemical characterization of expressed recombinant enzymes is discussed below.
FIG. 3.

Alignment of vertebrate sEH homologs with the urchin enzymes. The mammalian epoxide hydrolase catalytic triad residues are marked with circles. The residues involved in the coordination of a magnesium atom important for human sEH phosphatase activity are marked with triangles. Homo sapiens = GenBank accession no. L05779; Gallus gallus = DQ120010; Xenopus tropicalis = BC075370.
sEH is likely to be the result of a gene fusion event between two ancestral enzymes related to HAD and HLD (Beetham et al., 1993). However, in the urchin there are two enzymes that share significant sequence identity with sEH, suggesting a gene duplication event before or after the gene fusion event. To better understand the evolutionary history of sEH, the urchin sequences were compared to earlier results obtained in a search of the genomic database of C. elegans (Harris et al., 2008).
Previously, the genome of C. elegans was searched with vertebrate sEH sequences, resulting in hits that aligned with either the N- or C-terminal domains, but no hits that aligned with both (Harris et al., 2008). Given the existence of multiple nematode enzymes that aligned with the separate domains of the vertebrate sEH, the two sEH homologs in urchin could have been produced by two general schemes. The gene fusion could have occurred between one C-terminal and one N-terminal sequence, followed by a duplication event (Scheme 1 in Fig. 4). Alternatively, the fusion could have occurred between different N- and C-terminal sequences, with no duplication event (Scheme 2 in Fig. 4). To determine the most likely of these possibilities, we examined local and global alignments of translated nucleotide sequences.
FIG. 4.
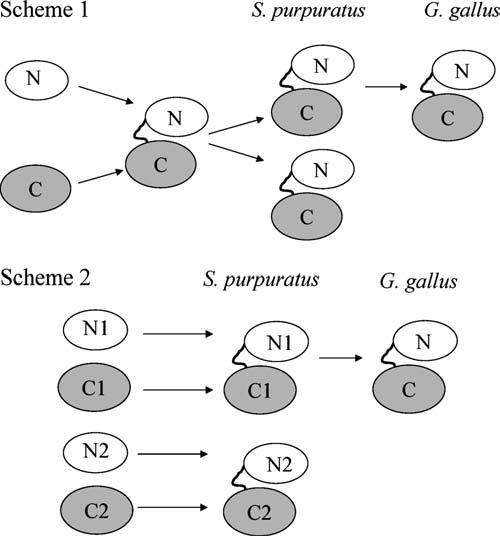
Two possible models to explain the existence of two multidomain urchin homologs of the mammalian sEH. A duplication event could be involved (Scheme 1), or the two enzymes could have been created through the fusion of some combination of different N-terminal and C-terminal domains (Scheme 2). These do not exhaust the logical possibilities, but represent the simplest models.
When aligned, the N-terminal regions of SPEH1 and SPEH2 shared a high identity, making it unlikely that they have different progenitors (Table 5).A TBLASTX search of the NCBI genome database for C. elegans using translated urchin sequences resulted in three hits for the N-terminal region of the urchin enzymes (Table 5).Supporting the existence of a common progenitor, the two N-terminal regions of SPEH1 and SPEH2 displayed higher identity with each other than with any of the nematode enzymes when aligned by CLUS-TALW (Table 5).
Table 5.
Pairwise Alignment Scores
| Sequence 1 | Sequence 2 | Score | Segment length | % identity |
|---|---|---|---|---|
| N-terminal sequences | ||||
| N-term SPEH1 | NM_072133 | 201 | 242 | 28.5 |
| N-term SPEH1 | NM_063993 | 246 | 251 | 29.9 |
| N-term SPEH1 | NM_072107 | 270.5 | 218 | 31.2 |
| N-term SPEH2 | NM_072133 | 195 | 238 | 26.5 |
| N-term SPEH2 | NM_063993 | 192.5 | 238 | 29.8 |
| N-term SPEH2 | NM_072107 | 214.5 | 222 | 30.2 |
| N-term SPEH2 | N-term SPEH1 | 619 | 228 | 52.6 |
| C-terminal sequences | ||||
| C-term SPEH1 | CEEH1 | 305 | 316 | 26.6 |
| C-term SPEH1 | CEEH2 | 307.5 | 329 | 27.1 |
| C-term SPEH2 | CEEH1 | 177 | 332 | 22.3 |
| C-term SPEH2 | CEEH2 | 151 | 253 | 21.3 |
| C-term SPEH2 | C-term SPEH1 | 476.5 | 350 | 32.6 |
| Full-length sequences | ||||
| SPEH1 | hsEH | 1210.5 | 557 | 43.6 |
| SPEH2 | hsEH | 823.5 | 563 | 35.2 |
Alignment of N- and C-terminal domains of the urchin enzyme with enzymes identified in a TBLASTX search of the genome of C. elegans. Values were calculated by EMBOSS-Align pairwise alignment program using the Smith–Waterman algorithm with the EBLOSUM62 matrix set for a gap penalty of 10 and a gap extension penalty of 0.5. N-term SPEH1 and SPEH2 refer to amino acids 1–237 of these enzymes. C-term SPEH1 and SPEH2 refer to amino acids 238 to the C-terminal ends. The nematode enzymes corresponding to GenBank accession nos. NM_072133, NM_063993, and NM_072107 align with the N-terminal domain of the urchin. CEEH1 and CEEH2 refer to GenBank accession nos. EU151493 and EU151492. These enzymes align with the C-terminal domain. hsEH refers to GenBank accession no. L05779.
The urchin C-terminal domains were only 32% identical when aligned (Table 5).This raised the possibility that the C-terminal regions of SPEH1 and SPEH2 were the result of two different domains fusing with a single promiscuous N-terminal domain. A TBLASTX search of the genomic database for C. elegans using translated urchin sequences resulted in two hits for the C-terminal regions of SPEH1 and SPEH2, the previously characterized CEEH1 and CEEH2 (Harris et al., 2008). When local pairwise alignments using the Smith-Waterman algorithm were performed, CEEH1 scored higher with SPEH2 than CEEH2 scored (Table 5).However, the nematode enzymes scored roughly the same when aligned with SPEH1 (Table 5).Of the two nematode enzymes, CEEH1 displayed the most sEH-like EH activity, having a higher activity on t-DPPO than CEEH2, and hydrolyzing proposed natural substrates of the mammalian sEH at a higher rate as well. To determine if one or both of the enzymes were ancestors of the urchin C-terminal domains, local alignments were examined more closely.
Regions of high identity in vertebrate sEH homologs were aligned in the invertebrate enzymes. These regions consisted of all C-terminal sequences of 10 or more amino acids that displayed 90% identity in human, frog, and chicken sEH after a multiple sequence alignment by CLUSTALW. The corresponding sequences were located in SPEH1 and SPEH2 after a multiple sequence alignment with the vertebrate enzymes, and then a pairwise alignment was performed with the urchin and nematode enzymes. Compared to CEEH1, CEEH2 displayed higher identity with the urchin C-terminal domains in only one of the five C-terminal regions in each urchin enzyme (Table 6).
Table 6.
Pairwise Alignment of Short Segments
| SPEH1 | SPEH2 | Aligned sequences | |
|---|---|---|---|
| CEEH1 | 47.6 | 33.3 | SPEH1: IFCHGFPESWYEWKSQIPAVA |
| SPEH2: VLCHDFEEDWEAWRSLMPELA | |||
| CEEH2 | 42.9 | 28.6 | CEEH1: LFIHGYPEFWYSWRFQLKEFA |
| CEEH2: LMVHGFPEFWYSWRFQLEHFK | |||
| CEEH1 | 38.9 | 33.3 | SPEH1: YATHYPDRVSAVGGICT |
| SPEH2: FANHTTDRVRAVAGINTS | |||
| CEEH2 | 16.7 | 27.8 | CEEH1: FAEQYPEMVDKLICCNIP |
| CEEH2: VAMLHSNLIDRLVICNVP | |||
| CEEH1 | 50 | 31.2 | SPEH1: YQLYFNEVGPPEAEIE |
| SPEH2: YIKFRHCDRNNNNNPD | |||
| CEEH2 | 25 | 37.5 | CEEH1: YMFFYQNEKIPEMLCS |
| CEEH2: YIYLFQSQYIPEIAMR | |||
| CEEH1 | 36.4 | 42.9 | SPEH1: GLRSM—LNWYRT |
| SPEH2: SKSRG—ANWFRN | |||
| CEEH2 | 45.4 | – | CEEH1: GASFKYPINYYRN |
| CEEH2: GGTTG-PLNYYRD | |||
| CEEH1 | 45.4 | 36.4 | SPEH1: KLYMPALMVTC |
| SPEH2: MLLIPALVVTS | |||
| CEEH2 | 27.3 | – | CEEH1: LEMP-TLIIWG |
| CEEH2: IVQPKVLILWG |
Percent identity of short segments after pairwise alignment of the nematode and urchin enzymes. The corresponding regions in vertebrate sEH homologs display a greater than 90% identity over 10 amino acids after alignment by CLUSTALW. Percent identity was calculated after pairwise alignments using EMBOSS Water algorithm with the EBLOSUM62 matrix set for a gap penalty of 10 and a gap extension penalty of 0.5. CEEH1 and CEEH2 refer to GenBank accession nos. EU151493 and EU151492, respectively. SPEH1 and SPEH2 refer to GenBank accession nos. EU642645 and EU642646, respectively.
These results support the hypothesis that the ancestor of both urchin sEHs was the product of a single fusion event between a CEEH1-like gene and a gene for the N-terminal domain. This fusion event was followed by at least one duplication event to produce the urchin enzymes (Scheme 1 in Fig. 4). SPEH1 is the likely ancestor of mammalian sEH when judged by local pairwise alignment as well as alignment of key catalytic residues mentioned above (Table 5).Whether or not these urchin EHs preserved the sEH-like EH activity observed with CEEH1 was next determined.
When kinetic parameters were determined with t-DPPO, it was found that SPEH1 displayed a higher specificity than either the human or nematode enzyme, having a kcat/Km ratio of 1.3, compared to 0.07 and 0.7 for nematode and human, respectively. SPEH2 did not display activity when assayed with t-DPPO, making it unlikely that the enzyme possessed sEH-like EH activity.
The mammalian sEH hydrolyzes lipid messenger molecules. Thus, SPEH1 was assayed with five of these proposed natural substrates. The EETs are cytochrome p450 metabolites of arachidonic acid that play a role in physiological processes such as the regulation of hypertension, pain, and inflammation. The mammalian enzyme hydrolyzes the epoxide moiety of the EETs to create 1,2 diols. The diols (or DHETs) are more polar, are more easily conjugated, and resist incorporation into membranes (Weintraub et al., 1999). In most assays evaluated to date, DHETs have dramatically reduced biological activities compared to EETs (Spector and Norris, 2007). SPEH1 hydrolyzed 11,12-EET at the highest rate, unlike the nematode enzyme, which hydrolyzed 14,15-EET at the highest rate (Table 2).It hydrolyzed 8,9-EET at the lowest rate of the EETs analyzed. SPEH1 also hydrolyzed the EpOMEs, p450 metabolites of linoleic acid that, when injected, cause lung inflammation and are correlated with the onset of acute respiratory distress syndrome (Ozawa et al., 1991; Zheng et al., 2001). When hydrolyzed by sEH, the EpOMEs are converted into a diol species (Moghaddam et al., 1997). It is possible that both the EpOMEs and their diols are natural endogenous chemical mediators. Their diols, for example, can greatly increase vascular permeability (Zheng et al., 2001). However, at high levels they can be very toxic (Zheng et al., 2001). Like the nematode enzyme, SPEH1 did not show a preference between the two regioisomers. However, it hydrolyzed these substrates at over 10 times the rate of the nematode sEH (Table 2).
Small molecules with urea-based structures are potent transition-state inhibitors of the mammalian sEH activity. Five such urea-based inhibitors with a range of potencies on the mammalian enzyme were selected. These were unsymmetrical 1,3 disubstituted ureas, except for N,N′-dicyclohexylurea. The 3 substituted group will be referred to here as the right side. Like the nematode and human enzymes, inhibitors with long fatty acid or hydrocarbon right sides displayed nanomolar potency, while a shorter alkyl group greatly reduced potency (Table 4).With both the urchin and nematode enzyme, a bulky ring structure on the right side also reduced potency, perhaps indicating a steric constraint in the invertebrate active site (Table 4).As with the nematode and human enzymes, AUDA was the most potent inhibitor, having an IC50 of 160 nM under these assay conditions. The nanomolar potency makes it a good first choice for in vivo inhibition of the enzyme in future studies.
SPEH1 hydrolyzed epoxide-containing lipid messenger molecules in vitro, as well as the surrogate substrate t-DPPO. These results indicate that an EH activity comparable to the EH activity of the mammalian sEH is present in this invertebrate homolog of sEH. The enzyme contains both the C-terminal and N-terminal domains present in the vertebrate enzymes; however, the enzyme did not display phosphatase activity under assay conditions.
Because a sEH-like EH activity was displayed by both CEEH1 and SPEH1, the hydrolysis of lipid messenger molecules such as the EETs or EpOMEs may have functional roles in these systems. However, because the function of the N-terminal domain in invertebrates is unknown, the selective benefit of the gene fusion cannot be determined.
It is unlikely that the N-terminal domain performs a subcellular targeting role because no signal sequences can be identified in the N-terminal domain of either SPEH1 or SPEH2 using the PSORT family of subcellular localization prediction programs (http://www.psort.org/). Another possible function of the N-terminal domain is stabilization of the EH activity. When expressed independently, the C-terminal domain of human sEH has reduced activity (Tran et al., 2005). However, the fusion of an approximately 26 kDa N-terminal domain is a seemingly inefficient manner to stabilize the C-terminal domain.
The presence of the N-terminal domain might also promote dimerization of the enzyme. This has an interesting consequence in the urchin. SPEH2 might affect SPEH1 EH activity, even though it does not possess EH activity itself. The mammalian sEH forms a dimer in solution. A dimer between SPEH1, which contains a functioning EH catalytic site, and SPEH2, which does not, might alter sEH-like EH activity in tissues where both enzymes are expressed. Determination of the function of the urchin N-terminal domain, as well as the selective benefit of the gene fusion, awaits further studies.
Acknowledgments
We would like to thank Christophe Morisseau, George Kamita, and Hiromasa Tanaka for advice at various stages of this project. T.R.H. and P.A.A were supported by NIEHS Advanced Training in Environmental Toxicology Grant T32 ES007059. Funding was also provided by NIEHS Grant R37 ES02710 and NIEHS Superfund Basic Research Program Grant P42 ES004699.
References
- Arand M. Wagner H. Oesch F. Asp333, Asp495, and His523 form the catalytic triad of rat soluble epoxide hydrolase. J Biol Chem. 1996;271:4223–229. doi: 10.1074/jbc.271.8.4223. [DOI] [PubMed] [Google Scholar]
- Beetham J.K. Grant D. Arand M. Garbarino J. Kiyosue T. Pinot F. Oesch F. Belknap W.R. Shinozaki K. Hammock B.D. Gene evolution of epoxide hydrolases and recommended nomenclature. DNA Cell Biol. 1995;14:61–71. doi: 10.1089/dna.1995.14.61. [DOI] [PubMed] [Google Scholar]
- Beetham J.K. Tian T. Hammock B.D. cDNA cloning and expression of a soluble epoxide hydrolase from human liver. Arch Biochem Biophys. 1993;305:197–201. doi: 10.1006/abbi.1993.1411. [DOI] [PubMed] [Google Scholar]
- Borhan B. Mebrahtu T. Nazarian S. Kurth M.J. Hammock B.D. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem. 1995;231:188–200. doi: 10.1006/abio.1995.1520. [DOI] [PubMed] [Google Scholar]
- Cronin A. Mowbray S. Durk H. Homburg S. Fleming I. Fisslthaler B. Oesch F. Arand M. The N-terminal domain of mammalian soluble epoxide hydrolase is a phosphatase. Proc Natl Acad Sci USA. 2003;100:1552–1557. doi: 10.1073/pnas.0437829100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S.J. Kinsey W.H. Characterization of cortical secretory vesicles from the sea urchin egg. Dev Biol. 1983;96:37–45. doi: 10.1016/0012-1606(83)90309-3. [DOI] [PubMed] [Google Scholar]
- Fang X. Kaduce T.L. Weintraub N.L. Harmon S. Teesch L.M. Morisseau C. Thompson D.A. Hammock B.D. Spector A.A. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- Gill S.S. Hammock B.D. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem Pharmacol. 1980;29:389–395. doi: 10.1016/0006-2952(80)90518-3. [DOI] [PubMed] [Google Scholar]
- Goldstone J.V. Hamdoun A. Cole B.J. Howard-Ashby M. Nebert D.W. Scally M. Dean M. Epel D. Hahn M.E. Stegeman J.J. The chemical defensome: environmental sensing and response genes in the Strongylocentrotus purpuratus genome. Dev Biol. 2006;300:366–384. doi: 10.1016/j.ydbio.2006.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G.A. Morisseau C. Hammock B.D. Christianson D.W. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004;43:4716–4723. doi: 10.1021/bi036189j. [DOI] [PubMed] [Google Scholar]
- Harris T.R. Aronov P.A. Jones P.D. Tanaka H. Arand M. Hammock B.D. Identification of two epoxide hydrolases in Caenorhabditis elegans that metabolize mammalian lipid signaling molecules. Arch Biochem Biophys. 2008;472:139–149. doi: 10.1016/j.abb.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.R. Morisseau C. Walzem R.L. Ma S.J. Hammock B.D. The cloning and characterization of a soluble epoxide hydrolase in chicken. Poult Sci. 2006;85:278–287. doi: 10.1093/ps/85.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino T. Loza-Coll M. Messier C. Majeske A.J. Cohen A.H. Terwilliger D.P. Buckley K.M. Brockton V. Nair S.V. Berney K. Fugmann S.D. Anderson M.K. Pancer Z. Cameron R.A. Smith L.C. Rast J.P. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300:349–365. doi: 10.1016/j.ydbio.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Imig J.D. Zhao X. Zaharis C.Z. Olearczyk J.J. Pollock D.M. Newman J.W. Kim I.-H. Watanabe T. Hammock B.D. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.D. Wolf N.M. Morisseau C. Whetstone P. Hock B. Hammock B.D. Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Anal Biochem. 2005;343:66–75. doi: 10.1016/j.ab.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H. Molecular phylogenies and divergence times of sea urchin species of Strongylocentrotidae, Echinoida. Mol Biol Evol. 2003;20:1211–1221. doi: 10.1093/molbev/msg125. [DOI] [PubMed] [Google Scholar]
- Merrington C.L. King L.A. Posse R.D. Baculovirus expression systems. In: Higgins S.J., editor; Hames B.D., editor. Protien Expression: A Practical Approach. Oxford University Press; Oxford, England: 1999. pp. 101–127. [Google Scholar]
- Moghaddam M.F. Grant D.F. Cheek J.M. Greene J.F. Williamson K.C. Hammock B.D. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C. Hammock B.D. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- Newman J.W. Morisseau C. Harris T.R. Hammock B.D. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA. 2003;100:1558–1563. doi: 10.1073/pnas.0437724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T. Hayakawa M. Kosaka K. Sugiyama S. Ogawa T. Yokoo K. Aoyama H. Izawa Y. Leukotoxin, 9,10-epoxy-12-octadecenoate, as a burn toxin causing adult respiratory distress syndrome. Adv Prostaglandin Thromboxane Leukot Res. 1991;21B:569–572. [PubMed] [Google Scholar]
- Pinot F. Grant D.F. Beetham J.K. Parker A.G. Borhan B. Landt S. Jones A.D. Hammock B.D. Molecular and biochemical evidence for the involvement of the Asp-333-His-523 pair in the catalytic mechanism of soluble epoxide hydrolase. J Biol Chem. 1995;270:7968–7974. doi: 10.1074/jbc.270.14.7968. [DOI] [PubMed] [Google Scholar]
- Rast J.P. Smith L.C. Loza-Coll M. Hibino T. Litman G.W. Genomic insights into the immune system of the sea urchin. Science. 2006;314:952–956. doi: 10.1126/science.1134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer K.R. Kubala L. Newman J.W. Kim I.-H. Eiserich J.P. Hammock B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.R. Pinkerton K.E. Watanabe T. Pedersen T.L. Ma S.J. Hammock B.D. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A.A. Norris A.W. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- Tran K.L. Aronov P.A. Tanaka H. Newman J.W. Hammock B.D. Morisseau C. Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian soluble epoxide hydrolase. Biochemistry. 2005;44:12179–12187. doi: 10.1021/bi050842g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub N.L. Fang X. Kaduce T.L. VanRollins M. Chatterjee P. Spector A.A. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. Am J Physiol. 1999;277:H2098–H2108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- Yu Z. Xu F. Huse L.M. Morisseau C. Draper A.J. Newman J.W. Parker C. Graham L. Engler M.M. Hammock B.D. Zeldin D.C. Kroetz D.L. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- Zheng J. Plopper C.G. Lakritz J. Storms D.H. Hammock B.D. Leukotoxindiol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2001;25:434–438. doi: 10.1165/ajrcmb.25.4.4104. [DOI] [PubMed] [Google Scholar]